Abstract
The well-being of dogs can be affected by changes in human lifestyle, eating habits and increased stressors that lead to behavioural disorders including fear, hyperactivity and anxiety, followed by negative affective moods and poor welfare. This randomised, controlled clinical evaluation involved 69 dogs, 38 males and 31 females, of different breeds, with behavioural disorders related to anxiety and chronic stress. They were fed a control diet or a nutraceutical diet (ND group) for 45 days. Neuroendocrine (serotonin, dopamine, β-endorphins, noradrenaline and cortisol) and stress (derivatives of reactive oxygen metabolites (dROMs) and biological antioxidant potential (BAP)) parameters related to behavioural disorders were evaluated at the beginning and end of the study period. Results showed a significant increase in serotonin, dopamine and β-endorphins plasma concentrations (*P<0.05, *P<0.05 and **P<0.01, respectively) and a significant decrease in noradrenaline and cortisol plasma concentrations in the ND group (*P<0.05). dROMs significantly decreased in the ND group (*P<0.05) while BAP was not affected. This study demonstrated for the first time that a specific diet significantly and positively affected neuroendocrine parameters and dROMs. These results open significant perspectives concerning the use of diet and nutraceuticals in the treatment of behavioural disorders.
Keywords: nutraceutical diet, Neuroendocrine parameters, stress parameters, anxiety, chronic stress, behavioral disorders
Animal behaviour is the result of interplay between genotype and environment (Overall and others 2002, 2006, Sih and others 2004) and is, at the neuroendocrine level, characterised by neuromediators like dopamine and serotonin (5-HT2A and γ-aminobutyric acid reduced binding activity) or endocrine (cortisol) pathways imbalance (Paredes and Agmo 1992, Inagawa and others 2005, Peremans and others 2006, Vermeire and others 2009, 2012), Chronic anxiety status (Frank 2014, Overall and others 2006) and nutrition can significantly affect behaviour (Dodman and others 1996, DeNapoli and others 2000, Bosch and others 2007). It was, as an example, demonstrated that a diet high in tryptophan can lower territorial aggression score while a high-protein diet without tryptophan supplementation can induce a high dominance aggression score (DeNapoli and others 2000). A specific diet supplementation with amino acids, n-3 and n-6 polyunsaturated fatty acids (PUFAs) and a well-balanced amount of proteins and fibre was considered as beneficial in dogs with evident behavioural disorders (Bosch and others 2007). Similarly, several reports showed the role of such compounds in modulating animal behaviour (Kantak and others 1980, Reinstein and others 1984, 1985, Lasley and Thurmond 1985, Raleigh and others 1985, Chamberlain and others 1987, Jewell and Toll 1996, Butterwick and Markwell 1997, Chalon and others 1998, Carrie and others 2000, Moriguchi and others 2000, Koopmans and others 2005).
The benefit of nutraceuticals was demonstrated in different species. Punica granatum has been extensively used to treat chronic anxiety and insomnia in rats (Riaz and Khan 2014, Swarnamoni and Phulen 2014). Mild sleep disorders, but also nervous tension, have been treated with roots and rhizomes of Valeriana officinalis in mice (Carlini 2003, Hattesohl and others 2008, Sudati and others 2009, Wang and others 2010). On the other hand, antianxiety and antidepressant activities were observed for Rosmarinus officinalis (Machado and others 2009, Ulbricht and others 2010), Tilia species in mice (Viola and others 1994, Coleta and others 2001) and Crataegus oxyacantha L. in human beings and mice (Hanus and others 2004, Ernst 2007, Lakhan and Vieira 2010). l-Theanine, one of the green tea constituents, has been shown, in human beings, to play a role in reducing stress and decreasing the heart rate in chronic anxiety (Juneja and others 1999, Miodownik and others 2011). As to l-tryptophan, many published reports have also described the presence of anxiety, mood and depressive symptoms associated with its depletion (Delgado and others 1990, 1999). Finally, there are several evidences suggesting that omega-3 deficiency may be associated with mood and behavioural disorders. In dogs, all these pathologies have been consistently reported to be associated with oxidative stress (Stoll and others 1999, Owen and others 2008, Valvassori and others 2015).
The biological effects of oxidative stress are often related to the production of free radicals, rapidly reacting with other molecules and triggering the oxidation process. Free radicals, such as peroxide ion, nitrogen monoxide and hydroxyl radical, are physiologically produced in cells and released during inflammatory processes (Pasquini and others 2010). Free radicals can also be generated by drug metabolism (Wang and others 2012), following exposure to environmental pollutants (Ademiluyi and Oboh 2013) and when fear and anxiety-related behaviours are present (Dreschel 2010). Once homeostasis is compromised, a progressive oxidation of biological substrates including lipids, DNA and proteins occurs. This is followed by the production of oxidant intermediates, such as hydroperoxides, referred to as reactive oxygen metabolites. As a consequence, this cascade mechanism progressively increases the biological damage. Several other reports also pointed out the role of other factors in the production of oxidant intermediates including physical exercise, characterised by an increase in body oxygen consumption and is associated with an increase production of reactive oxygen species to a point sometime exceeding antioxidant defence mechanisms and causing major oxidative stress (Alessio and others 2000, Watson and others 2005, Ji 2008). Exercise-related oxidative stress contributes to increase muscle fatigue and muscle fibre damages, leading to reduced performances (Baskin and others 2000, Piercy and others 2000, Moller and others 2001, Hargreaves and others 2002, Kirschvink and others 2002, Berzosa and others 2011) and decreased immune defence of the organism (Nieman 1997, Sen and Packer 2000). Several recent studies have shown an improvement of overall tissue stability and protection when the animals are fed with additional antioxidants (Cherian and others 1996, Lopez-Bote and others 1997, Castellini and others 1998, Alessio and others 2000, Watson and others 2005, Ji 2008, Sechi and others 2015).
For these reasons, the evaluation of specific oxidative stress-related factors, such as derivatives of reactive oxygen metabolites (dROMs) and biological antioxidant potential (BAP), is of interest and should be performed to monitor the welfare and health of dogs under stressful conditions (Passantino and others 2014, Sechi and others 2015).
While dROMs measure the oxidant level within the blood (Gletsu-Miller and others 2009), BAP matches the total antioxidant capability of plasma and includes either exogenous (ascorbate, tocopherols, carotenoids) or endogenous components (protein, glutathione peroxidase, superoxide dismutase, catalase) involved in the overall reactive oxygen species balance (Benzie and Strain 1996).
The objectives of the present study were, in a controlled study, to evaluate for the first time the oxidative stress and neuroendocrine parameters in dogs with behavioural problems administered a specific nutraceutical-based diet.
Materials and methods
Sixty-nine dogs (31 females and 38 males) aged 3.2±0.2 years (mean±sem) of different breeds (29 crossbreds, 9 labradors, 2 German shepherds, 3 boxers, 1 Maremma sheepdog, 3 German dachshunds, 1 bull terrier, 1 Coton de Tulear, 2 Border collie, 3 Jack Russells, 2 pinschers, 1 Cirneco of Etna, 1 Maltese, 1 Pekingese, 1 pug, 1 English setter, 1 poodle, 2 Fonni's dogs, 3 American Staffordshire terriers, 1 golden retriever, 1 grey hound) were used in a randomised controlled clinical evaluation performed at the University of Sassari, Department of Veterinary Medicine, Pathology and Veterinary Clinic Section.
The dogs were randomly assigned to the control diet (CD) group (n=34) or to the nutraceutical diet (ND) group (n=35) and fed over a period of 45 days following manufacturers’ instructions (Table 1).
TABLE 1:
Daily amount of diet suggested by the manufacturer
| Daily ratio | |
|---|---|
| Weight (kg) | Amount (g) |
| 1–10 | 30–180 |
| 11–20 | 190–300 |
| 21–35 | 310–455 |
| 36–50 | 465–595 |
Operative procedures and animal care were performed in compliance with the national and international regulations (Italian regulation D.L. vo 116/1992 and EU regulation 86/609/EC). The recommendations of CONSORT 2010 statement in randomised controlled trials were also consulted and considered (Bian and Shang 2011).
The diets
Both diets fulfilled the recommendations for protein, carbohydrate and fat regarding dog daily requirements (Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs—The European Pet Food Industry Federation). Briefly, the nutrient composition was 24 per cent of crude protein, 12 per cent of crude oils and fats, 3.7 per cent, of crude fibre, 5 per cent of crude ash, 9 per cent of moisture and a metabolised energy of 3.477 kcal/kg (or 14.6 MJ/kg). Both diets were in the form of kibbles industrially produced and had the same amount of vitamins (A, C and E), trace elements (choline chloride, zinc sulfate monohydrate and cupric chelate glycine hydrate) and amino acids (dl-methionine) (Table 2).
TABLE 2:
Vitamins, essential fatty acids, trace elements and amino acids amount per kilogram of complete food in nutraceutical diet and control diet
| Essential fatty acids | Amount per kilogram of complete food |
|---|---|
| Omega-6 | 12.5 g/kg |
| Omega-3 | 16 g/kg |
| Vitamins | |
| A | 18,500 UI/kg |
| E | 120 mg/kg |
| C | 250 mg/kg |
| Trace elements | |
| Choline chloride | 1000 mg/kg |
| Zinc sulfate monohydrate | 137 mg/kg |
| Cupric chelate glycine hydrate | 39 mg/kg |
| Amino acids | |
| dl-Methionine | 500 mg/kg |
The ND was also characterised by the presence of cold-pressed tablets composed by 60–80 per cent of protein hydrolysed (fish and vegetable ones), 20–40 per cent of minerals used as glidants and nutraceutical substances: P granatum, V officinalis, R officinalis, Tilia species, C oxyacantha, green tea extract rich in l-theanine and l-tryptophan (Table 3).
TABLE 3:
Nutraceutical substances amount per kilogram of complete food in nutraceutical diet (ND)
| Nutraceutical substances | Amount per kilogram of complete food (mg/kg) |
|---|---|
| Punica granatum | 457 |
| Valeriana officinalis | 260 |
| Rosmarinus officinalis | 0.44 |
| Tilia species | 635 |
| Crataegus oxyacantha | 392 |
| l-Theanine | 310 |
| l-Tryptophan | 329 |
Sample collection and biochemical analysis
Cephalic vein blood samples were collected from each dog before (T0) and after 45 days (T1) of diet administration. Heparinised plasma and serum samples were centrifuged at 4000×g for 1.5 min at 37°C and stored at −20°C up to evaluation.
Parameters evaluated
dROMs and BAP, as indicators of oxidative stress, were measured spectrophotometrically at 505 nm (Free Radical Analytical System FRAS 4, H&D s.r.l., Langhirano PR, Italy) on serum samples (Sechi and others 2015).
Concentration of dopamine, noradrenaline, serotonin, cortisol (MyBioSource, San Diego, USA; dopamine, catalogue no. MBS494632; cortisol, catalogue no. MBS703711; noradrenaline, catalogue no. MBS739721; serotonin, catalogue no. MBS283892) and β-endorphins (antibodies-online GmbH, Aachen, Germany; catalogue no. ABIN364677) were assessed by ELISA.
Statistical analysis
Data were analysed using Prism 6 (GraphPad Software, San Diego, USA). All data are presented as the means±sem and were first checked for normality using the D'Agostino-Pearson normality test. Differences in dROMs, BAP, serotonin, dopamine, noradrenaline, cortisol and β-endorphins serum concentrations between the two diets before (T0) and at the end of the evaluation period (T1) or between groups were analysed using a two-way analysis of variance followed by Sidak's multiple comparisons test. *P<0.05 was considered significant.
Results
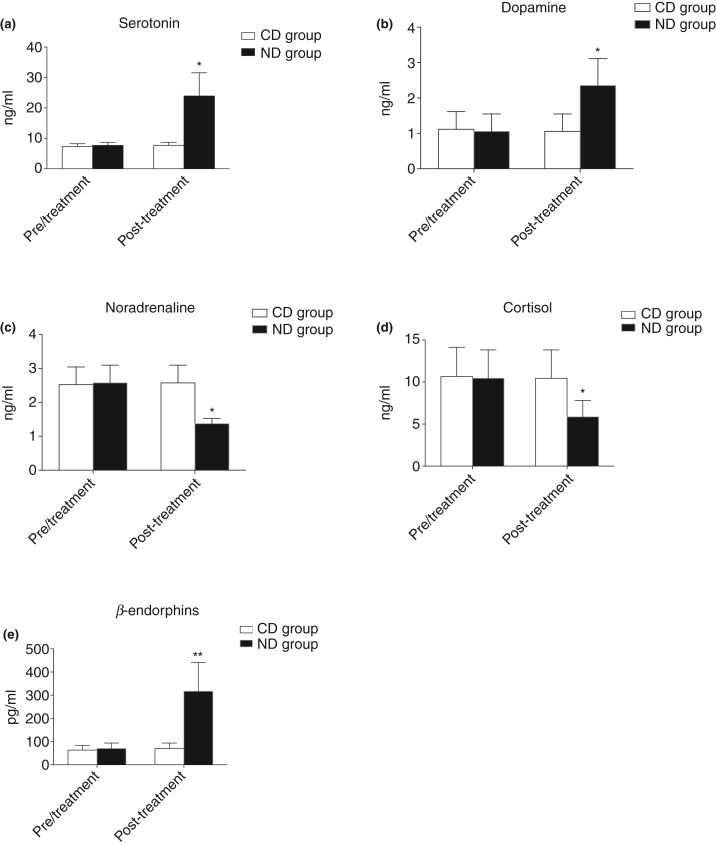
In Fig 1, the neurotransmitter’s serum concentrations before (T0) and at the end of the evaluation period are shown for the two groups of animals (Fig 1).
FIG 1:
Graphical representation of serum neurotransmitters concentration of dogs belonging to control diet (CD) and nutraceutical diet (ND) groups before (T0) and after 45 days (T1) of diet supplementation. (a) Serotonin, (b) dopamine, (c) noradrenaline, (d) cortisol and (e) β-endorphins.
In the nutraceutical treated group (ND), serotonin, dopamine and β-endorphins concentration significantly increased from 7.67±1.01 ng/ml to 23.91±7.64 ng/ml, 1.05±0.49 to 2.35±0.76 ng/ml, 70.20±23.82 ng/ml to 317.0±124.1 ng/ml, respectively, for T0 to T1 (Fig 1a–c). In the same group, noradrenaline and cortisol significantly decreased from 2.57±0.52 ng/ml to 1.36±0.15 ng/ml, or 10.44±3.38 ng/ml to 5.86±1.95 ng/ml at T0 and T1, respectively (Fig 1d, e). No significant variations for any of the evaluated parameters were observed in the CD group.
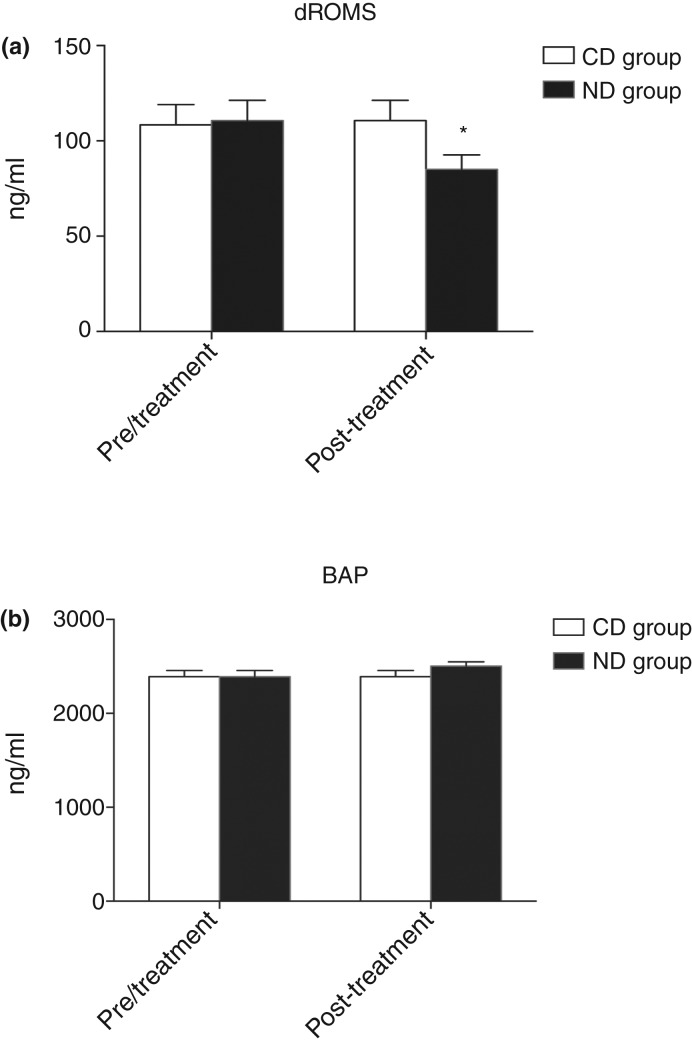
dROMs values presented a significant decrease from T0 to T1 (110.7±10.64 to 85.06±7.6 U. CARR) in ND group (*P<0.05) (Fig 2a).
FIG 2:
Graphical representation of serum oxidative stress parameters concentration of dogs. belonging to control diet (CD) and nutraceutical diet (ND) groups before (T0) and after 45 days (T1) of diet supplementation. (a) derivatives of reactive oxygen metabolites (dROMs) values and (b) biological antioxidant potential (BAP).
Discussion
In this randomised, controlled evaluation assessing neuroendocrine blood parameters in dogs with behavioural disorders, the authors demonstrated the significant positive effects, obtained within a short 45-day period, of a combination of hydrolysed fish proteins, rice carbohydrates, P granatum, V officinalis, R officinalis, Tilia species, C oxyacantha, green tea extract and l-tryptophan and a omega-3/6 (1:0.8 ratio).
According to Bosh et al, normal behaviour is characterised by a stable neurotransmitter and hormone balance; however, it is easily affected by stress, anxiety or any behavioural disorders (Bosch and others 2007). Low serotonin plasma concentrations have been associated with aggressive behaviour (Reisner and others 1996, Badino and others 2004, Cakiroglu and others 2007). Similarly, impulsivity, defined as an abnormal over-reactivity to normal stimuli (Wright and others 2011), has been usually associated with reduced monoaminergic (dopamine and serotonin) circulating levels (Reisner and others 1996, Wright and others 2012). In addition, a dopamine increase was associated with satiety and reward expectation (Tobler and others 2003).
In the present work, serotonin and dopamine, used as behavioural markers, significantly increased while cortisol and norepinephrine, used as stress markers, decreased: all returning to values expected in normal animals demonstrating the positive and beneficial effects of the nutraceutical diet on overall homeostasis balance.
Different factors included in this diet may be responsible for the positive and significant observations made in the present work including l-tryptophan known to affect general mood and behaviour (Fernstrom and Wurtman 1972, Lucki 1998, Barnes and Sharp 1999, Koopmans and others 2005). Similarly, cortisol reduction is also observed after administration of l-theanine known to have beneficial clinical effects in stress and anxiety management (Miodownik and others 2011).
Diet clearly influences the overall health status in dogs like in other species (Stein and others 1994, Odore and others 2015). For instance, seasonal allergies are generally associated with the onset of skin disorders including symptoms like intense itching, dandruff or flushes and have also been linked to obvious changes in behaviour (Nuttall and others 2013). Similarly, bone meal including chicken meat and bones derived from intensive poultry farming (one of the main ingredients of dry pet food; Maine and others 2015) has been shown to induce pro-inflammatory cytokines (i.e. interferon-γ) release in vitro (Di Cerbo and others 2015, Odore and others 2015, Guidetti and others 2016). Thus, the chronic intake of contaminated food is suggested to affect overall homeostasis and possibly induce a chronic inflammatory status in healthy animals, triggering potentially behavioural changes, such as anxiety and depression (Maier and Watkins 1998), dermatological changes, such as itching and erythema (Noli and others 2015), paving the way with depressed immunity for secondary infections, that is, by Malassezia pachydermatis and Candida parapsilosis (Yurayart and others 2013).
We recently published data supporting the immune-modulatory and anti-inflammatory effect of a specific diet, which shared part of the present formula including hydrolysed fish protein, rice carbohydrates Tilia cordata and P granatum, in dogs affected by chronic otitis externa, characterised by an overall ear overgrowth of M pachydermatis, Leishmania and keratoconjunctivitis sicca, both characterised by an overall inflammatory status, respectively (Cortese and others 2015, Destefanis and others 2016, Di Cerbo and others 2016). Low concentrations of aqueous T cordata extract have been shown to stimulate a T lymphocyte proliferation (Anesini and others 1999), potentially neutralising the constant solicitation exerted by the food contaminants. Moreover, monoterpenes such as eugenol and geraniol have also been detected in the flowers of T cordata (Toker and others 2001) and, along with carvacrol, thymol and also R officinalis, have been demonstrated to exert antioxidant and antiradical activities (Horvathova and others 2014).
Oxidative stress has been also suggested to contribute to the aetiology of anxiety disorders and depression becoming a consequence of either increased generation of reactive oxygen species or impaired enzymatic or non-enzymatic defence against it (Hovatta and others 2010). An overload of reactive oxygen metabolites can lead to damages of all major cellular functions and contribute to cognitive decline (Hovatta and others 2010, Sechi and others 2015).
dROMs were significantly decreased in dogs receiving the nutraceutical diet (Pasquini and others 2010). However, the antioxidant status revealed by BAP was not affected by the diet and values remained optimal throughout the whole observation period. It is speculated that the diet only affected dROMs species but not the endogenous antioxidant components, which remained in the initial optimal level.
The high and balanced content of PUFAs in the nutraceutical diet may also have played a role in the neuroendocrine changes observed in the study. Indeed, PUFA are known to exert anti-inflammatory effects (Hokari and others 2013, Attaman and others 2014) and PUFA concentrations have also been shown to modulate behaviour in aggressive dogs (Re and others 2008). Re and others in 2008 indeed observed in aggressive dogs lower docosahexaenoic acid concentrations and higher omega-6/omega-3 ratio (Re and others 2008).
Aggressive behaviour is generally seen as a major behavioural problem in dogs; however, nutrition is rarely considered as one possible contributing factor to this issue (Bosch and others 2007). In the present study, the authors demonstrated, in a randomised, control clinical study using dogs with abnormal behaviour, that an equilibrated nutraceutical diet was highly tolerated without any adverse effects.
In conclusion, this study demonstrated the positive effects of a nutraceutical diet on neuroendocrine parameters associated with stress, anxiety, aggression and numerous behavioural disorders. If a better understanding of dog behaviour and psychological and clinical signs associated with suffering is warranted, the authors demonstrated that the use of adapted and appropriate diet, devoid of contaminants and including specific nutraceuticals, may help ensuring a better quality of life of animals and improving behavioural disorders (Sonntag and Overall 2014). An easy and medication-free approach to behavioural issue treatment can be proposed.
Acknowledgments
The authors thank Joseph Paul for his language revision.
Footnotes
Contributors: SS and ADC contributed equally to the article.
Funding: This study was supported by Fondazione Banco di Sardegna (Italy).
Competing interests: This research was performed in collaboration with some scientists from the Division of Research and Development, Sanypet SpA, Padua, Italy (as indicated in the author's affiliation) according to scientific and ethical principles of the scientific community.
References
- ADEMILUYI A. O. & OBOH G. (2013) Soybean phenolic-rich extracts inhibit key-enzymes linked to type 2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting enzyme) in vitro. Experimental and Toxicologic Pathology 65, 305–309 10.1016/j.etp.2011.09.005 [DOI] [PubMed] [Google Scholar]
- ALESSIO H. M., HAGERMAN A. E., FULKERSON B. K., AMBROSE J., RICE R. E. & WILEY R. L. (2000) Generation of reactive oxygen species after exhaustive aerobic and isometric exercise. Medicine & Science in Sports & Exercise 32, 1576–1581 10.1097/00005768-200009000-00008 [DOI] [PubMed] [Google Scholar]
- ANESINI C., WERNER S. & BORDA E. (1999) Effect of Tilia cordata Mill. flowers on lymphocyte proliferation: participation of peripheral type benzodiazepine binding sites. Fitoterapia 70, 361–367 10.1016/S0367-326X(99)00049-0 [DOI] [Google Scholar]
- ATTAMAN J. A., STANIC A. K., KIM M., LYNCH M. P., RUEDA B. R. & STYER A. K. (2014) The anti-inflammatory impact of omega-3 polyunsaturated Fatty acids during the establishment of endometriosis-like lesions. American Journal of Reproductive Immunology 72, 392–402 10.1111/aji.12276 [DOI] [PubMed] [Google Scholar]
- BADINO P., ODORE R., OSELLA M. C., BERGAMASCO L., FRANCONE P., GIRARDI C. & RE G. (2004) Modifications of serotonergic and adrenergic receptor concentrations in the brain of aggressive Canis familiaris. Comp Biochem Physiol A Mol Integr Physiol 139, 343–350 [DOI] [PubMed] [Google Scholar]
- BARNES N. M. & SHARP T. (1999) A review of central 5-HT receptors and their function. Clinical Neuropharmacology 38, 1083–1152 [DOI] [PubMed] [Google Scholar]
- BASKIN C. R., HINCHCLIFF K. W., DISILVESTRO R. A., REINHART G. A., HAYEK M. G., CHEW B. P., BURR J. R. & SWENSON R. A. (2000) Effects of dietary antioxidant supplementation on oxidative damage and resistance to oxidative damage during prolonged exercise in sled dogs. American Journal of Veterinary Research 61, 886–891 10.2460/ajvr.2000.61.886 [DOI] [PubMed] [Google Scholar]
- BENZIE I. F. & STRAIN J. J. (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry 239, 70––76 10.1006/abio.1996.0292 [DOI] [PubMed] [Google Scholar]
- BERZOSA C., GOMEZ-TRULLEN E. M., PIEDRAFITA E., CEBRIAN I., MARTINEZ-BALLARIN E., MIANA-MENA F. J., FUENTES-BROTO L. & GARCIA J. J. (2011) Erythrocyte membrane fluidity and indices of plasmatic oxidative damage after acute physical exercise in humans. European Journal of Applied Physiology 111, 1127––1133 10.1007/s00421-010-1738-6 [DOI] [PubMed] [Google Scholar]
- BIAN Z. X. & SHANG H. C. (2011) CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 154, 290–291; author reply 291-292 10.7326/0003-4819-154-4-201102150-00016 [DOI] [PubMed] [Google Scholar]
- BOSCH G., BEERDA B., HENDRIKS W. H., VAN DER POEL A. F. & VERSTEGEN M. W. (2007) Impact of nutrition on canine behaviour: current status and possible mechanisms. Nutrition Research Reviews 20, 180–194 10.1017/S095442240781331X [DOI] [PubMed] [Google Scholar]
- BUTTERWICK R. F. & MARKWELL P. J. (1997) Effect of amount and type of dietary fiber on food intake in energy-restricted dogs. American Journal of Veterinary Research 58, 272–276 [PubMed] [Google Scholar]
- CAKIROGLU D., MERAL Y., SANCAK A. A. & CIFTI G. (2007) Relationship between the serum concentrations of serotonin and lipids and aggression in dogs. Vet Rec 161, 59–61 [DOI] [PubMed] [Google Scholar]
- CARLINI E. A. (2003) Plants and the central nervous system. Pharmacology, Biochemistry, and Behavior 75, 501–512 10.1016/S0091-3057(03)00112-6 [DOI] [PubMed] [Google Scholar]
- CARRIE I., CLEMENT M., DE JAVEL D., FRANCES H. & BOURRE J. M. (2000) Specific phospholipid fatty acid composition of brain regions in mice. Effects of n-3 polyunsaturated fatty acid deficiency and phospholipid supplementation. Journal of Lipid Research 41, 465–472 [PubMed] [Google Scholar]
- CASTELLINI C., DAL BOSCO A., BERNARDINI M. & CYRIL H. W. (1998) Effect of dietary vitamin e on the oxidative stability of raw and cooked rabbit meat. Meat Science 50, 153–161 10.1016/S0309-1740(98)00026-6 [DOI] [PubMed] [Google Scholar]
- CHALON S., DELION-VANCASSEL S., BELZUNG C., GUILLOTEAU D., LEGUISQUET A. M., BESNARD J. C. & DURAND G. (1998) Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. British Journal of Nutrition 128, 2512–2519 [DOI] [PubMed] [Google Scholar]
- CHAMBERLAIN B., ERVIN F. R., PIHL R. O. & YOUNG S. N. (1987) The effect of raising or lowering tryptophan levels on aggression in vervet monkeys. Pharmacology Biochemistry and Behavior 28, 503–510 10.1016/0091-3057(87)90513-2 [DOI] [PubMed] [Google Scholar]
- CHERIAN G., WOLFE F. W. & SIM J. S. (1996) Dietary oils with added tocopherols: effects on egg or tissue tocopherols, fatty acids, and oxidative stability. British Poultry Science 75, 423–431 10.3382/ps.0750423 [DOI] [PubMed] [Google Scholar]
- COLETA M., CAMPOS M. G., COTRIM M. D. & PROENCA DA CUNHA A. (2001) Comparative evaluation of Melissa officinalis L., Tilia europaea L., Passiflora edulis Sims. and Hypericum perforatum L. in the elevated plus maze anxiety test. Modern Problems of Pharmacopsychiatry 34(Suppl 1), S20–S21 10.1055/s-2001-15460 [DOI] [PubMed] [Google Scholar]
- CORTESE L., ANNUNZIATELLA M., PALATUCCI A. T., LANZILLI S., RUBINO V., DI CERBO A., CENTENARO S., GUIDETTI G., CANELLO S. & TERRAZZANO G. (2015) An immune-modulating diet increases the regulatory T cells and reduces T helper 1 inflammatory response in Leishmaniosis affected dogs treated with standard therapy. BMC Veterinary Research 11, 295 10.1186/s12917-015-0610-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELGADO P. L., CHARNEY D. S., PRICE L. H., AGHAJANIAN G. K., LANDIS H. & HENINGER G. R. (1990) Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Archives of General Psychiatry 47, 411–418 10.1001/archpsyc.1990.01810170011002 [DOI] [PubMed] [Google Scholar]
- DELGADO P. L., MILLER H. L., SALOMON R. M., LICINIO J., KRYSTAL J. H., MORENO F. A., HENINGER G. R. & CHARNEY D. S. (1999) Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: implications for the role of serotonin in the mechanism of antidepressant action. Biological Psychiatry 46, 212–220 10.1016/S0006-3223(99)00014-1 [DOI] [PubMed] [Google Scholar]
- DENAPOLI J. S., DODMAN N. H., SHUSTER L., RAND W. M. & GROSS K. L. (2000) Effect of dietary protein content and tryptophan supplementation on dominance aggression, territorial aggression, and hyperactivity in dogs. Journal of the American Veterinary Medical Association 217, 504–508 10.2460/javma.2000.217.504 [DOI] [PubMed] [Google Scholar]
- DESTEFANIS S., GIRETTO D., MUSCOLO M. C., DI CERBO A., GUIDETTI G., CANELLO S., GIOVAZZINO A., CENTENARO S. & TERRAZZANO G. (2016) Clinical evaluation of a nutraceutical diet as an adjuvant to pharmacological treatment in dogs affected by Keratoconjunctivitis sicca. BMC Veterinary Research 12, 214 10.1186/s12917-016-0841-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI CERBO A., CENTENARO S., BERIBE F., LAUS F., CERQUETELLA M., SPATERNA A., GUIDETTI G., CANELLO S. & TERRAZZANO G. (2016) Clinical evaluation of an antiinflammatory and antioxidant diet effect in 30 dogs affected by chronic otitis externa: preliminary results. Veterinary Research Communications 40, 29–38 10.1007/s11259-015-9651-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI CERBO A., PALATUCCI A. T., RUBINO V., CENTENARO S., GIOVAZZINO A., FRACCAROLI E., CORTESE L., RUGGIERO G., GUIDETTI G., CANELLO S. & TERRAZZANO G. (2015) Toxicological implications and inflammatory response in human lymphocytes challenged with oxytetracycline. Journal of Biochemical and Molecular Toxicology 30, 170–177 10.1002/jbt.21775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODMAN N. H., REISNER I., SHUSTER L., RAND W., LUESCHER U. A., ROBINSON I. & HOUPT K. A. (1996) Effect of dietary protein content on behavior in dogs. Journal of the American Veterinary Medical Association 208, 376–379 [PubMed] [Google Scholar]
- DRESCHEL N. A. (2010) The effects of fear and anxiety on health and lifespan in pet dogs. Applied Animal Behaviour Science 125, 157–162 10.1016/j.applanim.2010.04.003 [DOI] [Google Scholar]
- ERNST E. (2007) Herbal remedies for depression and anxiety. Advances in Psychiatric Treatment 13, 312–316 10.1192/apt.bp.105.001735 [DOI] [Google Scholar]
- FERNSTROM J. D. & WURTMAN R. J. (1972) Brain serotonin content: physiological regulation by plasma neutral amino acids. Acta Agriculturae Scandinavica Section A Animal Science 178, 414–416 10.1126/science.178.4059.414 [DOI] [PubMed] [Google Scholar]
- FRANK D. (2014) Recognizing behavioral signs of pain and disease: a guide for practitioners. The Veterinary Clinics of North America. Small Animal Practice 44, 507–524 10.1016/j.cvsm.2014.01.002 [DOI] [PubMed] [Google Scholar]
- GLETSU-MILLER N., HANSEN J. M., JONES D. P., GO Y. M., TORRES W. E., ZIEGLER T. R. & LIN E. (2009) Loss of total and visceral adipose tissue mass predicts decreases in oxidative stress after weight-loss surgery. Obesity (Silver Spring) 17, 439–446 10.1038/oby.2008.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUIDETTI G., DI CERBO A., GIOVAZZINO A., RUBINO V., PALATUCCI A. T., CENTENARO S., FRACCAROLI E., CORTESE L., BONOMO M. G., RUGGIERO G., CANELLO S. & TERRAZZANO G (2016) In vitro effects of some botanicals with anti-inflammatory and antitoxic activity. Journal of Immunology Research 2016, 5457010 10.1155/2016/5457010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANUS M., LAFON J. & MATHIEU M. (2004) Double-blind, randomised, placebo-controlled study to evaluate the efficacy and safety of fixed combination containing two plant extracts8Crataegus oxiacantha and Eshscholtzia california) and magnesium in mild-to moderate anxiety disorders. Current Medical Research and Opinion 20, 63–71 10.1185/030079903125002603 [DOI] [PubMed] [Google Scholar]
- HARGREAVES B. J., KRONFELD D. S., WALDRON J. N., LOPES M. A., GAY L. S., SAKER K. E., COOPER W. L., SKLAN D. J. & HARRIS P. A. (2002) Antioxidant status and muscle cell leakage during endurance exercise. Equine Veterinary Journal. Supplement 34, 116–121 10.1111/j.2042-3306.2002.tb05402.x [DOI] [PubMed] [Google Scholar]
- HATTESOHL M., FEISTEL B., SIEVERS H., LEHNFELD R., HEGGER M. & WINTERHOFF H. (2008) Extracts of Valeriana officinalis L. s.l. show anxiolytic and antidepressant effects but neither sedative nor myorelaxant properties. Phytomedicine 15, 2–15 10.1016/j.phymed.2007.11.027 [DOI] [PubMed] [Google Scholar]
- HOKARI R., MATSUNAGA H. & MIURA S. (2013) Effect of dietary fat on intestinal inflammatory diseases. Journal of Gastroenterology and Hepatology 28(Suppl 4), 33–36 10.1111/jgh.12252 [DOI] [PubMed] [Google Scholar]
- HORVATHOVA E., NAVAROVA J., GALOVA E., SEVCOVICOVA A., CHODAKOVA L., SNAHNICANOVA Z., MELUSOVA M., KOZICS K. & SLAMENOVA D. (2014) Assessment of antioxidative, chelating, and DNA-protective effects of selected essential oil components (eugenol, carvacrol, thymol, borneol, eucalyptol) of plants and intact Rosmarinus officinalis oil. Journal of Agricultural and Food Chemistry 62, 6632–6639 10.1021/jf501006y [DOI] [PubMed] [Google Scholar]
- HOVATTA I., JUHILA J. & DONNER J. (2010) Oxidative stress in anxiety and comorbid disorders. Journal of Neuroscience Research 68, 261–275 10.1016/j.neures.2010.08.007 [DOI] [PubMed] [Google Scholar]
- INAGAWA K, SEKI S., BANNAI M., TAKEUCHI Y., MORI Y. & TAKAHASHI M. (2005) Alleviative effects of gamma- acid (GABA) on behavioral abnormalities in aged dogs. Journal of Veterinary Medical Science 67, 1063–1066 10.1292/jvms.67.1063 [DOI] [PubMed] [Google Scholar]
- JEWELL D. E. & TOLL P. W. (1996) Effects of fiber on food intake in dogs. Veterinary Clinical Nutrition 3, 115–118 [Google Scholar]
- JI L. L. (2008) Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radical Biology & Medicine 44, 142–152 10.1016/j.freeradbiomed.2007.02.031 [DOI] [PubMed] [Google Scholar]
- JUNEJA L. R., CHU D.-C., OKUBO T., NAGATO Y. & YOKOGOSHI H. (1999) L-theanine a unique amino acid of green tea and its relaxation e\x80ect in humans. Trends in Food Science & Technology 10, 199–204 10.1016/S0924-2244(99)00044-8 [DOI] [Google Scholar]
- KANTAK K. M., HEGSTRAND L. R., WHITMAN J. & EICHELMAN B. (1980) Effects of dietary supplements and a tryptophan-free diet on aggressive behavior in rats. Pharmacology Biochemistry and Behavior 12, 173–179 10.1016/0091-3057(80)90351-2 [DOI] [PubMed] [Google Scholar]
- KIRSCHVINK N., ART T., DE MOFFARTS B., SMITH N., MARLIN D., ROBERTS C. & LEKEUX P (2002) Relationship between markers of blood oxidant status and physiological variables in healthy and heaves-affected horses after exercise. Equine Veterinary Journal. Supplement 159–164 [DOI] [PubMed] [Google Scholar]
- KOOPMANS S. J., RUIS M., DEKKER R., VAN DIEPEN H., KORTE M. & MROZ Z. (2005) Surplus dietary tryptophan reduces plasma cortisol and noradrenaline concentrations and enhances recovery after social stress in pigs. Journal of Entomology Series A Physiology & Behavior 85, 469–478 10.1016/j.physbeh.2005.05.010 [DOI] [PubMed] [Google Scholar]
- LAKHAN S. E. & VIEIRA K. F. (2010) Nutritional and herbal supplements for anxiety and anxiety-related disorders: systematic review. Nutrition Journal 9, 42 10.1186/1475-2891-9-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASLEY S. M. & THURMOND J. B. (1985) Interaction of dietary tryptophan and social isolation on territorial aggression, motor activity, and neurochemistry in mice. Psychopharmacology 87, 313–321 10.1007/BF00432714 [DOI] [PubMed] [Google Scholar]
- LOPEZ-BOTE C. J., REY A. I., SANZ M., GRAY J. I. & BUCKLEY D. J. (1997) Dietary vegetable oils and alpha-tocopherol reduce lipid oxidation in rabbit muscle. British Journal of Nutrition 127, 1176–1182 [DOI] [PubMed] [Google Scholar]
- LUCKI I. (1998) The spectrum of behaviors influenced by serotonin. Biological Psychiatry 44, 151–162 10.1016/S0006-3223(98)00139-5 [DOI] [PubMed] [Google Scholar]
- MAIER S. F. & WATKINS L. R. (1998) Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Neuropsychology Review 105, 83–107 10.1037/0033-295X.105.1.83 [DOI] [PubMed] [Google Scholar]
- MAINE I. R., ATTERBURY R. & CHANG K. C. (2015) Investigation into the animal species contents of popular wet pet foods. Acta Veterinaria Scandinavica 57, 7 10.1186/s13028-015-0097-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACHADO D. G., BETTIO L. E., CUNHA M. P., CAPRA J. C., DALMARCO J. B., PIZZOLATTI M. G. & RODRIGUES A. L. (2009) Antidepressant-like effect of the extract of Rosmarinus officinalis in mice: involvement of the monoaminergic system. Progress in Neuro-psychopharmacology & Biological Psychiatry 33, 642–650 10.1016/j.pnpbp.2009.03.004 [DOI] [PubMed] [Google Scholar]
- MIODOWNIK C., MAAYAN R., RATNER Y., LERNER V., PINTOV L., MAR M., WEIZMAN A., RITSNER M. S. (2011) Serum levels of brain-derived neurotrophic factor and cortisol to sulfate of dehydroepiandrosterone molar ratio associated with clinical response to L-theanine as augmentation of antipsychotic therapy in schizophrenia and schizoaffective disorder patients. Clinical Neuropharmacology 34, 155–160 10.1097/WNF.0b013e318220d8c6 [DOI] [PubMed] [Google Scholar]
- MOLLER P., LOFT S., LUNDBY C. & OLSEN N. V. (2001) Acute hypoxia and hypoxic exercise induce DNA strand breaks and oxidative DNA damage in humans. FASEB Journal 15, 1181–1186 10.1096/fj.00-0703com [DOI] [PubMed] [Google Scholar]
- MORIGUCHI T., GREINER R. S. & SALEM N. Jr (2000) Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. Journal of Neurochemistry 75, 2563–2573 10.1046/j.1471-4159.2000.0752563.x [DOI] [PubMed] [Google Scholar]
- NIEMAN D. C. (1997) Immune response to heavy exertion. Journal of Applied Physiology (Bethesda, MD. : 1985) 82, 1385–1394 [DOI] [PubMed] [Google Scholar]
- NOLI C., DELLA VALLE M. F., MIOLO A., MEDORI C., SCHIEVANO C. & SKINALIA G. (2015) Efficacy of ultra-micronized palmitoylethanolamide in canine atopic dermatitis: an open-label multi-centre study. Veterinary Dermatology 26, 432–e101 10.1111/vde.12250 [DOI] [PubMed] [Google Scholar]
- NUTTALL T., URI M. & HALLIWELL R (2013) Canine atopic dermatitis - what have we learned? The Veterinary Record 172, 201–207 10.1136/vr.f1134 [DOI] [PubMed] [Google Scholar]
- ODORE R., DE MARCO M., GASCO L., ROTOLO L., MEUCCI V., PALATUCCI A. T., RUBINO V., RUGGIERO G., CANELLO S., GUIDETTI G., CENTENARO S., QUARANTELLI A., TERRAZZANO G. & SCHIAVONE A. (2015) Cytotoxic effects of oxytetracycline residues in the bones of broiler chickens following therapeutic oral administration of a water formulation. British Poultry Science 94, 1979–1985 10.3382/ps/pev141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- OVERALL K. L. & DUNHAM A. (2002) Clinical features and outcome in dogs and cats with obsessive-compulsive disorder: 126 cases (1989–2000). Journal of the American Veterinary Medical Association 221, 1445–1452 10.2460/javma.2002.221.1445 [DOI] [PubMed] [Google Scholar]
- OVERALL K. L., HAMILTON S. P. & CHANG M. L. (2006) Understanding the genetic basis of canine anxiety: phenotyping dogs for behavioral, neurochemical, and genetic assessment. Journal of Veterinary Behavior 1, 124–141 10.1016/j.jveb.2006.09.004 [DOI] [Google Scholar]
- OWEN C., REES A. M. & PARKER G. (2008) The role of fatty acids in the development and treatment of mood disorders. Current Opinion in Psychiatry 21, 19–24 10.1097/YCO.0b013e3282f29841 [DOI] [PubMed] [Google Scholar]
- PAREDES R. G. & AGMO A. (1992) GABA and behavior: the role of receptor subtypes. Neuroscience & Biobehavioral Reviews 16, 145–170 10.1016/S0149-7634(05)80177-0 [DOI] [PubMed] [Google Scholar]
- PASQUINI A., LUCHETTI E. & CARDINI G. (2010) Evaluation of oxidative stress in hunting dogs during exercise. Research in Veterinary Science 89, 120–123 10.1016/j.rvsc.2010.01.004 [DOI] [PubMed] [Google Scholar]
- PASSANTINO A., QUARTARONE V., PEDILIGGERI M. C., RIZZO M. & PICCIONE G. (2014) Possible application of oxidative stress parameters for the evaluation of animal welfare in sheltered dogs subjected to different environmental and health conditions. Journal of Veterinary Behavior 9, 290–294 10.1016/j.jveb.2014.06.009 [DOI] [Google Scholar]
- PEREMANS K., GOETHALS I., DE VOS F., DOBBELEIR A., HAM H., VAN BREE H., VAN HEERINGEN C. & AUDENAERT K. (2006) Serotonin transporter and dopamine transporter imaging in the canine brain. Nuclear Medicine and Biology 33, 907–913 10.1016/j.nucmedbio.2006.07.013 [DOI] [PubMed] [Google Scholar]
- PIERCY R. J., HINCHCLIFF K. W., DISILVESTRO R. A., REINHART G. A., BASKIN C. R., HAYEK M. G., BURR J. R. & SWENSON R. A. (2000) Effect of dietary supplements containing antioxidants on attenuation of muscle damage in exercising sled dogs. American Journal of Veterinary Research 61, 1438–1445 10.2460/ajvr.2000.61.1438 [DOI] [PubMed] [Google Scholar]
- RALEIGH M. J., BRAMMER G. L., MCGUIRE M. T. & YUWILER A. (1985) Dominant social status facilitates the behavioral effects of serotonergic agonists. Behavioural Brain Research 348, 274–282 10.1016/0006-8993(85)90445-7 [DOI] [PubMed] [Google Scholar]
- RE S., ZANOLETTI M. & EMANUELE E. (2008) Aggressive dogs are characterized by low omega-3 polyunsaturated fatty acid status. Veterinary Research Communications 32, 225–230 10.1007/s11259-007-9021-y [DOI] [PubMed] [Google Scholar]
- REINSTEIN D. K., LEHNERT H., SCOTT N. A. & WURTMAN R. J. (1984) Tyrosine prevents behavioral and neurochemical correlates of an acute stress in rats. Asia Life Sciences 34, 2225–2231 10.1016/0024-3205(84)90209-1 [DOI] [PubMed] [Google Scholar]
- REINSTEIN D. K., LEHNERT H. & WURTMAN R. J. (1985) Dietary tyrosine suppresses the rise in plasma corticosterone following acute stress in rats. Asia Life Sciences 37, 2157–2163 10.1016/0024-3205(85)90566-1 [DOI] [PubMed] [Google Scholar]
- REISNER I. R., MANN J. J., STANLEY M., HUANG Y. Y. & HOUPT K. A. (1996) Comparison of cerebrospinal fluid monoamine metabolite levels in dominant-aggressive and non-aggressive dogs. Behavioural Brain Research 714, 57–64 10.1016/0006-8993(95)01464-0 [DOI] [PubMed] [Google Scholar]
- RIAZ A. & KHAN R. A. (2014) Effect of Punica Granatum on behavior in rats. African Journal of Pharmacy and Pharmacology 8, 1118–1126 [Google Scholar]
- SECHI S., CHIAVOLELLI F., SPISSU N., DI CERBO A., CANELLO S., GUIDETTI G., FIORE F. & COCCO R. (2015) An antioxidant dietary supplement improves brain-derived neurotrophic factor levels in serum of aged dogs: preliminary results. Journal of Veterinary Medicine 2015, 9 10.1155/2015/412501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN C. K. & PACKER L. (2000) Thiol homeostasis and supplements in physical exercise. American Journal of Clinical Nutrition 72, 653S–669S [DOI] [PubMed] [Google Scholar]
- SIH A., BELL A. & JOHNSON J. C. (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends in Ecology & Evolution 19, 372–378 10.1016/j.tree.2004.04.009 [DOI] [PubMed] [Google Scholar]
- SONNTAG Q. & OVERALL K. L. (2014) Key determinants of dog and cat welfare: behaviour, breeding and household lifestyle. Revue Scientifique et Technique 33, 213–220 [DOI] [PubMed] [Google Scholar]
- STEIN D. J., DODMAN N. H., BORCHELT P. & HOLLANDER E. (1994) Behavioral disorders in veterinary practice: relevance to psychiatry. Comprehensive Psychiatry 35, 275–285 10.1016/0010-440X(94)90019-1 [DOI] [PubMed] [Google Scholar]
- STOLL A. L., SEVERUS W. E., FREEMAN M. P., RUETER S., ZBOYAN H. A., DIAMOND E., CRESS K. K. & MARANGELL L. B. (1999) Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Archives of General Psychiatry 56, 407–412 10.1001/archpsyc.56.5.407 [DOI] [PubMed] [Google Scholar]
- SUDATI J. H., FACHINETTO R., PEREIRA R. P., BOLIGON A. A., ATHAYDE M. L., SOARES F. A., DE VARGAS BARBOSA N. B. & ROCHA J. B. (2009) In vitro antioxidant activity of valeriana officinalis against different neurotoxic agents. Neurochemical Research 34, 1372–1379 [DOI] [PubMed] [Google Scholar]
- SWARNAMONI D. & PHULEN S. (2014) A study on the anticonvulsant and anti anxiety activity of ethanolic extract of Punica granatum Linn. International Journal of Pharmaceutical Sciences 6, 389–392 [Google Scholar]
- TOBLER P. N., DICKINSON A. & SCHULTZ W. (2003) Coding of predicted reward omission by dopamine neurons in a conditioned inhibition paradigm. European Journal of Neuroscience 23, 10402–10410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOKER G., ASLAN M., YESILADA E., MEMISOGLU M. & ITO S. (2001) Comparative evaluation of the flavonoid content in officinal Tiliae flos and Turkish lime species for quality assessment. Journal of Pharmaceutical and Biomedical Analysis 26, 111–121 10.1016/S0731-7085(01)00351-X [DOI] [PubMed] [Google Scholar]
- ULBRICHT C., ABRAMS T. R., BRIGHAM A., CEURVELS J., CLUBB J., CURTISS W., KIRKWOOD C. D., GIESE N., HOEHN K., IOVIN R., ISAAC R., RUSIE E., SERRANO J. M., VARGHESE M., WEISSNER W. & WINDSOR R. C. (2010) An Evidence-Based Systematic Review of Rosemary (Rosmarinus officinalis) by the Natural Standard Research Collaboration. Journal of Dietary Supplements 7, 351–413 10.3109/19390211.2010.525049 [DOI] [PubMed] [Google Scholar]
- VALVASSORI S. S., DAL-PONT G. C., STECKERT A. V., VARELA R. B., LOPES-BORGES J., MARIOT E., RESENDE W. R., ARENT C. O., CARVALHO A. F. & QUEVEDO J. (2015) Sodium butyrate has an antimanic effect and protects the brain against oxidative stress in an animal model of mania induced by ouabain. Psychiatry Research 235, 154–159 10.1016/j.psychres.2015.11.017 [DOI] [PubMed] [Google Scholar]
- VERMEIRE S., AUDENAERT K., DE MEESTER R., VANDERMEULEN E., WAELBERS T., DE SPIEGELEER B., EERSELS J., DOBBELEIR A. & PEREMANS K. (2012) Serotonin 2A receptor, serotonin transporter and dopamine transporter alterations in dogs with compulsive behaviour as a promising model for human obsessive-compulsive disorder. Psychiatry Research 201, 78–87 10.1016/j.pscychresns.2011.06.006 [DOI] [PubMed] [Google Scholar]
- VERMEIRE S. T., AUDENAERT K. R., DOBBELEIR A. A., DE MEESTER R. H., DE VOS F. J. & PEREMANS K. Y. (2009) Evaluation of the brain 5-HT2A receptor binding index in dogs with anxiety disorders, measured with 123I-5I-R91150 and SPECT. Journal of Nuclear Medicine 50, 284–289 10.2967/jnumed.108.055731 [DOI] [PubMed] [Google Scholar]
- VIOLA H., WOLFMAN C., LEVI DE STEIN M., WASOWSKI C., PENA C., MEDINA J. H. & PALADINI A. C. (1994) Isolation of pharmacologically active benzodiazepine receptor ligands from Tilia tomentosa (Tiliaceae). Journal of Ethnopharmacology 44, 47–53 10.1016/0378-8741(94)90098-1 [DOI] [PubMed] [Google Scholar]
- WANG J., ZHAO J., LIU H., ZHOU L., LIU Z., WANG J., HAN J., YU Z. & YANG F. (2010) Chemical Analysis and Biological Activity of the Essential Oils of Two Valerianaceous Species from China: Nardostachys chinensis and Valeriana officinalis. Biomacromolecules 15, 6411–6422 10.3390/molecules15096411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y., HUANG S., SHAO S., QIAN L. & XU P. (2012) Studies on bioactivities of tea (Camellia sinensis L.) fruit peel extracts: Antioxidant activity and inhibitory potential against α-glucosidase and α-amylase in vitro. Industrial Crops and Products 37, 520–526 10.1016/j.indcrop.2011.07.031 [DOI] [Google Scholar]
- WATSON T. A., MACDONALD-WICKS L. K. & GARG M. L. (2005) Oxidative stress and antioxidants in athletes undertaking regular exercise training. International Journal of Sport Nutrition and Exercise Metabolism 15, 131–146 [DOI] [PubMed] [Google Scholar]
- WRIGHT H. F., MILLS D. S. & POLLUX P. M. J. (2011) Development and Validation of a Psychometric Tool for Assessing Impulsivity in the Domestic Dog (Canis familiaris). International Journal of Comparative Psychology 24, 210–225 [Google Scholar]
- WRIGHT H. F., MILLS D. S. & POLLUX P. M. (2012) Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Journal of Entomology Series A Physiology & Behavior 105, 676–682 10.1016/j.physbeh.2011.09.019 [DOI] [PubMed] [Google Scholar]
- YURAYART C., NUCHNOUL N., MOOLKUM P., JIRASUKSIRI S., NIYOMTHAM W., CHINDAMPORN A., KAJIWARA S. & PRAPASARAKUL N. (2013) Antifungal agent susceptibilities and interpretation of Malassezia pachydermatis and Candida parapsilosis isolated from dogs with and without seborrheic dermatitis skin. Medical Mycology 51, 721–730 10.3109/13693786.2013.777165 [DOI] [PubMed] [Google Scholar]