Abstract
We assessed the outcome of patients with drug resistant epilepsy and neuronal antibodies who underwent epilepsy surgery. Retrospective study, information collected with a questionnaire sent to epilepsy surgery centers. Thirteen patients identified, with antibodies to GAD (8), Ma2 (2), Hu (1), LGI1 (1) or CASPR2 (1). Mean age at seizure onset: 23 years. Five patients had an encephalitic phase. Three had testicular tumors and five had autoimmune diseases. All had drug resistant temporal lobe epilepsy (median: 20 seizures/month). MRI showed unilateral temporal lobe abnormalities (mainly hippocampal sclerosis) in 9 patients, bilateral abnormalities in 3, and was normal in 1. Surgical procedures included anteromesial temporal lobectomy (10 patients), selective amygdalohippocampectomy (1), temporal pole resection (1) and radiofrequency ablation of mesial structures (1). Perivascular lymphocytic infiltrates were seen in 7/12 patients. One year outcome available in all patients, at 3 years in 9. At last visit 5/13 patients (38.5%) (with Ma2, Hu, LGI1, and 2 GAD antibodies) were in Engel`s classes I or II. Epilepsy surgery may be an option for patients with drug resistant seizures associated with neuronal antibodies. Outcome seems to be worse than that expected in other etiologies, even in the presence of unilateral HS. Intracranial EEG may be required in some patients.
Keywords: hippocampal sclerosis, GAD, Ma2, LGI1, temporal lobectomy
Introduction
Autoimmunity is being increasingly recognized as a cause of drug resistant epilepsy. Some patients develop drug resistant seizures after an episode of well-characterized autoimmune encephalitis (Pillai et al., 2016). There is also evidence that autoimmune (paraneoplastic or non-paraneoplastic) limbic encephalitis may present as temporal lobe epilepsy, sometimes associated with hippocampal sclerosis (HS) (Bien et al., 2007;Gaspard, 2016). As neuronal antibody determination becomes more widely available, they are increasingly identified in a subset of patients with chronic epilepsy during the diagnostic workup.
Patients with autoimmune epilepsy usually have high seizure frequency and often display MRI abnormalities, such as HS. In this setting, when antiepileptic and immunosuppressive drugs fail, surgical treatment may be a therapeutic option. The HS observed in these patients probably represents a “burn out” inflammatory process and it is often considered the underlying “structural” cause of the seizures. Less frequently, surgery is performed when the autoimmune etiology has not been suspected yet and the mesial temporal swelling is mistaken for cortical dysplasia or a low-grade tumor (Almeida et al., 2012). The potential role of epilepsy surgery has been only assessed in isolated case reports and small series of patients with neuronal antibodies (Almeida et al., 2012;Kerling et al., 2008;Malter et al., 2015;Muehlebner et al., 2010). The objective of our study was to determine the outcome of a series of patients with epilepsy associated with neuronal antibodies who underwent epilepsy surgery.
Methods
This retrospective multicenter study was approved by the Ethics committee of Hospital Clínic, University of Barcelona (Barcelona, Spain). Patients were routinely consented for their clinical information to be included in clinical studies. A questionnaire was sent to 60 tertiary epilepsy surgery centers in Europe, USA, Canada, and Japan to find out if they had any patients who met the inclusion criteria: resective surgery for drug resistant epilepsy and positive neuronal antibodies. We asked about clinical features, acute encephalitic phase if present, immunosuppressive treatment, type and titres of autoantibodies if available, results of presurgical evaluation, pathological findings, and seizure outcome at 1 and 3 years. Surgically treated patients in whom pathological findings were suggestive of chronic encephalitis but no specific antibody was found were excluded from the study. Outcome was assessed by the Engel classification, a widely used tool to categorize the outcome of epilepsy surgery: class I (free of disabling seizures), class II (rare disabling seizures, almost seizure free), class III (worthwhile improvement) and class IV (not worthwhile improvement). Satisfactory outcome included classes I and II.
Results
Most epilepsy centers could not provide any patients who met the inclusion criteria. Finally, a total of 13 patients from 8 centers in Europe and the United States were included in the study. Two patients have been previously reported (Almeida et al., 2012;Haberlandt et al., 2011). Mean age at epilepsy onset was 23 years (range: 2–46 years). The following antibodies were detected in serum: GAD (8 patients), Ma2 (2) patients), Hu (1), LGI1 (1), and CASPR2 (1). Antibodies were also detected in CSF in 5/11 patients (CSF not analyzed in 2). Oligoclonal bands in CSF were found in 3 patients. The antibodies were found after surgery in 5 patients (Table 1), most often because of the pathological findings. Eight patients had drug resistant epilepsy without a previous episode of encephalitis (6 GAD, 1 LGI1, 1 CASPR2), whereas the other five patients developed drug resistant epilepsy after a typical episode of autoimmune encephalitis. Associated comorbidities included 3 testicular tumors (2 anti-Ma2, 1 anti-Hu), and several autoimmune disorders, all in patients with GAD antibodies (Table 1). Six patients (five who had encephalitis and one with chronic epilepsy) were treated with immunosuppressive drugs (intravenous corticosteroids ± immunoglobulins). Three patients were still taking maintenance corticosteroids at presurgical evaluation.
Table 1.
Clinical characteristics and seizure outcome after epilepsy surgery of patients with drug resistant epilepsy with neuronal antibodies
| Patien t |
Age*/ sex |
Encephal itic phase |
Antibod y, titres (if GAD) |
CSF features |
Comorbi dity |
MRI | Immuno therapy |
Ictal EEG |
Surgery (delay) |
Outcome** (Engel) |
Inflammato ry infiltrates (additional findings) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34/M | yes | Ma2 (serum, CSF) |
Oligoclonal bands * |
Testicula r tumor |
Unilater al MTS |
CS, Ig | Unilater al |
AMTL (1y) |
III | Yes (neuronal loss, gliosis) |
| 2 | 27/M | yes | Ma2 (serum, CSF) |
N.A. | Testicula r tumor |
Bilateral MTS |
CS, Ig | Bilateral | AMTL (7m) |
II | Yes (neuronal loss) |
| 3 | 37/M | yes | Hu (serum) |
Nomal | Testicula r tumor |
Unilater al MTS |
CS | Unilater al |
AMTL (11y) |
I | No (neuronal loss) |
| 4*** | 15/F | yes | GAD (serum, CSF) |
N.A. | Type I DM, Stiff person |
Bilateral LE |
CS | Bilateral | AMTL (6y) |
IV | Yes (hippocamp al sclerosis) |
| 5 | 9/F | yes | GAD (serum) |
N.A. | Stiff person |
Normal | CS | Bilateral | AMTL (8y) |
III | Yes |
| 6 | 24/F | No | GAD (serum, CSF) |
Oligoclonal bands ++ |
RA, cutaneou s vasculitis |
Unilater al MTS |
CS | Unilater al |
AMTL (8y) |
III | No (hippocamp al sclerosis) |
| 7 | 26/F | No | GAD (serum) |
Not done | None | Unilater al MTS |
No | Bilateral | AMTL (3y) |
IV | No (gliosis, satellitosis) |
| 8 | 19/F | No | GAD (serum) |
Oligoclonal bands ++ |
No | Unilater al MTs |
No | Unilater al |
AMTL (10y) |
III | No (abnormal cortical lamination) |
| 9 | 2/F | No | GAD (serum, CSF) **** |
N.A. | Type I DM |
Unilater al MTS |
No | Unilater al |
AMTL (61 y) |
IV | No (hippocamp al sclerosis) |
| 10 | 16/F | No | GAD (serum) **** |
N.A. | None | Unilater al LE |
No | Unilater al |
T pole resectio n (4y) |
II | Yes (gliosis) |
| 11 | 20/M | No | CASPR 2 (serum) **** |
Normal | None | Bilateral MTS |
No | Unilater al |
STRA (18y) |
III | Not available |
| 12*** | 19/M | No | LGI1 (serum) **** |
Not done | None | Unilater al LE |
No | Unilater al |
SAH (1y) |
I | Yes (gliosis, microglial activation) |
| 13 | 46/F | No | GAD (serum) **** |
Not done | Type I DM, celiac, thyroid |
Unilater al LE |
No | Unilater al |
AMTL (9m) |
II | Yes (gliosis, neuronal loss and cytomegaly |
M: male, F: female, CS: corticosteroids, RT: right temporal AMTL: anteromedial temporal lobectomy, RA: rheumathoid arthritis, STRA: stereotactic radiofrequency ablation, SAH: selective amygadalohipocampectomy, LE: limbic encephalitis, HS: hippocampal sclerosis,
at seizure onset.
N.A.: not available
at last follow up,
patient previously reported
antibodies found after surgery
Mean time to presurgical evaluation was 9.4 years (range 1–61 years). All patients underwent prolonged video-EEG monitoring with surface electrodes, MRI and neuropsychological testing. No patients underwent monitoring with invasive electrodes. Seizure frequency was daily or monthly (median number: 20 per month, range 2–720). Mean number of antiepileptic drugs was 2.1 (range 1–3). All patients had seizures with temporal lobe semiology. Ten patients had auras, being abdominal (5 patients), and psychic (5) the most common. Four patients displayed multiple types of auras. The most frequently recorded seizures were automotor (with oral or distant hand automatisms and alteration of awareness: 11 patients). Two patients had autonomic seizures (objective piloerection). In addition, four patients had complex motor seizures with irregular movements involving trunk and proximal parts of the limbs and 3 had secondarily generalized tonic-clonic seizures. Interictal surface EEG showed unilateral temporal epileptiform discharges in 9/13 patients, and bilateral temporal discharges in the other 4. Surface ictal EEG showed seizure onset zone in one temporal lobe in 9/13 patients and independently in both temporal lobes in 4/13 patients. Neuropsychological evaluation demonstrated bilateral temporal dysfunction in 4 patients (one with severe anterograde amnesia), unilateral temporal dysfunction in 7 and executive deficits only in 2 patients.
MRI was normal in 1 patient, showed unilateral HS in 6/13 patients, bilateral asymmetric HS in 2/13 patients, unilateral increase in size and signal of mesial structures thought to be a malformation or a low grade tumor in 3/13 patients and bilateral increase in size and signal of mesial temporal structures in 1 patient (Table 1).
Surgical procedures included standard anteromesial temporal resection in 10/13 patients, selective amygdalohippocampectomy in 1, resection of the temporal pole sparing the hippocampus in 1, and stereotactic radiofrequency ablation of mesial structures in 1. Complications after surgery included depression (2 patients), semantic memory deficits (1) and quadrantanopsia (1). Neuropathological findings included lymphocytic infiltrates in 7/12 patients (Figure 1), along with variable amounts of neuronophagic nodules, microglia activation, neuronal loss, hippocampal sclerosis, and abnormal cortical lamination (Table 1). Seizure outcome was available at 1 year for all patients and at 3 years for 9. At the last follow-up, 5/13 patients (38.4%) were in Engel classes I or II; 8 patients were in classes III or IV. Two patients became seizure free after surgery. Two patients (CASP2R and GAD) who were initially seizure free moved to a class III outcome during follow-up. We did not find any correlation between outcome of surgery and antibody type, previous history of encephalitis, or presence of uni or bilateral HS in the MRI (only 1 out of 6 patients −16%- with HS had a good outcome).
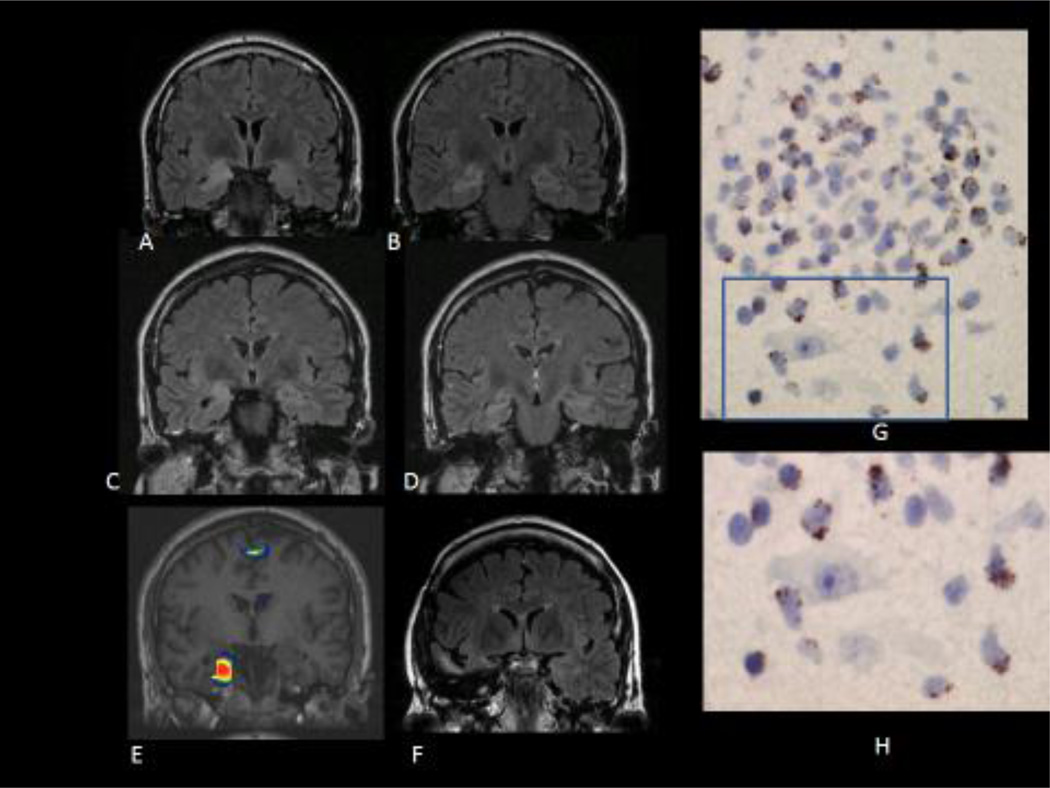
Figure 1.
A, B: Coronal FLAIR MRI images showing increased signal and volumen of right amígdala and hippocampus, suggestive of limbic encephalitis, in a patient with acute onset of seizures. The patient had anti Ma2 antibodies and a past history of seminoma (patient 1, see table).
C, D: Coronal FLAIR images one year later, showing atrophy of right mesial temporal structures with persistence of the increased signal (right MTS) (patient 1)
E: SISCOM showing increased ictal perfusion over the right hippocampus and parahippocampal gyrus during a right temporal lobe seizure with epigastric aura, piloerection and loss of awareness (patient 1).
F: Coronal FLAIR image showing resection of the temporal pole and the right mesial temporal structures. The patient continued to have seizures after surgery although with some seizure reduction (Engel´s class III) (patient 1)
G: Inflammatory infiltrate in the surgical specimen. The tissue section was immunostained with TIA-1 antibody, a marker of cytotoxic T cells. There are positive cells clearly showing the characteristic TIA-1 granular pattern in the inflammatory infiltrate. Some TIA-1 positive cells are in close apposition with neurons (H). (patient 1)
Discussion
This retrospective multicenter study shows that seizure outcome after temporal lobe surgery in patients with neuronal antibodies seems to be worse than that in other clinical settings. In temporal lobe epilepsy with unilateral HS not associated with neuronal antibodies, up to 70% of patients become seizure free after surgery (Mathon et al., 2015), whereas only 16% of patients with similar MRI findings but with neuronal antibodies became seizure free in the current study. We acknowledge that the number of patients is small and this could have led to an underestimation of the efficacy of the surgical procedure. However, in view of these results it seems reasonable to be cautious when discussing the possibility of seizure freedom with patients and their families.
In such a limited sample it is difficult to extract valuable conclusions regarding the influence of specific types of neuronal antibodies in surgical outcome. A good clinical outcome occurred with different types of onconeural and surface-directed antibodies. Only 2 of 8 patients with GAD antibodies became free or almost seizure free in the long-term. This is in agreement with previously published series reporting improvement in seizure frequency but not seizure freedom in patients with GAD autoimmunity after surgery (Malter et al., 2015).
There are several possible explanations for this type of outcome. First, we have found that active signs of inflammation are present not only in patients undergoing surgery during the “acute” phase of the disease, but also in some patients with HS, reflecting that residual lesions and active inflammation may occur simultaneously in the same patient. Second, the ongoing inflammatory process may well extend beyond the surgically resected area (for example to the insula) and contribute to seizure generation after surgery. The occurrence of prominent autonomic symptoms in some patients suggests involvement of the insular cortex in seizure generation or epileptic network. Consequently, failure to identify a “temporal plus” epilepsy could explain failure of surgery in some cases (Barba et al., 2016). Third, the setting of neuronal antibodies it is not uncommon to observe bilateral involvement of the temporal lobes, both structurally (MRI) and functionally (e.g. memory deficits, bilateral interictal epileptiform discharges, bilateral seizure onset in the ictal EEG). Therefore, the non-resected temporal lobe may become epileptogenic, particularly if there is an ongoing inflammatory process in the brain. (Kerling et al., 2008). Intracranial EEG should be considered in patients with a possible temporal plus or bitemporal epilepsy to delineate better the seizure onset zone and increase the likelihood of successful epilepsy surgery.
Our study has several limitations. The main one is the small number of patients. However these patients are extremely difficult to find. We managed to include 13 after contacting with 60 of the most active epilepsy surgery centers in the world. The reason is that they are very rarely considered for surgery, even after antiepileptic and immunosuppressive drugs have failed. In fact, in 4 patients the resection was performed before the antibodies were discovered, when a possible autoimmune etiology had not been suspected yet. It is possible that epileptologists consider autoimmune epilepsy as an entity less likely to result in seizure freedom after surgery, due to its widespread epileptogenic effects. Our series seems to confirm this belief.
Due to its retrospective design, the study has the inherent bias that only patients with selected “favorable” clinical and MRI features underwent presurgical evaluation (presumably those deemed to have some benefit from surgery). In addition, the titers of GAD antibodies were not available for many patients. However, we believe our findings add valuable information to the decision algorithm in patients with drug resistant seizures and neuronal antibodies that we encounter with increasing frequency in our practice. Taking into account the high seizure frequency and the low rate of surgery complications, the possible benefit of a palliative resection may be considered. Presurgical evaluation may require invasive EEG studies in some patients.
HIGHLIGHTS.
Surgery is sometimes performed for temporal lobe epilepsy with neuronal antibodies
Surgery outcome is poorer than in patients without neuronal antibodies
No clear relationship between outcome and antibody type or MRI lesion was found
Presence of unilateral HS does not necessarily associate to good outcome
Invasive studies may help in some patients to define better the seizure onset zone
Acknowledgments
Funding sources: This study was supported in part by NIH RO1NS077851 (JD), Fondo de Investigaciones Sanitarias, FEDER, Spain (14/00203, JD), and Fundació CELLEX (JD).
Disclosure of conflicts of interest: Mar Carreño has participated in advisory boards and pharmaceutical industry-sponsored symposia for Eisai, UCB, Bial, Esteve, Medtronic and Cyberonics, and received research grants from UCB and Eisai. She reports no financial relationships relevant to the manscript. Christian Bien reports no financial relationships relevant to the manuscript. Aliakbar Asadi-Pooya reports no financial relationsips relevant to the manuscript. Michael Sperling reports: Research: Eisai, UCB Pharma, Sunovion, SK Life Sciences, Marinus, Lundbeck, Medtronics, Accorda, Upsher-Smith, Brain Sentinel, Glaxo, Pfizer. Dr. Sperling serves as the Editor-in-Chief of Epilepsia Petr Marusic reports no financial relationships relevant to the manuscript. Martin Elisak reports no financial relationships relevant to the manuscript. José Pimentel reports no financial relationships relevant to the manuscript. Tim Wehner reports no financial relationships relevant to the manuscript. Rajiv Mohanraj reports no financial relationship relevant to the manuscript. Juan Uranga reports no financial relationships relevant to the manuscript. Asier Gómez-Ibáñez reports no financial relationships relevant to the manuscript. Vicente Villanueva has participated in advisory boards and pharmaceutical industry-sponsored symposia for Eisai, UCB, Merck Sharp & Dohme, Bial, Pfizer, GlaxoSmithKline (GSK), Esteve, Medtronic and Cyberonics. He reports no financial relationships relevant to the manuscript. Francisco Gil reports no financial relationships relevant to the manuscript. Antonio Donaire reports no financial relationships relevant to the manuscript. Nuria Bargalló reports no financial relationships relevant to the manuscript. Jordi Rumià reports no financial relationships relevant to the manuscript. Pedro Roldán reports no financial relationships relevant to the manuscript. Xavier Setoain reports no financial relationships relevant to the manuscript. Luis Pintor reports no financial relationships relevant to the manuscript. Teresa Boget reports no financial relationships relevant to the manuscript. Eva Bailles reports no financial relationships relevant to the manuscript. Mercè Falip reports no financial relationships relevant to the manuscript. Javier Aparicio reports no financial relationships relevant to the manuscript. Josep Dalmau has a research grant from Euroimmun, and receives royalties from patents for the use of Ma2, NMDAR, and GABAbR as autoantibody tests. Francesc Graus reports no financial relationships relevant to the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Almeida V, Pimentel J, Campos A, Bentes C, Maruta C, Morgado C, Martins IP. Surgical control of limbic encephalitis associated with LGI1 antibodies. Epileptic Disord. 2012;14:345–348. doi: 10.1684/epd.2012.0515. [DOI] [PubMed] [Google Scholar]
- Barba C, Rheims S, Minotti L, Guenot M, Hoffmann D, Chabardes S, Isnard J, Kahane P, Ryvlin P. Temporal plus epilepsy is a major determinant of temporal lobe surgery failures. Brain. 2016;139:444–451. doi: 10.1093/brain/awv372. [DOI] [PubMed] [Google Scholar]
- Bien CG, Urbach H, Schramm J, Soeder BM, Becker AJ, Voltz R, Vincent A, Elger CE. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy. Neurology. 2007;69:1236–1244. doi: 10.1212/01.wnl.0000276946.08412.ef. [DOI] [PubMed] [Google Scholar]
- Gaspard N. Autoimmune Epilepsy. Continuum (Minneap Minn) 2016;22:227–245. doi: 10.1212/CON.0000000000000272. [DOI] [PubMed] [Google Scholar]
- Haberlandt E, Bast T, Ebner A, Holthausen H, Kluger G, Kravljanac R, Kroll-Seger J, Kurlemann G, Makowski C, Rostasy K, Tuschen-Hofstatter E, Weber G, Vincent A, Bien CG. Limbic encephalitis in children and adolescents. Arch Dis Child. 2011;96:186–191. doi: 10.1136/adc.2010.183897. [DOI] [PubMed] [Google Scholar]
- Kerling F, Blumcke I, Stefan H. Pitfalls in diagnosing limbic encephalitis - a case report. Acta Neurol Scand. 2008;118:339–342. doi: 10.1111/j.1600-0404.2008.01020.x. [DOI] [PubMed] [Google Scholar]
- Malter MP, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C, Elger CE, Bien CG. Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure. 2015;30:57–63. doi: 10.1016/j.seizure.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Mathon B, Bedos UL, Adam C, Baulac M, Dupont S, Navarro V, Cornu P, Clemenceau S. Surgical treatment for mesial temporal lobe epilepsy associated with hippocampal sclerosis. Rev Neurol (Paris) 2015;171:315–325. doi: 10.1016/j.neurol.2015.01.561. [DOI] [PubMed] [Google Scholar]
- Muehlebner A, Groeppel G, Pahs G, Hainfellner JA, Prayer D, Czech T, Feucht M. Beneficial effect of epilepsy surgery in a case of childhood non-paraneoplastic limbic encephalitis. Epilepsy Res. 2010;90:295–299. doi: 10.1016/j.eplepsyres.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Pillai SC, Mohammad SS, Hacohen Y, Tantsis E, Prelog K, Barnes EH, Gill D, Lim MJ, Brilot F, Vincent A, Dale RC. Postencephalitic epilepsy and drug-resistant epilepsy after infectious and antibody-associated encephalitis in childhood: Clinical and etiologic risk factors. Epilepsia. 2016;57:e7–e11. doi: 10.1111/epi.13253. [DOI] [PubMed] [Google Scholar]