Abstract
Human neuronal cells differentiated from induced pluripotent cells have emerged as a new model system for the study of disease pathophysiology and evaluation of drug efficacy. Differentiated neuronal cells are more similar in genetics and biological content to the human brain cells than other animal disease models. However, culture of neuronal cells in assay plates requires a labor-intensive procedure of plate pre-coating, hampering its applications in high throughput screening (HTS). We developed a simplified method with one-step seeding of neural stem cells in assay plates by supplementing the medium with a recombinant human vitronectin (VTN), thus avoiding plate pre-coating. Robust results were obtained from cell viability, calcium response, and neurite outgrowth assays using this new method. Our data demonstrate that this approach greatly simplifies high throughput assays using neuronal cells differentiated from human stem cells for translational research.
Keywords: High content screening, high throughput screening, HTS, induced pluripotent stem cells, iPSC, neuronal cells, neural stem cells, plate coating, vitronectin
Introduction
Disease modeling using induced pluripotent stem cells (iPSCs) has recently been applied to a variety of diseases in the study of disease phenotype and pathophysiology.1 The iPSCs are self-renewing and can be differentiated into various types of human cells such as neurons, cardiomyocytes, and hepatocytes. Disease models play a critical role in preclinical drug development in the evaluation of drug efficacy. However, animal models, particularly rodent models, may not mimic certain human diseases appropriately. Insufficient disease models for neurodegenerative and neuropsychiatric diseases have hindered development of new therapeutics for these maladies in the last two decades.2 Recent advancement in iPSC technology has enabled large-scale production of neuronal cells differentiated from patient iPSCs that models neurological disorders including Parkinson’s disease (PD),3 Alzheimer’s disease (AD),3 Amyotrophic lateral sclerosis (ALS),3 spinal muscular atrophy (SMA),4 and familial dysautonomia.5 Neuronal cells differentiated from patient iPSCs exhibited specific disease phenotypes such as decrease of mitochondrial function in PD dopaminergic neurons,6 accumulation of amyloid β and p-tau/total tau in AD neurons,7 hyper excitability in ALS motor neurons,8 apoptosis in SMA motor neurons,9 and cholesterol accumulation in Niemann Pick disease type C (NPC).10 Different types of neuronal cells have been generated from iPSCs including neural stem cells, astrocytes, oligodendrocytes, motor neurons, and dopaminergic neurons. These human neuronal cells, particularly patient derived cells, can serve as cell-based disease models to evaluate compound efficacy and to screen compound libraries for drug development in addition to studying disease pathophysiology.
Practically, use of neuronal cells differentiated from iPSCs for various experiments involves labor-intensive laboratorial work. Culturing neuronal cells in assay plates requires plate precoating with extracellular matrix proteins and/or positively charged polymers to support cell attachment and growth. The procedure of plate pre-coating involves multiple steps of reagent addition and plate washes which not only reduces screening throughput but also yields large well-to-well and plate-to-plate variations.11 Pre-coating of plates is also a bottleneck in HTS using neuronal cells.11
Here we report development of a simple method of one-step seeding and culturing of neuronal cells in assay plates using a medium containing a truncated recombinant human vitronectin (rhVTN-N), which has been used as a plate coating substrate for iPSC feeder free culture.12 Because plate pre-coating and plate-washing are not needed in this method, it greatly simplifies experiments using neuronal cells differentiated from iPSCs (Fig. 1A). We have validated this method with several assays including cell viability, calcium response, and neurite outgrowth. The results demonstrate that this method enables high throughput screening using neural stem cells and neurons differentiated from stem cells. Therefore, this method of one-step seeding of neural stem cells in assay plates with the rhVTN-N-supplemented medium is useful for HTS employed to evaluate compound efficacy, to measure compound neural toxicity, and to identify new leads by screening of compound libraries.
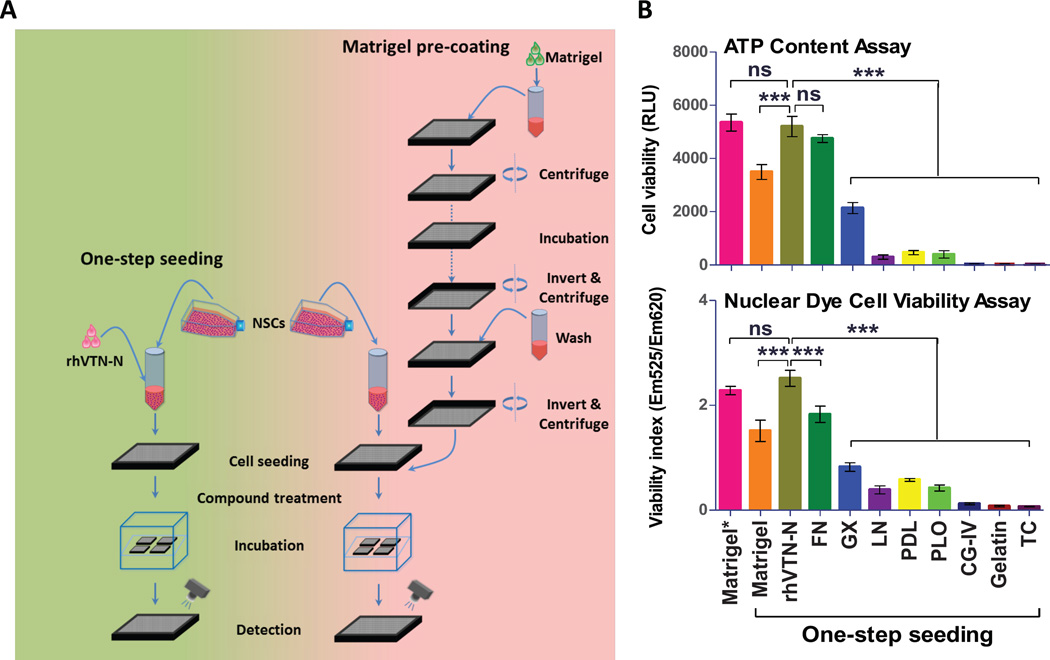
Figure 1.
Development of the method for directly seeding neural stem cells (NSCs) without plate pre-coating in 1536-well plates. (A): Schematic comparison of a new method of directly plating NSCs suspended in the rhVTN-N-supplemented medium with the traditional method using Matrigel pre-coating plates for compound screening assays in 1536-well plates. This new method avoids plate pre-coating and plate-washing steps and thus simplifies experiments that employ neuronal cells. (B): Results of cell viabilities determined in the ATP content assay and nuclear dye staining assay. NSCs were seeded in a medium containing one plate coating material. The cell viability in each substrate was compared with that obtained from the traditional Matrigel-pre-coated plate. The cell viability with the rhVTN-N-supplemented medium was similar to that of the control (p>0.5). Data are represented as the mean ± SEM of at least triplicates. (C): The bright field images of neural stem cells in various media supplemented with different coating materials. Cells cultured in the rhVTN–N- or fibronection-supplemented medium exhibited the similar health cell growth morphology as the cells cultured in the Matrigel pre-coated plate. (D): Results of immunofluorescence profiles of neural stem cell markers. Cells cultured in the rhVTN-N-supplemented medium showed a similar profile of neural stem cell markers as the control (cells cultured in the Matrigel pre-coated plate). Matrigel*: Matrigel Pre-coated, rhVTN-N: truncated recombinant human vitronectin, FN: Fibronectin, GX: Geltrex, LN: Laminin, PDL: poly-D-Lysine, PLO: poly-L-Ornithine, CG-IV: Collagen Type IV, TC: Tissue culture treated.
Materials and Methods
Cell lines and cell culture
Wild type (WT) iPSCs generated from normal fibroblasts (GM23450, Coriell Cell Repositories) were cultured in Matrigel (Catalog # 354277, Corning) pre-coated 6-well plates with Essential 8 Medium (A1517001, Life Technologies). After culturing at least 10 passages, neural stem cell (NCS) induction (Suppl. Materials and Methods) was initiated using the Neural Induction Medium kit (Life Technologies). The resulting NSCs were cultured in the complete StemPro NSC Serum Free Medium (A1050901, Life Technologies) containing knockout DMEM/F12, StemPro neural supplement, 20ng/ml bFGF, 20ng/ml EGF and 1× GlutaMAX on the Matrigel pre-coated flasks.10 Immunohistochemistry staining with antibodies against Nestin, SOX1, SOX2, PAX6, vimentin, and SSEA4 was performed for characterization of the NSCs induced from human iPSCs.
One-step NSCs seeding optimization in 1536-well plates
First, nine frequently used substrates (Suppl. Table S1) were tested in an effort to find a best substrate that could be supplemented to medium for directly seeding cells without plate precoating and plate-wash steps. Each substrate was handled according to the manufacturer’s instruction and added to NSCs suspended in the complete StemPro NSC Serum Free Medium with the indicated concentration (Suppl. Table S1). In 1536-well regular tissue-culture treated plates, 5µl/well of the neural stem cell (NSC) suspension supplemented with a plate coating reagent was dispensed at a density of 3000 cells/well. The plates were incubated at 37 °C supplied with 5% CO2 for 48 hours, 5µl/well of the reagent mixture of ATP content assay or nuclear dye cell viability assay was added to the assay plates followed by incubation for 30 minutes at room temperature and a luminescence detection of assay plates. Cells seeded in the Matrigel pre-coated plates were used as a control. After the initial test, a truncated recombinant human vitronectin (rhVTN-N) (A14700, Life Technologies) was selected from these nine commonly used substrates for the one-step seeding of NSCs in 1536-well plates. Briefly, after thawing on ice, rhVTN-N was added to the medium at a final concentration of 10µg/ml (5µg/ml of rhVTN-N also worked similarly). NSCs suspended in rhVTN-N-supplemented medium were seeded at 3000 cells/well in 5µl in tissue culture treated 1536-well plates. After four hours or overnight incubation, the cells were treated with compounds and incubated for indicated times, followed by plate detection. Immunofluorescence staining of NSCs with markers and Karyotyping were performed to characterize NSCs grown in the rhVTN-N-supplemented medium (Supplemental online Materials and Methods).
We also found that the neural expansion medium composed of Neurobasal medium, Advanced DMEM/F-12 medium and GIBCO Neural Induction Supplement (21103-049, 12634010 and A16477-01, Life Technologies) could not be used in this method because the neural stem cells were not attach to the assay plates and unable to grow when they were seeded in this medium supplemented with rhVTN-N (Suppl. Fig. S1F). It is possible that the function of vitronectin may be interfered by an unrevealed component in this medium.
One-step NSCs seeding with rhVTN-N-supplemented medium for neuronal differentiation in 384-well plates
Neuronal differentiation was conducted as previously described.10 The medium was composed of Neurobasal medium (21103-049, Life Technologies), B27, GlutaMAX, 10ng/ml of BDNF, 10ng/ml GDNF, 1uM cAMP and 200ng/ml of L-Ascorbic. Briefly, 50 µl of NSCs in the rhVTN-N-supplemented medium were seeded in PDL-coated 384-well plates (Catalog # 3845, Corning) or other plates as indicated at 3000 cells/well (cell density of 5×104cells/cm2). As a control group, NSCs were seeded in the poly-L-ornithine (PLO) and laminin (LN) dual pre-coated plates.13 Cells were cultured for 4 weeks in the neural differentiation medium and a half of the medium was replaced twice a week during the continuous culturing. Cell viability was measured by ATP content assay (CellTiter-Glo, Promega) and nuclear dye cell viability assay (Cell Mete Kit, AAT Bioquest) after 1, 2, and 4 weeks of neural differentiation.
Neurite Outgrowth Assay
NSCs were seeded in 384-well plates under the same condition as described above. After 4 week differentiation, neuronal cells were stained with anti-β-III Tubulin antibody and Hoechst 33342 nuclear dye as described in the immunofluorescence staining section. Fluorescence imaging was performed in the IN Cell 2200 imaging system with a 40× objective lens using standard FITC and DAPI filter sets. Images were analyzed using INCell Developer V1.9.2 analysis software (GE Healthcare). Briefly, the DAPI channel was used to identify nuclei using an intensity segmentation algorithm (objects greater than 650 RFU ranging in size from 5 to 75µm). β-III Tubulin (FITC) stained neurites were also identified by intensity segmentation (greater than 1150 RFU) with a post processing sieve to remove debris objects less than 2µm in size. Mean neurite length, measured in microns, was averaged to the number of nuclei per image.
High throughput cytotoxicity assays in iPSC NSCs
ATP content assay, nuclear dye cell viability assay, and Caspase 3/7 assay (Caspase-Glo, Promega) were conducted according to the manufactures’ protocols using the one-step seeded NSCs in the rhVTN-N-supplemented medium in 1536-well plates. NSCs were plated at 3000 cells/well in 5µl of StemPro NSC SFM complete culture medium with 10µg/ml rhVTN-N in 1536-well plates (white, solid bottom assay plates for ATP content assay and Caspase 3/7 assay; black, clear bottom plates for nuclear dye cell viability assay) and incubated 4 hours at 37 °C. Compounds were added to the assay plates at 23nl/well using an NX-TR pintool station (WAKO Scientific Solutions, San Diego, CA). After 16 (Caspase 3/7 assay) or 48 hours (ATP content assay and nuclear dye cell viability assay) incubation at 37 °C with 5% CO2, the assay reagents at 3 µl/well were added to the assay plates. After incubation for the indicated times, the plates were detected by luminescence for the ATP content assay and Caspase 3/7 assay or fluorescence for the nuclear dye cell viability assay detection in a plate reader.
Half maximal inhibitory (IC50) or activating (EC50) concentration values of compound confirmation data were calculated using Prism software (GraphPad Software, San Diego, CA). The percent cell viability in the ATP content and nuclear dye cell viability assays was normalized using the positive control compound thapsigargin (270µM) treated cells as 0% in cell viability, which has been shown to greatly reduce neuronal cell viability by depleting of intracellular calcium stores,14 and DMSO solvent treated wells for the 100% viable cells. The percent activities of the caspase-3/7 assay were normalized using the positive control compound staurosporine (75µM) treated cells as 0% in cell viability, which has been shown to increase caspase-3/7 activities, and DMSO treated wells for the 100% viable cells. Signal to basal ratio (S/B), CV and Z-factor were calculated and used to evaluate the assay (Suppl. Materials and Methods). For cell viability evaluation of differentiated neuronal cells in 384-well plates, the cell viability measurement was conducted after the cells were cultured for 1, 2, and 4 weeks with ATP content assay and nuclear dye cell viability assay.
Homogenous LysoTracker dye staining assay for measurement of enlarged lysosomes in NPC cells
Cells derived from patients with Niemann Pick disease Type C (NPC) exhibit the NPC disease phenotype of enlarged lysosome due to cholesterol accumulation in lysosomes that can be measured by the LysoTracker dye staining assay in a fluorescence imaging detection mode or in a fluorescence intensity mode by a plate reader.10, 15, 16 NSCs were plated at 1000 cells/well in 5µl of complete StemPro NSC Serum Free Medium supplemented with 10µg/ml rhVTN-N in 1536-well, black, clear bottom assay plates and incubated 4 hours at 37 °C and 5% CO2. Columns 1 and 2 were used as the negative control with the wild type NSCs and columns 3 through 48 were NPC NSCs. Methyl-β-cyclodextrin (MΒCD) and hydroxypropyl-β-cyclodextrin (HPBCD) which reduce cholesterol accumulation in NPC cells and thus decrease the enlarged lysosomes in NPC cells were added to the assay plate at 23nL/well as positive controls. After a 96 hours incubation, 200 nM Lysotracker-red dye (Life Technologies) was added to the assay plates to stain lysosomes. The fluorescence intensity (Ex = 570 nm, Em = 590 nm) was measured with a bottom read mode in the Tecan plate reader. The data from the homogenous LysoTracker staining assay was normalized using the WT NSCs as a negative control and DMSO-treated NPC NSCs as a positive control to calculate IC50 values, S/B ratio, CV, and Z-factor.
High throughput fluorescent calcium response assay
ATP (a purinergic receptor agonist), carbachol (a muscarinic receptor agonist), and calcium ionophore ionomycin were used as stimuli for intracellular calcium release (referred as agonists). The purinergic receptor antagonists suramin hexasodium and AR-C 118925XX, and muscarinic acetylcholine receptor antagonists tolterodine and telenzepine, as well as calcium chelators EGTA-AM and BAPTA-AM were used as the antagonist compounds to relevant stimulus (referred as antagonist). The 25 µl NSCs were plated at 10,000 cells/well in the 384 well, black/clear bottom plates in StemPro NSC SFM complete culture medium supplemented with 10 µg/ml rhVTN-N. The neurons were differentiated for four weeks as described above in the NSCs one-step seeding with rhVTN-N-supplemented medium in commercial PDL pre-coated 384-well plates. The fluorescence calcium dye (Cal-52-AM, AAT Bioquest) was prepared in DMSO at 2.5mg/ml. The calcium dye loading solution was prepared by adding 10µl to a mixture of 9 ml Hanks’ Balanced Salt solution (HBSS) and 1 ml 10× calcium assay buffer with quencher dye (Screen Quest 10× calcium assay buffer with Phenol Red Plus, AAT Bioquest). The loading solution was added at 25 µl/well and incubated for 3 hours at 37° C with 5% CO2 followed by a 30 minute incubation at room temperature. For calcium agonist response experiments, agonists were prepared at 3× of the final concentration in HBSS buffer for a sixteen concentration titration (1:2 dilution) in a 384-well compound plate in triplicate. The assay plate and compound plate were then placed into the FDSS µCELL kinetic fluorescence plate reader (Hamamatsu, Japan) to measure fluorescence intensity kinetically for 5 minutes at 1 read/sec (1 Hz). After the baseline fluorescent signals were measured for 15 seconds, 25 µl/well 3× agonists solutions in a titration were added to the assay plate from the compound plate each well at the speed of 50µl/s and the fluorescent intensity was continually recorded at 1 Hz for 300 seconds in total. The ratio (F/F0) of fluorescent intensity at each time point (F) after adding agonists to the average fluorescence intensity of baseline (F0) was calculated. The maximum of ratio (Fmax/F0) of each well was used for calculation of EC50 values.
For the antagonist experiments, the EC80 concentration of each agonist was added as the stimulus to induce the calcium response. Prior to the calcium response experiment, the cells were treated with the antagonists for 30 minutes at 25µl/well with sixteen concentrations of compound titration at 1:2 ratio (Suppl. Fig. S3A). The final DMSO concentration in both the antagonists and DMSO control well were 0.5%. The 4 × agonist compound solutions (at their EC80 values) were prepared in HBSS in 384-well compound plates. The dye loading procedure and detection method were same as described above.
Single-cell calcium response recording
The NSCs and differentiated neurons were loaded with a calcium indicator as described above. The fluorescence images before and after addition of calcium releasing agonists were recorded at 1 Hz in an INCell2200 automated imaging station equipped with an integrated liquid handling system (GE Healthcare). All fluorescence images were acquired kinetically at 37 °C in the INCell2200 automated imaging system using a 20× objective lens with standard DAPI and FITC excitation/emission filter sets. Cells were imaged at 1 Hz (1 frame per second) for a total of 180 seconds with autofocus enabled at each time point. At time point 1, both DAPI (for nuclei) and FITC (for calcium response) images were acquired. But for the remaining 179 cycles, only FITC images were acquired. At time point 5, 25µl/well of agonist compound solution or control solution was added via an on board liquid handling system. Images were collected at standard 1×1 binning and analyzed with the INCell Workstation software version 3.7.2 using the Multi Target Analysis algorithm. Briefly, Hoechst stained Nuclei (Channel 1) were identified using top-hat segmentation looking for objects with a typical area of 75µm2 and green Cal-520 dye stained cells (Chanel 2) were identified using multiscale top-hat segmentation looking for objects with a typical size of 200µm2. For the kinetic analysis, the nuclear images acquired at time point 1 were used as a unique cell ID for all subsequent time points and the Cal-520 images were analyzed at each time point for each cell. The average cellular fluorescence intensity ratio (F/F0) for individual cells was plotted over the time. F0 was the mean of fluorescence intensity of 10 initial reads without addition of a stimulus and F is the fluorescence intensity at a given time. The ratio of maximum fluorescence intensity (Fmax/F0) collected from each cell was used for determination of positive responded cells. To define the positive calcium in a cell, an average of the maximal intensity ratio (Fmax/F0) plus 3 times of the standard deviation (mean+3*s.d.) in the DMSO control was used as a threshold. The percent cells with calcium response was calculated using the following equation: Calcium responding cells (%) = (number of cells with their (Fmax/F0) ratios >= (Fmax (control) /F0) +3 × s.d.) / total cell numbers in the well that were imaged.
Data analysis and statistics
Half maximal inhibitory (IC50) or activating (EC50) concentration values of compound confirmation data were calculated using Prism software (GraphPad Software, San Diego, CA). Unless otherwise noted, all values are expressed as the mean ± s.d. (n = 3). For the statistical analysis, results were analyzed using one-way or two-way ANOVA and differences were considered statistically significant if p< 0.05.
Results
rhVTN-N supplemented medium for one-step seeding of neural stem cells
Plate pre-coating with positively charged synthetic polymers and/or biological reagents containing extracellular matrix proteins such as Matrigel, fibronectin (FN), and laminin (LN) is required for culture and growth of neuronal cells in assay plates. The plate pre-coating process involves multiple steps of reagent addition, incubation, and plate-washing which are labor-intensive and time-consuming, as well as a possibility of microbe contamination. Although synthetic polymers such as poly-D-lysine (PDL) and poly-L-ornithine (PLO) can be coated on plates in advance, the extracellular matrix proteins must be freshly coated in a thin layer to assay plates prior to the experiments because the coated proteins cannot be dried in the plates. To simplify the experiments using neuronal cells, we designed a method of one-step seeding of neuronal cells by adding extracellular matrix proteins to cell culture medium without a need for plate pre-coating (Fig. 1A). We first tested addition of one of nine commonly used coating reagents in cell culture medium for one-step seeding of neural stem cells in tissue culture-treated plates without plate pre-coating (Suppl. Table S1 and Fig. S1A–C). We found that the direct seeding of cells suspended in the medium supplemented with rhVTN-N or FN exhibited the highest cell viability as measured by a homogenous ATP content assay (Fig. 1B). The results were comparable to that obtained with Matrigel pre-coated plates (positive control). The cells seeded and cultured in the rhVTN-N-supplemented medium showed a better live-to-dead cell fluorescent intensity ratio than these in the FN-supplemented medium as determined by a nuclei dye assay (Fig. 1B). The neural stem cell spreading and growth patterns observed from cells seeded in the rhVTN-N-supplemented medium were similar to these cultured in the Matrigel precoated control plate, in contrast to cells cultured in FN-supplemented medium, which did not spread as completely (Fig. 1C). The cells seeded in the rhVTN-N-supplemented medium also exhibited similar positive neural stem cell markers compared to the cells cultured in the Matrigel pre-coated plate (Fig. 1D). We found that the neuronal stem cells (NSC) seeded in rhVTN-N-supplemented medium exhibited normal attachment, spreading morphology, and a profile of neural stem cell markers after 10 passages (Suppl. Fig. S1D), as well as the normal chromosome morphology (Suppl. Fig. 1E). Therefore, the addition of 10 µg/ml of rhVTN-N in medium for one-step seeding of neural stem cells in assay plates was selected as an optimal condition and used in the subsequent experiments.
One-step seeding of neural stem cells for neuron differentiation
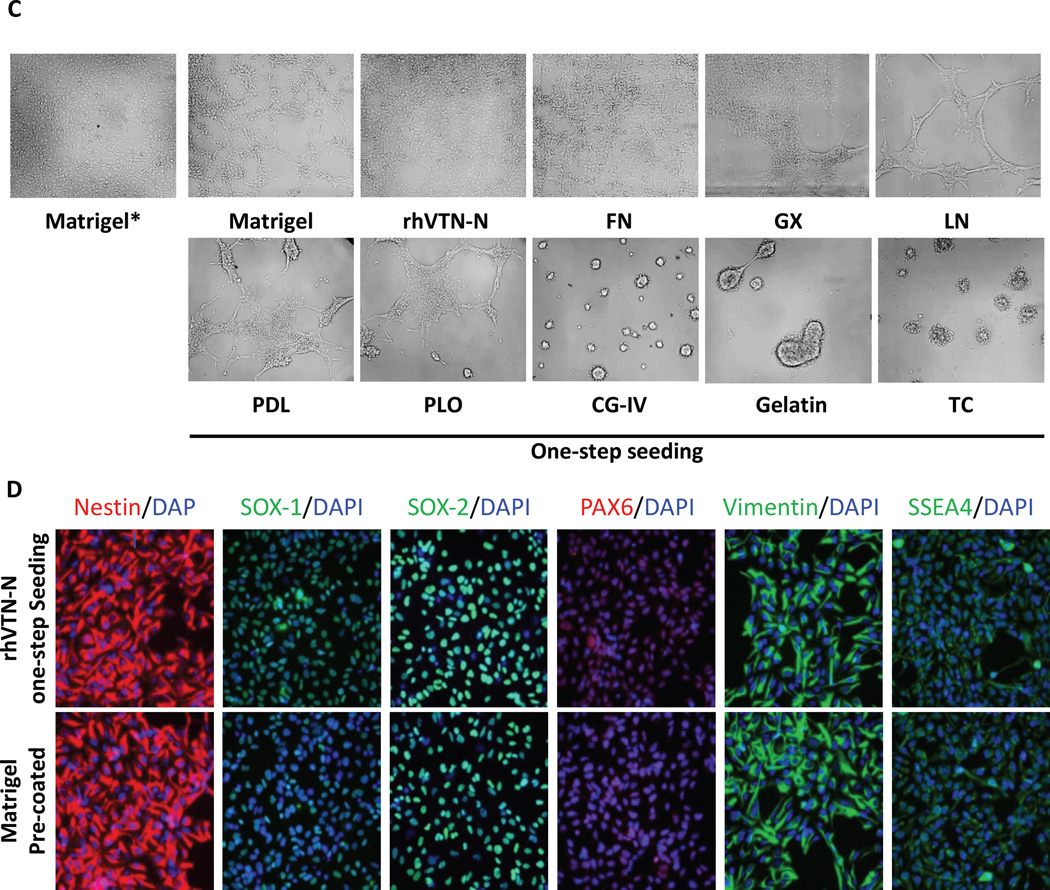
In order to determinate if a one-step seeding method could be applied to neuron differentiation in assay plates, we plated neural stem cells in 384-well assay plates. We initiated the neuron differentiation during one-step seeding by adding the rhVTN-N-supplemented neuronal differentiation medium to commercial PDL-coated plates or tissue culture-treated plates. Neurons differentiated in the LN and PLO doubly pre-coated plates were used as a control.10 For neurons cultured in the rhVTN-N-supplemented medium in commercial PDL-coated plates, the cell growth rate and cell viability were similar to these cultured in the control plates pre-coated with LN and PLO. However, most neuronal cells died after 2-weeks in culture in the rhVTN-N-supplemented medium in plates without PDL coatings (Fig. 2A). After 4-week differentiation in the assay plates, the cells showed positive staining of β-III Tubulin, microtubule associated protein (MAP2), neurofilament-L (NF-L) with negative Nestin, sex determining region Y-box 2 (SOX-2), and glial fibrillary acidic protein (GFAP) staining (Fig. 2B), indicating relatively mature neurons. The neurite outgrowth rate of these neurons was also similar to that of the control (Fig. 2C), as measured by a mean length of neurite projections stained by β-III Tubulin. The results indicate that neuronal cell differentiation in plates requires both rhVTN-N-supplemented medium and the use of commercial PDL-coated plates.
Figure 2.
Results of direct seeding of NSCs for neuron differentiation. (A): Results of cell viability determined in the ATP content and nuclear dye assay in neurons differentiated from NSCs. Cells directly seeded in commercial 384-well PDL-coated plates with the rhVTN-N-supplemented medium showed similar cell viability as those control cells plated in the traditional LN + PLO dual-coated plates (p > 0.05). However, neurons differentiated from NSCs directly seeded in regular (non-coated) 384-well plates with the rhVTN-N-supplemented medium exhibited low cell viability (***p<0.05). Data are represented as the mean ± SEM of at least triplicates. (B): Images of neurons differentiated from the direct seeding of NSCs in rhVTN-N-supplemented medium compared to the control condition of LN + POL plate pre-coating neurons. The NSCs differentiated to neurons with similar positive staining of β-III Tubulin, MAP2, and NF-L neuronal markers as the control cells cultured in LN + PLO dual-coated plates. The neurons also exhibited positive SOX-2 staining; they were negative for the markers Nestin, and GFAP (an astrocyte marker). (C): rhVTN-N The β-III Tubulin stained neurites were measured after 4 weeks of differentiation in assay plates. The neurite outgrowth rate of neurons differentiated from NSCs directly seeded in commercial 384-well PDL-plates with rhVTN-N-supplemented medium was similar to that of NSCs seeded in LN + PLO dual-coated plates (p>0.05). Data are represented as the mean ± SEM of at least triplicates.
One-step seeding of neural stem cells in rhVTN-N-supplemented medium for high throughput cell viability assays
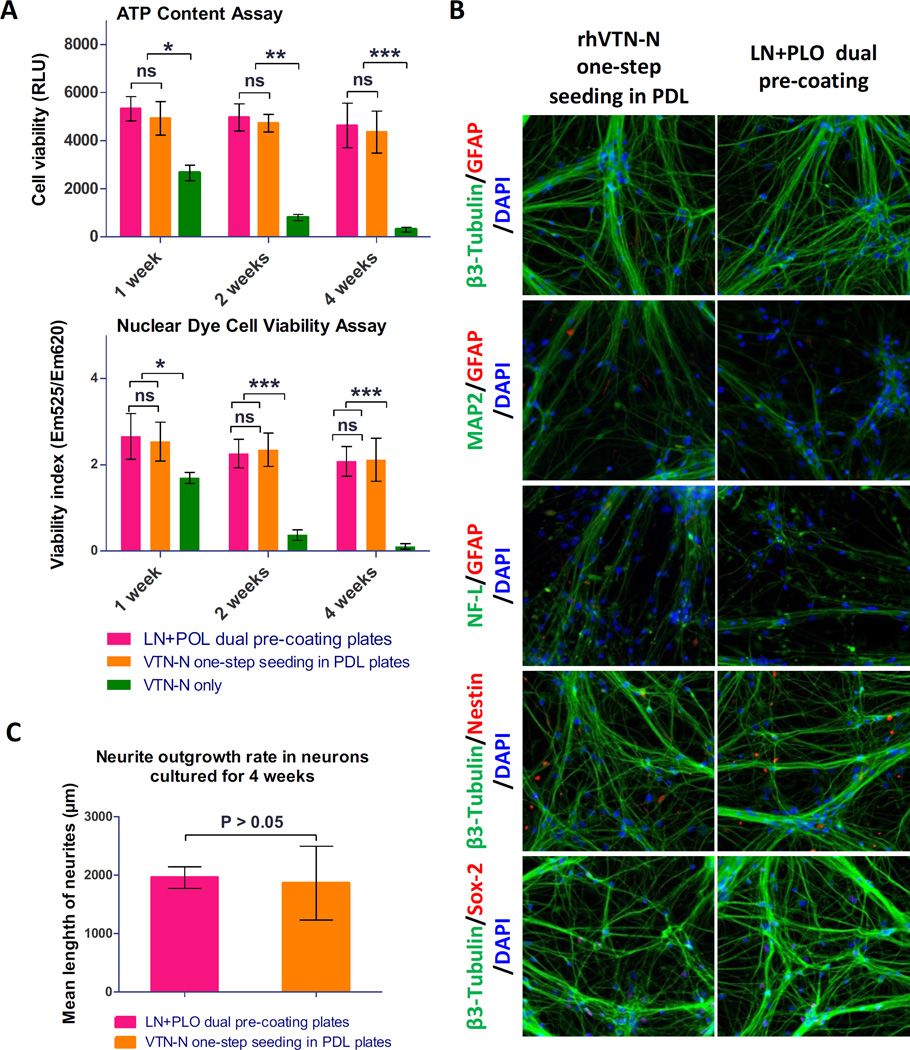
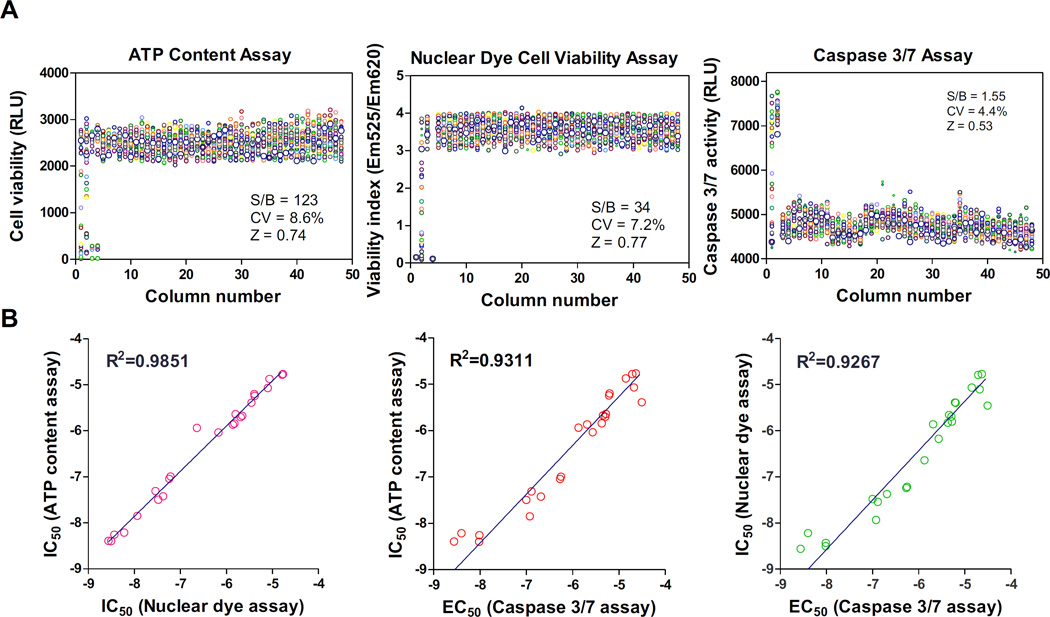
To validate this method, we first determined viability of neural stem cells directly seeded in 1536-well plates with the rhVTN-N-supplemented medium. A set of parameters for high throughput screening assays were first determined with cell viability experiments including the ATP content cell growth assay, nuclear dye cell viability assay, and cell apoptosis assay for caspase-3/7 activity. The scatter plots of these three assays exhibited robust assay profiles (Fig. 3A). The S/B ratios of ATP content, nuclear dye, and Caspase-3/7 assays were 123.8, 34.3 and 1.6 fold; coefficient of variation (CV) values were 8.6%, 7.2% and 4.4%; and Z factors were 0.74, 0.73 and 0.57, respectively. The results indicated that these assays are robust and suitable for HTS.
Figure 3.
Assay performances of the direct plating of NSC in 1536-well plates. (A): Scatter plots of DMSO plate tests in the ATP content, nuclear dye, and caspase 3/7 assays using NSCs directly seeded in rhVTN-N-supplemented medium in 1536-well plates. The results of HTS assays (Z score > 0.5) indicated statistically robust performances in all three assays. Each circle was the data from a single well in assay plates. The first column was a concentration titration of the control compound and the next column was the highest concentration of control compound. The rest of columns were added with DMSO, a solvent control for compounds. (B): Correlation cytotoxicity activities (IC50 or EC50 values) of 24 reported neurotoxic compounds determined in the ATP content, nuclear dye, and caspase 3/7 assays. The activities of these compounds correlated well in all three assays with R2 values from 0.93 to 0.98.
We also selected 24 neuronal toxic compounds from literatures (Suppl. Table S2) to determine the cytotoxicity in these assays using neural stem cells. These compounds exhibited cytotoxic effects in a concentration-dependent manner in neural stem cells (Suppl. Fig. S2). The cytotoxic half maximal inhibitory concentration (IC50) values of these 24 compounds correlated well: R2=0.98 for ATP and nuclear dye assays, R2=0.93 for ATP and caspase-3/7 assays, and R2=0.92 for nuclear dye and caspase-3/7 assays (Fig. 3B). Together, the results indicate that the method of neural stem cells directly seeded in the rhVTN-N-supplemented medium in 1536-well plates produced high quality results and is useful for high throughput cell viability assays.
Homogenous Lysotracker staining assay for measurement of enlarged lysosomes in NPC cells
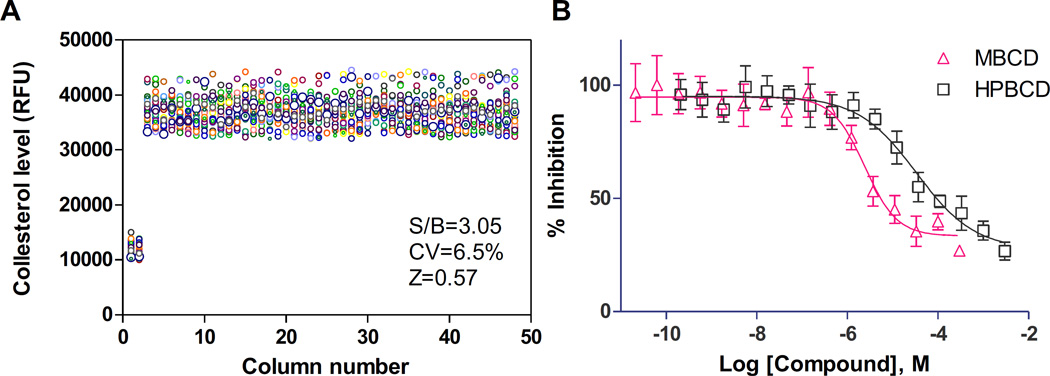
We also applied this method to a phenotypic compound screening assay using neural stem cells differentiated from iPSCs of a NPC patient. NPC NSCs exhibit a NPC disease phenotype of enlarged lysosome due to cholesterol accumulation; this can be detected by the Lysotracker dye staining assay.10, 15 The fluorescence signal of Lysotracker dye staining in NPC cells can be measured by fluorescence microscopy and also by a fluorescence plate reader.15 We found that the signal to background (S/B) ratio was 3.05 fold and Z-factor was 0.57 as determined by a Lysotracker dye staining assay in 1536-well plates when measured using a plate reader (Fig. 4A). The IC50 values of two control compounds, methyl-β-cyclodextrin (MΒCD) and hydroxypropyl-beta-cyclodextrin (HPBCD), were 2.4 and 33.7µM, respectively (Fig. 4B), similar to previous report.10 The results demonstrated that the one-step seeding method can also be used with patient derived neural stem cells in a HTS context.
Figure 4.
Results of the homogenous Lysotracker dye staining assay using Niemann Pick disease type C (NPC) patient NSCs directly seeded in 1536-well plates with rhVTN-N-supplemented medium. (A): Scatter plot of results from a DMSO plate test. Column 1 and 2 were wild type NSCs and column 3-through 48 were NPC NSCs that showed increased Lysotracker dye starting (enlarged lysosomes) due to the lysosomal cholesterol accumulation in the patient cells. (B): Concentration-response curves of two positive control compounds: methyl-beta-cyclodextrin (MΒCD) and hydroxypropyl-beta-cyclodextrin (HPBCD). The IC50 values of MΒCD and HPBCD were 2.4 and 33.7µM in the Lysotracker assay in the NPC NSCs, respectively. Data are represented as mean ± SEM of at least three biological replicates.
High throughput fluorescent calcium response assays using neural stem cells and neurons
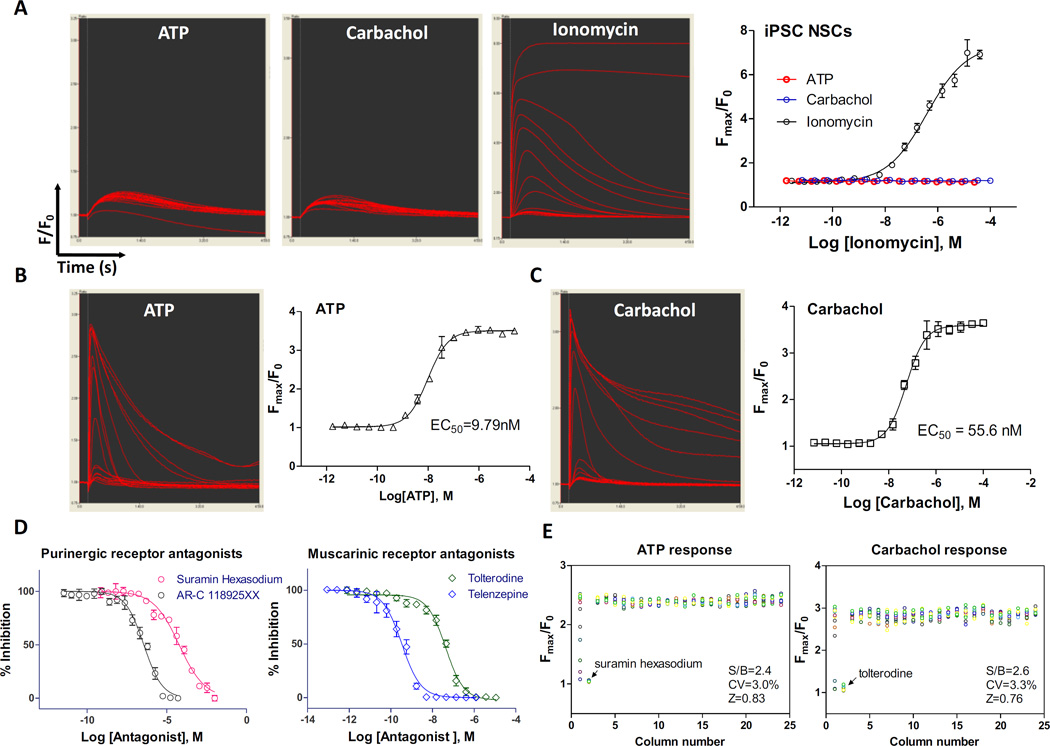
To further validate this method, we tested it with a high throughput calcium response assay in a 384-well assay plates (Suppl. Fig. S3A). We first measured the total calcium response using a fluorescence kinetic plate reader in a homogeneous format without the plate-washing steps. In the neural stem cells, ATP (a purinergic receptor agonist) and carbachol (a muscarinic receptor agonist) did not stimulate significant intracellular calcium responses, whereas the calcium ionophore ionomycin concentration dependently induced a calcium response with a half maximal effective concentration (EC50) of 0.39 µM (Fig. 5A, Suppl. Fig. S3B), similar to that previously reported.17 The calcium response of ionomycin in these cells was blocked by calcium chelators tetra (acetoxymethyl ester) (EGTA-AM) and 1, 2-Bis (2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetrakis (acetoxymethyl ester) (BAPTA-AM) with IC50 values of 3.3 and 2.8µM, respectively (Suppl. Fig. S3C). The S/B ratio, CV, and Z factor with the ionomycin stimulated calcium response and EGTA blocked were 4.68 fold, 6.32%, and 0.75, respectively (Suppl. Fig. S3D), indicating a robust high throughput screening assay.
Figure 5.
Results of calcium response assay and neurite outgrowth using NSCs directly seeded in rhVTN-N-supplemented medium in 384-plates. (A): Kinetic fluorescence intensity tracers of intracellular calcium responses of NSCs stimulated by ATP (a purinergic receptor agonist), carbachol (an acetylcholine muscarinic receptor agonist), and ionomycin (calcium ionophore). In the NSCs, only small and insignificant responses to ATP and carbachol were observed, while ionomycin showed a robust signal with an EC50 of 0.39µM. (B) and (C): Kinetic fluorescence intensity tracers and concentration response curves of intracellular calcium determined in neurons differentiated from NSCs stimulated by ATP (EC50 = 9.8nM) and carbachol (EC50 = 55.7nM). (D): Inhibitory concentration-response curves of two purinergic receptor antagonists and two muscarinic acetylcholine receptor antagonists determined in neurons differentiated from NSCs. Data are represented as mean ± SEM of at least three biological replicates. (E): Scatter plots of ATP induced and Carbachol induced intracellular calcium responses. The parameters of S/B ratio, CV, and Z-factor determined in a DMSO plate test exhibited robust assay parameters (S/B ratio > 2 fold and Z score > 0.5). Wells in column-1 were antagonist titration and in column-2 were the highest concentration of antagonist.
While neural stem cells only express low levels of receptors and ion channels,18 the differentiated neurons express much higher levels of ion channels and receptors including calcium channels, purinergic receptors, glutamate receptors, and ryanodine receptors.19 We then measured the intracellular calcium responses to ATP and carbachol in differentiated neurons plated in commercial PDL-coated 384-well plates with the rhVTN-N-supplemented medium. The EC50 of the ATP and carbachol were 9.8 and 55.7nM, respectively (Fig. 5B and C). The calcium responses stimulated by ATP and carbachol were concentration-dependently inhibited by the purinergic receptor antagonist suramin hexasodium (IC50=59.5µM) and AR-C 118925XX (IC50=359.8nM), and muscarinic acetylcholine receptor antagonist tolterodine (IC50=42.8nM) and telenzepine (IC50=0.3nM), respectively (Fig. 5D). These calcium responses in neurons were robust with the favorable HTS assay parameters (Fig. 5E and Suppl. Fig. S4). Together, the results demonstrate the excellence of the high throughput calcium response using our new method with the rhVTN-N-supplemented medium.
Single-cell calcium response in neural stem cells and neurons
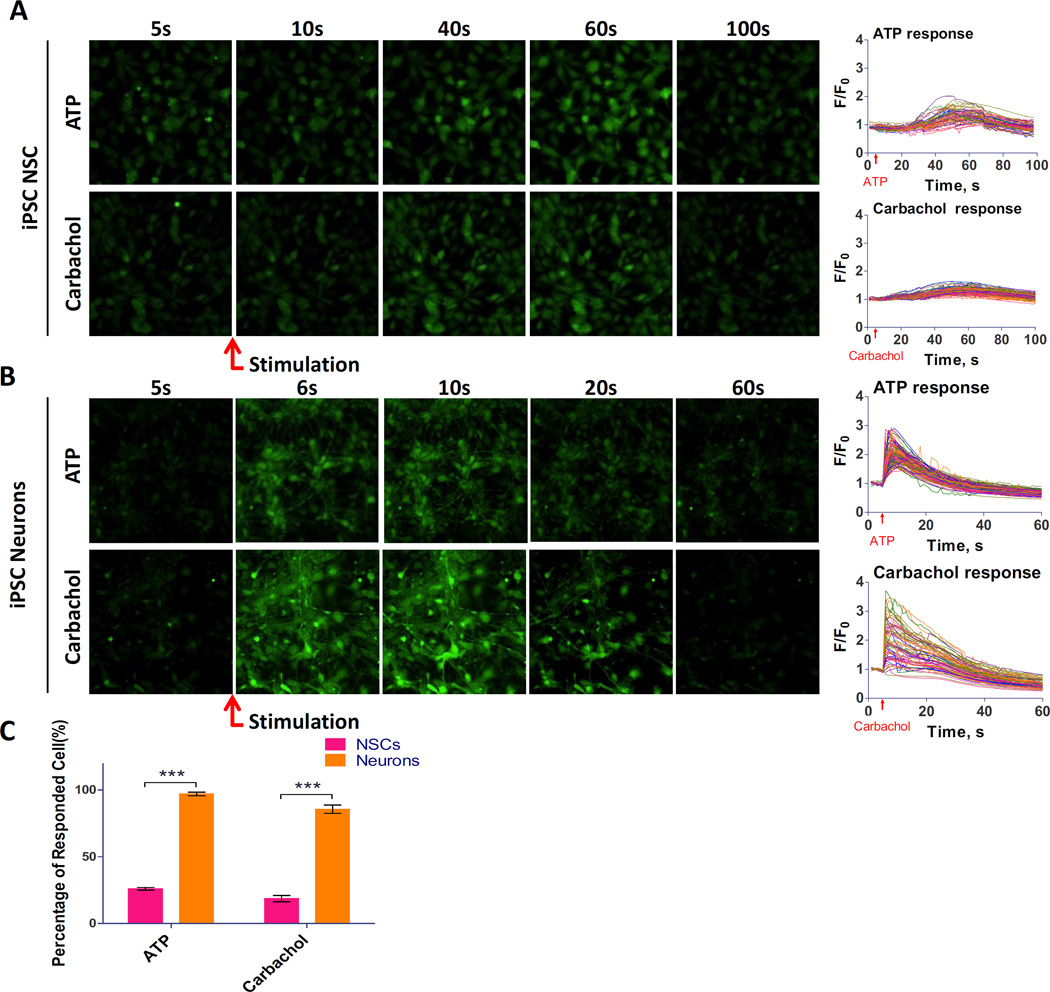
Because not all of the cells in a population respond equally to a stimulus, a single cell recording of calcium response offers a much more sensitive detection system that distinguishes the responding cells from non-responding cells in a cell population. Using a fast kinetic fluorescence imaging system, we measured the single-cell calcium response in neural stem cells and neurons in addition to the above whole-well calcium response. In neural stem cells, only small portion of cells responded to the ATP or carbachol stimulation (Fig. 6A), while the majority of cells responded to Ionomycin (Suppl. Fig. S5). The percentage of positive responding neural stem cells was 26% for ATP and 18% for carbachol. The results suggested that the weak calcium response in neural stem cells is more distinguishable by the single cell calcium response than that which is otherwise not detectable by the total calcium response in a well of assay plates as described above. In differentiated neurons, ATP and carbachol induced extensive calcium responses measured at the single cell level (Fig. 6B). Contrarily, the majority of neurons responded to agonists: 97% of the responded to ATP and 86% responded to carbachol stimulation (p<0.01) (Fig. 6C). The results indicated that the neurons differentiated from neural stem cells in the rhVTN-N-supplemented medium directly seeded in commercial PDL-coated plates responded well to ATP and carbachol stimulations.
Figure 6.
Results of single cell calcium responses in NSCs and neurons with NSCs directly seeded in rhVTN-N-supplemented medium in 384-plates. (A): Serial fluorescence images and kinetic tracers of NSCs recorded in a single cell calcium response assay. (B): Serial fluorescence images and kinetic tracers of neurons recorded in a single cell calcium response assay. (C): Comparison of percentage of responding cells determined from the single call calcium response in NSCs and neurons. A positive responding cell was defined by a cell responded to a stimulus with > 3 × SD increase in mean calcium signal of the control cells. The positive responding neural stem cells were 26% for ATP stimulation and 18% for carbachol stimulation, whereas positive neurons were 97%for ATP and 86% for carbachol. Data are represented as mean ± SEM of at least three biological replicates.
Discussion
We described here a simple method (Suppl. Fig. S6) for directly seeding neural stem cells in assay plates without the need for pre-coating. By addition of a recombinant vitronectin protein to the medium, the labor-intensive plate pre-coating and plate-wash steps are eliminated from the workflow. Neural stem cells can be directly seeded in regular tissue culture treated plates with the VNT-N-supplemented medium. Neuronal cells can be differentiated from neural stem cells that were seeded in commercial PDL-coated plates with the VNT-N-supplemented neuronal differentiation medium. Because no additional reagent addition and plate-wash steps are needed, this new method simplifies the experimental procedure, improves assay throughput, and increases reproducibility.
Additional surface treatment is often needed for culturing primary cells and neurons although tissue culture treated plastic ware prepared by gas plasma are generally used.20 The additional surface treatment provides an environment similar to the extracellular matrix (ECM) environment the cells experience in vivo. ECM consists of secreted proteins that mediate proper attachment, spreading, growth, and differentiation of neuronal cells.21 In many types of cell culture, fetal bovine serum (FBS) supplementation provides extracellular matrix proteins and various growth factors to cells. But FBS is usually not added to culture medium for stem cells and neurons differentiated from iPSCs, because only a few specific growth factors can be added in the medium to prevent uncontrolled differentiation. Therefore, pre-coating of dishes and plates with extracellular matrix proteins and polymers is a common practice for the culturing of stem cells and neurons although the process is labor-intensive with a high reagent cost. Neuronal cell culture requires freshly pre-coated plates, covered with thin layers of both extracellular matrix proteins and positively charged polymers. Because the ECM protein like LN is very easy to be absorbed by plastic and tends to form aggregates in plates at room temperature, precautions such as keeping the coating material on ice are usually needed for the plate pre-coating.22
Positively charged synthetic polymers such as PDL, polyethyleneimine, and PLO are commonly used to coat dishes and plates that are commercially available. They provide electrostatic interaction with the negatively charged cell membrane, supporting cell attachment.23 These materials are usually stable and can be pre-coated in assay plates in advance or can be obtained from commercial venders. On the other hand, extracellular proteins such as Matrigel, FN, LN, and VTN have to be freshly coated onto assay plates. A dual pre-coating of with LN and PLO in assay plates is commonly used for neuronal cell culture and neuronal cell differentiation. The process involves dispensing of a coating reagent to plates, incubation, and multiple plate-wash steps.24, 25 A recent protocol for using the rhVTN-N to pre-coat plates describes unnecessary to wash plates after the plate coating but it still requires removal of vitronectin (http://tools.thermofisher.com/content/sfs/manuals/vitronectin_man.pdf).
To eliminate the need for plate pre-coating, we first tested addition of ECM proteins and polymers into cell culture medium for directly seeding cells onto plates without plate precoating. Nine reagents were examined including rhVTN-N, Matrigel, FN, Geltrex, Matrix, LN, collagen Type IV and gelatin, as well as two polymers, PDL and PLO (Suppl. Table S1). We found that neural stem cells seeded in rhVTN-N-supplemented medium exhibited a similar viability, attachment, and spreading morphology when compared to those cultured in the traditional Matrigel pre-coated plates (Fig. 1B, 1C). The cells displayed a normal profile of neural stem cell markers and normal chromosome morphology. The results measured in the cell viability and calcium response assays were robust with high reproducibility (Fig. 2–4). Therefore, this method is useful for HTS assays used to identify lead compounds using neural stem cells for the cell-based disease model. It also can be applied to cell viability assays and cytotoxicity assays that employ neural stem cells to determine compound neurotoxicity.26 In addition, we utilized it on a few assays with neurons differentiated from neural stem cells (Fig. 2A). We found that only the addition of rhVNT-N to the medium is not enough for a proper neuron differentiation. A combination of rhVNT-N-supplemented medium and PDL-coated plates are needed. PDL has been commonly used for plate coating. PDL-coated plates can be sterilized and stored for 12 months or longer and are readily available from commercial vendors. On the other hand, we found cytotoxicity in two polymer-supplemented mediums, suggesting that the polymer-supplemented medium is not suitable for seeding of cells without plate-washes (Fig. 1B).
Neural stem cells,27 neural progenitor cells,28 and neurons differentiated from stem cells13 have recently been employed for compound screens, but the screening throughput of these reported experiments was limited due to the requirement of plate pre-coating to support cell attachment and growth. Matrigel, FN, LN, VTN, PLO and PDL have been used as substrates for plate pre-coating in neuronal cell culture (Suppl. Table S3). Matrigel is a protein mixture secreted by Engelbreth-Holm-Swarm mouse sarcoma cells which supports cell attachment on plastic ware. As a mixture, Matrigel suffers from large batch-to-batch variations and potential xenogeneic contamination. Both FN and LN are ECM glycoproteins with high molecular weight. However, human VTN is a relatively small (75 KDa) glycoprotein that is present in serum and supports cell adhesion and spreading.29 It has also been reported that vitronectin supported hESC self-renewal via an interaction with αVβ5 Integrin and stem cells maintained normal morphology and functions in the presence of VTN.29, 30 rhVTN-N is a truncated recombinant vitronectin corresponding to the amino acid fragment 62–478 of human vitronectin expressed in E. coli.12 rhVTN-N has recently been used in iPSC culture medium for supporting human iPSCs attachment and growth.12 The size of rhVTN-N is smaller than other ECM proteins and its cost is relatively low: a few dollars per plate. Therefore, rhVTN-N is a good choice as a supplement to cell culture medium for one-step seeding of neural stem cells compared to other extracellular matrix proteins.
Although the plate pre-coating can be handled in these experiments with a few plates, it is a burden for high throughput screening with large amount of compounds. Reductions of robotic steps in a screening assay can significantly increase compound screening throughput, reduce the chances of interruptions in robotic operation due to errors and malfunctions of liquid handlers, and increase data quality by decrease in well-to-well and plate-to-plate variations. This method of one-step seeding of neural stem cells significantly reduces assay steps, which is specifically useful for robotic screening of large compound collections using neuronal cells.
In conclusion, we have developed a simple method for one-step seeding of neural stem cells in assay plates with an rhVTN-N-supplemented medium without the need of plate pre-coating. This method greatly increases the throughput and reproducibility of experiments using neuronal cells derived from human iPSCs for translational research. Additionally, the principle of this method has a potential to be extended to seed iPSCs and iPSC-derived progenitor cells, which in turn can be differentiated into other cell types for various experiments.
Supplementary Material
Acknowledgments
This work was funded by the Intramural Research Programs of the National Center for Advancing Translational Sciences, National Institutes of Health. The authors thank Paul Shinn and compound management team at NCATS for their assistance. The authors also thank DeeAnn Visk for editing and critiquing the manuscript.
Abbreviations
- ECM
extracellular matrix
- FN
fibronectin
- hESC
human embryonic stem cell
- HTS
high throughput screening
- iPSC
induced pluripotent stem cell
- LN
laminin
- NSCs
neural stem cells
- PDL
poly-D-lysine
- PLO
poly-L-ornithine
- VTN
vitronectin
- rhVTN-N
truncated recombinant human vitronectin
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Bellin M, Marchetto MC, Gage FH, et al. Induced pluripotent stem cells: the new patient? Nature reviews. Molecular cell biology. 2012;13:713–726. doi: 10.1038/nrm3448. [DOI] [PubMed] [Google Scholar]
- 2.Wegener G, Rujescu D. The current development of CNS drug research. Int J Neuropsychopharmacol. 2013;16:1687–1693. doi: 10.1017/S1461145713000345. [DOI] [PubMed] [Google Scholar]
- 3.Xie YZ, Zhang RX. Neurodegenerative diseases in a dish: the promise of iPSC technology in disease modeling and therapeutic discovery. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2015;36:21–27. doi: 10.1007/s10072-014-1989-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert AD, Yu J, Rose FF, Jr, et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan SD, Dolatabadi N, Chan SF, et al. Isogenic human iPSC Parkinson's model shows nitrosative stress-induced dysfunction in MEF2-PGC1alpha transcription. Cell. 2013;155:1351–1364. doi: 10.1016/j.cell.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Israel MA, Yuan SH, Bardy C, et al. Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482:216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wainger BJ, Kiskinis E, Mellin C, et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7:1–11. doi: 10.1016/j.celrep.2014.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sareen D, Ebert AD, Heins BM, et al. Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS One. 2012;7:e39113. doi: 10.1371/journal.pone.0039113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu D, Swaroop M, Wang M, et al. Niemann-Pick Disease Type C: Induced Pluripotent Stem Cell-Derived Neuronal Cells for Modeling Neural Disease and Evaluating Drug Efficacy. J Biomol Screen. 2014;19:1164–1173. doi: 10.1177/1087057114537378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villa-Diaz LG, Ross AM, Lahann J, et al. Concise review: The evolution of human pluripotent stem cell culture: from feeder cells to synthetic coatings. Stem Cells. 2013;31:1–7. doi: 10.1002/stem.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen G, Gulbranson DR, Hou Z, et al. Chemically defined conditions for human iPSC derivation and culture. Nature methods. 2011;8:424–429. doi: 10.1038/nmeth.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Lei Y, Luo J, et al. Prevention of beta-amyloid induced toxicity in human iPS cell-derived neurons by inhibition of Cyclin-dependent kinases and associated cell cycle events. Stem cell research. 2013;10:213–227. doi: 10.1016/j.scr.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Wei H, Wei W, Bredesen DE, et al. Bcl-2 protects against apoptosis in neuronal cell line caused by thapsigargin-induced depletion of intracellular calcium stores. Journal of neurochemistry. 1998;70:2305–2314. doi: 10.1046/j.1471-4159.1998.70062305.x. [DOI] [PubMed] [Google Scholar]
- 15.Xu M, Liu K, Swaroop M, et al. delta-Tocopherol reduces lipid accumulation in Niemann-Pick type C1 and Wolman cholesterol storage disorders. J Biol Chem. 2012;287:39349–39360. doi: 10.1074/jbc.M112.357707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M, Liu K, Swaroop M, et al. A phenotypic compound screening assay for lysosomal storage diseases. J Biomol Screen. 2014;19:168–175. doi: 10.1177/1087057113501197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geng Y, Lotz M. Increased intracellular Ca2+ selectively suppresses IL-1-induced NO production by reducing iNOS mRNA stability. The Journal of cell biology. 1995;129:1651–1657. doi: 10.1083/jcb.129.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young A, Machacek DW, Dhara SK, et al. Ion channels and ionotropic receptors in human embryonic stem cell derived neural progenitors. Neuroscience. 2011;192:793–805. doi: 10.1016/j.neuroscience.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forostyak O, Romanyuk N, Verkhratsky A, et al. Plasticity of Calcium Signaling Cascades in Human Embryonic Stem Cell-Derived Neural Precursors. Stem cells and development. 2013;22:1506–1521. doi: 10.1089/scd.2012.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker SL, LaRocca PJ. Method of production and control of a commoncial tissue culture surface. Journal of Tissue Culture Methods. 1994;16:151–153. [Google Scholar]
- 21.Michel G, Tonon T, Scornet D, et al. The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. The New phytologist. 2010;188:82–97. doi: 10.1111/j.1469-8137.2010.03374.x. [DOI] [PubMed] [Google Scholar]
- 22.Hu BY, Zhang SC. Directed differentiation of neural-stem cells and subtype-specific neurons from hESCs. Methods in molecular biology. 2010;636:123–137. doi: 10.1007/978-1-60761-691-7_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harnett EM, Alderman J, Wood T. The surface energy of various biomaterials coated with adhesion molecules used in cell culture. Colloids and surfaces. B, Biointerfaces. 2007;55:90–97. doi: 10.1016/j.colsurfb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 24.Fedoroff S, Richardson A. Protocols for Neural Cell Culture [Online] Second. Totowa, NJ: Humana Press : Imprint: Humana Press; 1997. SpringerLink (Online service) http://dx.doi.org/10.1007/978-1-4757-2586-5. [Google Scholar]
- 25.Kirkeby A, Nelander J, Parmar M. Generating regionalized neuronal cells from pluripotency, a step-by-step protocol. Frontiers in cellular neuroscience. 2012;6:64. doi: 10.3389/fncel.2012.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sirenko O, Hesley J, Rusyn I, et al. High-content high-throughput assays for characterizing the viability and morphology of human iPSC-derived neuronal cultures. Assay and drug development technologies. 2014;12:536–547. doi: 10.1089/adt.2014.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumari D, Swaroop M, Southall N, et al. High-Throughput Screening to Identify Compounds That Increase Fragile X Mental Retardation Protein Expression in Neural Stem Cells Differentiated From Fragile X Syndrome Patient-Derived Induced Pluripotent Stem Cells. Stem cells translational medicine. 2015;4:800–808. doi: 10.5966/sctm.2014-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao WN, Cheng C, Theriault KM, et al. A high-throughput screen for Wnt/beta-catenin signaling pathway modulators in human iPSC-derived neural progenitors. J Biomol Screen. 2012;17:1252–1263. doi: 10.1177/1087057112456876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lambshead JW, Meagher L, O'Brien C, et al. Defining synthetic surfaces for human pluripotent stem cell culture. Cell Regen (Lond) 2013;2:7. doi: 10.1186/2045-9769-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braam SR, Zeinstra L, Litjens S, et al. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cells. 2008;26:2257–2265. doi: 10.1634/stemcells.2008-0291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.