Abstract
Background
As population lead levels decrease, the toxic effects of lead may be distributed to more sensitive populations, such as infants with poor fetal growth.
Objectives
To determine the association of prenatal lead exposure and fetal growth; and to evaluate whether infants with poor fetal growth are more susceptible to lead toxicity than those with normal fetal growth.
Methods
We examined the association of second trimester maternal blood lead levels (BLL) with birthweight-for-gestational age (BWGA) z-score in 944 mother-infant participants of the PROGRESS cohort. We determined the association between maternal BLL and BWGA z-score by using both linear and quantile regression. We estimated odds ratios for small-for-gestational age (SGA) infants between maternal BLL quartiles using logistic regression. Maternal age, body mass index, socioeconomic status, parity, household smoking exposure, hemoglobin levels, and infant sex were included as confounders.
Results
While linear regression showed a negative association between maternal BLL and BWGA z-score (β=−0.06 z-score units per log2 BLL increase; 95% CI: −0.13, 0.003; P=0.06), quantile regression revealed larger magnitudes of this association in the <30th percentiles of BWGA z-score (β range [−0.08, −0.13] z-score units per log2 BLL increase; all P values <0.05). Mothers in the highest BLL quartile had an odds ratio of 1.62 (95% CI: 0.99–2.65) for having a SGA infant compared to the lowest BLL quartile.
Conclusions
While both linear and quantile regression showed a negative association between prenatal lead exposure and birthweight, quantile regression revealed that smaller infants may represent a more susceptible subpopulation.
1. INTRODUCTION
Poor fetal growth precedes ~60% of neonatal deaths (Black et al. 2008) and leads to adverse fetal growth outcomes such as low birthweight and small-for-gestational age (SGA) (Victora et al. 2008). Low birthweight (<2,500 grams) and SGA (<10th percentile of the birthweight-for-gestational age distribution) infants, term and preterm, have an increased risk of chronic developmental and cardiometabolic disorders later in life (Lawn et al. 2014), and impose a substantial socioeconomic burden worldwide (Bhutta et al. 2014). The prevalence of low birthweight globally is estimated to be 20 million infants and of SGA about 32 million infants (of whom ~10 million are term low birthweight), with particularly high prevalence in low and middle income countries (Lee et al. 2013). Numerous preventable risk factors have been linked to poor fetal growth, including prenatal lead exposure (Jelliffe-Pawlowski et al. 2006).
Lead is a toxic heavy metal that is widespread in the environment. While exposure to lead has dropped dramatically in the last 30 years, toxic effects are still reported even in populations with blood lead levels (BLL) once believed to be safe (i.e. <5 µg/dL). During pregnancy, maternal lead can cross the placenta and enter the fetal blood circulation (Lin et al. 1998). Due to similar physicochemical properties, lead competes with calcium for deposition into the bone, which might lead to impaired fetal growth (Potula and Kaye 2005). Lead also binds to sulfhydryl groups and inhibits enzymes involved in heme synthesis, which is important for cellular respiration and metabolism, as well as hemoglobin synthesis (Flora et al. 2012). Several epidemiological studies have shown inconsistent associations of prenatal lead exposure and fetal growth (Burris et al. 2011; Cantonwine et al. 2010; Gonzalez-Cossio et al. 1997; Jelliffe-Pawlowski et al. 2006; Nishioka et al. 2014; Wigle et al. 2007; Zhang et al. 2015; Zhu et al. 2010). These studies used traditional regression methods, also known as ordinary least squares (OLS) regression, to estimate the conditional mean response of the association between prenatal lead exposure and fetal growth. Because OLS methods model the mean response of an outcome in relation to the exposure, any differences that occur in the magnitude and direction of the exposure-outcome association (e.g., prenatal lead exposure and birthweight) across different percentiles of the outcome may not be captured (Koenker 2005).
The value of quantile regression, which allows for effects of the exposure (e.g., lead) to vary across the distribution of the outcome (e.g., birthweight), has been demonstrated previously in lead poisoning with regards to school performance. Burgette et al., showed that childhood lead exposure is predictive of poorer performance on standardized state tests, with more pronounced effects in the lower percentiles of the test score distribution (Burgette et al. 2011). We built upon this research by testing whether prenatal lead exposure predicts lower birthweight more prominently at the lower range of birthweight-for-gestational age distribution. In other words: are infants with poor fetal growth more susceptible to lead toxicity than infants with normal fetal growth? Fetal growth is a complex and dynamic process, with infants at the tails of the outcome (e.g., birthweight) distribution suffering a disproportionate burden of perinatal morbidities (Barker et al. 2002; Barker 2006; Fabricius-Bjerre et al. 2011). We hypothesized that OLS regression analysis may not capture any differences in the associations between prenatal lead exposure and birthweight-for-gestational age that occur for instance at the tails of the outcome distribution (e.g., small-for-gestational age infants), and that these associations could be revealed by using quantile regression.
We used data from the Programming Research in Obesity, Growth Environment and Social Stress (PROGRESS) prospective cohort study of 946 mother-infant pairs in Mexico City to determine the association between prenatal lead exposure at second trimester and fetal growth as measured by birthweight-for-gestational age and risk of SGA. Lead exposure is still a major public health problem in Mexico (Caravanos et al. 2014) and the prevalence of both low birthweight and SGA, which are measures of poor fetal growth, is relatively high (~10%) in the Mexican population (Lee et al. 2013).
2. MATERIAL AND METHODS
2.1. Study population
Study participants were enrolled as part of the PROGRESS birth cohort project in Mexico City, Mexico, between 2007 and 2011. Details of the cohort’s profile and enrollment are described in previous publications (Braun et al. 2014; Burris et al. 2013). In brief, pregnant women who attended the Mexican Social Security Institute (Instituto Mexicano del Seguro Social) clinics for their prenatal care were enrolled. Eligibility criteria for participation in the study were singleton pregnancy, gestational age <20 weeks, maternal age of ≥18 years, expectation to live in Mexico City for the following three years, and have access to a telephone. Exclusion criteria at screening were chronic medical conditions such as heart or kidney disease; use of steroids or anti-epilepsy drugs; drug addiction; and daily consumption of alcoholic beverages due to its association with adverse fetal outcomes. We screened 3,898 women who presented to the IMSS clinics during this time, and we enrolled 1054 who agreed to participate by providing written informed consent and who met the eligibility criteria. All eligible participants were Hispanic. Of these, 946 gave birth to a live infant; this represents our base population. Two participants had missing blood lead measurements and were excluded, resulting in 944 participants included in the statistical analysis. The study was approved by the Institutional Review Boards of Brigham and Women’s Hospital (#14265-101; #14706-101; #2006-P-001416; #2006-P-001792) and the National Institute of Public Health in Mexico (#560) according to the Declaration of Helsinki.
2.2. Data collection
Baseline information on demographics, anthropometric characteristics, and health status was collected from all participants at first visit (<20 weeks of gestation), and at every subsequent visit until delivery. We calculated gestational age at enrollment using the date of last menstrual period (LMP) and at birth using a standardized physical examination assessment (Capurro et al. 1978). In cases where the estimated gestational age from the two methods differed by more than three weeks (n=40), the physical examination assessment data were used. Second trimester (<20 weeks of gestation) maternal BLL were used, which was the earliest time point lead was measured. Pre-pregnancy body mass index (BMI) was calculated using self-reported maternal body weight. Because we noticed that there was some error associated with self-reported body weight data, we decided to use the second trimester body weight to calculate BMI, as measured by professional staff at enrollment. Pearson correlation between pre-pregnancy and second trimester BMI was rho=0.88. Infants’ birthweight was adjusted for gestational age at delivery, and percentiles and z-scores were calculated according to the international infant growth charts developed by Fenton et al. (Fenton et al. 2013). We defined infants with a birthweight-for-gestational age z-score <10th percentile as SGA. Socioeconomic status (SES) was calculated based on an index created by the Mexican Association of Market and Public opinion Research Agencies (Spanish acronym AMAI) using the following 13 variables derived from questionnaire results: (1) education of the head of household, (2) number of rooms, (3) number of bathrooms with showers, (4) type of floor, (5) number of light bulbs, ownership of: (6) car, (7) hot water heater, (8) automatic washing machine, (9) videocassette recorder, (10) toaster, (11) vacuum cleaner, (12) microwave oven, and (13) personal computer (Carrasco 2002). Other lifestyle information such as alcohol consumption, maternal smoking, and household smoking exposure was also attained from the validated in-person questionnaire at first visit. We defined household smoking exposure as the reported cases of indoor smoking by the participants’ spouse and/or other household members based on relevant questions from the questionnaire administered at enrollment.
2.3. Measurement of maternal blood lead levels
Blood samples were collected from participants at enrollment in trace metal-free tubes and stored at −20 C. Upon thaw on ice, blood samples (1mL) were weighed, digested in 2 mL of ultra-pure concentrated HNO3 acid (1mL) for 48 hours, and diluted to 10 mL with deionized water after the addition 0.5 ml of 30% hydrogen peroxide. Samples were handled in ISO Class 5 laminar flow clean hood in an ISO Class 6 clean room. Digested samples were analyzed using external calibration with seven calibration points using an Agilent 8800 ICP Triple Quad (ICP-QQQ) instrument (Agilent technologies, Inc., Delaware, USA) in MS/MS mode with Lutetium as the internal standard. Quality control and quality assurance procedures included analyses of calibration verification standards and continuous calibration verification standards (CCVS) [1 ng/mL and 5 ng/mL standards, National Institute of Standard and Technology Standard Reference Material (NIST SRM) 1640a (trace elements in natural water, Gaithersburg, MD)], procedural blanks, duplicates, spiked samples, NIST SRM 955c (Toxic metals in Caprine blood), Seronorm, Trace Elements Whole Blood L-3 (SERO, Billingstad, Norway), and in house pooled blood sample (IHB) to monitor the accuracy, recovery rates, and reproducibility of the procedure for each analytic batch. CCVS and IHB were run after analysis of every 10 samples. Laboratory recovery rates for quality control standards and spiked samples were 85 to 115% and precision (given as % relative standard deviation) was <10% for samples with concentrations above the limit of quantitation. The limit of detection for lead by this procedure was 0.02 ng/mL and limit of quantitation was 0.07 ng/mL.
2.4. Statistical analysis
We quantified descriptive characteristics of the study participants at second trimester using mean ± standard deviation (SD). Linear regression was used to estimate the association between maternal BLL (continuous) and birthweight-for-gestational age z-score (continuous). We log-transformed (log2) maternal BLL to meet homoscedasticity assumption of model’s residuals (Figure S1; Supplementary Material). To adjust for the association between maternal BLL and birthweight-for-gestational age z-score, we selected a priori the following covariates that were previously found to be associated with poor fetal growth: maternal age (continuous), second trimester BMI (continuous), SES (low, medium, and high), hemoglobin levels (continuous), and infant sex. Other covariates such as parity, maternal smoking, alcohol consumption, as well as household smoking exposure were also considered in the analysis; however, they were not included in the final models as they did not confound the association between maternal BLL and birthweight-for-gestational age z-score (assessed by a change of >10% of the β coefficient estimate), and did not improve model’s fit (assessed by an increase in model’s R2 value). To further investigate the association between maternal BLL and percentiles of the birthweight-for-gestational age z-score distribution, we used quantile regression adjusting for the same covariates as in the linear regression. We used the β coefficient estimates from both linear and quantile regression models to calculate the study population’s distributional shift in birthweight-for-gestational age associated with a log2 increase in maternal BLL. To calculate the predicted birthweight-for-gestational age distributions assuming five log2 increments in maternal BLL, we used the calculated estimates from linear and quantile regression. Last, we used logistic regression to determine the association [odds ratio (OR) and 95% confidence intervals (CIs)] between maternal BLL and SGA, and we tested for trend using the median value of the maternal BLL quartiles. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
3. RESULTS
Second trimester maternal BLL was 3.7 ± 2.7 µg/dL (median 2.8, IQR: 1.9–4.5, range: 0.5–22.9 µg/dL) (Table 1), with 21% of the participants with BLL above the reference level (>5 µg/dL) for pregnant women recommended by the U.S. Centers for Disease Control and Prevention ((CDC) 2010). When we log2-transformed the maternal BLL data, the range spanned through five log2 unit increments. Women with higher BLL had lower socioeconomic status, were older in age and shorter in height, and had elevated levels of hemoglobin. Infants’ birthweight-for-gestational age z-score was −0.5 ± 0.9, and 165 infants (17.5%) were diagnosed as SGA.
Table 1.
Characteristics of study participants at second trimester visit.
| Maternal blood lead levels (µg/dL) | ||||||
|---|---|---|---|---|---|---|
| Mean ± SDa |
Q1 <1.93 |
Q2 1.93–2.79 |
Q3 2.80–4.53 |
Q4 >4.53 |
P valuec |
|
| Characteristics | N=944 | N=236 | N=236 | N=236 | N=236 | |
| Maternal | ||||||
| Age (years) | 27.2 ± 5.5 | 26.6 ± 5.6 | 27.0 ± 5.2 | 27.4 ± 5.6 | 27.5 ± 5.5 | 0.008 |
| Height (cm) | 155.1 ± 5.5 | 155.3 ± 5.9 | 155.5 ± 5.6 | 154.7 ± 5.2 | 154.4 ± 5.4 | 0.003 |
| Body weight (kg) | 64.7 ± 4.1 | 65.0 ± 11.1 | 65.3 ± 10.9 | 63.7 ± 10.5 | 65.0 ± 11.9 | 0.29 |
| Body mass index (kg/m2) | 26.9 ± 4.2 | 26.9 ± 4.2 | 26.9 ± 4.1 | 26.6 ± 4.0 | 27.2 ± 4.5 | 0.84 |
| Hemoglobin levels (g/dL) | 12.9 ± 0.9 | 12.8 ± 0.8 | 12.9 ± 0.8 | 13.0 ± 0.9 | 13.0 ± 0.9 | 0.006 |
| Blood lead levels (µg/dL) | 3.7 ± 2.7 | 1.4 ± 0.3 | 2.4 ± 0.2 | 3.6 ± 0.5 | 7.3 ± 2.8 | - |
| Multiparous; n (%) | 591 (62.6) | 141 (14.9) | 151 (16.0) | 149 (15.8) | 150 (15.9) | 0.68 |
| Alcohol consumption; n (%) | 0.38 | |||||
| Yes | 78 (8.3) | 18 (7.6) | 22 (9.3) | 17 (7.2) | 21 (8.9) | |
| Socioeconomic status; n (%) | 0.009 | |||||
| Low | 484 (51.3) | 108 (11.5) | 121 (12.8) | 120 (12.7) | 135 (14.3) | |
| Medium | 354 (37.5) | 96 (10.2) | 86 (9.1) | 92 (9.7) | 80 (8.5) | |
| High | 106 (11.2) | 32 (3.4) | 29 (3.1) | 24 (2.5) | 21 (2.2) | |
| Household smoking exposured; n (%) | 0.84 | |||||
| Yes | 295 (31.3) | 64 (27.5) | 80 (34.0) | 81 (34.6) | 70 (29.8) | |
| Infant | ||||||
| Birthweight (g) | 3043 ± 482 | 3032 ± 497 | 3063 ± 439 | 3024 ± 510 | 3052 ± 481 | 0.82 |
| Gestational age (weeks) | 38.3 ± 1.9 | 38.1 ± 1.9 | 38.4 ± 1.8 | 38.2 ± 2.1 | 38.3 ± 1.8 | 0.13 |
| Birthweight-for-gestational age z- scoreb |
−0.5 ± 0.9 | −0.4 ± 0.8 | −0.5 ± 0.9 | −0.5± 1.0 | −0.5 ± 0.9 | 0.08 |
| Small-for-gestational age | 165 (17.5) | 33 (14.0) | 41 (17.4) | 43 (18.2) | 48 (20.3) | 0.01 |
| Infant sex; n (%) | 0.79 | |||||
| Males | 498 (52.7) | 119 (52.7) | 127 (53.8) | 124 (52.5) | 128 (54.2) | |
All values are shown as mean ± SD, unless otherwise noted.
Birthweight-for-gestational age z-score is adjusted for gestational age and calculated based on the Fenton growth charts.
P value corresponds to the univariate association between maternal blood lead levels and variables considered in the analysis.
Household smoking exposure was defined as the reported cases of indoor smoking exposure by the participant’s spouse and/or other household members. Data are missing for seven participants.
In the multivariable linear regression analysis adjusted for maternal age, BMI, SES, hemoglobin levels, and infant sex, we found a negative association between maternal BLL and birthweight-for-gestational age z-score (β=−0.06, 95% CI: −0.13, 0.003; P=0.06) (Table 2). Quantile regression analysis, adjusted for maternal age, BMI, SES, infant sex, and hemoglobin, confirmed the negative association between maternal BLL and birthweight-for-gestational age z-score, and revealed that the magnitude of the association was larger in the lowest (<30th) z-score percentiles (Table 3). We found a substantially larger left shift and change of the shape of the predicted birthweight-for-gestational age distribution in the lower tail of the distribution per log2 increase in maternal BLL (Figure 1). For example, while in linear regression analysis a log2 increase in maternal BLL was associated with a mean [95% confidence interval (CI)] z-score difference of −0.06 (95% CI: −0.13, 0.003; Table 2), in quantile regression the estimates were −0.12 (95% CI: −0.20, −0.03; Table 3) z-score difference in the 20th percentile, and −0.02 (95% CI: −0.1, 0.06; Table 3) z-score difference in the 80th percentile of the birthweight-for-gestational age distribution.
Table 2.
Association between second trimester maternal blood lead levels (per log2 increment) and birthweight-for-gestational age z-score from linear regression.
| Model | R2 (%) | Difference in z-score | SE | 95% CI | P value |
|---|---|---|---|---|---|
| Unadjusted | 0.3 | −0.06 | 0.03 | (−0.12, 0.007) | 0.08 |
| Adjusteda | 42.3 | −0.06 | 0.03 | (−0.13, 0.003) | 0.06 |
Model adjusted for maternal age, body mass index, socioeconomic status, hemoglobin levels, and infant sex.
Table 3.
Quantile regression analysis of the association of maternal blood lead levels (per log2 increment) and birthweight-for-gestational age z-score.
| Quantile level |
Fenton percentile |
Difference in z-scorea |
95% CI | P value |
|---|---|---|---|---|
| 0.05 | 0.03 | −0.08 | (−0.19, 0.03) | 0.17 |
| 0.10 | 0.05 | −0.13 | (−0.25, −0.004) | 0.04 |
| 0.15 | 0.08 | −0.11 | (−0.22, −0.002) | 0.05 |
| 0.20 | 0.12 | −0.12 | (−0.20, −0.03) | 0.007 |
| 0.25 | 0.15 | −0.10 | (−0.19, −0.02) | 0.01 |
| 0.30 | 0.19 | −0.11 | (−0.18, −0.04) | 0.003 |
| 0.35 | 0.23 | −0.04 | (−0.12, 0.04) | 0.32 |
| 0.40 | 0.27 | −0.06 | (−0.14, 0.03) | 0.19 |
| 0.45 | 0.30 | −0.05 | (−0.13, 0.04) | 0.26 |
| 0.50 | 0.35 | −0.07 | (−0.16, 0.01) | 0.10 |
| 0.55 | 0.40 | −0.07 | (−0.16, 0.01) | 0.10 |
| 0.60 | 0.45 | −0.07 | (−0.15, 0.01) | 0.08 |
| 0.65 | 0.50 | −0.04 | (−0.12, 0.04) | 0.32 |
| 0.70 | 0.56 | −0.04 | (−0.12, 0.03) | 0.27 |
| 0.75 | 0.61 | −0.01 | (−0.08, 0.06) | 0.80 |
| 0.80 | 0.68 | −0.02 | (−0.1, 0.06) | 0.64 |
| 0.85 | 0.74 | −0.06 | (−0.16, 0.04) | 0.23 |
| 0.90 | 0.85 | −0.07 | (−0.16, 0.02) | 0.15 |
| 0.95 | 1.00 | −0.02 | (−0.13, 0.09) | 0.77 |
Model adjusted for maternal age, body mass index, socioeconomic status, hemoglobin levels, and infant sex.
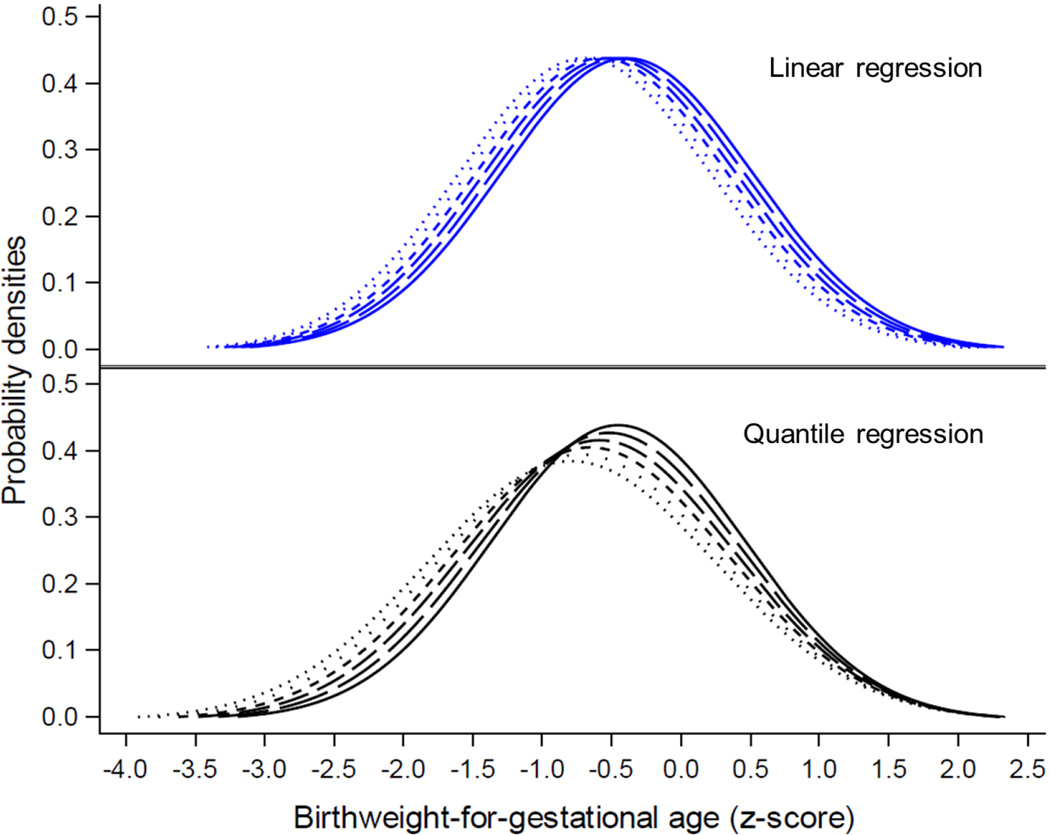
Figure 1. Empirical distribution of birthweight-for-gestational age and associated predicted distributions assuming a log2 increment in maternal blood lead levels.
This figure illustrates the empirical (solid line) and predicted (dashed and dotted lines) distributions from the association of prenatal lead exposure and birthweight-for-gestational age z-score as calculated by both linear (top) and quantile (bottom) regression assuming a range of five increments (log2) in maternal blood lead levels. Quantile regression estimates revealed that prenatal lead exposure is associated with a heterogeneous shift to left of the birthweight-for-gestational age z-score distribution, increasing in particular the probabilities infants would have been diagnosed as SGA or had lower birthweight-for-gestational age z-scores, had they been exposed to higher levels of lead prenatally.
In the multivariable logistic regression analysis, adjusted for maternal age, BMI, SES, hemoglobin levels, and infant sex, the odds ratio (OR) (95% CI) for having a SGA infant in mothers with higher BLL was 1.30 (0.79–2.15) for Q2, 1.37 (0.83–2.25) for Q3, and 1.62 (0.99–2.65) for Q4, as compared to mothers with the lowest BLL (Q1) (Table 4). We also found a marginally significant linear dose-response relationship between prenatal lead exposure at second trimester and increased odds for having a SGA infant (P for trend <0.06).
Table 4.
Odds ratios (95 % confidence interval) of small-for-gestational age infants across quartiles of maternal blood lead levels.
| Maternal blood lead levels (µg/dL) |
P for trend |
||||
|---|---|---|---|---|---|
| Q1 (<1.93) | Q2 (1.93–2.79) | Q3 (2.80–4.53) | Q4 (>4.53) | ||
| Cases/total | 33/229 | 41/231 | 43/229 | 48/228 | |
| Unadjusted | 1 | 1.28 (0.78–2.10) | 1.37 (0.84–2.24) | 1.58 (0.97–2.56) | 0.07 |
| Adjusteda | 1 | 1.30 (0.79–2.15) | 1.37 (0.83–2.25) | 1.62 (0.99–2.65) | 0.06 |
Model adjusted for maternal age, body mass index, socioeconomic status, hemoglobin levels, and infant sex.
4. DISCUSSION
We found a negative association of prenatal lead exposure at the second trimester with birthweight-for-gestational age. Quantile regression revealed different magnitudes of the association across the birthweight-for-gestational age distribution, suggesting that susceptibility to lead varied based on differences in fetal growth conditions. In particular, the magnitude of the association was larger in the lower percentiles of the birthweight-for-gestational age distribution indicating that smaller infants may be more sensitive to prenatal lead exposure. Consistent with our findings from linear and quantile regression, we found that prenatal exposure to elevated maternal BLL was associated with increased odds of SGA birth, suggesting a linear dose-response relationship with no safe threshold for lead exposure.
Our findings are consistent with previous epidemiological studies that also found negative associations between prenatal lead exposure and birthweight-for-gestational age. Using linear regression estimates and the international infant growth charts by Fenton et al. (Fenton et al. 2013), we estimated a decrease in birthweight for a 39-week male infant equal to 10 g per 1µg/dL increase in maternal BLL. Taylor et al. showed a comparable decrease in birthweight (13 g per 1 µg/dL increase in maternal BLL) of infants in a UK cohort of 4,285 infants whose mothers had second trimester maternal BLL of 3.67 ± 1.47 µg/dL (mean ± SD) (Taylor et al. 2015). Additionally, Zhu et al. reported a decrease in birthweight of 4–27 g per 1 µg/dL in a cohort of 43,288 mother-infant pairs in New York, USA, with second trimester maternal BLL of <10 µg/dL (Zhu et al. 2010).
Similarly, quantile regression revealed a negative association between prenatal lead exposure and birthweight-for-gestational age. Quantile regression analysis showed stronger associations at the lower tail of the distribution, whereas in higher percentiles the magnitude and statistical significance were considerably attenuated. Using quantile regression estimates, the decrease in birthweight for the same 39-week male infant would have been 50 g had the infant been in the 20th percentile, and 20 g had the infant been in the 80th percentile of the birthweight-for-gestational age distribution. Not only do our findings suggest that the magnitude of the association between prenatal lead exposure and birthweight is higher among infants in the lower birthweight-for-gestational age percentiles, but they also suggest that the public health burden associated with lead exposure on fetal growth may have been underestimated by the use of linear regression only, as in previous investigations.
When we examined the odds of SGA births across different quartiles of second trimester maternal BLL, we observed a linear dose-response relationship. Our findings are in agreement with other studies with respect to the direction of the association; however, in our study we found that mothers had increased odds to deliver SGA infant at much lower BLL than what has been previously reported. For example, in a study of 1,611 participants Chen et al. found that mothers with BLL ≥20 µg/dL during pregnancy had an increased risk ratio (RR) of 2.15 (95% CI: 1.15–3.83) of SGA as compared to mothers with BLL <10 µg/dL (Chen et al. 2006). In another study with smaller sample size (n=262), Jelliffe-Pawlowski et al. found increased odds for SGA births of 4.2 (95% CI: 1.3–13.9) in women with BLL ≥10 µg/dL as compared to those with BLL ≥10 µg/dL (Jelliffe-Pawlowski et al. 2006). In our study, we found increased odds for SGA births of 1.62 (95% CI: 0.99–2.65) in women with BLL >4.53 µg/dL (Q4) as compared to those with <1.93 µg/dL (Q1). Our findings suggest that prenatal lead exposure has a negative impact on fetal growth even at lower concentrations than those previously reported, with no safe threshold for lead exposure. This is in agreement with the findings by Zhu et al. that suggested a no threshold effect of lead on birthweight (Zhu et al. 2010).
Several plausible biological mechanisms may account for the negative association we observed between prenatal lead exposure and adverse fetal growth outcomes. Lead in the maternal blood circulation is a potent inhibitor of heme synthesis and can induce hypoxic conditions (Flora et al. 2012). Lead can also induce the generation of reactive oxygen species and the inactivation of important antioxidant enzymes (e.g., glutathione), which lead to increased oxidative stress (Ahamed and Siddiqui 2007; Hultberg et al. 2001). Both hypoxia and oxidative stress disturb human placentation, which often leads to adverse fetal growth outcomes (Burton et al. 2009; Jauniaux et al. 2006; Jauniaux and Burton 2016). Therefore, smaller babies who experience such conditions may be more susceptible to the effects of prenatal lead exposure. Last, due to its similar physicochemical characteristics, lead competes with calcium for their deposition into the bones (Potula and Kaye 2005). Deposition of calcium into the bones is an important process during fetal growth, therefore, increased lead levels in the maternal blood circulation can lead to poor fetal growth conditions.
Our study has certain limitations. The lack of ultrasound measurements and use of the LMP method to estimate gestational age at enrollment may have added some measurement error. In previous research, we collected ultrasound data on 98 women and validated the LMP method, which yielded a high correlation (Spearman’s Rho: 0.9, P<0.0001) (Burris et al. 2015). We also believe that any measurement error may have been introduced by the use of LMP data would be non-differential and most likely would drive effect estimates towards the null. We acknowledge that some residual measurement error from using the LMP data might still be present. Additionally, the use of only second trimester maternal BLL data may not precisely reflect prenatal exposure throughout the course of pregnancy. As we enrolled participants at second trimester, first trimester maternal BLL could not be measured. However, we measured maternal BLL at third trimester (n=788) and the Spearman’s correlation between second and third trimester maternal BLL was rho = 0.79. We performed additional analyses by using third trimester or mean (from third and second trimester) maternal BLL in our linear regression models but our findings were unchanged as compared to when we used second trimester BLL (Tables S1 and S2; Supplementary Material). Rabito et al. measured maternal BLL in the first, second, and third trimester and found that second trimester is possibly the most critical window for the association between prenatal lead exposure and birthweight (Rabito et al. 2014). Last, to ensure our findings were robust by using second trimester BMI instead of pre-pregnancy BMI, we performed additional sensitivity analysis which showed no changes in the β coefficient when we used pre-pregnancy BMI (β=−0.06, 95% CI: −0.13, 0.001; P=0.05), as compared to when we used second trimester BMI (β=−0.06, 95% CI: −0.13, 0.003; P=0.06) (Table S3; Supplementary Material). Our findings should be carefully interpreted in the context of an observational study where the possibility of unmeasured residual confounding precludes causal inference.
Strengths of our study include its prospective design, population size, and detailed data collection. Furthermore, the use of birthweight-for-gestational age z-score allows for comparison with other international cohorts. Last, we demonstrated for the first time the advantages of using quantile regression in examining the association between prenatal lead exposure and birthweight. Quantile regression is a method that estimates the association independently of any outcome distribution, compared to linear regression that makes certain assumptions and calculates the mean estimate across the whole outcome distribution. We demonstrated that quantile regression is a useful tool to ascertain environmental effects which may vary at the tail ends of continuous outcomes, such as birthweight, cognitive test scores, or BMI. When used together with linear regression, quantile regression can help to more precisely estimate the impact of environmental exposures on health outcomes and identify vulnerable subpopulations.
5. CONCLUSIONS
Quantile regression revealed significant shifts in birthweight-for-gestational age distribution associated with prenatal lead exposure, which were not fully captured by linear regression models. Our findings indicate that the magnitude of this association is larger in the lower percentiles of the birthweight-for-gestational age distribution and provide evidence that infants with poorer fetal growth may represent a sensitive subpopulation for lead exposure. Improvements in maternal lead exposure early in pregnancy may significantly improve fetal growth, especially among infants who are at highest risk of long-term sequelae from poor fetal growth.
HIGHLIGHTS.
Existing evidence on the association between prenatal lead exposure and fetal growth is inconsistent.
We showed that infants prenatally exposed to higher lead levels had lower birthweight and increased odds to be born small-for-gestational age.
We demonstrated, by using quantile regression, that infants with poorer fetal growth may represent a sensitive subpopulation for lead exposure.
We encourage the use of quantile regression, together with linear regression, when assessing the impact of prenatal exposures on fetal growth.
Acknowledgments
This work was supported by the Harvard T. H. Chan School of Public Health-NIEHS Center for Environmental Health (ES000002) and the National Institute of Environmental Health Science grants ES013744, ES014930, ES021357, ES020268, ES023515, and ES009089. We thank the faculty and staff of the American British Cowdray (ABC) Hospital who provided space and valuable assistance in the data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests: Authors have no competing financial interests
REFERENCES
- Centers for Disease Control and Prevention (CDC) Guidelines for the identification and management of lead exposure in pregnant and lactating women. [accessed 6 June 2016];2010 Available: http://www.cdc.gov/nceh/lead/publications/leadandpregnancy2010.pdf.
- Ahamed M, Siddiqui MK. Low level lead exposure and oxidative stress: Current opinions. Clin Chim Acta. 2007;383:57–64. doi: 10.1016/j.cca.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: Strength of effects and biological basis. Int J Epidemiol. 2002;31:1235–1239. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Adult consequences of fetal growth restriction. Clinical obstetrics and gynecology. 2006;49:270–283. doi: 10.1097/00003081-200606000-00009. [DOI] [PubMed] [Google Scholar]
- Bhutta ZA, Das JK, Bahl R, Lawn JE, Salam RA, Paul VK, et al. Can available interventions end preventable deaths in mothers, newborn babies, and stillbirths, and at what cost? Lancet. 2014;384:347–370. doi: 10.1016/S0140-6736(14)60792-3. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Braun JM, Wright RJ, Just AC, Power MC, Tamayo YOM, Schnaas L, et al. Relationships between lead biomarkers and diurnal salivary cortisol indices in pregnant women from mexico city: A cross-sectional study. Environ Health. 2014;13:50. doi: 10.1186/1476-069X-13-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgette LF, Reiter JP, Miranda ML. Exploratory quantile regression with many covariates: An application to adverse birth outcomes. Epidemiology. 2011;22:859–866. doi: 10.1097/EDE.0b013e31822908b3. [DOI] [PubMed] [Google Scholar]
- Burris HH, Collins JW, Jr, Wright RO. Racial/ethnic disparities in preterm birth: Clues from environmental exposures. Curr Opin Pediatr. 2011;23:227–232. doi: 10.1097/MOP.0b013e328344568f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Braun JM, Byun HM, Tarantini L, Mercado A, Wright RJ, et al. Association between birth weight and DNA methylation of igf2, glucocorticoid receptor and repetitive elements line-1 and alu. Epigenomics. 2013;5:271–281. doi: 10.2217/epi.13.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burris HH, Baccarelli AA, Byun HM, Cantoral A, Just AC, Pantic I, et al. Offspring DNA methylation of the aryl-hydrocarbon receptor repressor gene is associated with maternal bmi, gestational age, and birth weight. Epigenetics. 2015;10:913–921. doi: 10.1080/15592294.2015.1078963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Yung HW, Cindrova-Davies T, Charnock-Jones DS. Placental endoplasmic reticulum stress and oxidative stress in the pathophysiology of unexplained intrauterine growth restriction and early onset preeclampsia. Placenta. 2009;30(Suppl A):S43–S48. doi: 10.1016/j.placenta.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantonwine D, Hu H, Tellez-Rojo MM, Sanchez BN, Lamadrid-Figueroa H, Ettinger AS, et al. Hfe gene variants modify the association between maternal lead burden and infant birthweight: A prospective birth cohort study in mexico city, mexico. Environ Health. 2010;9:43. doi: 10.1186/1476-069X-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capurro H, Konichezky S, Fonseca D, Caldeyro-Barcia R. A simplified method for diagnosis of gestational age in the newborn infant. J Pediatr. 1978;93:120–122. doi: 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- Caravanos J, Dowling R, Tellez-Rojo MM, Cantoral A, Kobrosly R, Estrada D, et al. Blood lead levels in mexico and pediatric burden of disease implications. Ann Glob Health. 2014;80:269–277. doi: 10.1016/j.aogh.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Carrasco AV. The amai system of classifying households by socio-economic level: The experience of mexico and its comparison with brazil and argentina. ESOMAR. 2002 [Google Scholar]
- Chen PC, Pan IJ, Wang JD. Parental exposure to lead and small for gestational age births. Am J Ind Med. 2006;49:417–422. doi: 10.1002/ajim.20313. [DOI] [PubMed] [Google Scholar]
- Fabricius-Bjerre S, Jensen RB, Faerch K, Larsen T, Molgaard C, Michaelsen KF, et al. Impact of birth weight and early infant weight gain on insulin resistance and associated cardiovascular risk factors in adolescence. PLoS One. 2011;6:e20595. doi: 10.1371/journal.pone.0020595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92. doi: 10.1186/1471-2431-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012;5:47–58. doi: 10.2478/v10102-012-0009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cossio T, Peterson KE, Sanin LH, Fishbein E, Palazuelos E, Aro A, et al. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- Hultberg B, Andersson A, Isaksson A. Interaction of metals and thiols in cell damage and glutathione distribution: Potentiation of mercury toxicity by dithiothreitol. Toxicology. 2001;156:93–100. doi: 10.1016/s0300-483x(00)00331-0. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Poston L, Burton GJ. Placental-related diseases of pregnancy: Involvement of oxidative stress and implications in human evolution. Hum Reprod Update. 2006;12:747–755. doi: 10.1093/humupd/dml016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauniaux E, Burton GJ. the role of oxidative stress in placental-related diseases of pregnancy. J Gynecol Obstet Biol Reprod (Paris) 2016 doi: 10.1016/j.jgyn.2016.02.012. [DOI] [PubMed] [Google Scholar]
- Jelliffe-Pawlowski LL, Miles SQ, Courtney JG, Materna B, Charlton V. Effect of magnitude and timing of maternal pregnancy blood lead (pb) levels on birth outcomes. J Perinatol. 2006;26:154–162. doi: 10.1038/sj.jp.7211453. [DOI] [PubMed] [Google Scholar]
- Koenker R. Quantile regression. Cambridge ; New York: Cambridge University Press; 2005. [Google Scholar]
- Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every newborn: Progress, priorities, and potential beyond survival. Lancet. 2014;384:189–205. doi: 10.1016/S0140-6736(14)60496-7. [DOI] [PubMed] [Google Scholar]
- Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1:e26–e36. doi: 10.1016/S2214-109X(13)70006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Hwang SA, Marshall EG, Marion D. Does paternal occupational lead exposure increase the risks of low birth weight or prematurity? Am J Epidemiol. 1998;148:173–181. doi: 10.1093/oxfordjournals.aje.a009621. [DOI] [PubMed] [Google Scholar]
- Nishioka E, Yokoyama K, Matsukawa T, Vigeh M, Hirayama S, Ueno T, et al. Evidence that birth weight is decreased by maternal lead levels below 5mug/dl in male newborns. Reprod Toxicol. 2014;47:21–26. doi: 10.1016/j.reprotox.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Potula V, Kaye W. Report from the cdc. Is lead exposure a risk factor for bone loss? J Womens Health (Larchmt) 2005;14:461–464. doi: 10.1089/jwh.2005.14.461. [DOI] [PubMed] [Google Scholar]
- Rabito FA, Kocak M, Werthmann DW, Tylavsky FA, Palmer CD, Parsons PJ. Changes in low levels of lead over the course of pregnancy and the association with birth outcomes. Reprod Toxicol. 2014;50:138–144. doi: 10.1016/j.reprotox.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Taylor CM, Golding J, Emond AM. Adverse effects of maternal lead levels on birth outcomes in the alspac study: A prospective birth cohort study. BJOG. 2015;122:322–328. doi: 10.1111/1471-0528.12756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet. 2008;371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle DT, Arbuckle TE, Walker M, Wade MG, Liu S, Krewski D. Environmental hazards: Evidence for effects on child health. J Toxicol Environ Health B Crit Rev. 2007;10:3–39. doi: 10.1080/10937400601034563. [DOI] [PubMed] [Google Scholar]
- Zhang B, Xia W, Li Y, Bassig BA, Zhou A, Wang Y, et al. Prenatal exposure to lead in relation to risk of preterm low birth weight: A matched case-control study in china. Reprod Toxicol. 2015;57:190–195. doi: 10.1016/j.reprotox.2015.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Fitzgerald EF, Gelberg KH, Lin S, Druschel CM. Maternal low-level lead exposure and fetal growth. Environ Health Perspect. 2010;118:1471–1475. doi: 10.1289/ehp.0901561. [DOI] [PMC free article] [PubMed] [Google Scholar]