Abstract
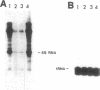
The rapid induction of thionein (apometallothionein) by many endogenous stimuli such as steroid hormones, cytokines, and second messengers suggests that this cysteine-rich, metal binding protein participates in an as yet undefined role in cellular regulatory processes. This study demonstrates with DNA and RNA binding assays and in vitro transcription measurements that thionein suppresses the binding of the Xenopus laevis zinc finger transcription factor IIIA (TFIIIA) to 5S RNA and to the 5S RNA gene and abrogates the capacity of TFIIIA to initiate the RNA polymerase III-catalyzed synthesis of 5S RNA. The effect is reversed by the addition of zinc and is not observed in the TFIIIA-independent transcription of a tRNA gene by the same RNA polymerase. In view of the strong tendency of thionein to complex posttransition metals such as zinc, one effect of its enhanced synthesis in vivo could be to reduce the intracellular disposability of zinc and thus modulate the actions of zinc-dependent enzymes and proteins, most notably those of the zinc finger transcription factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg J. M. Zinc finger domains: hypotheses and current knowledge. Annu Rev Biophys Biophys Chem. 1990;19:405–421. doi: 10.1146/annurev.bb.19.060190.002201. [DOI] [PubMed] [Google Scholar]
- Bohm S., Berghard A., Pereswetoff-Morath C., Toftgård R. Isolation and characterization of complementary DNA clones corresponding to genes induced in mouse epidermis in vivo by tumor promoters. Cancer Res. 1990 Mar 1;50(5):1626–1633. [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Cousins R. J., Leinart A. S. Tissue-specific regulation of zinc metabolism and metallothionein genes by interleukin 1. FASEB J. 1988 Oct;2(13):2884–2890. doi: 10.1096/fasebj.2.13.2458983. [DOI] [PubMed] [Google Scholar]
- Csermely P., Szamel M., Resch K., Somogyi J. Zinc can increase the activity of protein kinase C and contributes to its binding to plasma membranes in T lymphocytes. J Biol Chem. 1988 May 15;263(14):6487–6490. [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Fairall L., Rhodes D., Klug A. Mapping of the sites of protection on a 5 S RNA gene by the Xenopus transcription factor IIIA. A model for the interaction. J Mol Biol. 1986 Dec 5;192(3):577–591. doi: 10.1016/0022-2836(86)90278-0. [DOI] [PubMed] [Google Scholar]
- Falchuk K. H. Effect of acute disease and ACTH on serum zinc proteins. N Engl J Med. 1977 May 19;296(20):1129–1134. doi: 10.1056/NEJM197705192962001. [DOI] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Hanas J. S., Bogenhagen D. F., Wu C. W. Cooperative model for the binding of Xenopus transcription factor A to the 5S RNA gene. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2142–2145. doi: 10.1073/pnas.80.8.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanas J. S., Hazuda D. J., Bogenhagen D. F., Wu F. Y., Wu C. W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J Biol Chem. 1983 Dec 10;258(23):14120–14125. [PubMed] [Google Scholar]
- Herschman H. R. Extracellular signals, transcriptional responses and cellular specificity. Trends Biochem Sci. 1989 Nov;14(11):455–458. doi: 10.1016/0968-0004(89)90105-9. [DOI] [PubMed] [Google Scholar]
- KAGI J. H., VALEE B. L. Metallothionein: a cadmium- and zinc-containing protein from equine renal cortex. J Biol Chem. 1960 Dec;235:3460–3465. [PubMed] [Google Scholar]
- KAGI J. H., VALLEE B. L. Metallothionein: a cadmium and zinc-containign protein from equine renal cortex. II. Physico-chemical properties. J Biol Chem. 1961 Sep;236:2435–2442. [PubMed] [Google Scholar]
- Karin M. Metallothioneins: proteins in search of function. Cell. 1985 May;41(1):9–10. doi: 10.1016/0092-8674(85)90051-0. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Schäffer A. Biochemistry of metallothionein. Biochemistry. 1988 Nov 15;27(23):8509–8515. doi: 10.1021/bi00423a001. [DOI] [PubMed] [Google Scholar]
- Laurin D. E., Klasing K. C. Roles of synthesis and degradation in the regulation of metallothionein accretion in a chicken macrophage-cell line. Biochem J. 1990 Jun 1;268(2):459–463. doi: 10.1042/bj2680459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. Y., Kraker A. J., Shaw C. F., 3rd, Petering D. H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., McLachlan A. D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985 Jun;4(6):1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson P. E., Zafarullah M., Gedamu L. A role of metallothionein in zinc regulation after oestradiol induction of vitellogenin synthesis in rainbow trout, Salmo gairdneri. Biochem J. 1989 Jan 15;257(2):555–559. doi: 10.1042/bj2570555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck L. J., Millstein L., Eversole-Cire P., Gottesfeld J. M., Varshavsky A. Transcriptionally inactive oocyte-type 5S RNA genes of Xenopus laevis are complexed with TFIIIA in vitro. Mol Cell Biol. 1987 Oct;7(10):3503–3510. doi: 10.1128/mcb.7.10.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Wegnez M. Isolation of a 7S particle from Xenopus laevis oocytes: a 5S RNA-protein complex. Proc Natl Acad Sci U S A. 1979 Jan;76(1):241–245. doi: 10.1073/pnas.76.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakonju S., Bogenhagen D. F., Brown D. D. A control region in the center of the 5S RNA gene directs specific initiation of transcription: I. The 5' border of the region. Cell. 1980 Jan;19(1):13–25. doi: 10.1016/0092-8674(80)90384-0. [DOI] [PubMed] [Google Scholar]
- Shang Z. G., Windsor W. T., Liao Y. D., Wu C. W. Purification of Xenopus transcription factor IIIA and 5 S RNA from 7 S ribonucleoprotein particle by ammonium sulfate precipitation. Anal Biochem. 1988 Jan;168(1):156–163. doi: 10.1016/0003-2697(88)90023-1. [DOI] [PubMed] [Google Scholar]
- Shang Z., Liao Y. D., Wu F. Y., Wu C. W. Zinc release from Xenopus transcription factor IIIA induced by chemical modifications. Biochemistry. 1989 Dec 12;28(25):9790–9795. doi: 10.1021/bi00451a037. [DOI] [PubMed] [Google Scholar]
- Shastry B. S., Honda B. M., Roeder R. G. Altered levels of a 5 S gene-specific transcription factor (TFIIIA) during oogenesis and embryonic development of Xenopus laevis. J Biol Chem. 1984 Sep 25;259(18):11373–11382. [PubMed] [Google Scholar]
- Silverman S., Schmidt O., Söll D., Hovemann B. The nucleotide sequence of a cloned Drosophila arginine tRNA gene and its in vitro transcription in Xenopus germinal vesicle extracts. J Biol Chem. 1979 Oct 25;254(20):10290–10294. [PubMed] [Google Scholar]
- Udom A. O., Brady F. O. Reactivation in vitro of zinc-requiring apo-enzymes by rat liver zinc-thionein. Biochem J. 1980 May 1;187(2):329–335. doi: 10.1042/bj1870329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Coleman J. E., Auld D. S. Zinc fingers, zinc clusters, and zinc twists in DNA-binding protein domains. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):999–1003. doi: 10.1073/pnas.88.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L. Implications and inferences of metallothionein structure. Experientia Suppl. 1987;52:5–16. doi: 10.1007/978-3-0348-6784-9_1. [DOI] [PubMed] [Google Scholar]
- Vallee B. L. Introduction to metallothionein. Methods Enzymol. 1991;205:3–7. doi: 10.1016/0076-6879(91)05077-9. [DOI] [PubMed] [Google Scholar]
- Vallee B. L. Metallothionein: historical review and perspectives. Experientia Suppl. 1979;34:19–39. doi: 10.1007/978-3-0348-6493-0_1. [DOI] [PubMed] [Google Scholar]
- Zeng J., Heuchel R., Schaffner W., Kägi J. H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991 Feb 25;279(2):310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]