Abstract
Mitochondria play a key role in maintaining cellular metabolic homeostasis. These organelles have a high plasticity and are involved in dynamic processes such as mitochondrial fusion and fission, mitophagy and mitochondrial biogenesis. Type 2 diabetes is characterised by mitochondrial dysfunction, high production of reactive oxygen species (ROS) and low levels of ATP. Mitochondrial fusion is modulated by different proteins, including mitofusin-1 (MFN1), mitofusin-2 (MFN2) and optic atrophy (OPA-1), while fission is controlled by mitochondrial fission 1 (FIS1), dynamin-related protein 1 (DRP1) and mitochondrial fission factor (MFF). PARKIN and (PTEN)-induced putative kinase 1 (PINK1) participate in the process of mitophagy, for which mitochondrial fission is necessary. In this review, we discuss the molecular pathways of mitochondrial dynamics, their impairment under type 2 diabetes, and pharmaceutical approaches for targeting mitochondrial dynamics, such as mitochondrial division inhibitor-1 (mdivi-1), dynasore, P110 and 15-oxospiramilactone. Furthermore, we discuss the pathophysiological implications of impaired mitochondrial dynamics, especially in type 2 diabetes.
Abbreviations: AMPK, AMP-activated protein kinase; ATF6, activating transcription factor 6; BNIP3, BCL2/adenovirus E1B 19 kDa interacting protein 3; CHOP, C/EBP homologous protein; DRP1, dynamin-related protein 1; FIS1, fission protein 1; GRP78, 78 kDa glucose-regulated protein; IMM, inner mitochondrial membrane; LC3, 1 A/1B-light chain 3; mdivi-1, mitochondrial division inhibitor-1; MFF, mitochondrial fission factor; MFN1, mitofusin 1; MFN2, mitofusin 2; MiD49, mitochondrial dynamics proteins of 49; MiD51, mitochondrial dynamics proteins of 51 kDa; mtDNA, mitochondrial DNA; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor kappa B; Nox 4, NADPH oxidase-4; OMM, outer mitochondrial membrane; OPA1, optic atrophy 1; p38 MAPK, p38 mitogen-activated protein kinase; PINK1, (PTEN)-induced putative kinase 1; ROS, reactive oxygen species; S3, 15-Oxospiramilactone; SIRT1/3, sirtuin 1/3; SOD, superoxide dismutase; sXBP1, spliced X-box binding protein 1; TGF-β1, transforming growth factor-β1; TRX2, thioredoxin 2; TXNIP, thioredoxin interacting protein; p62/SQSTM1, ubiquitin and sequestosome-1; UCP-1, uncoupling protein-1; ΔΨm, mitochondrial membrane potential
Keywords: Mitochondrial dynamics, Type 2 diabetes, Redox biology, Oxidative stress
1. Introduction
Hyperglycemia and type 2 diabetes are directly related to oxidative stress. In fact, a high production of reactive oxygen species (ROS) and a subsequent change in redox state and cellular homeostasis has been described in type 2 diabetes. Mitochondria are one of the main sources of ROS and the major site of ATP production. When levels of glucose are high, mitochondria enhance ROS production and induce oxidative stress and tissue damage as a result [1].
Mitochondrial impairment can also contribute to the development of age-dependent insulin resistance [2]. Mitochondria biogenesis contributes to modulate the energy balance, and an enhanced production of ROS by the electron transport chain under hyperglycemic conditions is thought to exacerbate pathological pathways, leading to diabetic microvascular (nephropathy, retinopathy and neuropathy) and macrovascular (stroke, myocardial ischemia) complications [3].
All of the aforementioned characteristics suggest that mitochondria play a key role in insulin resistance in general and in type 2 diabetes in particular. Therefore, mitochondrial quality must be very well controlled. Different mechanisms have been used by mitochondria to control their homeostasis; for example, mitochondrial fusion and fission are key to the repair of mitochondrial damaged components, allowing the exchange of material between damaged and non-damaged mitochondria via the fusion process, or segregation of damaged components via the fission process [4], [5]. Other mechanisms for maintaining mitochondrial homeostasis are the proteolytic system, the proteasome, and the formation of mitochondria-derived vesicles under oxidative stress conditions which can be degraded by lysosomes. Damaged mitochondria can also form autophagosomes to trigger their degradation into the lysosomes via mitophagy [[6], [7], [8]]. All of these aspects suggest that mitochondria may play a critical role in the pathophysiology of diabetes. It is important to take into account that mitochondria are not static; in fact they are highly dynamic and constantly changing in shape, size and location within the cells, which confers them high plasticity.
In type 2 diabetic patients, high levels of glucose can induce glucose oxidation, thereby generating pyruvate and NADH. Furthermore, ROS are released from mitochondrial complex I and III. In these conditions different antioxidant systems are triggered, such as manganese superoxide dismutase (SOD) or uncoupling protein-1 (UCP-1), to prevent ROS production and inhibit the formation of advanced glycation end products or nuclear factor kappa beta (NF-κB) activation [9], thus impeding a chronic proinflammatory state.
In this context, it is important to highlight the concept of mitochondrial hormesis in type 2 diabetes [10], which involves a reduction of ROS production and ATP synthesis in different tissues in response to high levels of glucose or excess of nutrient intake, constituting a compensatory response to overnutrition. This effect can activate sirtuin 1/3 (SIRT1/3), AMP-activated protein kinase (AMPK) and PGC-1α, therefore restoring mitochondrial function and increasing insulin sensitivity in β-cells, liver and muscle, which, in turn, prevents vascular complications [10].
In summary, mitochondrial function depends on their quality control, and an essential characteristic of this quality control is the high level of plasticity in their dynamic structures, which allows them to constantly change by fusion and fission processes [11]. Mitochondrial dysfunction, on the other hand, can play a critical role in the development of type 2 diabetes and insulin resistance-related diseases.
In the present review, mitochondrial fusion and fission dynamics, their alteration in type 2 diabetes, and the targeting of mitochondrial dynamics will be discussed, in addition to the pathophysiological implications of these aspects.
2. Mitochondrial fusion and fission dynamics
During physiological conditions mitochondria undergo morphologic changes in order to adapt to cellular energetic demands. These changes can occur through the continuous cycles of mitochondrial fusion and fission that allow an adequate distribution of mitochondria within the cells. Therefore, mitochondria are not static organelles; they change their shape and location depending on physiological stimuli. Mitochondrial fission produces small individual mitochondria, whereas large interconnected networks of mitochondria are generated through fusion. The morphology of mitochondria varies widely across different cell types; hepatocytes, for example, have small spherical or oval mitochondria, whereas fibroblast mitochondria are long filaments [[12], [13]].
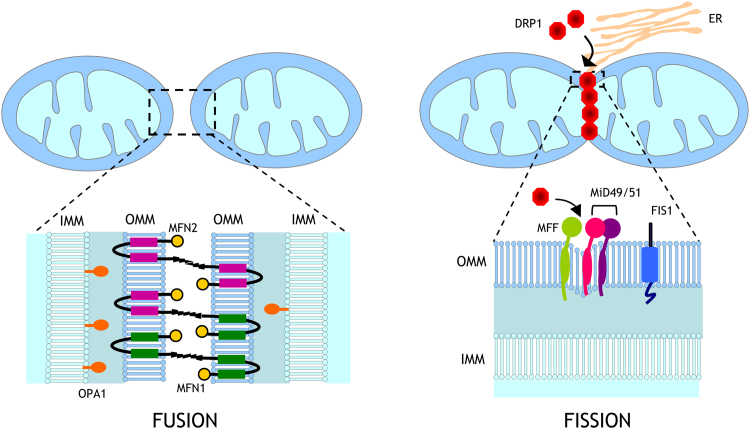
Several dynamin-related GTPases constitute the core machinery of mitochondrial fusion/fission processes. Mitofusin (MFN) 1 and 2 are responsible for outer mitochondrial membrane (OMM) fusion. MFN1 proteins can interact intermitochondrially, tethering two opposing mitochondria through their HR2 domains [14]. As for MFN1, MFN2 proteins interact among themselves, but they also heterooligomerize with MFN1 to promote mitochondrial fusion (Fig. 1) [15]. MFN2 also participates in the physical interaction between endoplasmic reticulum and the mitochondria, which is essential for Ca2+ signalling [16]. Whereas mitofusins mediate fusion of the OMM, fusion of the inner mitochondrial membrane (IMM) is orchestrated by optic atrophy 1 (OPA1) protein, which is also involved in maintenance of the mitochondrial cristae structure [17]. On the other hand, fission proteins include dynamin-related protein 1 (DRP1) and fission protein 1 (FIS1). DRP1 molecules assemble into a ring-like structure to constrict mitochondrial membranes in a GTP-dependent manner, while FIS1 is anchored to the OMM and seems to participate in the recruitment of DRP1 through its cytosolic domain [18]. Other OMM proteins that mediate the recruitment of DRP1 are mitochondrial fission factor (MFF) and the mitochondrial dynamic proteins of 49 (MiD49) and 51 kDa (MiD51), the latter two being sufficient to mediate fission in the absence of FIS1 and MFF [19] (Fig. 1).
Fig. 1.
Regulation of mitochondrial fusion and fission. Mitochondrial fusion is mediated by homo- and heterotypic interactions between mitofusin (MFN) 1 and 2 at the outer mitochondrial membrane and optic atrophy protein 1 (OPA1) at the inner mitochondrial membrane. Receptor-mediated recruitment by dynamin-related protein-1 (DRP1) from the cytosol to the outer mitochondrial membrane by fission protein 1 (FIS1), mitochondrial fission factor (MFF), and mitochondrial dynamics proteins of 49 and 51 kDa (MiD49/51) to sites of division marked by endoplasmic reticulum drives mitochondrial fission. DRP-1, dynamin-related protein-1; FIS 1, fission protein 1; IMM, inner mitochondrial membrane; OMM, outer mitochondrial membrane; MFN 1/2, mitofusins 1 and 2; OPA1, optical atrophy 1; MFF, mitochondrial fission factor; MiD49, mitochondrial dynamics protein of 49 kDa; MiD51, mitochondrial dynamics protein of 51 kDa; ER, endoplasmic reticulum.
Fusion and fission processes are essential for the maintenance of important cellular functions such as mitochondrial respiratory activity, mitochondrial DNA (mtDNA) distribution, apoptosis, cell survival or calcium signalling. ATP production is modulated by mitochondrial networks generated by fusion and this pathway is controlled by transmitting the membrane potential from areas of high O2 availability to those with low availability, thus allowing the dissipation of energy [12]. Whereas this pro-fusion state is typical in situations of increased energy efficiency due to starvation or acute stress, the opposite occurs when cells are subjected to a large nutrient supply such as in obesity or type 2 diabetes. Exposure to an excess nutrient environment promotes mitochondrial fission and decreases mitochondrial fusion, which is related to uncoupled respiration [20]. In addition, mitochondrial fission is crucial for the removal of damaged mitochondria by mitophagy [21], as discussed later.
Therefore, the regulation of mitochondrial dynamics is a complex process involving different dynamin-related GTPases that maintain a balance between mitochondrial fusion and fission. Any alteration of this balance can involve oxidative stress, mitochondrial dysfunction and metabolic alterations, eventually promoting the development of mitochondria-related diseases, such as insulin resistance and type 2 diabetes. In this sense, several studies suggest that genetic ablation of fusion proteins alters glucose homeostasis and promotes insulin resistance and obesity in mice [22], [23]. In addition, recent evidence shows that genetic ablation of Drp1 or Mfn1 in the liver protects mice against HFD-induced obesity and insulin resistance [24], [25]. These findings highlight the important role of mitochondrial dynamics in the regulation of glucose metabolism and insulin signalling, and, in turn, in the development of obesity and type 2 diabetes.
2.1. Mitochondrial dynamics and mitophagy
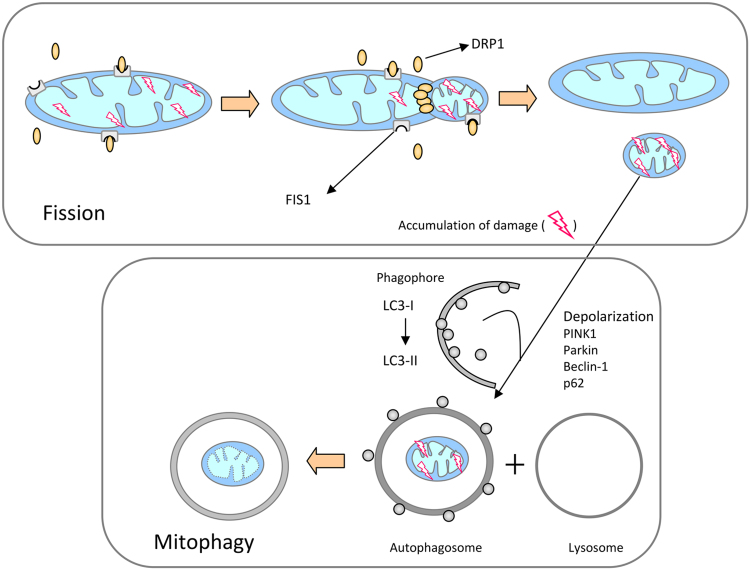
The maintenance of a healthy mitochondrial population is essential for cell survival. Cells employ the mechanism of autophagy to remove defective organelles and recycle their essential components through their encapsulation by a double-membrane structure known as the autophagosome. In the case of mitochondria, this mechanism is known as mitophagy [26]. The importance of a proper regulation of mitophagy lies in the fact that when a damaged mitochondrion fuses with a healthy one, the result is not a larger healthy organelle, but rather a larger damaged mitochondrion, which could expand the damage by releasing high amounts of ROS [27]. In this sense, mitochondrial fission plays a central role, since mitophagy is preceded by mitochondrial division, which generates individual mitochondrial fragments of manageable size for encapsulation [8] (Fig. 2). To achieve this pro-fission state, the fusion proteins MFN1 and MFN2 are degraded by the ubiquitin proteasome system during the induction of mitophagy, whereas OPA1 is degraded by the IMM zinc metalloprotease OMA1 and AAA proteases [28]. The main orchestrators of the process of mitophagy are (PTEN)-induced putative kinase 1 (PINK1), the ubiquitin ligase PARKIN, ubiquitin and sequestosome-1 (p62/SQSTM1). PINK1 and PARKIN are both indispensable for mitophagy, since loss of any of these proteins results in failure of selective mitochondrial clearance [29]. Upon malfunctioning of a mitochondrion, it suffers a depolarization, which interrupts normal proteolytic processing of PINK1, leading to PINK1 accumulation in the mitochondrion and phosporylation of its targets, which include ubiquitine and PARKIN. Afterwards, PARKIN mediates the ubiquitination of the OMM, tagging it for p62 binding, which is linked to the autophagosomal microtubule-associated protein 1 A/1B-light chain 3 (LC3), leading eventually to the targeting of the mitochondrion for mitophagy [30].
Fig. 2.
Schematic representation of mitochondrial fission and mitophagy. Mitophagy is preceded by mitochondrial division, which generates individual mitochondrial fragments of feasible size for encapsulation. Upon malfunctioning of a mitochondrion, it suffers a depolarization, which interrupts normal proteolytic processing of PINK1, leading to PINK1 accumulation in the mitochondrion and phosporylation of its targets, which include ubiquitine and PARKIN. Afterwards, PARKIN mediates the ubiquitination of the OMM, tagging it for p62 binding, which is linked to the autophagosomal LC3 protein, leading eventually to the targeting of the mitochondrion for mitophagy. DRP-1, dynamin-related protein-1; FIS1, fission 1 protein 1; PINK1, (PTEN)-induced putative kinase 1; LC3, microtubule-associated protein 1 A/1B-light chain 3; p62, ubiquitin-binding protein 62.
Alterations in the expression of some of the aforementioned proteins alter mitochondrial dynamics and mitophagic recycling, and can have an impact on the development of certain diseases. Examples of this are mutations in parkin, which cause early-onset familial Parkinson's disease through defects in mitophagy, and the loss of function of pink1, which is involved in a recessive familial form of Parkinson's disease, by causing an elongation of mitochondrial filaments. Both types of genetic mutation lead to neuronal alterations related to the development of parkinsonism [31], [32].
3. Type 2 diabetes and mitochondrial dynamics
Mitochondrial dynamics is crucial in type 2 diabetes and its vascular complications. In fact, mitochondria are master regulators of insulin secretion, and mtDNA mutations have been related to the development of type 2 diabetes. In fact, a novel mutation m.8561 C>G in MT-ATP6/8 (subunits of mitochondrial ATP synthase) has recently been reported to cause diabetes mellitus and hypergonadotropic hypogonadism [33]. In the study in question, the authors demonstrated that this mutation leads biochemically to impaired assembly and decreased ATP production of mitochondrial ATP synthase. Furthermore, it is important to take into account that mitochondria are one of the main sources of ROS and also mediate cellular apoptosis and cell death.
β-cells synthesize insulin, and this secretion is modulated mainly by glucose levels. In relationship to this, an enhanced level of glucose induces oxidative phosphorylation in β- cells, increasing the ATP/ADP ratio and inhibiting K+ channels that can depolarize the plasma membrane and lead to enhanced Ca2+ levels inside the cells. This effect triggers the secretion of insulin into the blood stream in order to maintain adequate levels of glucose [[34], [35]]. Therefore, insulin secretion is modulated by the bioenergetic state of mitochondria.
Insulin resistance is a common characteristic of type 2 diabetes and mitochondrial dysfunction has been associated with it [[36], [37], [38], [39]]. An inhibition of mitochondrial function and an increase in lipid peroxidation have been described in skeletal muscle of insulin-resistant patients [40]. Furthermore, a downregulation of genes involved in mitochondrial biogenesis and oxidative phosphorylation has been observed in type 2 diabetes [41]. Recently, it has been demonstrated that obese patients presented a global expressional downregulation of mitochondrial oxidative pathways with concomitant downregulation of mtDNA, mtDNA-dependent translation system and protein levels of the oxidative phosphorylation machinery, when compared with lean co-twins. In fact, a downshifting of fatty acid oxidation, ketone body production and breakdown, and tricarboxylic acid cycle was observed and inversely correlated with insulin resistance, adiposity and inflammatory cytokines [42].
There is evidence that exercise, resveratrol, mitochondrial-targeted antioxidants and caloric restriction improve insulin sensitivity and mitochondrial function in type 2 diabetes [[43], [44], [45], [46], [47]] (Fig. 3). For example, metformin and resveratrol have demonstrated beneficial effects by protecting mitochondrial integrity through the inhibition of DRP1 activity, and preventing NLRP3 inflammasome activation by suppressing endoplasmic reticulum stress, thereby protecting cell function under hyperglycemic conditions [48].
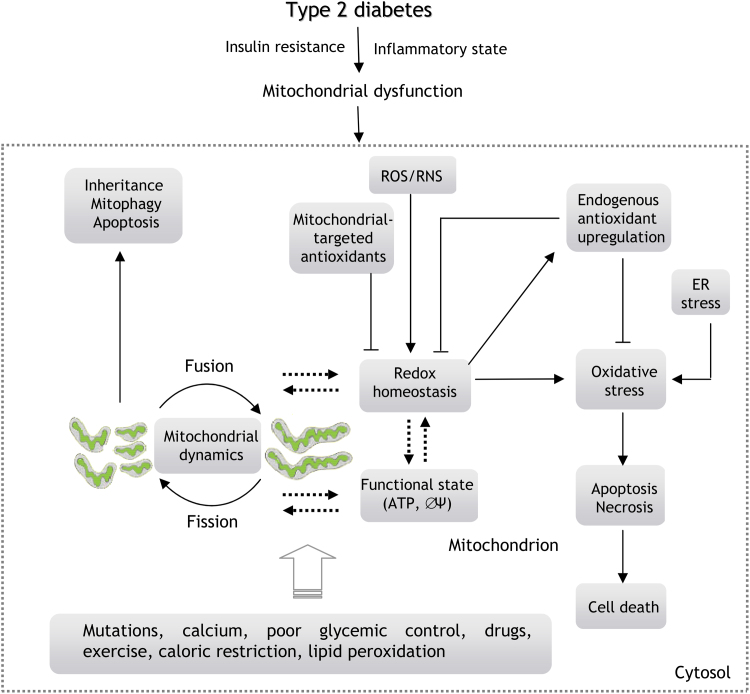
Fig. 3.
Interaction between mitochondrial dynamics, oxidative stress and mitochondrial function in type 2 diabetes. Mitochondria are one of the main sources of ROS/RNS and also mediate cellular apoptosis and cell death. Oxidative stress and type 2 diabetes are related to changes in the ΔΨm that could be related to altered mitochondrial dynamics. There is evidence that exercise, drugs, mitochondrial-targeted antioxidants and caloric restriction improve insulin sensitivity and mitochondrial function in type 2 diabetes. In contrast, mtDNA mutations, enhanced calcium levels, poor glycemic control or lipid peroxidation enhance oxidative stress and can be related to the development of type 2 diabetes.
Mitochondrial-targeted antioxidants such as SS-31 have also been shown to exert beneficial effects by modulating mitochondrial membrane potential (ΔΨm) and ATP alterations and inhibiting the expression of NADPH oxidase-4 (Nox 4) and TGF-β1 (TGF-β1) and the activation of p38 mitogen-activated protein kinase (p38 MAPK) and NADPH oxidase activity in mesangial cells under hyperglycemia. SS-31 treatment also upregulated the expression of thioredoxin 2 (TRX2), demonstrating beneficial effects in the treatment of diabetic nephropathy [49].
Exercise can also improve mitochondrial function and insulin sensitivity, as demonstrated in numerous studies. In this sense, we would like to highlight a recently published review [50] that summarises the effect of a planned self-directed exercise intervention in individuals with type 2 diabetes; namely an improvement in HbA1c, cardiorespiratory fitness, physical characteristics and functional measures.
Finally, caloric restriction has also been demonstrated to exert beneficial effects in type 2 diabetic patients. For example, the study by Sathananthan et al. showed that six weeks of caloric restriction decreased fasting glucose and endogenous glucose production, with subsequent improvements in β cell function in type 2 diabetic patients [51]. An additional 6 weeks of caloric restriction maintained the improvement in glucose metabolism.
Oxidative stress and type 2 diabetes are related to changes in the ΔΨm of β-cells. This alteration in β-cells could be closely related to altered mitochondrial dynamics, leading to impaired glucose-stimulated insulin secretion, as suggested by previous studies [52], [53]. During type 2 diabetes, fusion and fission processes are continuously altered in β-cells, and it has been demonstrated that hyperglycemia and enhanced levels of palmitate decrease fusion and inhibit mitochondrial oxygen consumption [54], [55]. Furthermore, one recent study has demonstrated that PINK1 alleviates palmitate-induced insulin resistance in hepatic cells by suppressing ROS-mediated MAPK pathways [56].
Type 2 diabetes is related to a reduced expression of MFN2, which may be linked to an impairment of mitochondrial function in skeletal muscle [57]. Our group has recently demonstrated that mitochondrial fusion was reduced and fission enhanced in leukocytes from type 2 diabetic patients, while both features were accentuated in patients with poor glycemic control [36]. In addition, we demonstrated that leukocyte rolling flux increased in parallel to HbA1c levels, and that induction of leukocyte-endothelial interactions in diabetic patients was related to reduced mitochondrial fusion and higher mitochondrial fission. Our results suggest that mitochondrial dynamics is influenced by glycemic control in the leukocytes of diabetic patients, in which there is a decreased mitochondrial fusion and elevated fission related to enhanced leukocyte-endothelial interactions. We hypothesise that poor glycemic control during type 2 diabetes alters mitochondrial dynamics and eventually promotes leukocyte-endothelial interactions and the onset of cardiovascular diseases [36].
Other studies in insulin resistant patients have demonstrated that exercise promotes an improvement in fat oxidation and insulin sensitivity, as well as a decrease in DRP1 and an increase of MFN1 and MFN2 expression [58]. In addition, it has recently been shown that exercise prevents the decline in the MFN2/DRP1 ratio in the db/db mice hearts, and that exercise attenuates ΔΨm decline and cytochrome c leakage. The authors is question concluded that moderate intensity exercise produces significant cardiovascular benefits by improving mitochondrial function through restoration of mitochondrial biogenesis regulators (MFN2 and DRP1), Cx43 networks and mitochondrial ΔΨm and prevention of excessive mitochondrial fission [59].
Furthermore, in recent years it has been demonstrated that mitochondria-associated endoplasmic reticulum membranes reorganize under insulin resistance conditions and decrease O2 consumption, thereby enhancing oxidative stress [60]. In our laboratory, we have demonstrated that 78 kDa glucose-regulated protein (GRP78) levels are higher in type 2 diabetic patients than control subjects; whereas patients with good glycemic control (HbA1c <7%) displayed higher levels of spliced X-box binding protein 1 (sXBP1), and those with HbA1c >7% exhibited preferentially enhanced levels of C/EBP homologous protein (CHOP) and activating transcription factor 6 (ATF6). Our findings lead to the hypothesis of an association between poor glycemic control in type 2 diabetes and increased leukocyte ROS production and chronic endoplasmic reticulum stress. This eventually promotes leukocyte-endothelial interactions, which, in turn, poses a risk of vascular complications for these patients [61].
Changes in shape or size of mitochondria have been observed in diabetic patients and animal models. Mitochondria from type 2 diabetic patients are smaller than in healthy controls [40], and hyperglycemia induce mitochondrial fragmentation in different cell types, including heart, liver, cardiovascular or pancreas [62], [63]. Another example are hepatocytes from insulin resistant patients, in which mitochondria are swollen and disrupted [64].
Mitochondrial dynamics is related to ROS production under hyperglycemic conditions [[65], [63]]. In fact, high glucose levels induce ROS production and fragmentation, and when ROS decrease mitochondria recover their activity. Yu et al. have described that, in the presence of high levels of glucose, morphological alterations of mitochondria are an upstream causal factor in ROS production, highlighting the key role of mitochondrial dynamics as a master regulator of mitochondrial activity [63]. However, the mechanisms by which mitochondrial morphology disturb ROS under hyperglycemia are not clear. Furthermore, it is important to note that abnormal glucose homeostasis may not always be a cause, but rather a consequence, of altered mitochondrial dynamics, as suggested by several studies [22], [23], [25].
In relationship to this, it has recently been demonstrated that livers from mfn1 knockout mice display a highly fragmented mitochondrial network. This effect was coupled with an increased mitochondrial respiration capacity and a preference for lipids as the main energy source. mfn1 knockout mice were found to be protected in a high-fat diet-induced insulin resistance model. In fact, mfn1 deficiency increased Complex I abundance and sensitized animals to the hypoglycemic effect of metformin. The authors concluded that targeting mfn1 could provide novel improvements in glucose homeostasis in insulin-resistant patients and improve the effectiveness of metformin [24].
Montaigne et al. demonstrated that type 2 diabetes is associated with mitochondrial dysfunction, increased impairment of contraction and enhanced myocardial oxidative stress, regardless of body mass index [66]. Type 2 diabetes was related to mitochondrial network fragmentation in myocardium and a large decrease in mfn1 expression. In another study, Schultz et al. have confirmed that FIS1 is a key regulator in pancreatic β-cells [67]; the authors found that both glucose-stimulated insulin secretion and mitochondrial dynamics were clearly adapted to produce precise expression levels of this fission protein.
4. Targeting mitochondrial fusion and fission
The interest in the development of compounds to be directed to mitochondrial fusion, fission and mitophagy has increased exponentially in the last years [68], highlighting the importance of these targets for therapy against multiple diseases (Fig. 4). For example, mitochondrial division inhibitor-1 (mdivi-1), which can inhibit GTPase activity of DRP1 in cells, has been evaluated [69]. Furthermore, mdivi-1 can inhibit apoptosis by blocking cytochrome C release in HeLa cells [69], and can amielorate renal injury and myocardial infarction under ischemia [70]. Another study has demonstrated that mdivi-1 can reverse the mitochondrial impairment and ROS production induced by palmitic acid by inhibiting DRP1 [71]. Mdivi-1 can also improve insulin sensitivity in conditions of insulin resistance. Some authors have demonstrated that mdivi-1 reduces mitochondrial fragmentation, oxidative stress, inflammation, atherosclerosis and improves endothelial function in diabetic mice [71], [72]. In summary, the beneficial effects of mdivi-1 have been well demonstrated in short-term treatments, suggesting that this therapeutic approach could offer great benefits in the treatment of macrovascular complications in diabetic patients.
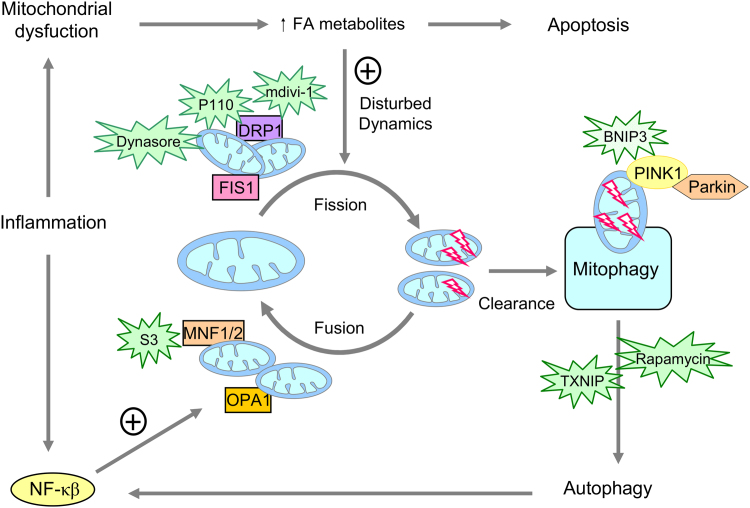
Fig. 4.
Targeting mitochondrial fusion and fission and mitophagy. The different compounds which inhibit mitochondrial fission and can have beneficial effects by favouring mitochondrial fusion are mdivi-1, dynasore, P110 and S3. Other compounds with beneficial effects for mitophagy are BNIP3, rapamycin and TXNIP. mdivi-1, mitochondrial division inhibitor-1; P110, peptide inhibitor; S3, 15-oxospiramilactone; BNIP3, BCL2/adenovirus E1B 19KDa interacting protein 3, TXNIP, thioredoxin interacting protein.
However, other studies have demonstrated that long-term treatment with this compound can inhibit mitochondrial function [73]. Treatment with mdivi-1 for more than 24-h was shown to reduce mitochondrial mass, induce apoptosis and decrease vascular cell bioenergetics and cell proliferation during myogenic differentiation [74]. Therefore, due to the inhibitory effect on cell division, mdivi-1 has the capacity to induce cell death in cancer cells [75], pointing to its potential as a therapy for cancer.
Another compound which can interfere with the GTPase activity of dynamin is dynasore, a small molecule which inhibits endocytic pathways that depend on dynamin [76]. In fact, dynasore can inhibit mitochondrial fission, as DRP1 is a dynamin-like protein. Dynasore can prevent oxidative stress and mitochondrial fission and increase cardiomyocyte survival under ischemic/reperfusion conditions [77]. It can also protect against cardiac lusitropy by maintaining mitochondrial function and morphology and levels of ATP under oxidative stress conditions, thus avoiding cell damage [77].
Taking into account the potential inhibitory effect of dynasore on dynamin-related proteins, its possible secondary effects are a matter of controversy.
Cytosolic DRP1 needs adaptors such as MFF and FIS1 to dock on mitochondria for mitochondrial fission; for this reason, it is important to develop compounds for inhibiting this interaction. P110 is a small peptide inhibitor which reduces DRP1 enzyme activity and is capable of blocking DRP1/FIS1 interactions in neurons [78], having beneficial effects for mitochondrial morphology and function. P110 has also demonstrated neuroprotective effects in Parkinson's disease by reducing neurite loss [78]. Furthermore, in an animal model of brain ischemia/reperfusion damage, it has been shown to inhibit the interaction between DRP1 and p53, thereby reducing brain infarction and mitochondria-related necrosis [79]. P110 can decrease DRP1 Thr595 phosphorylation and mitochondrial dysfunction by inhibiting the interaction between LRRK2 G2019S mutation and DRP1 [80]. Recently, it has been demonstrated that inhibition of DRP1 hyperactivation by P110 is neuroprotective in the MPTP animal model of Parkinson's disease [81]. This effect may be mediated by inhibiting p53-mediated apoptotic pathways in different cells such as neurons through decreasing of DRP1-dependent p53 mitochondrial translocation. In one study, it has been demonstrated that the proliferation-inducing capacity of DRP1-mediated mitochondrial fission is mediated via p53/p21 and NF-κB/cyclins pathways [82]. The authors demonstrated that the crosstalk between p53 and NF-κB pathways is involved in the regulation of mitochondrial fission-mediated cell proliferation, and concluded that DRP1-mediated mitochondrial fission plays a critical role in the regulation of cell cycle progression and cancer cell proliferation. In summary, P110 has demonstrated beneficial effects on mitochondrial-damage in brain diseases and in cancer. It effects on metabolic diseases remain to be studied.
Another compound with beneficial effects for mitochondrial dynamics is 15-Oxospiramilactone (S3), a diterpenoid derivate that acts as an anticancer agent by inhibiting Wnt/β-catenin signalling or inducing BAX/BAK- independent apoptosis [83]. S3 can target deubiquitinase USP30 in mitochondria, an isopeptidase that regulates mitochondrial morphology by deubiquitination of MFN1 and MFN2. This effect can enhance the nondegradative ubiquitination of MFN1/2 that increases MFN1/2 activity and induces mitochondrial fusion [84]. S3 can restore mitochondrial function and fusion in mfn1–/– or mfn2–/– knockout cells, suggesting the therapeutic potential of this compound for treatment of insulin resistance-related diseases [84].
The potential benefits of the abovementioned compounds have been well demonstrated, but it is necessary to consider their possible deleterious effects in long-term treatment; for example, perhaps a partial reduction of fission is more therapeutic than a complete inhibition.
Other strategies have implicated mitophagy in conditions such as myocardial infarction [85] and diabetic nephropathy [86]. The BCL2/adenovirus E1B 19 kDa interacting protein 3 (BNIP3), a mitochondrial BH3-only protein, can interact with PINK1 to promote accumulation of full-length PINK1 on the OMM, and this effect can facilitate PARKIN recruitment and PINK1/PARKIN-mediated mitophagy [87]. Therefore, BNIP3 may be a target for controlling mitochondrial function, enhancing mitophagy and recovery following myocardial ischemia and reperfusion injuries [85]. The impairment of autophagy/mitophagy is a feature of diabetic nephropathy, and various compounds have been shown to modulate autophagy/mitophagy, such as sirolimus (rapamycin), a mammalian target of rapamycin (mTOR) complex inhibitor [86]. Rapamycin protects against glomerulosclerosis and proteinuria [88], [89], renal hypertrophy [90] and mesangial expansion in rodent models [91]. In this sense, it has recently been described that hyperglycemia-induced thioredoxin interacting protein (TXNIP) can activate mTOR signalling pathway contributing to the dysregulation of tubular autophagy and mitophagy in diabetic nephropathy [92] (Fig. 4).
5. Concluding remarks and future perspectives
Mitochondria are highly dynamic organelles and play a very important role in maintaining homeostasis. Furthermore, they are the main source of ROS in the organism and control cellular homeostasis, cell death and apoptosis. Therefore, their functionality is vital for life. Mitochondrial dynamics and mitochondrial biogenesis are processes which are impaired in conditions of insulin resistance in general, and in type 2 diabetes in particular. Therefore, these processes are promising pharmaceutical targets for the treatment of this group of age-related diseases.
The role of mitochondrial dynamics in energy expenditure and nutrient utilization is emerging, and mitochondrial biogenesis, dynamics and mitophagy are related to adaptation to metabolic demands. Nutrient excess induces mitochondrial impairment and fragmentation and can inhibit autophagic flux and promote ROS production. This review has discussed how disturbances in mitochondrial dynamics contribute to the development of insulin resistance condition and type 2 diabetes. Furthermore, we have described compounds which inhibit mitochondrial fission and can have beneficial effects by favouring mitochondrial fusion, such as mdivi-1, dynasore, P110 and S3. Although the molecular mechanisms of mitochondrial dynamics and their relationship with diseases require clarification, the development and design of pharmacological therapies that target mitochondrial dynamics promises benefits in the treatment of cardiometabolic diseases.
Acknowledgements
The authors thank Brian Normanly (University of Valencia-CIBERehd) for his editorial assistance. This study was financed by grants PI15/1424, PI16/1083, PI16/0301 and CIBERehd CB06/04/0071 by Carlos III Health Institute and by the European Regional Development Fund (ERDF “A way to build Europe”); UGP15-193 and UGP15-220 by FISABIO and GV/2016/169 and PROMETEO 2014/035 by the Ministry of Education of the Valencian Regional Government. V.M.V. and M.R. are recipients of contracts from the Ministry of Health of the Valencian Regional Government and Carlos III Health Institute (CES10/030 and CPII16/00037, respectively). C.B. is recipient of a Sara Borrell contract from Carlos III Health Institute (CD14/00043). S.R-L. is recipient of a Juan de la Cierva-Formación contract from the Spanish Ministry of Economy and Competitiveness (FJCI-2015–25040).
No potential conflicts of interest relevant to this article are reported. The authors have nothing to disclose.
References
- 1.Szendroedi J., Phielix E., Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2011;8(2):92–103. doi: 10.1038/nrendo.2011.138. [DOI] [PubMed] [Google Scholar]
- 2.Petersen K.F., Befroy D., Dufour S., Dziura J., Ariyan C., Rothman D.L., DiPietro L., Cline G.W., Shulman G.I. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.Twig G., Elorza A., Molina A.J., Mohamed H., Wikstrom J.D., Walzer G., Stiles L., Haigh S.E., Katz S., Las G., Alroy J., Wu M., Py B.F., Yuan J., Deeney J.T., Corkey B.E., Shirihai O.S. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27(2):433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van der Bliek A.M., Shen Q., Kawajiri S. Mechanisms of mitochondrial fission and fusion. Cold Spring Harb. Perspect. Biol. 2013;5(6) doi: 10.1101/cshperspect.a011072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ding W.X., Yin X.M. Mitophagy: mechanisms, pathophysiological roles, and analysis. Biol. Chem. 2012;393(7):547–564. doi: 10.1515/hsz-2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemasters J.J. Variants of mitochondrial autophagy: Types 1 and 2 mitophagy and micromitophagy (Type 3) Redox Biol. 2014;2:749–754. doi: 10.1016/j.redox.2014.06.004. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011;12(1):9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kiritoshi S., Nishikawa T., Sonoda K., Kukidome D., Senokuchi T., Matsuo T., Matsumura T., Tokunaga H., Brownlee M., Araki E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: potential role in diabetic nephropathy. Diabetes. 2003;52(10):2570–2577. doi: 10.2337/diabetes.52.10.2570. [DOI] [PubMed] [Google Scholar]
- 10.Sharma K. Mitochondrial hormesis and diabetic complications. Diabetes. 2015;64(3):663–672. doi: 10.2337/db14-0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni H.M., Williams J.A., Ding W.X. Mitochondrial dynamics and mitochondrial quality control. Redox Biol. 2015;4:6–13. doi: 10.1016/j.redox.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11(12):872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 13.Youle R.J., van der Bliek A.M. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–1065. doi: 10.1126/science.1219855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galloway C.A., Yoon Y. Mitochondrial morphology in metabolic diseases. Antioxid. Redox Signal. 2013;19(4):415–430. doi: 10.1089/ars.2012.4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detmer S.A., Chan D.C. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J. Cell Biol. 2007;176(4):405–414. doi: 10.1083/jcb.200611080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Brito O.M., Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456(7222):605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 17.Otera H., Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J. Biochem. 2011;149(3):241–251. doi: 10.1093/jb/mvr002. [DOI] [PubMed] [Google Scholar]
- 18.Yoon Y., Galloway C.A., Jhun B.S., Yu T. Mitochondrial dynamics in diabetes. Antioxid. Redox Signal. 2011;14(3):439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loson O.C., Song Z., Chen H., Chan D.C. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell. 2013;24(5):659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wai T., Langer T. Mitochondrial Dynamics and Metabolic Regulation. Trends Endocrino. Metab. 2016;27(2):105–117. doi: 10.1016/j.tem.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Quirós P.M., Ramsay A.J., Sala D., Fernández-Vizarra E., Rodríguez F., Peinado J.R., Fernández-García M.S., Vega J.A., Enríquez J.A., Zorzano A., López-Otín C. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31(9):2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sebastián D., Hernández-Alvarez M.I., Segalés J., Sorianello E., Muñoz J.P., Sala D., Waget A., Liesa M., Paz J.C., Gopalacharyulu P., Orešič M., Pich S., Burcelin R., Palacín M., Zorzano A. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc Natl. Acad. Sci. USA. 2012;109(14):5523–5528. doi: 10.1073/pnas.1108220109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni S.S., Joffraud M., Boutant M., Ratajczak J., Gao A.W., Maclachlan C., Hernandez-Alvarez M.I., Raymond F., Metairon S., Descombes P., Houtkooper R.H., Zorzano A. Cantó C6. Mfn1 Deficiency in the liver protects against diet-induced insulin resistance and enhances the hypoglycemic effect of metformin. Diabetes. 2016 doi: 10.2337/db15-1725. [DOI] [PubMed] [Google Scholar]
- 25.Wang L., Ishihara T., Ibayashi Y., Tatsushima K., Setoyama D., Hanada Y., Takeichi Y., Sakamoto S., Yokota S., Mihara K., Kang D., Ishihara N., Takayanagi R., Nomura M. Disruption of mitochondrial fission in the liver protects mice from diet-induced obesity and metabolic deterioration. Diabetologia. 2015;58(10):2371–2380. doi: 10.1007/s00125-015-3704-7. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Dorn G.W. 2nd, Kitsis R.N. The mitochondrial dynamism-mitophagy-cell death interactome: multiple roles performed by members of a mitochondrial molecular ensemble. Circ. Res. 2015;116(1):167–182. doi: 10.1161/CIRCRESAHA.116.303554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stotland A., Gottlieb R.A. Mitochondrial quality control: Easy come, easy go. Biochim. Biophys. Acta. 2015;1853(10 Pt B):2802–2811. doi: 10.1016/j.bbamcr.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisler S., Holmström K.M., Skujat D., Fiesel F.C., Rothfuss O.C., Kahle P.J., Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010;12(2):119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 30.Shirihai O.S., Song M., Dorn G.W. 2nd. How mitochondrial dynamism orchestrates mitophagy. Circ. Res. 2015;116(11):1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee J.Y., Nagano Y., Taylor J.P., Lim K.L., Yao T.P. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell. Biol. 2010;189(4):671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liesa M., Palacín M., Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol. Rev. 2009;89(3):799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- 33.Kytövuori L., Lipponen J., Rusanen H., Komulainen T., Martikainen M.H., Majamaa K. A novel mutation m.8561C>G in MT-ATP6/8 causing a mitochondrial syndrome with ataxia, peripheral neuropathy, diabetes mellitus, and hypergonadotropic hypogonadism. J. Neurol. 2016;263(11):2188–2195. doi: 10.1007/s00415-016-8249-2. [DOI] [PubMed] [Google Scholar]
- 34.Hoeks J., Hesselink M.K., Russell A.P., Mensink M., Saris W.H., Mensink R.P., Schrauwen P. Peroxisome proliferator-activated receptor-gamma coactivator-1 and insulin resistance: acute effect of fatty acids. Diabetologia. 2006;49(10):2419–2426. doi: 10.1007/s00125-006-0369-2. [DOI] [PubMed] [Google Scholar]
- 35.Maechler P., Wollheim C.B. Mitochondrial function in normal and diabetic beta-cells. Nature. 2001;414(6865):807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 36.Diaz-Morales N., Rovira-Llopis S., Bañuls C., Escribano-Lopez I., de Marañon A.M., Lopez-Domenech S., Orden S., Roldan-Torres I., Alvarez A., Veses S., Jover A., Rocha M., Hernandez-Mijares A., Victor V.M. Are Mitochondrial Fusion and Fission Impaired in Leukocytes of Type 2 Diabetic Patients? Antioxid. Redox Signal. 2016;25(2):108–115. doi: 10.1089/ars.2016.6707. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Mijares A., Rocha M., Apostolova N., Borras C., Jover A., Bañuls C., Sola E., Victor V.M. Mitochondrial complex I impairment in leukocytes from type 2 diabetic patients. Free Radic. Biol. Med. 2011;50(10):1215–1221. doi: 10.1016/j.freeradbiomed.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Lowell B.B., Shulman G.I. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 39.Rovira-Llopis S., Díaz-Morales N., Bañuls C., Blas-García A., Polo M., López-Domenech S., Jover A., Rocha M., Hernández-Mijares A., Víctor V.M. Is Autophagy Altered in the Leukocytes of Type 2 Diabetic Patients? Antioxid. Redox Signal. 2015;23(13):1050–1056. doi: 10.1089/ars.2015.6447. [DOI] [PubMed] [Google Scholar]
- 40.Kelley D.E., He J., Menshikova E.V., Ritov V.B. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 41.Mootha V.K., Lindgren C.M., Eriksson K.F., Subramanian A., Sihag S., Lehar J., Puigserver P., Carlsson E., Ridderstråle M., Laurila E., Houstis N., Daly M.J., Patterson N., Mesirov J.P., Golub T.R., Tamayo P., Spiegelman B., Lander E.S., Hirschhorn J.N., Altshuler D., Groop L.C. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 42.Heinonen S., Buzkova J., Muniandy M., Kaksonen R., Ollikainen M., Ismail K., Hakkarainen A., Lundbom J., Lundbom N., Vuolteenaho K., Moilanen E., Kaprio J., Rissanen A., Suomalainen A., Pietiläinen K.H. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64(9):3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- 43.Apostolova N., Victor V.M. Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid. Redox Signal. 2015;22(8):686–729. doi: 10.1089/ars.2014.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escribano-Lopez I., Diaz-Morales N., Rovira-Llopis S., de Marañon A.M., Orden S., Alvarez A., Bañuls C., Rocha M., Murphy M.P., Hernandez-Mijares A., Victor V.M. The mitochondria-targeted antioxidant MitoQ modulates oxidative stress, inflammation and leukocyte-endothelium interactions in leukocytes isolated from type 2 diabetic patients. Redox Biol. 2016;10:200–205. doi: 10.1016/j.redox.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larson-Meyer D.E., Heilbronn L.K., Redman L.M., Newcomer B.R., Frisard M.I., Anton S., Smith S.R., Alfonso A., Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–1344. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimbert V., Boirie Y., Bedu M., Hocquette J.F., Ritz P., Morio B. Muscle fat oxidative capacity is not impaired by age but by physical inactivity: association with insulin sensitivity. FASEB J. 2004;18(6):737–739. doi: 10.1096/fj.03-1104fje. [DOI] [PubMed] [Google Scholar]
- 47.Toledo F.G., Menshikova E.V., Ritov V.B., Azuma K., Radikova Z., DeLany J., Kelley D.E. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56(8):2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 48.Li A., Zhang S., Li J., Liu K., Huang F., Liu B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell Endocrinol. 2016;434:36–47. doi: 10.1016/j.mce.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Hou Y., Li S., Wu M., Wei J., Ren Y., Du C., Wu H., Han C., Duan H., Shi Y. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2016;310(6):F547–F559. doi: 10.1152/ajprenal.00574.2014. [DOI] [PubMed] [Google Scholar]
- 50.Byrne H., Caulfield B., De Vito G. Effects of Self-directed Exercise Programmes on Individuals with Type 2 Diabetes Mellitus: A Systematic Review Evaluating Their Effect on HbA1c and Other Metabolic Outcomes, Physical Characteristics, Cardiorespiratory Fitness and Functional Outcomes. Sports Med. 2016 doi: 10.1007/s40279-016-0593-y. [DOI] [PubMed] [Google Scholar]
- 51.Sathananthan M., Shah M., Edens K.L., Grothe K.B., Piccinini F., Farrugia L.P., Micheletto F., Man C.D., Cobelli C., Rizza R.A., Camilleri M., Vella A. Six and 12 Weeks of Caloric Restriction Increases β Cell Function and Lowers Fasting and Postprandial Glucose Concentrations in People with Type 2 Diabetes. J. Nutr. 2015;145(9):2046–2051. doi: 10.3945/jn.115.210617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lo M.C., Chen M.H., Lee W.S., Lu C.I., Chang C.R., Kao S.H., Lee H.M. Nε-(carboxymethyl) lysine-induced mitochondrial fission and mitophagy cause decreased insulin secretion from β-cells. Am. J. Physiol. Endocrinol. Metab. 2015;309(10):E829–E839. doi: 10.1152/ajpendo.00151.2015. [DOI] [PubMed] [Google Scholar]
- 53.Reinhardt F., Schultz J., Waterstradt R., Baltrusch S. Drp1 guarding of the mitochondrial network is important for glucose-stimulated insulin secretion in pancreatic beta cells. Biochem. Biophys. Res. Commun. 2016 10;474(4):646–651. doi: 10.1016/j.bbrc.2016.04.142. [DOI] [PubMed] [Google Scholar]
- 54.Cerqueira F.M., Chausse B., Baranovski B.M., Liesa M., Lewis E.C., Shirihai O.S., Kowaltowski A.J. Diluted serum from calorie-restricted animals promotes mitochondrial β-cell adaptations and protect against glucolipotoxicity. FEBS J. 2016;283(5):822–833. doi: 10.1111/febs.13632. [DOI] [PubMed] [Google Scholar]
- 55.Molina A.J., Wikstrom J.D., Stiles L., Las G., Mohamed H., Elorza A., Walzer G., Twig G., Katz S., Corkey B.E., Shirihai O.S. Mitochondrial networking protects beta-cells from nutrient-induced apoptosis. Diabetes. 2009;58(10):2303–2315. doi: 10.2337/db07-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cang X., Wang X., Liu P., Wu X., Yan J., Chen J., Wu G., Jin Y., Xu F., Su J., Wan C., Wang X. PINK1 alleviates palmitate induced insulin resistance in HepG2 cells by suppressing ROS mediated MAPK pathways. Biochem. Biophys. Res. Commun. 2016;478(1):431–438. doi: 10.1016/j.bbrc.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Zorzano A., Liesa M., Palacín M. Mitochondrial dynamics as a bridge between mitochondrial dysfunction and insulin resistance. Arch. Physiol. Biochem. 2009;115(1):1–12. doi: 10.1080/13813450802676335. [DOI] [PubMed] [Google Scholar]
- 58.Fealy C.E., Mulya A., Lai N., Kirwan J.P. Exercise training decreases activation of the mitochondrial fission protein dynamin-related protein-1 in insulin-resistant human skeletal muscle. J. Appl. Physiol. 2014;117(3):239–245. doi: 10.1152/japplphysiol.01064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veeranki S., Givvimani S., Kundu S., Metreveli N., Pushpakumar S., Tyagi S.C. Moderate intensity exercise prevents diabetic cardiomyopathy associated contractile dysfunction through restoration of mitochondrial function and connexin 43 levels in db/db mice. J. Mol. Cell. Cardiol. 2016;92:163–173. doi: 10.1016/j.yjmcc.2016.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arruda A.P., Pers B.M., Parlakgül G., Güney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014;20(12):1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rovira-Llopis S., Bañuls C., Apostolova N., Morillas C., Hernandez-Mijares A., Rocha M., Victor V.M. Is glycemic control modulating endoplasmic reticulum stress in leukocytes of type 2 diabetic patients? Antioxid. Redox Signal. 2014;21(12):1759–1765. doi: 10.1089/ars.2014.6030. [DOI] [PubMed] [Google Scholar]
- 62.Paltauf-Doburzynska J., Malli R., Graier W.F. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J. Cardiovasc. Pharmacol. 2004;44(4):423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- 63.Yu T., Robotham J.L., Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA. 2006;103(8):2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vanhorebeek I., De Vos R., Mesotten D., Wouters P.J., De Wolf-Peeters C., Van, den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365(9453):53–59. doi: 10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 65.Gerber P.A., Rutter G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2016 doi: 10.1089/ars.2016.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Montaigne D., Marechal X., Coisne A., Debry N., Modine T., Fayad G., Potelle C., El Arid J.M., Mouton S., Sebti Y., Duez H., Preau S., Remy-Jouet I., Zerimech F., Koussa M., Richard V., Neviere R., Edme J.L., Lefebvre P., Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130(7):554–564. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 67.Schultz J., Waterstradt R., Kantowski T., Rickmann A., Reinhardt F., Sharoyko V., Mulder H., Tiedge M., Baltrusch S. Precise expression of Fis1 is important for glucose responsiveness of beta cells. J. Endocrinol. 2016;230(1):81–91. doi: 10.1530/JOE-16-0111. [DOI] [PubMed] [Google Scholar]
- 68.Jheng H.F., Huang S.H., Kuo H.M., Hughes M.W., Tsai Y.S. Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Ann. N. Y. Acad. Sci. 2015;1350:82–94. doi: 10.1111/nyas.12863. [DOI] [PubMed] [Google Scholar]
- 69.Cassidy-Stone A., Chipuk J.E., Ingerman E., Song C., Yoo C., Kuwana T., Kurth M.J., Shaw J.T., Hinshaw J.E., Green D.R., Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev. Cell. 2008;14(2):193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ong S.B., Subrayan S., Lim S.Y., Yellon D.M., Davidson S.M., Hausenloy D.J. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121(18):2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 71.Jheng H.F., Tsai P.J., Guo S.M., Kuo L.H., Chang C.S., Su I.J., Chang C.R., Tsai Y.S. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol. Cell Biol. 2012;32(2):309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Q, Zhang M., Torres G., Wu S., Ouyang C., Xie Z., Zou M.H. Metformin Suppresses Diabetes-Accelerated Atherosclerosis via the Inhibition of Drp1-Mediated Mitochondrial Fission. Diabetes. 2016 doi: 10.2337/db16-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim B., Kim J.S., Yoon Y., Santiago M.C., Brown M.D., Park J.Y. Inhibition of Drp1-dependent mitochondrial division impairs myogenic differentiation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305(8):R927–R938. doi: 10.1152/ajpregu.00502.2012. [DOI] [PubMed] [Google Scholar]
- 74.Salabei J.K., Hill B.G. Mitochondrial fission induced by platelet-derived growth factor regulates vascular smooth muscle cell bioenergetics and cell proliferation. Redox Biol. 2013;1:542–551. doi: 10.1016/j.redox.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Hansen K., Edwards R., Van Houten B., Qian W. Mitochondrial division inhibitor 1 (mdivi-1) enhances death receptor-mediated apoptosis in human ovarian cancer cells. Biochem. Biophys. Res. Commun. 2015;456(1):7–12. doi: 10.1016/j.bbrc.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macia E., Ehrlich M., Massol R., Boucrot E., Brunner C., Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell. 2006;10(6):839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 77.Gao D., Zhang L., Dhillon R., Hong T.T., Shaw R.M., Zhu J. Dynasore protects mitochondria and improves cardiac lusitropy in Langendorff perfused mouse heart. PLoS One. 2013;8(4):e60967. doi: 10.1371/journal.pone.0060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi X., Qvit N., Su Y.C., Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. J. Cell Sci. 2013;126(Pt 3):789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo X., Sesaki H., Qi X. Drp1 stabilizes p53 on the mitochondria to trigger necrosis under oxidative stress conditions in vitro and in vivo. Biochem. J. 2014;461(1):137–146. doi: 10.1042/BJ20131438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Su Y.C., Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum. Mol. Genet. 2013;22(22):4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]
- 81.Filichia E., Hoffer B., Qi X., Luo Y. Inhibition of Drp1 mitochondrial translocation provides neural protection in dopaminergic system in a Parkinson's disease model induced by MPTP. Sci. Rep. 2016;6:32656. doi: 10.1038/srep32656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhan L., Cao H., Wang G., Lyu Y., Sun X., An J., Wu Z., Huang Q., Liu B., Xing J. Drp1-mediated mitochondrial fission promotes cell proliferation through crosstalk of p53 and NF-κB pathways in hepatocellular carcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu J., Mu C., Yue W., Li J., Ma B., Zhao L., Liu L., Chen Q., Yan C., Liu H., Hao X., Zhu Y. A diterpenoid derivate compound targets selenocysteine of thioredoxin reductases and induces Bax/Bak-independent apoptosis. Free Radic. Biol. Med. 2013;63:485–494. doi: 10.1016/j.freeradbiomed.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 84.Yue W., Chen Z., Liu H., Yan C., Chen M., Feng D., Yan C., Wu H., Du L., Wang Y., Liu J., Huang X., Xia L., Liu L., Wang X., Jin H., Wang J., Song Z., Hao X., Chen Q. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24(4):482–496. doi: 10.1038/cr.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jimenez R.E., Kubli D.A., Gustafsson Å.B. Autophagy and mitophagy in the myocardium: therapeutic potential and concerns. Br. J. Pharmacol. 2014;171(8):1907–1916. doi: 10.1111/bph.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Higgins G.C., Coughlan M.T. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014;171(8):1917–1942. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang T., Xue L., Li L., Tang C., Wan Z., Wang R., Tan J., Tan Y., Han H., Tian R., Billiar T.R., Tao W.A., Zhang Z. BNIP Suppresses PINK1 Proteolytic Cleavage to Promote Mitophagy. J. Biol. Chem. 2016 doi: 10.1074/jbc.M116.733410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartleben B., Gödel M., Meyer-Schwesinger C., Liu S., Ulrich T., Köbler S., Wiech T., Grahammer F., Arnold S.J., Lindenmeyer M.T., Cohen C.D., Pavenstädt H., Kerjaschki D., Mizushima N., Shaw A.S., Walz G., Huber T.B. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120(4):1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mori H., Inoki K., Masutani K., Wakabayashi Y., Komai K., Nakagawa R., Guan K.L., Yoshimura A. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophys. Res. Commun. 2009;384(4):471–475. doi: 10.1016/j.bbrc.2009.04.136. [DOI] [PubMed] [Google Scholar]
- 90.Sakaguchi M., Isono M., Isshiki K., Sugimoto T., Koya D., Kashiwagi A. Inhibition of mTOR signaling with rapamycin attenuates renal hypertrophy in the early diabetic mice. Biochem. Biophys. Res. Commun. 2006;340(1):296–301. doi: 10.1016/j.bbrc.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 91.Yang Y., Wang J., Qin L., Shou Z., Zhao J., Wang H., Chen Y., Chen J. Rapamycin prevents early steps of the development of diabetic nephropathy in rats. Am. J. Nephrol. 2007;27(5):495–502. doi: 10.1159/000106782. [DOI] [PubMed] [Google Scholar]
- 92.Huang C., Zhang Y., Kelly D.J., Tan C.Y., Gill A., Cheng D., Braet, Park J.S., Sue C.M., Pollock C.A., Chen X.M. Thioredoxin interacting protein (TXNIP) regulates tubular autophagy and mitophagy in diabetic nephropathy through the mTOR signaling pathway. Sci. Rep. 2016;6:29196. doi: 10.1038/srep29196. [DOI] [PMC free article] [PubMed] [Google Scholar]