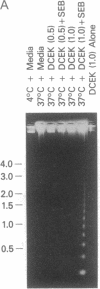
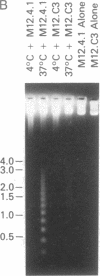
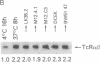Abstract
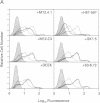
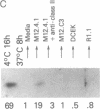
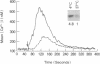
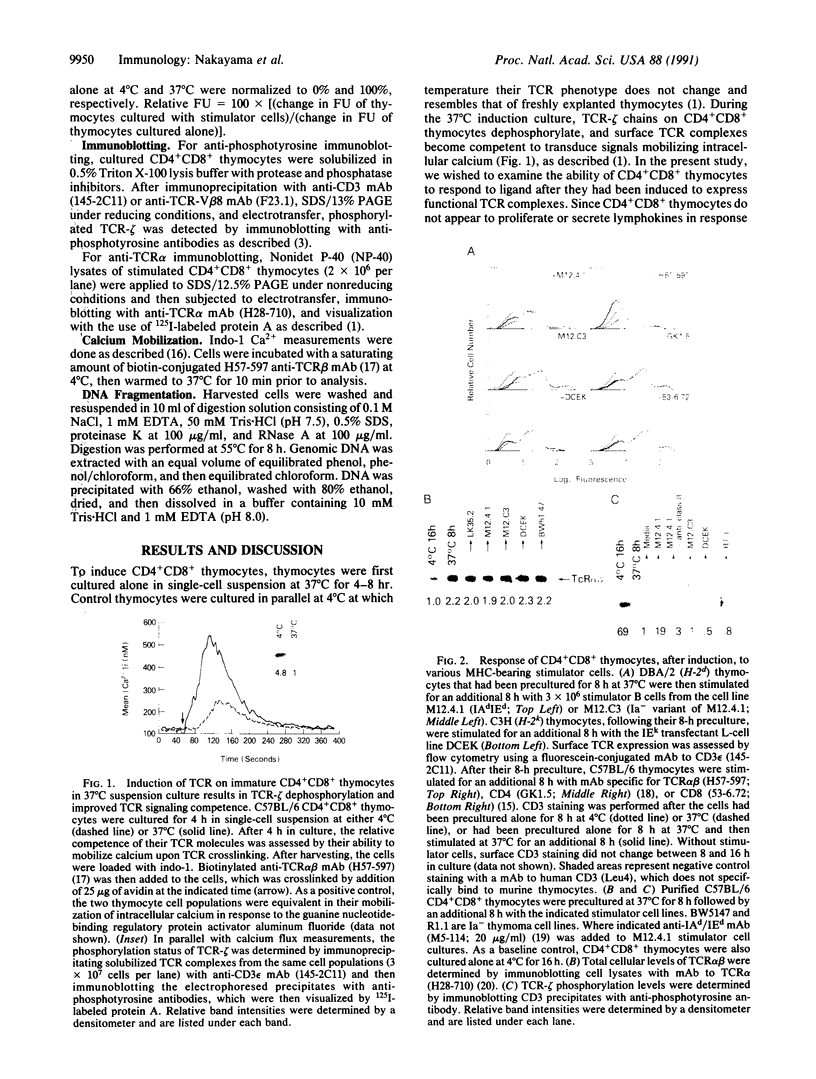
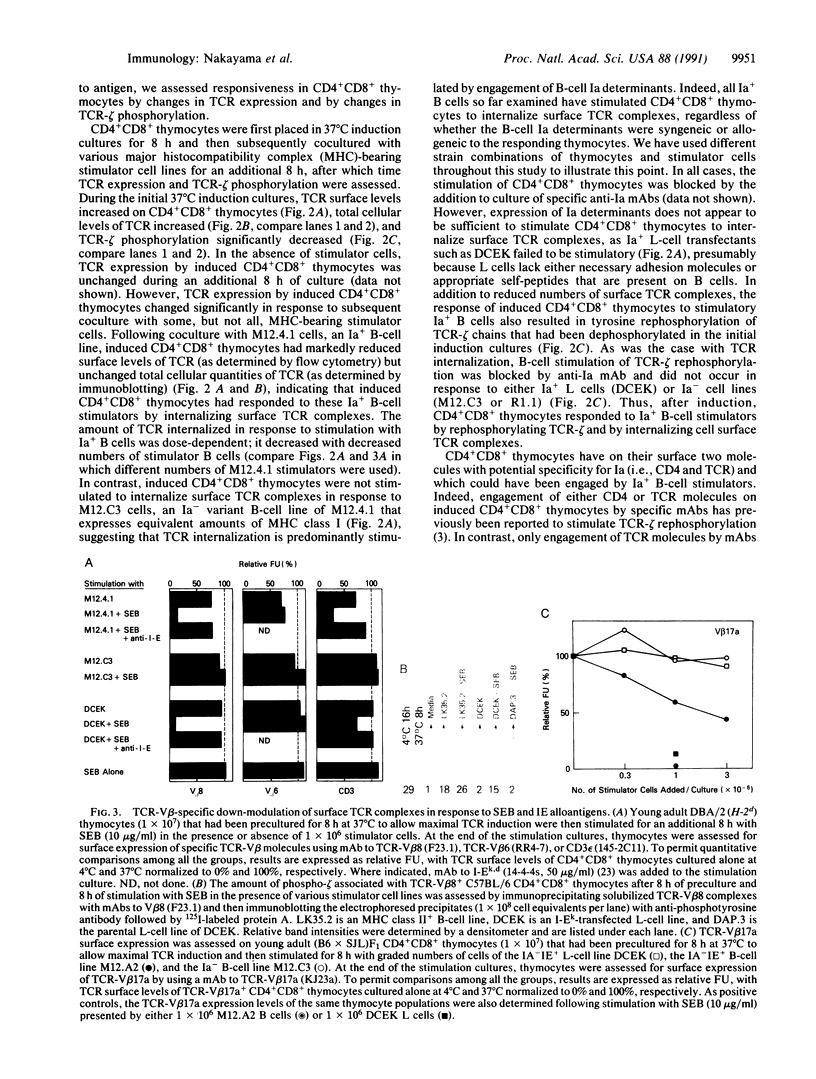
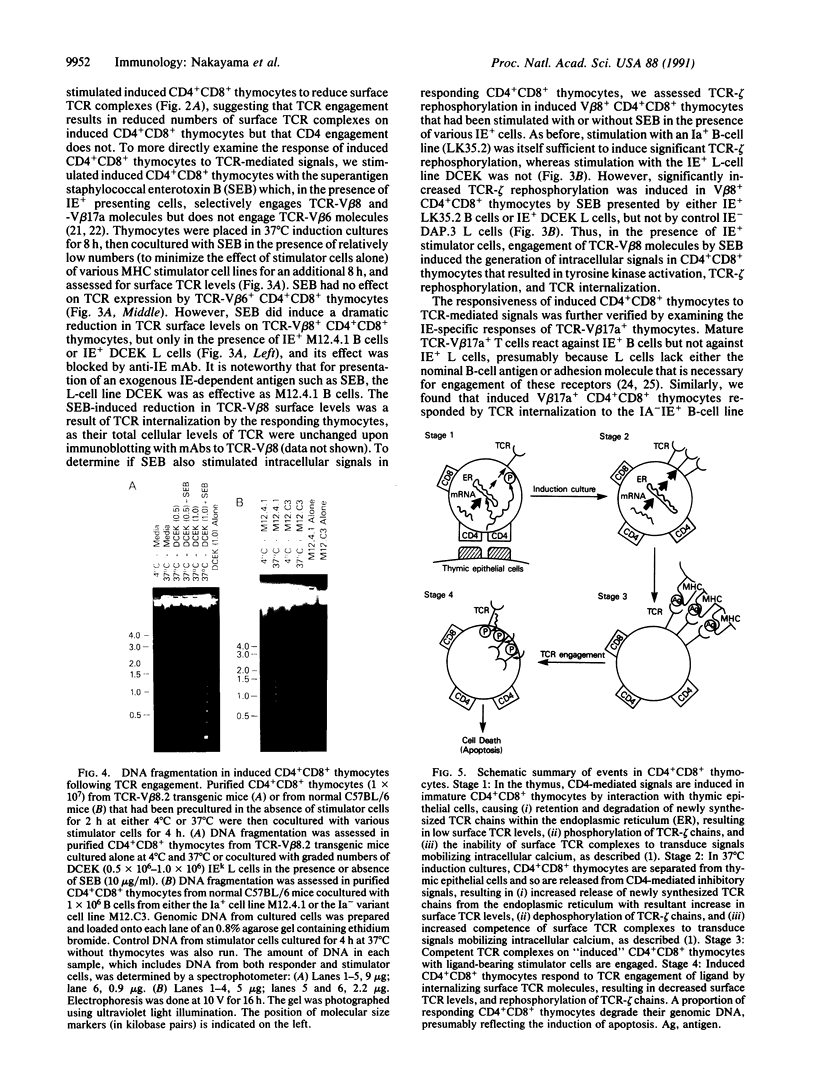
During thymic selection of the developing T-cell repertoire, the fate of individual CD4+CD8+ thymocytes is determined by the specificity of the T-cell antigen receptors (TCRs) they express. Paradoxically, most CD4+CD8+ thymocytes express few TCR molecules, and those they express are essentially incapable of transducing intracellular signals as measured by intracellular calcium mobilization. However, both TCR number and calcium-signaling capability are significantly induced in CD4+CD8+ thymocytes when the cells are released from intrathymic inhibitory signals that are mediated by their CD4 molecules. Here, the response to ligand engagement of TCR on "induced" CD4+CD8+ thymocytes that have been released from CD4-mediated inhibition was examined and was found to result in internalization of surface TCR complexes and rephosphorylation of zeta chains of the TCR complex. In addition, a proportion of induced CD4+CD8+ thymocytes were found to fragment their DNA upon ligand engagement. Thus, this study describes early events in immature CD4+CD8+ thymocytes resulting from TCR-mediated signals.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker M. L., Near R., Mudgett-Hunter M., Margolies M. N., Kubo R. T., Kaye J., Hedrick S. M. Expression of a hybrid immunoglobulin-T cell receptor protein in transgenic mice. Cell. 1989 Sep 8;58(5):911–921. doi: 10.1016/0092-8674(89)90943-4. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Bonifacino J. S., McCarthy S. A., Maguire J. E., Nakayama T., Singer D. S., Klausner R. D., Singer A. Novel post-translational regulation of TCR expression in CD4+CD8+ thymocytes influenced by CD4. Nature. 1990 Mar 15;344(6263):247–251. doi: 10.1038/344247a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. J., Duke R. C. Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to cell death. J Immunol. 1984 Jan;132(1):38–42. [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Griffith I. J., Nabavi N., Ghogawala Z., Chase C. G., Rodriguez M., McKean D. J., Glimcher L. H. Structural mutation affecting intracellular transport and cell surface expression of murine class II molecules. J Exp Med. 1988 Feb 1;167(2):541–555. doi: 10.1084/jem.167.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Yagi J., Conrad P. J., Katz M. E., Jones B., Vroegop S., Buxser S. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989 Feb;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- Kanagawa O., Palmer E., Bill J. The T cell receptor V beta 6 domain imparts reactivity to the Mls-1a antigen. Cell Immunol. 1989 Apr 1;119(2):412–426. doi: 10.1016/0008-8749(89)90255-4. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Roehm N., Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987 Apr 24;49(2):273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Wade T., White J., Kushnir E., Blackman M., Bill J., Roehm N., Marrack P. A T cell receptor V beta segment that imparts reactivity to a class II major histocompatibility complex product. Cell. 1987 Apr 24;49(2):263–271. doi: 10.1016/0092-8674(87)90567-8. [DOI] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. T cells can distinguish between allogeneic major histocompatibility complex products on different cell types. Nature. 1988 Apr 28;332(6167):840–843. doi: 10.1038/332840a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Lo D., Brinster R., Palmiter R., Burkly L., Flavell R. H., Kappler J. The effect of thymus environment on T cell development and tolerance. Cell. 1988 May 20;53(4):627–634. doi: 10.1016/0092-8674(88)90578-8. [DOI] [PubMed] [Google Scholar]
- McCarthy S. A., Kruisbeek A. M., Uppenkamp I. K., Sharrow S. O., Singer A. Engagement of the CD4 molecule influences cell surface expression of the T-cell receptor on thymocytes. Nature. 1988 Nov 3;336(6194):76–79. doi: 10.1038/336076a0. [DOI] [PubMed] [Google Scholar]
- Nakayama T., June C. H., Munitz T. I., Sheard M., McCarthy S. A., Sharrow S. O., Samelson L. E., Singer A. Inhibition of T cell receptor expression and function in immature CD4+CD8+ cells by CD4. Science. 1990 Sep 28;249(4976):1558–1561. doi: 10.1126/science.2120773. [DOI] [PubMed] [Google Scholar]
- Nakayama T., Singer A., Hsi E. D., Samelson L. E. Intrathymic signalling in immature CD4+CD8+ thymocytes results in tyrosine phosphorylation of the T-cell receptor zeta chain. Nature. 1989 Oct 19;341(6243):651–654. doi: 10.1038/341651a0. [DOI] [PubMed] [Google Scholar]
- Ohashi P. S., Pircher H., Bürki K., Zinkernagel R. M., Hengartner H. Distinct sequence of negative or positive selection implied by thymocyte T-cell receptor densities. Nature. 1990 Aug 30;346(6287):861–863. doi: 10.1038/346861a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rabinovitch P. S., June C. H., Grossmann A., Ledbetter J. A. Heterogeneity among T cells in intracellular free calcium responses after mitogen stimulation with PHA or anti-CD3. Simultaneous use of indo-1 and immunofluorescence with flow cytometry. J Immunol. 1986 Aug 1;137(3):952–961. [PubMed] [Google Scholar]
- Ronchese F., Schwartz R. H., Germain R. N. Functionally distinct subsites on a class II major histocompatibility complex molecule. Nature. 1987 Sep 17;329(6136):254–256. doi: 10.1038/329254a0. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Rammensee H. G., Benedetto J. D., Bevan M. J. Characterization of a murine monoclonal antibody specific for an allotypic determinant on T cell antigen receptor. J Immunol. 1985 Jun;134(6):3994–4000. [PubMed] [Google Scholar]
- Uematsu Y., Ryser S., Dembić Z., Borgulya P., Krimpenfort P., Berns A., von Boehmer H., Steinmetz M. In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell. 1988 Mar 25;52(6):831–841. doi: 10.1016/0092-8674(88)90425-4. [DOI] [PubMed] [Google Scholar]
- White J., Herman A., Pullen A. M., Kubo R., Kappler J. W., Marrack P. The V beta-specific superantigen staphylococcal enterotoxin B: stimulation of mature T cells and clonal deletion in neonatal mice. Cell. 1989 Jan 13;56(1):27–35. doi: 10.1016/0092-8674(89)90980-x. [DOI] [PubMed] [Google Scholar]