Abstract
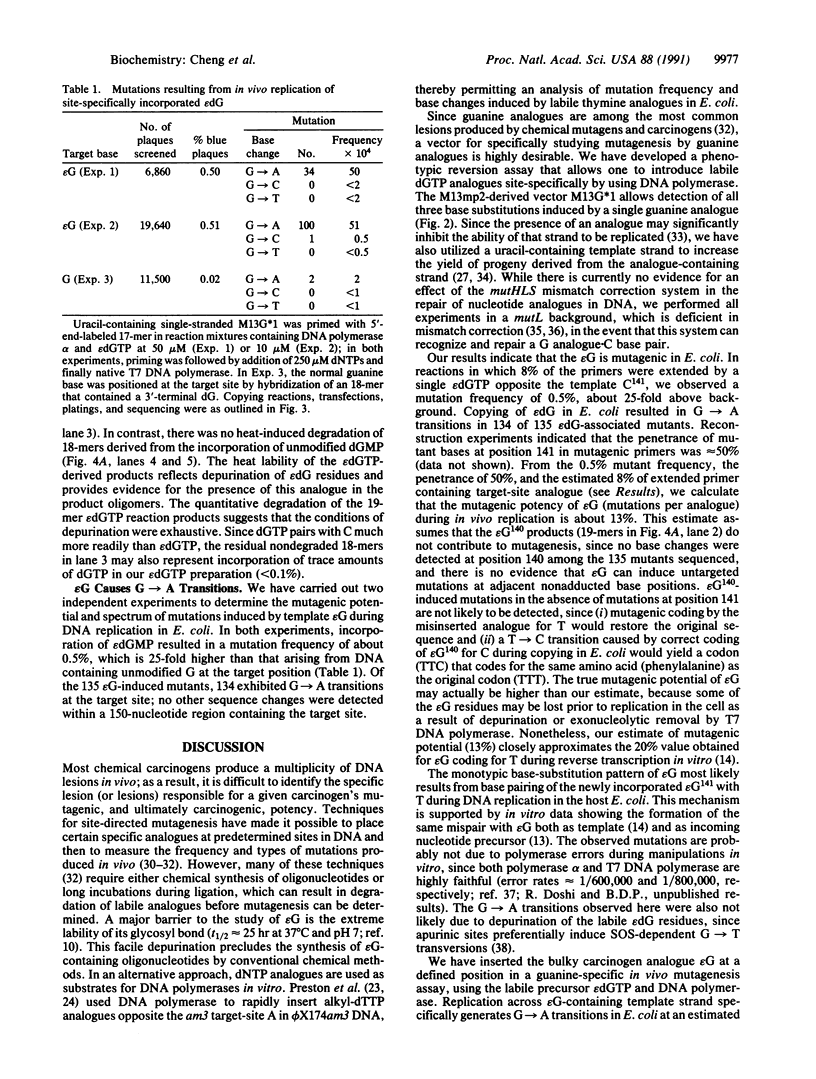
Vinyl chloride is a known human and rodent carcinogen that forms several cyclic base derivatives in DNA. The mutagenic potential of these derivatives has been examined in vitro but not in vivo. One of these derivatives, N2,3-ethenoguanine (epsilon G), is known to base pair with both cytosine and thymine during in vitro DNA synthesis, which would result in G----A transitions. To determine the base pairing specificity of this labile guanine derivative in Escherichia coli, we have developed a genetic reversion assay for guanine derivatives. The assay utilizes DNA polymerase-mediated analogue insertion into a bacteriophage vector, M13G*1, which detects all single-base substitutions at position 141 of the lacZ alpha gene by change in plaque color. After the insertion of a single epsilon G opposite the template cytosine at position 141 by use of epsilon dGTP and DNA polymerase and further extension with all four normal dNTPs, the DNA was transfected into E. coli. Transfection of M13G*1 containing epsilon G at the target site yielded 135 mutants among 26,500 plaques, 134 of which represented G----A transitions. The uncorrected mutation frequency was 0.5%, as compared with the control value, approximately 0.02%; when corrected for epsilon G content and penetrance, the calculated mutagenic potential of epsilon G (mutations/analogue) was about 13%. We thus conclude that epsilon G specifically induces G----A transitions during DNA replication in E. coli. The M13G*1 assay may permit the testing of other labile guanine derivatives not otherwise amenable to mutagenesis studies.
Full text
PDF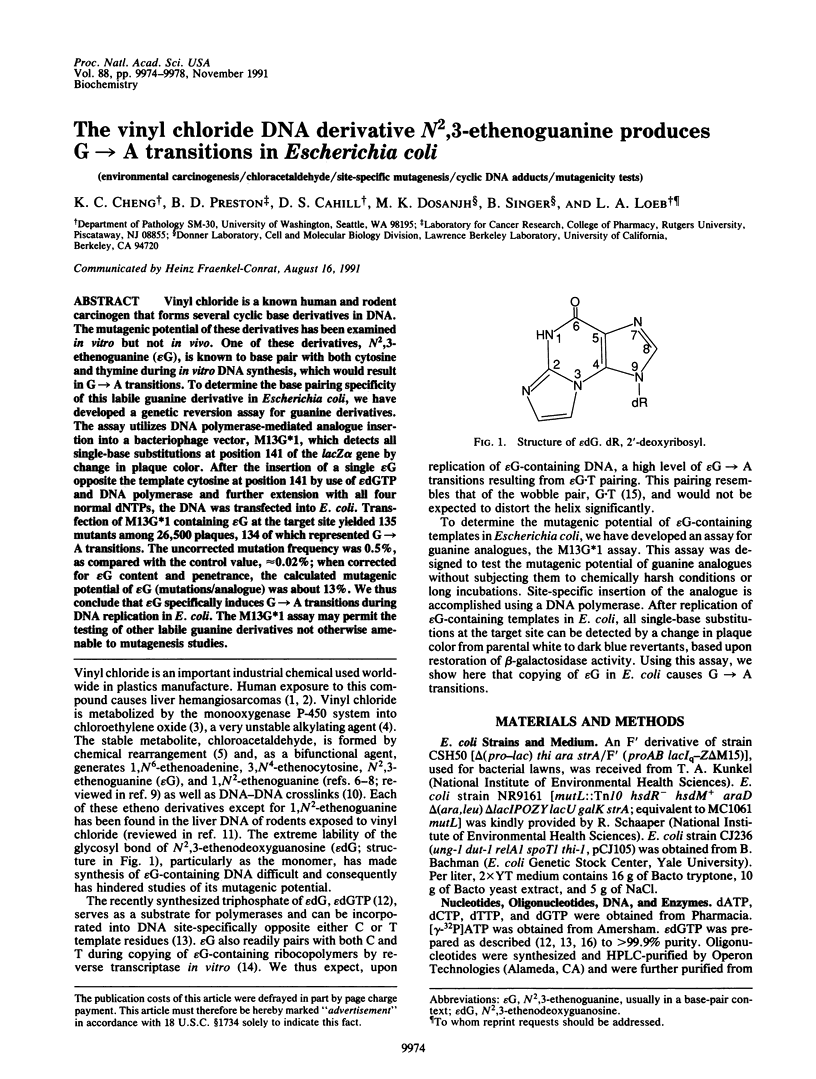



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Bebenek K., Kunkel T. A. The use of native T7 DNA polymerase for site-directed mutagenesis. Nucleic Acids Res. 1989 Jul 11;17(13):5408–5408. doi: 10.1093/nar/17.13.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Hunter W. N., Kneale G., Kennard O. Molecular structure of the G.A base pair in DNA and its implications for the mechanism of transversion mutations. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2402–2406. doi: 10.1073/pnas.83.8.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creech J. L., Jr, Johnson M. N. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Med. 1974 Mar;16(3):150–151. [PubMed] [Google Scholar]
- Doll R. Effects of exposure to vinyl chloride. An assessment of the evidence. Scand J Work Environ Health. 1988 Apr;14(2):61–78. doi: 10.5271/sjweh.1943. [DOI] [PubMed] [Google Scholar]
- Eberle G., Barbin A., Laib R. J., Ciroussel F., Thomale J., Bartsch H., Rajewsky M. F. 1,N6-etheno-2'-deoxyadenosine and 3,N4-etheno-2'-deoxycytidine detected by monoclonal antibodies in lung and liver DNA of rats exposed to vinyl chloride. Carcinogenesis. 1989 Jan;10(1):209–212. doi: 10.1093/carcin/10.1.209. [DOI] [PubMed] [Google Scholar]
- Fedtke N., Boucheron J. A., Walker V. E., Swenberg J. A. Vinyl chloride-induced DNA adducts. II: Formation and persistence of 7-(2'-oxoethyl)guanine and N2,3-ethenoguanine in rat tissue DNA. Carcinogenesis. 1990 Aug;11(8):1287–1292. doi: 10.1093/carcin/11.8.1287. [DOI] [PubMed] [Google Scholar]
- Fedtke N., Walker V. E., Swenberg J. A. Determination of 7-(2-oxoethyl)guanine and N2,3-ethenoguanine in DNA hydrolysates by HPLC. Arch Toxicol Suppl. 1989;13:214–218. doi: 10.1007/978-3-642-74117-3_33. [DOI] [PubMed] [Google Scholar]
- Folkman W., Kuśmierek J. T., Singer B. A new one-step method for the preparation of 3',5'-bisphosphates of acid-labile deoxynucleosides. Chem Res Toxicol. 1990 Nov-Dec;3(6):536–539. doi: 10.1021/tx00018a007. [DOI] [PubMed] [Google Scholar]
- Huisman O., Fox M. S. A genetic analysis of primary products of bacteriophage lambda recombination. Genetics. 1986 Mar;112(3):409–420. doi: 10.1093/genetics/112.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuśmierek J. T., Folkman W., Singer B. Synthesis of N2,3-ethenodeoxyguanosine, N2,3-ethenodeoxyguanosine 5'-phosphate, and N2,3-ethenodeoxyguanosine 5'-triphosphate. Stability of the glycosyl bond in the monomer and in poly(dG,epsilon dG-dC). Chem Res Toxicol. 1989 Jul-Aug;2(4):230–233. doi: 10.1021/tx00010a003. [DOI] [PubMed] [Google Scholar]
- Laib R. J., Gwinner L. M., Bolt H. M. DNA alkylation by vinyl chloride metabolites: etheno derivatives or 7-alkylation of guanine? Chem Biol Interact. 1981 Oct;37(1-2):219–231. doi: 10.1016/0009-2797(81)90179-4. [DOI] [PubMed] [Google Scholar]
- Leonard N. J. Etheno-substituted nucleotides and coenzymes: fluorescence and biological activity. CRC Crit Rev Biochem. 1984;15(2):125–199. doi: 10.3109/10409238409102299. [DOI] [PubMed] [Google Scholar]
- McBride T. J., Preston B. D., Loeb L. A. Mutagenic spectrum resulting from DNA damage by oxygen radicals. Biochemistry. 1991 Jan 8;30(1):207–213. doi: 10.1021/bi00215a030. [DOI] [PubMed] [Google Scholar]
- Modrich P. DNA mismatch correction. Annu Rev Biochem. 1987;56:435–466. doi: 10.1146/annurev.bi.56.070187.002251. [DOI] [PubMed] [Google Scholar]
- Perrino F. W., Loeb L. A. Differential extension of 3' mispairs is a major contribution to the high fidelity of calf thymus DNA polymerase-alpha. J Biol Chem. 1989 Feb 15;264(5):2898–2905. [PubMed] [Google Scholar]
- Preston B. D., Loeb L. A. Enzymatic synthesis of site-specifically modified DNA. Mutat Res. 1988 Jul-Aug;200(1-2):21–35. doi: 10.1016/0027-5107(88)90068-1. [DOI] [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Comparison of the relative mutagenicities of O-alkylthymines site-specifically incorporated into phi X174 DNA. J Biol Chem. 1987 Oct 5;262(28):13821–13827. [PubMed] [Google Scholar]
- Preston B. D., Singer B., Loeb L. A. Mutagenic potential of O4-methylthymine in vivo determined by an enzymatic approach to site-specific mutagenesis. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8501–8505. doi: 10.1073/pnas.83.22.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid T. M., Lee M. S., King C. M. Mutagenesis by site-specific arylamine adducts in plasmid DNA: enhancing replication of the adducted strand alters mutation frequency. Biochemistry. 1990 Jul 3;29(26):6153–6161. doi: 10.1021/bi00478a007. [DOI] [PubMed] [Google Scholar]
- Reyland M. E., Loeb L. A. On the fidelity of DNA replication. Isolation of high fidelity DNA polymerase-primase complexes by immunoaffinity chromatography. J Biol Chem. 1987 Aug 5;262(22):10824–10830. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Schaaper R. M., Kunkel T. A., Loeb L. A. Infidelity of DNA synthesis associated with bypass of apurinic sites. Proc Natl Acad Sci U S A. 1983 Jan;80(2):487–491. doi: 10.1073/pnas.80.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer B., Essigmann J. M. Site-specific mutagenesis: retrospective and prospective. Carcinogenesis. 1991 Jun;12(6):949–955. doi: 10.1093/carcin/12.6.949. [DOI] [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T., Folkman W., Chavez F., Dosanjh M. K. Evidence for the mutagenic potential of the vinyl chloride induced adduct, N2, 3-etheno-deoxyguanosine, using a site-directed kinetic assay. Carcinogenesis. 1991 Apr;12(4):745–747. doi: 10.1093/carcin/12.4.745. [DOI] [PubMed] [Google Scholar]
- Singer B., Spengler S. J., Chavez F., Kuśmierek J. T. The vinyl chloride-derived nucleoside, N2,3-ethenoguanosine, is a highly efficient mutagen in transcription. Carcinogenesis. 1987 May;8(5):745–747. doi: 10.1093/carcin/8.5.745. [DOI] [PubMed] [Google Scholar]
- Spengler S. J., Singer B. Formation of interstrand cross-links in chloroacetaldehyde-treated DNA demonstrated by ethidium bromide fluorescence. Cancer Res. 1988 Sep 1;48(17):4804–4806. [PubMed] [Google Scholar]
- Wood M. L., Dizdaroglu M., Gajewski E., Essigmann J. M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990 Jul 31;29(30):7024–7032. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- Zajdela F., Croisy A., Barbin A., Malaveille C., Tomatis L., Bartsch H. Carcinogenicity of chloroethylene oxide, an ultimate reactive metabolite of vinyl chloride, and bis(chloromethyl)ether after subcutaneous administration and in initiation-promotion experiments in mice. Cancer Res. 1980 Feb;40(2):352–356. [PubMed] [Google Scholar]




