Abstract
Background & Aims
Magnetic-resonance-imaging (MRI) techniques and ultrasound-based transient elastography (TE) can be used in noninvasive diagnosis of fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD). We performed a prospective study to compare the performance of magnetic resonance elastography (MRE) vs TE for in diagnosis of fibrosis, and MRI-based proton density fat fraction (MRI-PDFF) analysis vs TE-based controlled attenuation parameter (CAP) for diagnosis of steatosis in patients undergoing biopsy to assess NAFLD.
Methods
We performed a cross-sectional study of 104 consecutive adults (56.7% female) who underwent MRE, TE, and liver biopsy analysis (using the histological scoring system for NAFLD from the nonalcoholic steatohepatitis clinical research network scoring system) from October 2011 through May 2016 at a tertiary medical center. All patients received a standard clinical evaluation, including collection of history, anthropometric examination, and biochemical tests. The primary outcomes were fibrosis and steatosis. Secondary outcomes included dichotomized stages of fibrosis and NASH vs no NASH. Receiver operating characteristic (ROC) curve analyses were used to compare performances of MRE vs TE in diagnosis of fibrosis (stages 1–4 vs 0) and MRI-PDFF vs CAP for diagnosis of steatosis (grades 1–3 vs 0) with respect to findings from biopsy analysis.
Results
MRE detected any fibrosis (stage 1 or more) with an area under the ROC (AUROC) of 0.82 (95% CI, 0.74–0.91), which was significantly higher than that of TE (AUROC, 0.67; 95% CI, 0.56–0.78). MRI-PDFF detected any steatosis with an AUROC of 0.99 (95% CI, 0.98–1.00), which was significantly higher that of CAP (AUROC, 0.85; 95% CI, 0.75–0.96). MRE detected fibrosis of stages 2, 3, or 4 with AUROC values of 0.89 (95% CI, 0.83–0.96), 0.87 (95% CI, 0.78–0.96), and 0.87 (95% CI, 0.71-1.00); TE detected fibrosis of stages 2, 3, or 4 with AUROC values of 0.86 (95% CI, 0.77–0.95), 0.80 (95% CI, 0.67–0.93), and 0.69 (95% CI, 0.45–0.94). MRI-PDFF identified steatosis of grades 2 or 3 with AUROC values of 0.90 (95% CI, 0.82–0.97) and 0.92 (95% CI, 0.84–0.99); CAP identified steatosis of grades 2 or 3 with AUROC values of 0.70 (95% CI, 0.58–0.82) and 0.73 (95% CI, 0.58–0.89).
Conclusions
In a prospective, cross-sectional study of more than 100 patients, we found MRE to be more accurate than TE in identification of liver fibrosis (stage 1 or more), using biopsy analysis as the standard. MRI-PDFF is more accurate than CAP in detecting all grades of steatosis in patients with NAFLD.
Keywords: noninvasive, assessment, comparative, biomarker
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is increasingly emerging as the predominant cause of chronic liver disease around the world.1 In the United States, NAFLD is estimated to affect nearly 100 million adults, or one-third of the population, and its prevalence is predicted to rise along with increasing rates of obesity, diabetes, and metabolic syndrome.2, 3 NAFLD ranges from simple benign hepatic steatosis or nonalcoholic fatty liver (NAFL) to severe hepatocellular inflammation known as nonalcoholic steatohepatitis 4.5 This latter condition is estimated to affect 5% of the US population and carries higher risk of progressing to cirrhosis and hepatocellular carcinoma.6-8 Although liver biopsy is the current gold standard for assessing NAFLD, its accuracy has been questioned because of sampling errors and variable intra- and inter-observer agreement.9-11 Moreover, biopsy is invasive, which limits use as a population screening tool. Thus, there is a need for accurate, noninvasive methods that can clinically assess NAFLD.
Liver fibrosis and steatosis are two features of NAFLD that have been investigated by noninvasive imaging tests to assess NAFLD. Transient elastography (TE; FibroScan®) is an ultrasound-based imaging technique that allows rapid, bed-side measurements of tissue stiffness.12 TE-based liver stiffness measurements using the M Probe have shown to correlate with stages of fibrosis, particularly in severe fibrosis and cirrhosis.13-15 Additionally, the controlled attenuation parameter (CAP) allows TE to simultaneously assess steatosis.16-18 An important limitation of TE is the high failure rates in obese patients with BMI of > 28 kg/m2,19, 20 which limits reliable measurement of liver stiffness and steatosis in a significant portion of obese NAFLD patients. However, the new XL probe equipped with CAP has shown to reduce the failure rate for measuring fibrosis and steatosis in obese patients.21, 22
Magnetic resonance imaging (MRI)-based techniques such as magnetic resonance elastography (MRE) and proton density fat fraction (MRI-PDFF) have shown to accurately diagnose fibrosis and steatosis, respectively, in NAFLD patients.23-29 Although MRI-based techniques have shown to be accurate and effective in patients with obesity,30 they are more expensive and not widely available compared to TE.31 A recent Japanese study by Imajo et al.32 directly compared and demonstrated that MRE and MRI-PDFF have higher accuracy than TE and CAP, respectively, for diagnosing fibrosis and steatosis in NAFLD patients. However, this study assessed TE using only the M probe. Therefore, TE using M or XL probe, when indicated, has not been compared to MRE. Furthermore, MRI-based techniques and TE have not yet been compared in a western cohort of NAFLD patients, who are likely to have higher BMI and may have other characteristics that may affect the diagnostic performance of TE and MRE.
Using a well-characterized, prospective cohort of American adults with biopsy-proven NAFLD, we compared the accuracy of TE versus MRE for diagnosing fibrosis, and CAP versus MRI-PDFF for diagnosing steatosis in NAFLD patients. We hypothesize that MRE is superior to TE for diagnosing early fibrosis, and that MRI-PDFF is superior to CAP for diagnosing steatosis in NAFLD patients.
MATERIALS AND METHODS
Study Design
This was a prospective, cross-sectional study of patients with suspected NAFLD who underwent contemporaneous MRI and TE with a liver biopsy assessment. Between October 2011 and May 2016, 104 adult patients with clinical indication for liver biopsies for suspected NAFLD were consecutively enrolled with written informed consent. After undergoing evaluation for other causes of hepatic steatosis and liver disease, patients were invited to undergo standardized history, physical and anthropometric exam, laboratory testing, MRI at the UCSD MR3T Research Laboratory, and TE at the UCSD NAFLD Research Center.4, 33-37 This study was HIPAA compliant and approved by the UCSD Institutional Review Board and the Clinical and Translational Research Institute.
Inclusion/Exclusion criteria
We included patients ≥18 years old with suspected NAFLD patients who are willing and able to provide informed consent. Exclusion criteria were: history of significant alcohol intake within 2 years of recruitment (≥14 drinks/week for men or ≥7 drinks/week for women); any evidence of secondary causes of hepatic steatosis including nutritional, iatrogenic, or infectious etiology or HIV infection; evidence of liver diseases other than NAFLD, which include viral hepatitis (screened by positive serum hepatitis B surface antigen and hepatitis C RNA assays), autoimmune hepatitis, genetic or acquired disorders such as hemochromatosis, Wilson’s disease, glycogen storage disease, alpha-1 antitrypsin deficiency, and cholestatic or vascular liver disease; evidence of decompensated liver disease (defined as Child-Pugh score > 7 points); active substance use; major systemic illnesses; contraindication(s) to MRI; pregnant or trying to be pregnant; or any other conditions believed by the principal investigator to affect patient’s competence, compliance, or completion of the study.
Clinical Research Evaluation
All patients underwent a standardized clinical evaluation which included history, anthropometric exam, and biochemical tests at the UCSD NAFLD Research Center. Documented information from history and anthropometric exam included age, sex, height, weight, BMI, ethnic background, vital signs. Alcohol intake history was assessed in prior clinical visits and re-assessed at the research unit with the Alcohol Use Disorders Identification Test and the Skinner questionnaire. Other causes of liver diseases and secondary causes of hepatic steatosis such as steatogenic medications were ruled out systematically using history and biochemical tests. Biochemical tests included aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, total bilirubin, direct bilirubin, albumin, fasting glucose, hemoglobin A1c, insulin, triglycerides, total cholesterol, HDL, LDL, platelet, prothrombin time and international normalized ratio.
Histologic Evaluation
All patients underwent liver biopsy for assessment by an experienced liver pathologist who was blinded to the patient’s clinical and radiological data. Histologic scoring was done using the Nonalcoholic Steatohepatitis Clinical Research Network Histologic Scoring System.38 This scoring system is further described in the Supplementary Material.
Outcome measures
The primary outcomes were fibrosis (stage 1-4 versus 0) and steatosis (grade 1-3 versus 0). Secondary outcomes included dichotomized stages of fibrosis (stage 2-4 (significant fibrosis) versus 0-1, stage 3-4 (advanced fibrosis) versus 0-2, and stage 4 (cirrhosis) versus stage 0-3), grades of steatosis (grade 2-3 versus 0-1, and grade 3 versus 0-2), and NASH versus no NASH.
Magnetic Resonance Imaging
MRI of the abdomen was performed at the UCSD MR3T Research Laboratory on a single 3T MR scanner (GE Signa EXCITE HDxt, GE Healthcare, Waukesha, WI). MRI-PDFF sequences were acquired according to previously-published methods.28, 29, 39 The median time interval between MRI and biopsy was 42 days.
Magnetic Resonance Elastography
MRE was performed according to previously described methods25, 30, 40 on commercially available software and hardware (Resoundant, Inc., Rochester, MN) and is further described in the Supplementary Material.
Transient Elastography
Transient elastography was performed using the FibroScan® 502 Touch model (M Probe; XL Probe; Echosens, Paris, France) by a trained technician, blinded to clinical and histologic data, according to previously-described methods.12, 22 Briefly, patients were asked to fast at least 3 hours prior to the exam. The procedure was performed in the supine position with the right arm fully adducted during a 10 second breath hold. Based on the manufacturer’s recommendation, all patients were first scanned by applying the M probe (3.5 MHz) over the area of abdomen at the location of the right liver lobe. When indicated by the equipment upon initial assessment, patients were re-scanned using the XL probe (2.5 MHz). A minimum of 10 measurements was made to obtain the median valid liver stiffness measurements in kilopascals (kPa) and the interquartile range (IQR). Technical failure was defined as no stiffness measurement obtained or unreliable measurements (defined as success rate < 60% and/or IQR/med >30%).41 Simultaneous liver steatosis measurements were obtained using the CAP values in dB/m, co-localized to the valid liver stiffness measurements. All CAP data were collected prospectively. The median time interval between TE and biopsy was 107 days.
Statistical Analyses
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). Patients’ demographic, biochemical, and histological, and imaging characteristics were summarized as mean and standard deviation for continuous variables and numbers and percentages for categorical variables. A two-tailed P value ≤0.05 was considered statistically significant. Main analyses: Receiver operating characteristic (ROC) curve analyses were used to compare the performances of MRE versus TE for diagnosing fibrosis (stage 1-4 versus 0), and MRI-PDFF versus CAP for diagnosing steatosis (grade 1-3 versus 0) with respect to biopsy. For each ROC analysis, the area under the ROC curve (AUROC), the optimal thresholds, and the following performance parameters were calculated: sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). The optimal threshold of each modality was determined using the Youden index.42 The Delong test was used to compare the AUROCs of MRE versus TE for diagnosing fibrosis, and CAP versus MRI-PDFF for diagnosing steatosis.43 Multivariable ROC analyses were performed to assess the effect of biopsy-to-imaging time interval and probe type on the AUROCs.
Secondary analyses
The following additional ROC curve analyses were performed: MRE versus TE for diagnosing other dichotomized stages of fibrosis (stage 2-4 0-1, stage 3-4 versus 0-2, and stage 4 versus 0-3) and NASH versus no NASH; and MRI-PDFF versus CAP for diagnosing other dichotomized grades of steatosis (grade 2-3 versus 0-1, and grade 3 versus 0-2). The Kruskal-Wallis test was used to compare liver stiffness and steatosis measurements between groups at different stages of fibrosis and grades of steatosis, respectively.
RESULTS
Baseline Characteristics
In this prospective study, 104 patients with liver biopsy, MRI, and TE were consecutively enrolled. The mean (± SD) age and body mass index were 50.8 (± 14.6) and 30.4 (± 5.2), respectively. Baseline cohort characteristics are summarized in Table 1. A total of 110 patients with biopsy-proven NAFLD were initially seen at the NAFLD Research Center, although 6 patients were excluded because TE was not performed. Of the 104 TE examinations, 7 exams (6.7%) resulted in technical failure.
Table 1.
Demographical, biochemical, histological, and imaging characteristics of study cohort
| Patients (n=104) | |
|---|---|
| Demographic | |
| Age at biopsy, mean (s.d.) | 50.8 (14.6) |
| Male, n (%) | 45 (43.3) |
| Female, n (%) | 59 (56.7) |
| Height (m), mean (s.d.) | 1.7 (0.1) |
| Weight (kg), mean (s.d.) | 86.1 (17.9) |
| BMI (kg/m2), mean (s.d.) | 30.4 (5.2) |
| Race | |
| White, n (%) | 48 (47.1) |
| African American, n (%) | 0 (0) |
| Asian, n (%) | 21 (20.5) |
| Hispanic, n (%) | 32 (31.4) |
| Other, n (%) | 1 (1.0) |
| Diabetes, n (%) | 29 (27.9) |
| Biochemical profile | |
| AST (U/l), median (iqr) | 31.0 (15.0) |
| ALT (U/l), median (iqr) | 42.0 (34.0) |
| AST/ALT ratio, median (iqr) | 0.8 (0.4) |
| Alkaline Phosphatase (U/l), median (iqr) | 71.0 (30.5) |
| GGT (U/l), median (iqr) | 35.0 (37.0) |
| Total bilirubin (mg/dl), median (iqr) | 0.4 (0.3) |
| Direct bilirubin (mg/dl), median (iqr) | 0.2 (0.1) |
| Albumin (g/dl), median (iqr) | 4.5 (0.5) |
| Glucose (mg/dl), median (iqr) | 98.0 (31.5) |
| Hgb A1C (%), median (iqr) | 5.8 (0.8) |
| Insulin (u), median (iqr) | 21.5 (20.0) |
| Triglycerides (mg/dl), median (iqr) | 133.0 (92.0) |
| Total cholesterol (mg/dl), median (iqr) | 180.0 (56.0) |
| HDL (mg/dl), median (iqr) | 47.0 (19.0) |
| LDL (mg/dl), median (iqr) | 100.0 (52.0) |
| Platelet count (109/L), median (iqr) | 223000 (77000) |
| Prothombin time, median (iqr) | 10.8 (1.0) |
| INR, median (iqr) | 1.0 (0.1) |
| Histology | |
| Fibrosis | |
| 0 | 47 (45.6) |
| 1 | 24 (23.3) |
| 2 | 11 (10.7) |
| 3 | 13 (12.6) |
| 4 | 8 (7.8) |
| Steatosis | |
| 0 | 9 (8.7) |
| 1 | 49 (47.6) |
| 2 | 29 (28.2) |
| 3 | 16 (15.5) |
| Lobular inflammation | |
| 0 | 4 (3.9) |
| 1 | 53 (52.0) |
| 2 | 41 (40.2) |
| 3 | 4 (3.9) |
| Ballooning | |
| 0 | 43 (43.4) |
| 1 | 44 (44.4) |
| 2 | 12 (12.2) |
| NASH | |
| No NAFLD, n (%) | 4 (4.0) |
| NAFLD, not NASH, n (%) | 20 (20.0) |
| Borderline NASH, n (%) | 13 (13.0) |
| Definite NASH, n (%) | 63 (63.0) |
| NAS mean (SD) | 3.8 (1.4) |
| Imaging | |
| TE (kPa), median (IQR) | 6.1 (4.6) |
| CAP, median (IQR) | 299 (80.0) |
| MRE(kPa), median (IQR) | 2.7 (1.0) |
| MRI-PDFF (%), median (IQR) | 11.5 (10.4) |
| Use of M probe, n (%) | 51 (49.0) |
| Use of XL probe, n (%) | 53 (51.0) |
| Technical Failures of Fibroscan, n (%) | 7 (6.7) |
Abbreviations: ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; BMI, Body mass index; GGT, Gamma-glutamyl transpeptidase; HDL, high-density lipoprotein; Hgb, Hemoglobin; INR, international normalized ratio; kPa, kilopascal; NAFLD, LDL, low-density lipoprotein; Nonalcoholic fatty liver disease; NAS, NAFLD activity score; NASH, Nonalcoholic steatohepatitis; SD, Standard deviation
Distribution of fibrosis stages and steatosis grades
Respectively, 47, 24, 11, 13, and 8 patients had stage 0, 1, 2, 3, and 4 fibrosis; and 9, 49, 29, and 16 patients had grade 0, 1, 2, and 3 steatosis.
Comparison of MRE and TE for diagnosing fibrosis
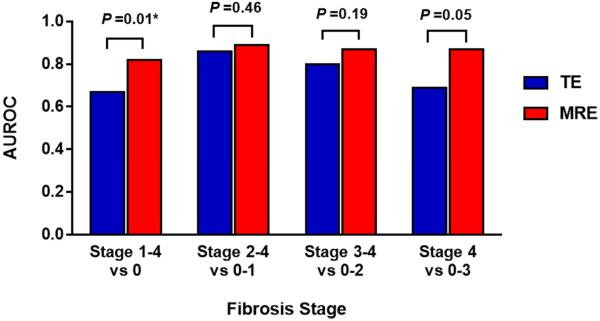
MRE had an AUROC of 0.82 (95% CI 0.74-0.91) for diagnosing fibrosis stage 1-4 versus 0. Using a threshold of 2.65 kPa, MRE had a sensitivity of 76.5%, specificity of 79.1%, PPV of 81.3%, and NPV of 73.9% (Figure 1). TE had an AUROC of 0.67 (95% CI 0.56-0.78) for diagnosing fibrosis. Using a threshold of 6.10 kPa, TE had a sensitivity of 66.7%, specificity of 65.1%, PPV of 69.4%, and NPV of 62.2%. Direct comparison using the Delong Test showed that MRE is significantly more accurate than TE (P = 0.0116) for diagnosing any fibrosis (Table 2).
Figure 1.
Diagnostic accuracy of MRE and TE for diagnosing dichotomized stages of fibrosis. MRE was significantly better than TE for diagnosis of any fibrosis with an AUROC of 0.82 (red bar) versus 0.67 (P = 0.01).
Table 2.
Diagnostic test characteristics of TE and MRE for the Diagnosis of Fibrosis
| Overall N=94 | AUROC (95% CI) | Threshold (kPa) |
Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
TE vs MRE (p)* |
|
|---|---|---|---|---|---|---|---|---|
| Primary analysis | ||||||||
| Stage 1-4 (n=51) versus stage 0 (n=43) |
MRE | 0.82 (0.74-0.91) | 2.65 | 76.5 | 79.1 | 81.3 | 73.9 | |
| TE | 0.67 (0.56-0.78) | 6.10 | 66.7 | 65.1 | 69.4 | 62.2 | 0.0116 | |
| Secondary analyses | ||||||||
| Stage 2-4 (n=29) versus stage 0-1 (n=65) |
MRE | 0.89 (0.83-0.96) | 2.86 | 79.3 | 81.8 | 65.7 | 89.8 | |
| TE | 0.86 (0.77-0.95) | 6.90 | 79.3 | 84.6 | 69.7 | 90.2 | 0.4596 | |
| Stage 3-4 (n=18) versus stage 0-2 (n=76) |
MRE | 0.87 (0.78-0.96) | 2.99 | 77.8 | 80.3 | 48.3 | 93.8 | |
| TE | 0.80 (0.67-0.93) | 7.30 | 77.8 | 77.6 | 45.2 | 93.7 | 0.1942 | |
| Stage 4 (n=8) versus stage 0-3 (n=86) |
MRE | 0.87 (0.71-1.00) | 3.35 | 75.0 | 81.4 | 27.3 | 97.2 | |
| TE | 0.69 (0.45-0.94) | 6.90 | 62.5 | 66.3 | 14.7 | 95.0 | 0.0546 | |
| NASH (n=72) versus no NASH (n=22) |
MRE | 0.70 (0.57-0.82) | 2.53 | 63.9 | 68.2 | 86.8 | 36.6 | |
| TE | 0.35 (0.22-0.49) | 5.60 | 61.1 | 59.1 | 83.0 | 31.7 | 0.0011 | |
p value: AUROC of TE versus MRE via Delong Test
AUROC: Area under receiver operating characteristic curve; CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Comparison of MRE and TE for diagnosing other dichotomized stages of fibrosis
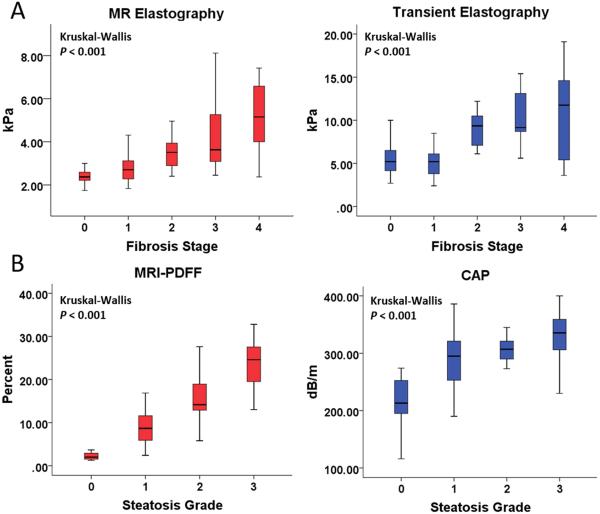
The AUROCs of MRE and TE for diagnosing other dichotomized stages of fibrosis are summarized in Table 2. For diagnosing stages 2-4 versus 0-1, stage 3-4 versus 0-2, and stage 4 versus 0-3 fibrosis, respectively, MRE had AUROCs of 0.89 (95% CI 0.83-0.96), 0.87 (95% CI 0.78-0.96), and 0.87 (95% CI 0.71-1.00), and TE had AUROCs of 0.86 (95% CI 0.77-0.95), 0.80 (95% CI 0.67-0.93), and 0.69 (95% CI 0.45-0.94). Direct comparisons showed that MRE is more accurate than TE for diagnosing any fibrosis (stage 1-4 versus 0), but no significant difference existed between MRE and TE for diagnosing other dichotomized stages of fibrosis. Distributions of liver stiffness measurements by MRE and TE are illustrated in Figure 3a.
Figure 3.
Figure 3a: Distribution of liver stiffness measurements by MRE and TE stratified by fibrosis stage. Stiffness measurements by both MRE and TE increased with increasing fibrosis stage (Kruskal-Wallis test P < 0.001)
Figure 3b: Distribution of steatosis measurements by MRI-PDFF and CAP stratified by steatosis grade. Steatosis measurements by both MRI-PDFF and CAP (Kruskal-Wallis P < 0.001) increased with increasing steatosis grade.
The mean (± SD) liver stiffness for stage 0, 1, 2, 3, and 4 fibrosis measured by MRE was 2.37 (± 0.38), 2.82 (± 0.65), 3.49 (± 0.71), 4.51 (± 1.74), and 5.16 (± 1.62) kPa, respectively. Similarly, the mean (± SD) liver stiffness for stage 0, stage 1, 2, 3, and 4 fibrosis by TE was 6.89 (± 10.37), 8.07 (± 13.48), 9.89 (± 2.67), 11.3 (± 4.93) and 10.39 (± 4.95), respectively.
Comparison of MRE and TE for diagnosing histologic NASH
For diagnosing NASH, MRE had AUROC of 1.70 (95% CI 0.57-0.82), which was significantly higher than TE AUROC (p = 0.0011) of 0.35 (95% CI 0.22-0.49).
Comparison of MRI-PDFF and CAP for diagnosing steatosis
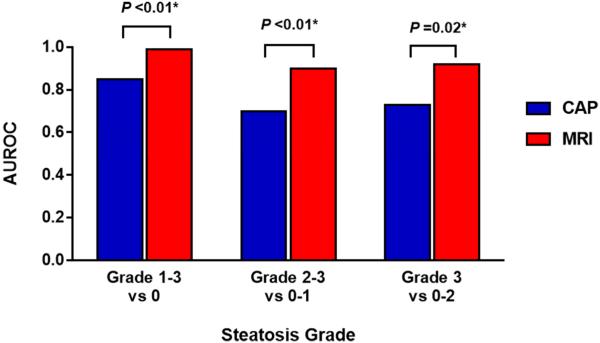
MRI-PDFF had an AUROC of 0.99 (95% CI 0.98-1.00) for diagnosing any steatosis (grades 1-3 versus 0). Using a threshold of 3.71 %, MRI-PDFF had a sensitivity of 95.8%, specificity of 100%, PPV of 100%, and NPV of 70.0% for diagnosing steatosis (Figure 2). CAP had an AUROC of 0.85 (95% CI 0.75-0.96). Using a threshold of 261 dB/m, CAP had a sensitivity of 71.8%, specificity of 85.7%, PPV of 98.1%, and NPV of 23.1%. Direct comparison showed that MRI-PDFF is more accurate than CAP for diagnosing (P = 0.0091) any steatosis (Table 3).
Figure 2.
Diagnostic accuracy of MRI-PDFF and CAP for diagnosing dichotomized grades of steatosis. MRI-PDFF was significantly better than CAP for all comparison including grade 0 versus grade 1-3, grade 0-1 versus grade 2-3, grade 0-2 versus grade 3.
Table 3.
Diagnostic test characteristics of CAP and MRI-PDFF for the Diagnosis of Steatosis
| Overall N=78 | AUROC (95% CI) | Threshold | Sensitivity (%) |
Specificity (%) |
PPV (%) |
NPV (%) |
PDFF vs CAP (p)* |
|
|---|---|---|---|---|---|---|---|---|
| Primary analysis | ||||||||
| Grade 1-3 (n=71) versus Grade 0 (n=7) |
MRI-PDFF | 0.99 (0.98-1.00) | 3.71 | 95.8 | 100 | 100 | 70.0 | |
| CAP | 0.85 (0.75-0.96) | 261 | 71.8 | 85.7 | 98.1 | 23.1 | 0.0091 | |
| Secondary analyses | ||||||||
| Grade 2-3 (n=30) versus Grade 0-1 (n=48) |
MRI-PDFF | 0.90 (0.82-0.97) | 13.03 | 80.0 | 83.3 | 75.0 | 87.0 | |
| CAP | 0.70 (0.58-0.82) | 305 | 63.3 | 68.8 | 55.9 | 75.0 | 0.0017 | |
| Grade 3 (n=11) versus Grade 0-2 (n=67) |
MRI-PDFF | 0.92 (0.84-0.99) | 16.37 | 81.8 | 83.6 | 45.0 | 96.6 | |
| CAP | 0.73 (0.58-0.89) | 312 | 63.6 | 70.1 | 25.9 | 92.2 | 0.0238 | |
p value: AUROC of CAP versus PDFF via Delong Test
AUROC: Area under receiver operating characteristic curve; CAP: Controlled attenuation parameter; CI: Confidence interval; PPV: Positive predictive value; NPV: Negative predictive value
Comparison of MRI-PDFF and CAP for diagnosing other dichotomized grades of steatosis
The AUROCs of MRI-PDFF and CAP for diagnosing other dichotomized grades of steatosis are summarized in Table 3. For diagnosing grade 2-3 versus 0-1 and grade 3-4 versus 0-2 steatosis, respectively, MRI-PDFF had AUROCs of 0.90 (95% CI 0.82-0.97) and 0.92 (95% CI 0.84-0.99), and CAP had AUROCs of 0.70 (95% CI 0.58-0.82) and 0.73 (95% CI 0.58-0.89). Direct comparison showed that MRI-PDFF was more accurate than CAP at all dichotomization cutoff points for diagnosing steatosis. Distributions of liver steatosis measurements by MRI-PDFF and CAP are illustrated in Figure 3b.
Multivariable-adjusted ROC analyses adjusted for biopsy-to-imaging time interval and probe type
The adjusted ROC analyses are summarized in Supplementary Tables 1 and 2. There was no significant difference in the performances of MRE, TE, MRI-PDFF, or CAP between unadjusted and adjusted models, when either biopsy-to-imaging time interval or type of probe were included as covariates.
DISCUSSION
Summary of main findings
Using a prospective, well-characterized, United States based cohort of patients, this study demonstrates that MRE is more accurate than TE for diagnosing liver fibrosis in patients with NAFLD. The key novelty of this study is that this is first study using XL-probe to perform head to head comparison between MRE versus TE, and MRI-PDFF versus CAP, and provided estimates of differences in diagnostic accuracy of these modalities in a Western NAFLD population that has a higher BMI than Asian NAFLD population so these results are more generalizable to Western cohorts.
Furthermore, this study showed that MRI-PDFF is significantly more accurate than CAP for diagnosing all dichotomized grades of hepatic steatosis. These results may have important implications in developing an optimal clinical approach for noninvasive assessment of NAFLD. Although cost-effectiveness studies are needed to determine the optimal approach, we propose that an MRI-based approach may be preferable to TE when accurate steatosis and fibrosis quantification is needed such as in the setting of a clinical trial because MR based methods have higher precision and accuracy than TE based assessment. TE may be preferable in routine clinical assessment at the level of population for screening out advanced fibrosis among low risk patient populations. However, further studies are needed to draw more definite conclusions.
In the context of published literature
This is the first prospective study to directly compare the accuracy of MRE and TE for diagnosing fibrosis, and MRI-PDFF versus CAP for diagnosing steatosis in a well-characterized cohort of American adults with biopsy-proven NAFLD. Both MRE and TE were not adequate for diagnosing NASH. Our study is consistent with prior studies showing MRI to have high diagnostic accuracy for fibrosis and steatosis in NAFLD patients.23-25, 28, 29 Our study is also consistent with prior studies showing TE to have high negative predictive value for diagnosing significant fibrosis (stages 2-4), severe fibrosis (stages 3-4), and cirrhosis,14, 15 and CAP to be accurate for diagnosing any steatosis, but not at higher dichotomized grades of steatosis.17, 18
A recent seminal study by Imajo et al.32 has shown that MRE is more accurate than TE for diagnosing significant fibrosis (stage 2-4 versus 0-1) and cirrhosis in Japanese NAFLD patients. In comparison, our study showed that MRE is more accurate than TE for diagnosing any fibrosis (stage 1-4 versus 0) but not cirrhosis (p = 0.0546) Although Imajo el al. assessed TE with using the M probe only, we also used the XL probe when indicated during our examination (n = 53). Our cohort demographical characteristics such as race and higher BMI (30.5 ± 5.2 kg/m2) may have reflected a more accurate assessment of the diagnostic performances and cutoffs of MRI and TE in a Western population. Future studies with larger cohort of patients may be needed to determine the optimal cutoff points for MRI-PDFF versus CAP for the grade of steatosis in NAFLD as well as MRE versus TE for the stage of fibrosis in NAFLD, which may be different for Western NAFLD population versus Asian NAFLD population.
Liver fibrosis and steatosis are clinically important features NAFLD that have been investigated by non-invasive tests such as MRI and TE. Steatosis alone is known to progress to NASH and fibrosis.8 Moreover, any fibrosis, even in the absence of severe fibrosis (stages 3-4), compared to no fibrosis was shown to be associated with increased mortality or liver transplantation rate in NAFLD patients.44 Therefore, early diagnosis and screening of fibrosis and steatosis before progression to severe fibrosis and/or NASH may benefit NAFLD patients. We acknowledge that liver histology, liver stiffness by TE, liver stiffness by MRE, ultrasound attenuation for CAP assessment, and steatosis quantification by MRI-PDFF all assess different properties using different physical properties. Therefore, although some of these would be co-linear with each other, they are not likely to be identical as each assesses different properties of liver tissue. Furthermore, the prognostic significance of changes in liver fat have not yet been assessed in long-term clinical trials, reduction in liver fat content by MRI-PDFF may have utility in short term trials, as previously shown. 34, 45, 46 Our study shows that MRI-based techniques are superior to TE for detecting any fibrosis and steatosis in NAFLD patients who may be at increased risk for mortality and other poor prognostic outcomes. Other advantages of MRI-based techniques over TE include larger area of the liver measured, which may reduce sampling variability secondary to heterogeneity of fibrosis,9, 11 and the utility of MRI-PDFF for assessing longitudinal changes in steatosis.47 Although TE has excellent inter- and intra-operator reproducibility48 and is accurate for diagnosing cirrhosis,12 its applicability is limited by high failure rates in patients with narrow intercostal space and ascites,12 interference of liver stiffness measurements by extrahepatic cholestasis and acute liver injury,49, 50 and reduced reproducibility in early stages of fibrosis and in the presence of steatosis.48, 51
Strengths and limitations
The strength of this study included use of well-characterized, prospective cohort of NAFLD patients undergoing liver biopsy for clinical indication. Liver biopsy, used as the reference standard for imaging, was scored using the NASH Clinical Research Network Histologic Scoring System, which is well-validated for assessing NAFLD patients. This study was performed by experienced investigators at a dedicated research center that is specialized for both clinical and radiologic research in NAFLD, and patients were carefully evaluated to exclude for other causes of liver disease before inclusion in the study.
However, this study also had the following limitations. The cross-sectional design of the study did not allow the assessment of MRE and TE for monitoring longitudinal changes in fibrosis. Since this was a single center study in a highly specialized setting, the generalizability of its findings in other clinical settings is unknown. The median time interval between TE and biopsy was 107 days. A recent meta-analysis of paired liver biopsy studies has shown that the rate of fibrosis progression is slow, with an average progression of one stage to take 14.3 years in patients with NAFL and 7.1 years in patients with NASH 6. Therefore, our time interval is reasonable as fibrosis stage is unlikely to change within a year. Furthermore, our analyses showed that the biopsy to imaging time interval did not affect the diagnostic accuracy of MRI and TE. Nevertheless, rapid changes in steatosis are possible, and ideally biopsy and imaging should be performed contemporaneously within 1 week, if feasible. MRI-based techniques, including MRE and MRI-PDFF, are often expensive, although at our center the cost of MRE is lower than that of biopsy without the associated morbidity. Although TE is more widely available in some parts of the world, MRI techniques are more widely deployed in the United States (US), therefore MRE can also be made available on commercially available MRI platforms throughout the US. While TE may be more useful for wide-spread screening, MRE may play a role in clinical trial assessments that require higher accuracy and precision. Further studies are needed to evaluate the cost-effectiveness of MRI over TE for diagnosing NAFLD-related fibrosis and steatosis in before implementing these competing noninvasive approaches in routine clinical practice.
Implication for future research
Using prospective, head-to-head comparisons, we showed that MRI-based MRE and MRI-PDFF are significantly more accurate than ultrasound-based TE and CAP, respectively, for diagnosing fibrosis and steatosis in an American cohort of patients with biopsy-proven NAFLD. MRI-based techniques may be preferable to TE for accurate noninvasive assessment of NAFLD. Future studies are necessary to assess the clinical utility of MRI and TE for diagnosing fibrosis and steatosis in a multicenter, longitudinal design, both in observational and intervention studies. The cost-effectiveness of utilizing MRE versus TE and/or biopsy must also be evaluated to develop optimal diagnostic strategies for diagnosing NAFLD-associated fibrosis and steatosis.
Supplementary Material
Acknowledgments
Grant support: The study was conducted at the Clinical and Translational Research Institute, University of California at San Diego. RL is supported in part by the American Gastroenterological Association (AGA) Foundation – Sucampo – ASP Designated Research Award in Geriatric Gastroenterology and by a T. Franklin Williams Scholarship Award; Funding provided by: Atlantic Philanthropies, Inc, the John A. Hartford Foundation, OM, the Association of Specialty Professors, and the American Gastroenterological Association and grant K23-DK090303. CS and RL serve as co-PIs on the grant R01-DK106419. CP is supported by NIH TL1 training grant TL1TR00098. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- AUROC
area under the receiver operator characteristic curve
- BMI
body mass index
- CAP
controlled attenuation parameter
- CI
confidence interval
- MRE
magnetic resonance imaging
- MRI-PDFF
magnetic resonance imaging-proton density fat fraction
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- TE
transient elastography
- UCSD
University of California at San Diego
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of study sponsor: The study sponsor(s) had no role in the study design, collection, analysis, interpretation of the data, and/or drafting of the manuscript. All authors report that no conflicts of interest exist.
Conflict of interests: Dr. Sirlin consults, advises, and is on the speakers’ bureau for Bayer. He received grants from GE Healthcare. All other authors report no other conflict of interests.
Author contributions:
Charlie C. Park: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, approved final submission.
Phirum Nguyen: patient visits, data collection, critical revision of the manuscript, approved final submission
Carolyn Hernandez: patient visits, data collection, critical revision of the manuscript, approved final submission.
Ricki Bettencourt: statistical analysis, critical revision of the manuscript, approved final submission
Kimberly Ramirez: patient visits, data collection, critical revision of the manuscript, approved final submission.
Lynda Fortney: patient visits, data collection, critical revision of the manuscript, approved final submission.
Jonathan Hooker: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Ethan Sy: data collection, imaging analysis, critical revision of the manuscript, approved final submission
Mosab H. Alquraish: patient visits, data collection, critical revision of the manuscript, approved final submission.
Mark A. Valasek: interpreted biopsies, critical revision of the manuscript, approved final submission
Emily Rizo: patient visits, critical revision of the manuscript, approved final submission
Lisa Richards: patient visits, critical revision of the manuscript, approved final submission
David Brenner: critical revision of the manuscript, study supervision, approved final submission
Claude B. Sirlin: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
Rohit Loomba: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding, study supervision, approved final submission
All authors approved the final version of this article.
REFERENCES
Author names in bold designate shared co-first authorship
- 1.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–50. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 3.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–31. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 4.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–65. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13:643–54. doi: 10.1016/j.cgh.2014.04.014. e1-9; quiz e39-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 8.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–7. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 9.Bonekamp S, Tang A, Mashhood A, et al. Spatial distribution of MRI-Determined hepatic proton density fat fraction in adults with nonalcoholic fatty liver disease. J Magn Reson Imaging. 2014;39:1525–32. doi: 10.1002/jmri.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merriman RB, Ferrell LD, Patti MG, et al. Correlation of paired liver biopsies in morbidly obese patients with suspected nonalcoholic fatty liver disease. Hepatology. 2006;44:874–80. doi: 10.1002/hep.21346. [DOI] [PubMed] [Google Scholar]
- 11.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- 12.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–13. doi: 10.1016/j.ultrasmedbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–74. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 14.Wong VW, Vergniol J, Wong GL, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51:454–62. doi: 10.1002/hep.23312. [DOI] [PubMed] [Google Scholar]
- 15.Pavlov CS, Casazza G, Nikolova D, et al. Systematic review with meta-analysis: diagnostic accuracy of transient elastography for staging of fibrosis in people with alcoholic liver disease. Aliment Pharmacol Ther. 2016;43:575–85. doi: 10.1111/apt.13524. [DOI] [PubMed] [Google Scholar]
- 16.Sasso M, Beaugrand M, de Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36:1825–35. doi: 10.1016/j.ultrasmedbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Myers RP, Pollett A, Kirsch R, et al. Controlled Attenuation Parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–10. doi: 10.1111/j.1478-3231.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 18.Chan WK, Nik Mustapha NR, Mahadeva S. Controlled attenuation parameter for the detection and quantification of hepatic steatosis in nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2014;29:1470–6. doi: 10.1111/jgh.12557. [DOI] [PubMed] [Google Scholar]
- 19.de Ledinghen V, Vergniol J, Capdepont M, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–31. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Foucher J, Castera L, Bernard PH, et al. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411–2. doi: 10.1097/00042737-200604000-00015. [DOI] [PubMed] [Google Scholar]
- 21.de Ledinghen V, Wong VW, Vergniol J, et al. Diagnosis of liver fibrosis and cirrhosis using liver stiffness measurement: comparison between M and XL probe of FibroScan(R) J Hepatol. 2012;56:833–9. doi: 10.1016/j.jhep.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Wong VW, Vergniol J, Wong GL, et al. Liver stiffness measurement using XL probe in patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2012;107:1862–71. doi: 10.1038/ajg.2012.331. [DOI] [PubMed] [Google Scholar]
- 23.Huwart L, Sempoux C, Vicaut E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32–40. doi: 10.1053/j.gastro.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 24.Talwalkar JA. Elastography for detecting hepatic fibrosis: options and considerations. Gastroenterology. 2008;135:299–302. doi: 10.1053/j.gastro.2008.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–8. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui J, Ang B, Haufe W, et al. Comparative diagnostic accuracy of magnetic resonance elastography vs. eight clinical prediction rules for non-invasive diagnosis of advanced fibrosis in biopsy-proven non-alcoholic fatty liver disease: a prospective study. Aliment Pharmacol Ther. 2015;41:1271–80. doi: 10.1111/apt.13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui J, Heba E, Hernandez C, et al. Magnetic resonance elastography is superior to acoustic radiation force impulse for the Diagnosis of fibrosis in patients with biopsy-proven nonalcoholic fatty liver disease: A prospective study. Hepatology. 2016;63:453–61. doi: 10.1002/hep.28337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Permutt Z, Le TA, Peterson MR, et al. Correlation between liver histology and novel magnetic resonance imaging in adult patients with non-alcoholic fatty liver disease - MRI accurately quantifies hepatic steatosis in NAFLD. Aliment Pharmacol Ther. 2012;36:22–9. doi: 10.1111/j.1365-2036.2012.05121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274:416–25. doi: 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–55. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imajo K, Kessoku T, Honda Y, et al. Magnetic Resonance Imaging More Accurately Classifies Steatosis and Fibrosis in Patients With Nonalcoholic Fatty Liver Disease Than Transient Elastography. Gastroenterology. 2016;150:626–637. doi: 10.1053/j.gastro.2015.11.048. e7. [DOI] [PubMed] [Google Scholar]
- 33.Le TA, Chen J, Changchien C, et al. Effect of colesevelam on liver fat quantified by magnetic resonance in nonalcoholic steatohepatitis: a randomized controlled trial. Hepatology. 2012;56:922–32. doi: 10.1002/hep.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loomba R, Sirlin CB, Ang B, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–50. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loomba R, Schork N, Chen CH, et al. Heritability of Hepatic Fibrosis and Steatosis Based on a Prospective Twin Study. Gastroenterology. 2015;149:1784–93. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doycheva I, Cui J, Nguyen P, et al. Non-invasive screening of diabetics in primary care for NAFLD and advanced fibrosis by MRI and MRE. Aliment Pharmacol Ther. 2016;43:83–95. doi: 10.1111/apt.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. Am J Gastroenterol. 2016;111:986–94. doi: 10.1038/ajg.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 39.Tang A, Tan J, Sun M, et al. Nonalcoholic fatty liver disease: MR imaging of liver proton density fat fraction to assess hepatic steatosis. Radiology. 2013;267:422–31. doi: 10.1148/radiol.12120896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207–1213. doi: 10.1016/j.cgh.2007.06.012. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835–47. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 43.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 44.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389–97. doi: 10.1053/j.gastro.2015.04.043. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui J, Philo L, Nguyen P, et al. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369–76. doi: 10.1016/j.jhep.2016.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel J, Bettencourt R, Cui J, et al. Association of noninvasive quantitative decline in liver fat content on MRI with histologic response in nonalcoholic steatohepatitis. Therap Adv Gastroenterol. 2016;9:692–701. doi: 10.1177/1756283X16656735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noureddin M, Lam J, Peterson MR, et al. Utility of magnetic resonance imaging versus histology for quantifying changes in liver fat in nonalcoholic fatty liver disease trials. Hepatology. 2013;58:1930–40. doi: 10.1002/hep.26455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraquelli M, Rigamonti C, Casazza G, et al. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–73. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–9. doi: 10.1111/j.1365-2893.2006.00811.x. [DOI] [PubMed] [Google Scholar]
- 50.Millonig G, Reimann FM, Friedrich S, et al. Extrahepatic cholestasis increases liver stiffness (FibroScan) irrespective of fibrosis. Hepatology. 2008;48:1718–23. doi: 10.1002/hep.22577. [DOI] [PubMed] [Google Scholar]
- 51.Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology. 2012;142:1293–1302. doi: 10.1053/j.gastro.2012.02.017. e4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.