Abstract
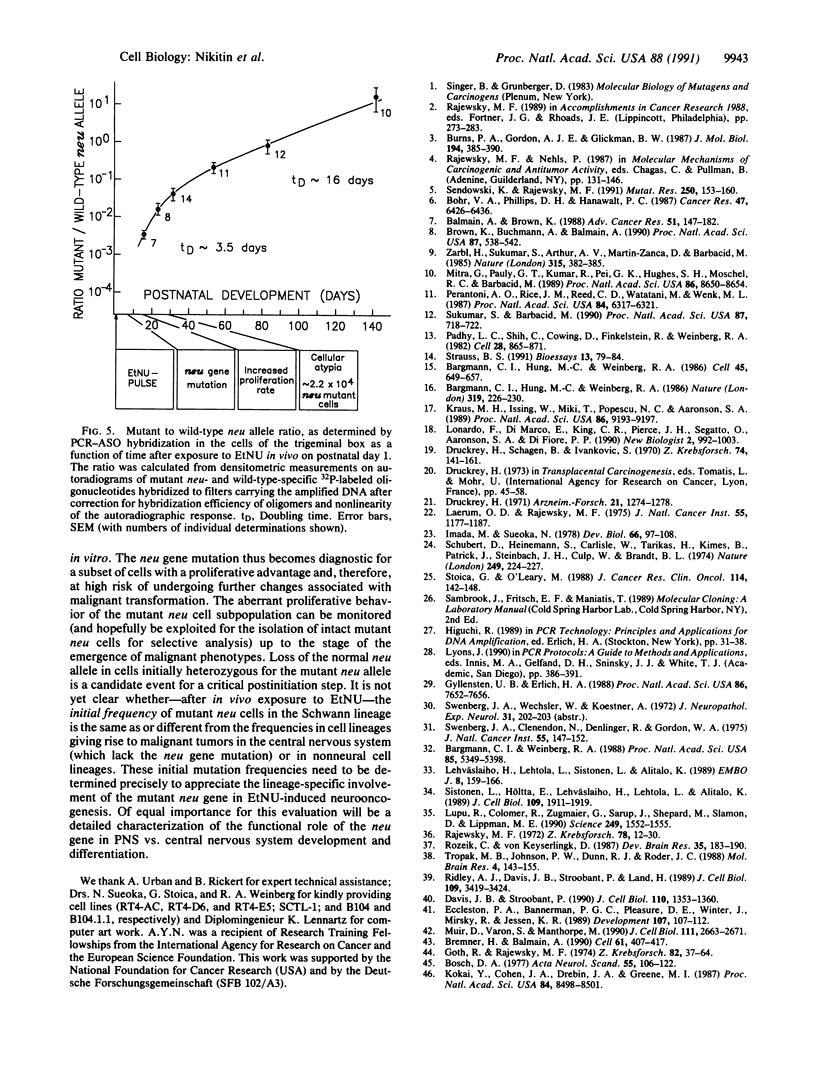
The development of malignant tumors of the peripheral nervous system (schwannomas) within a defined intracranial section of the rat trigeminal nerve ("trigeminal box") was used as a model to identify genetic alterations typically associated with the process of cell-lineage-specific oncogenesis induced by exposure to N-ethyl-N-nitrosourea on postnatal day 1. All 47 trigeminal schwannomas (and 12 extracranial neurinomas) investigated carried a T.A----A.T transversion mutation at nucleotide 2012 of the neu (erbB-2) gene sequence encoding the transmembrane domain of pg185neu. This mutation was absent in all 18 tumors in the brain and spinal cord (central nervous system) isolated from the same animals. Identical observations were made in cell lines derived from N-ethyl-N-nitrosourea-induced rat schwannomas vs. brain tumors. By asymmetric PCR and mutant-specific Mnl I restriction fragment length analyses, cells carrying the mutant neu allele became detectable and could be localized within the trigeminal box as early as 7 days after the carcinogen pulse. The proliferation rate of the mutant cells strongly exceeded that of the wild-type cells up to the time of maturation of the trigeminal nerve around postnatal day 30 and thereafter to a lesser extent until the appearance of schwannomas. A specific mutation of the neu gene thus represents a very early, probably the first, step in the malignant conversion of immature rat Schwann cells exposed to N-ethyl-N-nitrosourea in vivo and is diagnostic for a subset of proliferative cells at high risk of progressing toward the expression of fully malignant phenotypes. Loss of heterozygosity for the mutant neu allele is a candidate event for a critical second step in the process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. Multiple independent activations of the neu oncogene by a point mutation altering the transmembrane domain of p185. Cell. 1986 Jun 6;45(5):649–657. doi: 10.1016/0092-8674(86)90779-8. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Hung M. C., Weinberg R. A. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986 Jan 16;319(6050):226–230. doi: 10.1038/319226a0. [DOI] [PubMed] [Google Scholar]
- Bargmann C. I., Weinberg R. A. Increased tyrosine kinase activity associated with the protein encoded by the activated neu oncogene. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5394–5398. doi: 10.1073/pnas.85.15.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohr V. A., Phillips D. H., Hanawalt P. C. Heterogeneous DNA damage and repair in the mammalian genome. Cancer Res. 1987 Dec 15;47(24 Pt 1):6426–6436. [PubMed] [Google Scholar]
- Bosch D. A. Short and long term effects of methyl- and ethylnitrosourea (MNU & ENU) on the developing nervous system of the rat. II. Short term effects: concluding remarks on chemical neuro-oncogenesis. Acta Neurol Scand. 1977 Feb;55(2):106–122. doi: 10.1111/j.1600-0404.1977.tb05631.x. [DOI] [PubMed] [Google Scholar]
- Bremner R., Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- Brown K., Buchmann A., Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns P. A., Gordon A. J., Glickman B. W. Influence of neighbouring base sequence on N-methyl-N'-nitro-N-nitrosoguanidine mutagenesis in the lacI gene of Escherichia coli. J Mol Biol. 1987 Apr 5;194(3):385–390. doi: 10.1016/0022-2836(87)90668-1. [DOI] [PubMed] [Google Scholar]
- Davis J. B., Stroobant P. Platelet-derived growth factors and fibroblast growth factors are mitogens for rat Schwann cells. J Cell Biol. 1990 Apr;110(4):1353–1360. doi: 10.1083/jcb.110.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckrey H. Genotypes and phenotypes of ten inbred strains of BD-rats. Arzneimittelforschung. 1971 Aug;21(8):1274–1278. [PubMed] [Google Scholar]
- Druckrey H., Schagen B., Ivankovic S. Erzeugung neurogener Malignome durch einmalige Gabe von Athyl-nitrosoharnstoff (ANH) an neugeborene und junge BD IX-Ratten. Z Krebsforsch. 1970;74(2):141–161. [PubMed] [Google Scholar]
- Eccleston P. A., Bannerman P. G., Pleasure D. E., Winter J., Mirsky R., Jessen K. R. Control of peripheral glial cell proliferation: enteric neurons exert an inhibitory influence on Schwann cell and enteric glial cell DNA synthesis in culture. Development. 1989 Sep;107(1):107–112. doi: 10.1242/dev.107.1.107. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Molecular and cellular mechanisms associated with pulse-carcinogenesis in the rat nerbous system by ethyinitrosourea: ethylation of nucleic acids and elimination rates of ethylated bases from the DNA of different tissues. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1974;82(1):37–64. doi: 10.1007/BF00304382. [DOI] [PubMed] [Google Scholar]
- Gyllensten U. B., Erlich H. A. Generation of single-stranded DNA by the polymerase chain reaction and its application to direct sequencing of the HLA-DQA locus. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7652–7656. doi: 10.1073/pnas.85.20.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imada M., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. I. Isolation and characterization of cell lines and cell type conversion. Dev Biol. 1978 Sep;66(1):97–108. doi: 10.1016/0012-1606(78)90276-2. [DOI] [PubMed] [Google Scholar]
- Kokai Y., Cohen J. A., Drebin J. A., Greene M. I. Stage- and tissue-specific expression of the neu oncogene in rat development. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8498–8501. doi: 10.1073/pnas.84.23.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M. H., Issing W., Miki T., Popescu N. C., Aaronson S. A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9193–9197. doi: 10.1073/pnas.86.23.9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laerum O. D., Rajewsky M. F. Neoplastic transformation of fetal rat brain cells in culture after exposure to ethylnitrosourea in vivo. J Natl Cancer Inst. 1975 Nov;55(5):1177–1187. doi: 10.1093/jnci/55.5.1177. [DOI] [PubMed] [Google Scholar]
- Lehväslaiho H., Lehtola L., Sistonen L., Alitalo K. A chimeric EGF-R-neu proto-oncogene allows EGF to regulate neu tyrosine kinase and cell transformation. EMBO J. 1989 Jan;8(1):159–166. doi: 10.1002/j.1460-2075.1989.tb03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonardo F., Di Marco E., King C. R., Pierce J. H., Segatto O., Aaronson S. A., Di Fiore P. P. The normal erbB-2 product is an atypical receptor-like tyrosine kinase with constitutive activity in the absence of ligand. New Biol. 1990 Nov;2(11):992–1003. [PubMed] [Google Scholar]
- Lupu R., Colomer R., Zugmaier G., Sarup J., Shepard M., Slamon D., Lippman M. E. Direct interaction of a ligand for the erbB2 oncogene product with the EGF receptor and p185erbB2. Science. 1990 Sep 28;249(4976):1552–1555. doi: 10.1126/science.2218496. [DOI] [PubMed] [Google Scholar]
- Mitra G., Pauly G. T., Kumar R., Pei G. K., Hughes S. H., Moschel R. C., Barbacid M. Molecular analysis of O6-substituted guanine-induced mutagenesis of ras oncogenes. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8650–8654. doi: 10.1073/pnas.86.22.8650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir D., Varon S., Manthorpe M. Schwann cell proliferation in vitro is under negative autocrine control. J Cell Biol. 1990 Dec;111(6 Pt 1):2663–2671. doi: 10.1083/jcb.111.6.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhy L. C., Shih C., Cowing D., Finkelstein R., Weinberg R. A. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982 Apr;28(4):865–871. doi: 10.1016/0092-8674(82)90065-4. [DOI] [PubMed] [Google Scholar]
- Perantoni A. O., Rice J. M., Reed C. D., Watatani M., Wenk M. L. Activated neu oncogene sequences in primary tumors of the peripheral nervous system induced in rats by transplacental exposure to ethylnitrosourea. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6317–6321. doi: 10.1073/pnas.84.17.6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajewsky M. F. Proliferative parameters of mammalian cell systms and their rôle in tumor growth and carcinogenesis. Z Krebsforsch Klin Onkol Cancer Res Clin Oncol. 1972;78(1):12–30. doi: 10.1007/BF00284309. [DOI] [PubMed] [Google Scholar]
- Ridley A. J., Davis J. B., Stroobant P., Land H. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozeik C., Von Keyserlingk D. The sequence of myelination in the brainstem of the rat monitored by myelin basic protein immunohistochemistry. Brain Res. 1987 Oct;432(2):183–190. doi: 10.1016/0165-3806(87)90043-5. [DOI] [PubMed] [Google Scholar]
- Schubert D., Heinemann S., Carlisle W., Tarikas H., Kimes B., Patrick J., Steinbach J. H., Culp W., Brandt B. L. Clonal cell lines from the rat central nervous system. Nature. 1974 May 17;249(454):224–227. doi: 10.1038/249224a0. [DOI] [PubMed] [Google Scholar]
- Sendowski K., Rajewsky M. F. DNA sequence dependence of guanine-O6 alkylation by the N-nitroso carcinogens N-methyl- and N-ethyl-N-nitrosourea. Mutat Res. 1991 Sep-Oct;250(1-2):153–160. doi: 10.1016/0027-5107(91)90171-j. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Lehväslaiho H., Lehtola L., Alitalo K. Activation of the neu tyrosine kinase induces the fos/jun transcription factor complex, the glucose transporter and ornithine decarboxylase. J Cell Biol. 1989 Nov;109(5):1911–1919. doi: 10.1083/jcb.109.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoica G., O'Leary M. In vitro malignant transformation of in vivo ENU-induced rat ovarian Sertoli cell tumor (adenoma). J Cancer Res Clin Oncol. 1988;114(2):142–148. doi: 10.1007/BF00417828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss B. S. The 'A rule' of mutagen specificity: a consequence of DNA polymerase bypass of non-instructional lesions? Bioessays. 1991 Feb;13(2):79–84. doi: 10.1002/bies.950130206. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Barbacid M. Specific patterns of oncogene activation in transplacentally induced tumors. Proc Natl Acad Sci U S A. 1990 Jan;87(2):718–722. doi: 10.1073/pnas.87.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenberg J. A., Clendenon N., Denlinger R., Gordon W. A. Sequential development of ethylnitrosourea-induced neurinomas: morphology, biochemistry, and transplantability. J Natl Cancer Inst. 1975 Jul;55(1):147–152. doi: 10.1093/jnci/55.1.147. [DOI] [PubMed] [Google Scholar]
- Tropak M. B., Johnson P. W., Dunn R. J., Roder J. C. Differential splicing of MAG transcripts during CNS and PNS development. Brain Res. 1988 Sep;464(2):143–155. doi: 10.1016/0169-328x(88)90006-x. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]