Abstract
One of the promising host-directed chemotherapeutic interventions in tuberculosis (TB) is based on inducing autophagy as an immune effector. Here we consider the strengths and weaknesses of potential autophagy-based pharmacological intervention. Using the existing drugs that induce autophagy is an option, but it has limitations given the broad role of autophagy in most cells, tissues, and organs. Thus, it may be desirable that the agent being used to modulate autophagy is applied in a targeted manner, e.g. delivered to affected tissues, with infected macrophages being an obvious choice. This review addresses the advantages and disadvantages of delivering drugs to induce autophagy in M. tuberculosis-infected macrophages. One option, already being tested in models, is to design particles for inhalation delivery to lung macrophages. The choice of drugs, drug release kinetics and intracellular residence times, non-target cell exposure and feasibility of use by patients is discussed. We term here this (still experimental) approach, of compartment-targeting, autophagy-based, host-directed therapy as “Track-II antituberculosis chemotherapy.”
Keywords: Inhalations, Aerosols, Host-pathogen interaction, Phagocytosis, Apoptosis, Microautophagy
1. Introduction
A decade ago, Guiterrez et al. [1] demonstrated that induction of the process of autophagy in Mycobacterium tuberculosis (Mtb)-infected macrophages, by physiological, immunological or pharmacological means, could kill Mtb. The process of macroautophagy (henceforth: autophagy) is known to operate in vivo during Mtb infection. Autophagy-related (ATG) proteins are the “core machinery” responsible for different steps of the process [2]. Mice deficient in Atg5 in their myeloid linage, which includes macrophages, are more susceptible to establishment of Mtb infection and progression of the disease [3]. Since the initial report in 2004 [1] several research groups have extended these initial observations [4–25]. There is now emerging consensus that autophagy is an important and potentially a key host response in tuberculosis (TB) [26–30]. Furthermore, the ability to pharmacologically induce autophagy to control intracellular Mtb [1,31] calls for its investigation as a host-directed chemotherapeutic strategy in tuberculosis (TB) [32,33]. An unexpected but potentially highly significant observation is an apparent synergism between autophagy and classical frontline anti-TB chemotherapeutics [34], further strengthening the potential for autophagy applications in track-II anti-tuberculosis therapy, the term we propose here for this approach, which involves autophagy-based and compartment-targeting host-directed therapy.
Planned pharmacological intervention with known inducers of autophagy [35] has not, to our knowledge, been attempted to treat TB. There are many reasons why there is both a time-lag in and an impetus to investigate pro-autophagic drugs for management of TB and initiation of pre-clinical and clinical trials. First, although the discovery was made a decade ago [1] the field of TB drug development and therapeutics have been relatively slow in acceptance of this newly found host defense mechanism. Second, although the selection of current drugs that can induce autophagy include primary indications such as infection (tetracyclines, imidazoles, triazoles), many of the panel of drugs are known immunosuppressants (e.g. rapamycin) and thus could be considered a priori counter-indicated for TB. Other potential candidates are used to treat psychosis (haloperidol), bipolar disorders (lithium), epilepsy (valproate), cardiovascular disease (resveratrol, reserpine, digoxin), etc., and thus may have serious off-target, adverse effects.
Targeting proautophagic agents specifically to alveolar macrophages infected with Mtb, in principle, could overcome many of the concerns that restrain detailed investigation of their autophagy-inducing potential as anti-TB agents. The most facile way to achieve this is to use inhaled drug delivery systems that target lung macrophages by virtue of the innate ability of these cells to take up particulate matter deposited in the alveolar space [36]. An increasing body of work on particulate and vesicular inhaled formulations has demonstrated that drugs may indeed be preferentially targeted to lung and airway macrophages, minimizing drug exposure to systemic blood circulation and non-target cells. This review addresses some aspects of autophagy that are of immediate concern to drug delivery research in TB, suggests candidate agents that may be investigated for ‘repurposing’ or ‘repositioning’ small molecular weight drugs, and summarizes experiences suggesting that pulmonary delivery of macrophage-targeted particles may be suitable for safely investigating the efficacy of autophagy induction for Track-II, or host-directed therapy of TB.
1.1. The spectrum of host macrophage responses to infection with Mtb
Lung macrophages are considered to represent a key niche for Mtb parasitism, with the pathogen having evolved a number of mechanisms to evade detection and clearance by the host [37]. In the current view, macrophages successfully invaded by Mtb tend to display the alternative activation or M2 phenotype [38], such that their ability to mount a coordinated program for eliminating the intracellular bacterium is compromised [39]. In contrast, classically activated or M1 macrophages deploy a spectrum of mechanisms that either destroy the bacterium, or work to deny it sanctuary in the macrophage phagosome. Fig. 1 shows a simplified picture of the nature of responses elaborated by the macrophage during infection with Mtb.
Fig. 1.
A spectrum of macrophage responses to invasion by Mtb. (A) Sensing the presence of Mtb in a phagosome. (B) Mobilization of innate effector molecules such as free radicals and antimicrobial peptides. (C) Secretion of signaling molecules such as cytokines and chemokines. (D) Maturation of phagosomes into phagolysosomes and mobilization of innate immunity devices such as the inflammasome, both inhibited or manipulated by Mtb). (E) Autophagy. (F) Cell death.
In brief, macrophage phagocytosis of Mtb, through a variety of cell surface receptors, results in diverse outcomes [40]. Most pathogens that bind pattern recognition receptors on macrophages elicit rapid deployment of innate bactericidal mechanisms associated with recognition of pathogen-associated molecular patterns. These include, an oxidative burst, calcium flux, free radical generation, phagosome maturation leading to phagosome–lysosome fusion, proinflammatory cytokine and chemokine secretion, inflammasome activation, mobilization of antimicrobial peptides, macroautophagy, and failing all else, apoptosis. However, all of these macrophage responses are counteracted and often effectively neutralized by Mtb leading to successful and chronic infection. It is not clear whether these responses alone are sufficient to eliminate the bacterium if expressed to its full potential, and thus the initiative to employ host-directed Track II therapies for TB must be informed about which of the processes may be necessary and sufficient to eliminate the infection. Likewise, some of the above processes are highly inflammatory and tissue-damaging, and thus any host-directed therapy should be cognizant of the perils of causing uncontrolled tissue damage in TB, harking back to the days of the well-intended but ill-fated Koch’s effect [41]. Koch described severe adverse effects of the purified protein derivative of tuberculin injected as a host-directed therapy in TB patients who would have had a pre-existing immune response to Mtb.
1.2. An overview of macrophage autophagy in TB
As shown in Fig. 2, autophagy involves three phases: initiation, elongation and maturation [42]. Initiation culminates in internalization of the target phase-separated particle and leads to the formation of an autophagic vacuole. This step is controlled by class III phosphatidylinositol (PI) 3-kinase (PI3K Complex III). Elongation proceeds with the formation of a stable complex comprising ATG5, ATG12, and ATG16 and is characterized by the lipidation of microtubule associated protein 1 light chain 3 (LC3I-cytosolic form) with phosphatidylethanolamine (PE) to form membrane bound LC3II [43]. Maturation, the final step of autophagy, involves autophagosome fusion with late endosomal/lysosomal organelles through the association of the complex between the PI3K termed as the vacuolar protein sorting (VPS)-34 and BECN1, with the protein encoded by the UV irradiation resistance associated gene (UVRAG) or VPS38. Although UVRAG contributes to the activation of Rab7 that may promote eventual maturation of an autophagosome into the autolysosome [44], this process is more complicated and involves yet-to-be-fully understood control by a specific SNARE molecule, Syntaxin 17, which pivots the formation of the autolysosome step [45,46]. Autolysosomes degrade particulate content not only by lowering luminal pH and increasing the activity of lysosomal hydrolases, a property shared by the conventional phagolysosome, but also by the delivery of conventional [15,20] and neo-antimicrobial peptides [4,7] making the autolysosome environment particularly robust for eliminating the captured cargo.
Fig. 2.
Stages of autophagy and proposed Mtb countermeasures. Autophagy initiates with the internalization of the microbe. Ingested material is surrounded by double-membrane bound autophagosomes. Autophagosome contents are degraded by lysosomal hydrolases. Mtb cell wall components like Lipoarabinomannan (LAM), mannosylated LAM (ManLAM), secretory proteins and virulence factors inhibit fusion of phagosome with lysosome via suppression of the release of TACO or recruitment of V0H+ ATPases and prevent phagosome maturation.
Virulent Mtb can activate the mammalian target of rapamycin (mTOR) and thereby inhibit autophagy [14]. Furthermore, direct interference in the host macrophage metabolism by Mtb [47,48] results in accumulation of lipid droplets in the cytosol. Lipid droplets are proposed as a platform for ‘convergence’ of proteasomal and autophagic pathways [49], and direct evidence of localization of Atg-2 on lipid droplets convincingly suggests their role in regulation of autophagy [50,51]. Inhibition of autophagy protects intracellular Mtb from elimination [48].
Several Mtb-encoded factors, including enhanced intracellular survival (Eis) [16,34,52] may interact with specific autophagy factors or affect upstream signaling regulators. Eis acetylates a Janus kinase (JNK)-specific phosphatase, inhibiting the activation of the key autophagy regulator Beclin 1. Fig. 2 shows a few examples of Mtb factors that inhibit autophagy. Lipoarabinomannan (Man-Lam) is also reported as an inhibitor of autophagy [53]. A type VII secretion system of Mtb, ESX-1, releases ESAT-6 to block maturation of Mtb phagosomes [54,55], a process that can be pharmacologically reversed [54]. Drug-induced autophagy is thus similar to physiological or immunological stimulation of autophagy (e.g. by starvation or IFN-γ) [1,6] and can eliminate intracellular Mtb.
With reference to macrophages infected with Mtb, autophagic machinery serves three principal functions related to both innate and adaptive immunity. First, the recognition, capture and neutralization of intracellular bacteria through two distinct forms of anti-microbial autophagy [56]: (i) xenophagy [57], which entails recognition and autophagic capture of cytosolic or cytosol-exposed intracellular microbes, and (ii) LC3-associated phagocytosis (LAP) [58–60], which does not result in the formation of double membrane autophagosomes but assembles parts of the autophagic machinery (e.g. LC3) directly on the phagosomal membrane, with the final outcome of cargo delivery to the autolysosome being similar during conventional autophagy or xenophagy. The respective final degradative organelles are referred to as the autolysosome for xenophagy and the autophagolysosome for LAP respectively [61]. A specific role of LAP in immunity to TB remains to be elucidated. Autophagic adaptors such as the sequestosome 1/p62-like receptors (SLRs) [62,63] enable autophagic capture but can also lead to inflammation in cases when the microbe or its products are not eliminated through autophagy [56]. SLRs furthermore enable delivery of conventional (e.g., cathelicidin) as well as cryptic or neo-antimicrobial peptides to Mtb. Such novel bactericidal peptides are derived by digestion of cytosolic proteins through autophagy [63]. Second, autophagy enables presentation of mycobacterial peptides on major histocompatibility molecules [25]. The third, and probably least appreciated contribution of factors that participate in autophagy is their ‘moonlighting’ [64] as inhibitors of signaling through pathogen recognition receptors (PRRs) such as the retinoic acid inducible gene I (RIG-I)-like receptor (RLR) family [65].
2. Regulators of autophagy
A variety of immune mediators and mechanisms impacting autophagy have been reviewed in the context of TB [66]. These include host factors such as cytokines produced by macrophages displaying the classical (M1) or alternative (M2) activation, ROS, vitamin D3- and Toll-like receptor (TLR)-associated signaling molecules; pathogen products such as lipoproteins, and pharmacological agents such as rapamycin and nitazoxanide that can induce or inhibit autophagy.
An extremely valuable ‘curated census’ of host proteins and small molecules that regulate autophagy has recently become available for identifying potential targets of cancer therapy [67]. Lorenzi et al. undertook painstaking analysis of results published till August 2012. Based on siRNA screens, complemented with metabolic pathway analysis and finally reading through text enabled these authors to identify a number of positive and negative regulators of autophagy. This census is also important in that it identifies molecules capable of impacting autophagy under conditions of sufficient nutrition, i.e., when the cell is “nutrient replete.” The macrophage infected with Mtb, however, is likely to experience distortions in nutrient supply, for example, on account of sequestration of micronutrients within phagosomes [68] or even the nutritional status of the host organism [69,70]. The positive regulators of autophagy enumerated in this census of small molecules require updating on the basis of information appearing in subsequent literature.
Host molecules that inhibit autophagy may be considered as pharmacological targets, or siRNA-targets for development of Track-II host-directed TB therapies. A variety of nutritional (e.g., amino acids, lipids), hormonal (e.g., insulin), metabolic and stress signals regulate autophagy. TOR kinase functions to integrate many of these inputs, and represents a principal negative regulator of autophagy [71]. Progression of autophagy can also inhibited by lipid phosphatases, such as the phosphatidylinositol 3-phosphate (PI3P) phosphatase Jumpy [72].
Lorenzi et al. identified a set of 44 host molecules reported as negative regulators and another 16 that are reported as both positive and negative regulators of autophagy in different contexts [67]. While MTOR is unequivocally reported as a negative regulator, the next most-studied molecule (BCL2) was reported as a negative regulator in 159 studies but as a positive regulator in 170. Such observations serve to caution us about the pitfalls when trying to classify a continuum of host responses into a binary, positive/negative universe.
On the pathogen’s side, several factors have been implicated as regulators of autophagy. The early secreted antigenic target (ESAT)-6 of Mtb is a key component of a mechanism to inhibit autophagy at the stage of autophagosome-lysosome fusion in host dendritic cells [54]. Nabatov et al. showed that Mtb-specific sulfolipid 1 competitively inhibits NOD2 from binding with host sulfatide and that such loss of NOD2 function inhibits autophagy [73]. Using a deletion mutant of Mtb that lacks the enhanced intracellular survival (Eis) gene, Shin et al. demonstrated that autophagy is enhanced in macrophages infected with the mutant, and that complementation or supplementation with Eis protein resulted in inhibition of autophagy [74]. However, the effects of Eis on host cell biology are manifested through acetylation of a host JNK-specific phosphatise and are thus not restricted to autophagy [75]. Again, there is need for caution in identifying a pathogen-derived factor as exclusively a regulator of autophagy rather than an agent with several potential effects on host cell biology.
3. Identifying agents for Track-II chemotherapy of TB
Small molecular weight drugs that can induce or potentiate autophagy could also, in theory, be considered as TB therapeutics. Host metabolites such as 1,25 dihydroxy vitamin D3 are therefore attractive Track-II candidates for chemotherapy of TB [76] [77]. Following the demonstration that 1,25 D3 induces autophagic flux via the action of the antimicrobial peptide cathelicidin [17], it might also be feasible to use the peptide as the therapeutic agent, provided its delivery to the macrophage cytosol can be ensured.
A different set of molecules that positively regulate autophagy are difficult to imagine as viable therapeutics for TB. For example, signal transduction through Toll-like receptors (TLRs) by PAMPs and damage-associated molecular patterns (DAMPs) referred to earlier is a common mechanism for inducing a variety of host responses, including autophagy [78]. The use of pathogen-derived molecules that are clearly PAMPs (such as MDP [67,79]or lipoprotein [74]) as therapeutics for TB is counterintuitive, and seems likely to give rise to greater toxicity than therapeutic benefit. Similarly, among the 262 small molecules identified as positive regulators of autophagy by Lorenzi et al. [67], there are agents such as arsenic, sodium arsenite, phorbol ester, etc., that are difficult to imagine as anti-TB therapeutics. However, drug targeting is an acknowledged strategy for overcoming systemic toxicity. If extremely toxic substances can be targeted to, and retained within, infected lung macrophages, there is a window of opportunity to deploy host-directed therapies. Thus, molecules that are considered a priori toxic can only be used if they are formulated as drug delivery systems capable of targeting lung macrophages.
Before and since the meta-analysis by Lorenzi et al. [67], there have been several attempts to screen small molecules that induce autophagy for use in cancer and infectious diseases. The screened molecules include those that have regulatory approval by agencies like the US FDA, or libraries synthesized as part of drug discovery efforts.
Some compounds that synergize with rapamycin have been validated as anti-TB agents in primary human (M1) macrophages [31]. The prospect of ‘re-purposing’ drugs already enjoying regulatory approval to induce autophagy in macrophages infected with Mtb that seems to have captured the imagination of researchers worldwide. Rapamycin, nitazoxanide, etc. have been alluded to earlier. A tandem approach of informatics combined with high-content screening discovered two more agents [80]. Shaw et al. used a combination of genetic screens and a ‘chemical biology approach’ to identify small molecule inducers of autophagy [81]. Exploring mechanisms of action such as regulation of IL1-β, T cell differentiation, and monitoring autophagic flux induced by intracellular Salmonella, these authors identified 130 known and novel compounds that induce autophagy from a pool of 3713 bioactive small molecules. HeLa cells and mouse bone marrow derived macrophages, infected with Salmonella typhimurium were tested at a single concentration of <8 μM, and 16 classes of compounds were identified. Interestingly, some of these had been earlier reported as negative regulators of autophagy, while neither rapamycin nor nitazoxanide (tizoxanide) was identified as a hit in the investigation. Other imidazole and triazole antifungals were, however, picked up using stringent statistical criteria [81].
It is in this context that Lorenzi et al.’s curated list of compounds [67] becomes a valuable resource. Screens, by definition, generate ‘hits’ and certainly not a ready-made candidate for clinical testing. It is for the development scientist to interrogate the list of putative inducers of autophagy as candidates for lung macrophage-targeted Track-II therapies.
Table 1 summarizes some additional drugs that were shown to induce autophagy in at least one screen, including those that have been tested for use against intracellular Mtb and are prima facie good candidates to develop as Track-II therapies. Agents listed in Table 1 are not included in the curated census referred to earlier [67].
Table 1.
Some agents that induce autophagy and are not included in the census by Lorenzi et al. [67].
| Name | Primary indication | Mode induction of autophagy | Oral dose | Ref. |
|---|---|---|---|---|
| ABC294640 [3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide] | Kills PC-3 prostate and MDA-MB-231 breast adenocarcinoma cells | Novel sphingosine kinase inhibitor (SK2-selective inhibitor) | Cell-free IC50 60–85 μM | [82] |
| 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR) | TB | AMPK mediated activation of autophagy | 500 mg/kg i.p., treated for 3 consecutive days | [83,84] |
| Alkaloids: Lienshinine, Isoliensinine, Dauricine, Cepharanthine | Kill HeLa and multiple cancer cells | Activation of AMPK-mTOR pathway | – | [85] |
| Apicidin | Kills human oral squamous carcinoma cells, malaria parasite; | Histone deacetylase inhibition, IGF-1 R inhibition | Cell-free IC50 0.7 nM | [86,87] |
| ATP directed agent, 8-chloro-adenosine | Phase I clinical trial for hematological malignancies | Induces AMPK pathway | 100 mg/kg/d 3 times a week | [88] |
| Bromocriptine | Parkinson’s disease, galactorrhea, infertility | Unknown | 40 mg/d × > 3 yrs | [89] |
| Bufalin (A major component of Chan-Su, extracts from the venom of Bufo gargarizan) | Anti-inflammatory and antinociceptive activity | ATP depletion results in an increase in active form of AMPK, decreased phosphorylation of mTOR | – | [90,91] |
| Chlorpromazine | Hiccups, nausea, psychosis | IL1-β inhibition, inducing PI3K/AKT/mTOR pathway | 25 mg to 1 g/d × > 3 yrs | [81,92] |
| Clindamycin and Lincomycin | Serious anaerobic infections | Unknown | Up to 1.8 g/d × 7 d | [93] |
| Clomipramine | Depression, phobia, narcolepsy | Unknown | Up to 250 mg/d × > 3 yrs | [89] |
| Concavalin A | Kills hepatoma cells | PI3K/Akt/mTOR and MEK/ERK pathway | 7.5 mg/kg given twice at a 3 days interval | [94,95] |
| Cyclometalated iridium(III)–β-carboline complexes | Kills human breast cancer cells | ROS generation but caspase independent | – | [93] |
| Cyproheptadine and 44 antihistaminics | Allergy | IL1-β inhibition, +? | 32 mg/d × > 3 yrs | [89] |
| Digoxin | Heart failure, supraventricular arrhythmias | AMPK mediated inhibition of mTOR/ERK1/2 signaling | up to 500 μg/d × > 3 yrs | [89,96] |
| Doxycycline | Susceptible infection | Mg2+ chelation; inhibition of phosphorylation of mTOR substrates | 200 mg/d × 7 d | [97] |
| Fangchinoline | Hepatocellular carcinoma cells | p53/sestrin2/AMPK signaling | – | [98] |
| Fludrocortisone | Adrenocortical insufficiency | Unknown | 300 μg/d × > 1 yr | [89] |
| G226, novel epipolythiodioxo-piperazine derivative | Kills human breast cancer cells | – | – | [99] |
| Ginseng metabolite (Compound K) | Kills HCT-116 Colon cancer cells | ROS generation | – | [100] |
| (+)-Grandifloracin | Kills PANC-1 pancreatic cancer cells | Mechanism unclear | – | [101] |
| Imidazole and triazole antifungals, antiprotozoals anthelmintics (17) | Infection, infestation | Various mechanisms e.g. Ciclopirox (Fungicide): ROS generation, Monepental (anthelmintic): mTOR/p70S6K signaling | 1–3 g/d × 7 d | [89,102,103] |
| Isoniazid | TB | ROS generation | 300 mg/d × > 6 mo | [104] |
| Lithium, Haloperidol and 19 phenothiazines | Psychotropic | Downregulation of GSK-3β; inositol species and cytoplasmic p53 | up to 1.2 g of Li or 10 mg Haloperidol/day × > 3 yrs | [89,105] |
| NF449 | Antithrombotic | Gαs inhibitor; mTOR-independent | – | [106,107] |
| Nitazoxanide | Amebiasis | Inhibition of human quinone oxidoreductase and mTORC1 | 1 g/d × 3 d | [108] |
| Nitolinib | Chronic myelogenous leukemia | AMPK activation | 0.6–0.8 g/d | [109] |
| Noscapine and brominated derivatives | Cough | ROS generation | 7 mg/d × 7 d | [89,110] |
| Penitrem A | Fungal neurotoxin | Inhibits high conductance Ca2+-activated K+ channel; mTOR-independent | – | [111] |
| Psoralidin | Kills lung cancer A549 cells | ROS generation | – | [112] |
| Pyrazinamide | TB | ROS generation | 1.5 g/d × > 6 mo | [104] |
| Quinine sulfate | Falciparum malaria | K+ ATP channel blocker | 1.8 g/d × 7 d | [106] |
| Salirasib (S-trans, trans-farnesyl thiosalicylic acid; FTS) | Kills mouse embryonic fibroblasts and the human cancer cells | Ras inhibitor | Phase I clinical trial for solid tumor | [113,114] |
| SMER10, SMER18, SMER28, SMER analogs | – | Unknown; mTOR-independent | – | [115] |
| Suberoylanilide hydroxamic acid (SAHA) | Glioblastoma stem cells | Histone deacetylase inhibition | Cell-free IC50 10 nM | [116] |
| Temozolomide | Refractory anaplastic astrocytoma | ATM-AMPK-ULK1 pathway | 50 mg/m2 PO/IV qD for 5 d; repeat at 28-day cycles | [117,118] |
| Tetrandrine | Leukemia | ROS generation | – | [119] |
| Thioridazine | Schizophrenia, depression | AMPK-induced suppression of Raptor | 200 mg/d × > 3 yrs | [120] |
| Tolazamide | Diabetes | K+ ATP channel blocker | 0.1–1 g daily | [106] |
| Unsaturated fatty acids | Kill cancer cells | Induce non-canonical autophagy | – | [121] |
Apart from the possibility of generating toxicity on account of their primary indication and mechanisms of action, agents listed in Table 1 might give rise to toxicity simply on account of inducing autophagy in non-target cells. Shintani and Klionsky have raised this concern and pointed out possible mechanisms of induction of hepatic, neural or myoskeletal toxicity [122]. Systemic bioavailability and non-target tissue accumulation of autophagy-inducing agents is therefore best avoided, and targeted delivery might turn out to be a necessary condition for optimal clinical use.
4. Prospects of clinical intervention by pulmonary drug delivery
A pharmacopoeial armamentarium is thus at the disposal of clinicians interested in investigating whether induction of macrophage autophagy is of value in treating active or latent TB infection. Acknowledging that most of the drugs identified as inducers of autophagy have potent primary indications, it has been proposed that pharmacological inducers of autophagy may be delivered locally to the tubercular lung and targeted to macrophages [123]. The prospect of siRNA delivery for silencing genes that inhibit autophagy, or endogenous host molecules that induce it further expands the scope of agents that may potentially be clinically useful. However, there are many issues to be addressed before such therapies may be brought to human patients. Some of these are discussed below.
4.1. Dose
The in vitro screen employed by Shaw et al. tested compounds for induction of autophagy in cancer cells at a concentration of <8 μM [81]. The internal surface area of the human lungs is popularly estimated to lie between 70 and 100 m2 [124] and has been documented to range between 23.56 and 68.76 m2 in post-mortem anatomical examinations [125]. Assuming a layer of lung surfactant of dimensions 1 μl/μm bathing 100 m2 of the lung surface, the amount of a hypothetical drug with a molecular weight of 400 Da required to generate a concentration of 8 μM in the layer of lung surfactant would be about 3.2 g. Further, even if this amount were to be instantaneously deposited on the lung surface; it would be rapidly dissipated as diffusion, tissue penetration and perfusion remove it from the vicinity of the target cells—the infected alveolar macrophages. Thus a ‘generalized’ derivation of posology of targeted doses from in vitro results is difficult. The situation is further complicated by an appraisal of the doses of agents that are used for their ‘primary indication’ (Table 1). Since treatment of TB is likely to be long-term even if autophagy is sought to be induced as a part of therapeutic strategy, doses and IC50 values listed in Table 1 will in likelihood require drastic modification if targeted drug delivery is not envisaged.
Patient factors would also require serious consideration in deciding dosing regimens. Apart from differences arising out of age, ethnicity, gender, nutrition status, doses required to induce autophagy in infected macrophages while sparing surrounding tissue would be affected by the anatomy and physiology of the tubercular lung [126].
Dosing in some protocols is linked to delivery methods that can be linked to cell targeting. Notably, drug targeting directly to macrophages, the cell that is central to Mtb infection may facilitate achievement of the necessary dose and may be of particular significance when repurposing drugs where induction of autophagy is weak and side effects may be prohibitive. As an example, it has been recently demonstrated that the nitric oxide donor DETA/NO reduces the viability of intracellular Mtb by 3 logs of colony forming units (CFU)/ml if administered in a particulate form with particle size allowing them to be readily phagocytosed. An equivalent amount of the drug added into the culture wells in the form of a solution achieved only reduction of 1.5 logs of CFU [127, 128]. Dose-ranging studies, both pre-clinical and clinical will be required to arrive at an optimal inhaled dose for different autophagy-inducing compounds. Given the targeting of lung macrophages are present in amounts of about 100–1000/mm3 of lung volume [129], the optimal inhaled doses are likely to fall within the definition of ‘microdoses’ currently employed for establishing pharmacokinetics in Phase 0 clinical trials [130].
4.2. Formulation
The objective of targeting lung macrophages requires that host-directed therapies should be incorporated in phase-separated structures that retain their integrity for a few hours after deposition on the alveolar surface. Ideally, the payload should be retained until the delivery system is taken up by macrophages, with minimal burst release. Several kinds of particulate and vesicular systems have been formulated for pulmonary delivery, but their uptake or “clearance” by lung macrophages is conventionally viewed as a problem to be solved for the purpose of rapid or sustained systemic delivery of drugs [131]. Formulation and pulmonary delivery strategies have been recently reviewed in this journal [132] and need not be elaborated here. In respect of Track-II agents discussed here, however, uptake by macrophages is an advantage, since autophagy is sought to be induced in infected macrophages. Although we lack clinical evidence that inhaled particles are taken up by lung macrophages that harbor Mtb, results from mouse models of infection clearly show that this is indeed the case (Fig. 4). It is important to remember that the physico-chemical properties of phagocytosed particles have a clear bearing on the fate of the phagolysosome [133].
Fig. 4.
TEM of lung section from a mouse infected with Mtb and treated with inhaled particles. (A): an alveolar macrophage in which Mtb debris co-localizes with a particle (arrow). (B) a lysosome releasing its contents into a phagosome with an inhaled particle (arrow). (C) condensed mitochondria and (D) intense Golgi activity in the vicinity of the particle phagosome (arrows). Panels A and B reprinted from reference [149] with permission from Oxford University Press.
4.3. Method of administration
Pulmonary drug delivery may be achieved by nebulization, through pressurized metered dose inhalers (pMDI or MDI) and dry powder inhalations (DPI), the last of which is considered the most cost-effective and efficient [134,135]. In animal models of TB, powders are generally administered by positive pressure insufflation rather than ambient pressure inhalation [136]. As is well known, these different methods of administration lead to marked differences in the extent and pattern of lung deposition [134]. Thus, the formulation intended for lung delivery requires to be carefully matched with a device for pulmonary delivery.
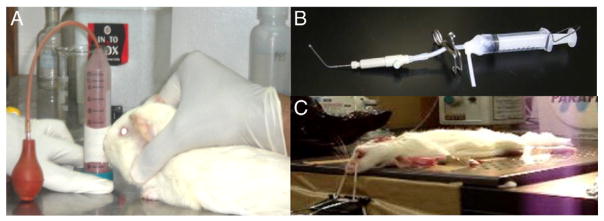
In this context, it is important to note that non-clinical testing of formulations that induce lung macrophage autophagy must take into account differences in outcome arising out of lung deposition of formulations, in addition to species differences when interpreting data. Apart from non-human primates, animal models of TB breathe in the prone position, while intratracheal intubation for insufflating formulations is carried out with the animal laid out on its back (Fig. 3).
Fig. 3.
(A): An upright, nose-only inhalation apparatus allows animals to inhale powders at ambient pressure. The animal is restrained with its nostrils inserted in a chamber which contains a powder aerosol. Powder properties and duration of exposure reproducibly control inhaled dose. The apparatus is suitable for use in an ABSL3 setting. (B): The DP-4M PennCentury™ insufflator is a positive-pressure apparatus inserted into the trachea of anesthetized animals using a bronchoscope. (C): Orientation of the test animal for receiving the DP-4M apparatus that delivers precise doses to deep lungs, without loss of material in the mouth and upper airway region. Photographs in Panels B and C: courtesy, Prof. Hiroshi Terada, Niigata University of Pharmacy and Applied Life Sciences.
The entry of aerosols into the airways under positive pressure while the animal is in an unusual orientation may result in patterns of deposition that are different from the kind of deposition achieved under inspiratory airflow in a human breathing in the upright position. Further, TB granulomas around which infected macrophages are present in large numbers, are preferentially localized to the posterior, upper lung lobes and superior parts of the lower lung lobes [137]. If the method used to introduce formulations into the airways results in sub-optimal deposition in these regions, the outcome may be poorer than predicted by cell culture studies.
There are also concerns regarding procedures and apparatus for lung delivery of formulations to lab animals that require attention, primarily related to aerosol performance. It is now clear that there are ‘pitfalls’ associated with concluding that results of in vitro tests on powders for inhalation will be borne out when positive-pressure apparatus are used in animals [138].
Progressing from pre-clinical to clinical testing, there are several issues relating to the development of formulations and ensuring their correct coordination with the inhalation device. These have been recently reviewed by Hoppentocht et al., with valuable comments on misconceptions associated with formulation and device development [139].
4.4. Lung pharmacokinetics and biodistribution
Pulmonary drug delivery is often compared to intravenous dosing in terms of the pharmacokinetics resulting from inhalation of ‘free’ drugs. The lag time of appearance of the drug in blood is only slightly longer than that observed with intravenous administration, hepatic first pass is avoided, and the absorption phase is virtually absent after a drug reaches the lungs. In contrast, formulations that are capable of targeting lung macrophages and sustaining drug release lead to prolonged retention of the drug in the lungs [140–142]. If the intention is to induce autophagy of infected alveolar macrophages, retention and targeting to lung macrophages would be essential for achieving intended effect and avoiding off-target effects. Therefore, lung pharmacokinetics of investigational agents would have to be established.
Next, an optimal dosing interval would need to be figured out so that autophagy could be induced in macrophages that are newly infected during the course of active disease; and also to reach macrophages in the vicinity of tubercular granulomas. Incidentally, many of the latter display the foamy macrophage phenotype, and could be an important sanctuary for latent Mtb [143]. The high lipid content in these cells suggests that lipid signaling could be recruited for induction of autophagy [80]. However, with reference to macrophage traffic within the lung, it may be anticipated that cells near the alveolar surface would tend to migrate towards granulomatous lesions after they take up inhaled particles. This surmise is supported by the observation that Mtb induces the macrophage migration inhibitory factor (MIF) in regions surrounding the granuloma [144]. Thus, it is possible to visualize a ‘shell’ of migrating alveolar macrophages around the granulomatous region. If many of these macrophages have significant cytosolic content of an autophagy-inducing drug, it would be interesting to investigate whether the drug could reach foamy macrophages by diffusing out of the newly-arrived migrating macrophages. Recent investigations on zebrafish embryos infected with Mycobacterium marinum indicate that macrophages that take up fluorescent particles loaded with rifampicin do in fact migrate to granulomatous lesions to create this kind of tissue architecture [145]. These and other mechanisms would need to be studied in order to elucidate the biodistribution of inhaled drugs and particles within lung tissue. Simplistic study designs that estimate drug concentrations in lung homogenates would not suffice for this purpose [146].
Finally, it would be ideal for the purpose if the agent selected for induction of autophagy could be retained in the lung macrophages for long periods, with minimal ‘leakage’ to surrounding tissue or systemic circulation. Clofazimine, a second-line anti-TB agent, is known to accumulate within macrophages when given orally, so much so that 90% of the administered drug is sequestered in macrophages [147]. When administered to mice infected with Mtb as inhalable particles, the drug was able to efficiently kill bacteria in the lungs, but not in the spleen [148]. This observation indicates that inhaled drug did not reach the spleen through systemic circulation following inhalation. An autophagy-inducing molecule with analogous biopharmaceutics would be very useful for Track-II chemotherapy of TB.
4.5. Macrophage activation by phagocytosis
Fig. 4 shows transmission electron micrographs of a mouse alveolar macrophages infected with Mtb and administered inhalable particles containing isoniazid and rifampicin [149]. Although autophagy induction was not investigated in these experiments, intense Golgi activity is repeatedly observed in macrophages when they take up inhaled particles, even if these are comprised of the innocuous poly(lactide) polymer used to make resorbable surgical sutures [149–153]. Fusion of the Mtb phagosome with lysosomes is also induced in the presence of intracellular particles [127].
Induction of autophagy by internalized poly(acrylamide) microspheres was demonstrated 30 years ago [154]. Using particles prepared from an acrylate polymer, Eidi et al. observed induction of autophagy and loss of viability in a rat macrophage cell line exposed to 15–100 μg/ml concentrations [155]. Although these particles were smaller in size and chemically distinct from the biodegradable particles reported for pulmonary delivery in TB, the potential of inducing autophagy as a consequence of particle phagocytosis is highlighted by these observations. A variety of mechanisms is likely to be implicated in induction of autophagy by phagocytosed particles, including induction of the oxidative burst, matrix remodeling, autocrine signaling, mitochondrial stress, etc. Thus, induction of macrophage autophagy by the drug carrier can confound results of experiments designed to study effects of the drug payload. Due attention would be required to dissecting out these effects during non-clinical and clinical testing.
5. Conclusions and recommendations
Targeting alveolar macrophages, especially those harboring Mtb, with drugs that induce autophagy has the potential to provide the means for clinical intervention in TB wherein host-directed therapies are deployed. Inhaled particles represent a demonstrably feasible modality to achieve such targeting. Drug discovery efforts aimed at host molecules responsible for inhibiting autophagy could add to the list of the drugs that can be tested for use in TB as well as other diseases. Known and novel molecules that induce macrophage autophagy require validation of their efficacy in TB; alone and/or in combination with classical anti-TB agents. Several critical issues relating to drug testing and development need attention if translation efforts are undertaken to develop inhaled particles that induce autophagy in Mtb-infected macrophages. These include an appreciation of dosimetry and dose-finding with reference to macrophage targeting on the one hand, and drug concentrations developed in systemic circulation on the other. Equally importantly, serendipitous induction of autophagy by drug carrying particles themselves needs to be identified if such a mechanism is indeed operative.
Acknowledgments
Funded by BSC 0112 grant and a Senior Research Fellowship to AG from the Council of Scientific and Industrial Research, this is CDRI Communication Number 9172. AG, AM and VD received travel and hospitality funds to present some of the information incorporated herein at the Second International Meeting on Inhaled Therapies for Tuberculosis, Tokyo, Oct. 1–3, 2013.
Footnotes
This review is part of the Advanced Drug Delivery Reviews theme issue on “antituberculosis_immunotherapeutics”.
References
- 1.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 2.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castillo EF, Dekonenko A, Arko-Mensah J, Mandell MA, Dupont N, Jiang S, Delgado-Vargas M, Timmins GS, Bhattacharya D, Yang H, Hutt J, Lyons CR, Dobos KM, Deretic V. Autophagy protects against active tuberculosis by suppressing bacterial burden and inflammation. Proc Natl Acad Sci U S A. 2012;109:E3168–E3176. doi: 10.1073/pnas.1210500109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rekha RS, Rao Muvva SS, Wan M, Raqib R, Bergman P, Brighenti S, Gudmundsson GH, Agerberth B. Phenylbutyrate induces LL-37-dependent autophagy and intracellular killing of Mycobacterium tuberculosis in human macrophages. Autophagy. 2015;11:1688–1699. doi: 10.1080/15548627.2015.1075110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris J, De Haro SA, Master SS, Keane J, Roberts EA, Delgado M, Deretic V. T helper 2 cytokines inhibit autophagic control of intracellular Mycobacterium tuberculosis. Immunity. 2007;27:505–517. doi: 10.1016/j.immuni.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Ponpuak M, Davis AS, Roberts EA, Delgado MA, Dinkins C, Zhao Z, Virgin HWt, Kyei GB, Johansen T, Vergne I, Deretic V. Delivery of cytosolic components by autophagic adaptor protein p62 endows autophagosomes with unique antimicrobial properties. Immunity. 2010;32:329–341. doi: 10.1016/j.immuni.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SB, Ornatowski W, Vergne I, Naylor J, Delgado M, Roberts E, Ponpuak M, Master S, Pilli M, White E, Komatsu M, Deretic V. Human IRGM regulates autophagy and cell-autonomous immunity functions through mitochondria. Nat Cell Biol. 2010;12:1154–1165. doi: 10.1038/ncb2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, Lammas DA. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol. 2008;9:35. doi: 10.1186/1471-2172-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghadimi D, de Vrese M, Heller KJ, Schrezenmeir J. Lactic acid bacteria enhance autophagic ability of mononuclear phagocytes by increasing Th1 autophagy-promoting cytokine (IFN-gamma) and nitric oxide (NO) levels and reducing Th2 autophagy-restraining cytokines (IL-4 and IL-13) in response to Mycobacterium tuberculosis antigen. Int Immunopharmacol. 2010;10:694–706. doi: 10.1016/j.intimp.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D, Nath L, Kamal MA, Varshney A, Jain A, Singh S, Rao KV. Genome-wide analysis of the host intracellular network that regulates survival of Mycobacterium tuberculosis. Cell. 2010;140:731–743. doi: 10.1016/j.cell.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Petruccioli E, Romagnoli A, Corazzari M, Coccia EM, Butera O, Delogu G, Piacentini M, Girardi E, Fimia GM, Goletti D. Specific T cells restore the autophagic flux inhibited by Mycobacterium tuberculosis in human primary macrophages. J Infect Dis. 2012;205:1425–1435. doi: 10.1093/infdis/jis226. [DOI] [PubMed] [Google Scholar]
- 13.Juarez E, Carranza C, Hernandez-Sanchez F, Leon-Contreras JC, Hernandez-Pando R, Escobedo D, Torres M, Sada E. NOD2 enhances the innate response of alveolar macrophages to Mycobacterium tuberculosis in humans. Eur J Immunol. 2012;42:880–889. doi: 10.1002/eji.201142105. [DOI] [PubMed] [Google Scholar]
- 14.Zullo AJ, Lee S. Old antibiotics target TB with a new trick. Cell Host Microbe. 2012;11:419–420. doi: 10.1016/j.chom.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell GR, Spector SA. Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy. 2012;8:1523–1525. doi: 10.4161/auto.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anandaiah A, Sinha S, Bole M, Sharma SK, Kumar N, Luthra K, Li X, Zhou X, Nelson B, Han X, Tachado SD, Patel NR, Koziel H. Vitamin D rescues impaired Mycobacterium tuberculosis-mediated tumor necrosis factor release in macrophages of HIV-seropositive individuals through an enhanced Toll-like receptor signaling pathway in vitro. Infect Immun. 2013;81:2–10. doi: 10.1128/IAI.00666-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klug-Micu GM, Stenger S, Sommer A, Liu PT, Krutzik SR, Modlin RL, Fabri M. CD40 ligand and interferon-gamma induce an antimicrobial response against Mycobacterium tuberculosis in human monocytes. Immunology. 2013;139:121–128. doi: 10.1111/imm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabri M, Realegeno SE, Jo EK, Modlin RL. Role of autophagy in the host response to microbial infection and potential for therapy. Curr Opin Immunol. 2011;23:65–70. doi: 10.1016/j.coi.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zugel U, Cheng G, Jo EK, Bloom BR, Modlin RL. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watson RO, Manzanillo PS, Cox JS. Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell. 2012;150:803–815. doi: 10.1016/j.cell.2012.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Jagannath C, Liu XD, Sharafkhaneh A, Kolodziejska KE, Eissa NT. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity. 2007;27:135–144. doi: 10.1016/j.immuni.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagannath C, Lindsey DR, Dhandayuthapani S, Xu Y, Hunter RL, Jr, Eissa NT. Autophagy enhances the efficacy of BCG vaccine by increasing peptide presentation in mouse dendritic cells. Nat Med. 2009;15:267–276. doi: 10.1038/nm.1928. [DOI] [PubMed] [Google Scholar]
- 26.Bradfute SB, Castillo EF, Arko-Mensah J, Chauhan S, Jiang S, Mandell M, Deretic V. Autophagy as an immune effector against tuberculosis. Curr Opin Microbiol. 2013;16:355–365. doi: 10.1016/j.mib.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deretic V. Autophagy, an immunologic magic bullet: Mycobacterium tuberculosis phagosome maturation block and how to bypass it. Future Microbiol. 2008;3:517–524. doi: 10.2217/17460913.3.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goletti D, Petruccioli E, Romagnoli A, Piacentini M, Fimia GM. Autophagy in Mycobacterium tuberculosis infection: a passepartout to flush the intruder out? Cytokine Growth Factor Rev. 2013;24:335–343. doi: 10.1016/j.cytogfr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Jo EK. Autophagy as an innate defense against mycobacteria. Pathog Dis. 2013;67:108–118. doi: 10.1111/2049-632X.12023. [DOI] [PubMed] [Google Scholar]
- 30.Harris J, Hope JC, Lavelle EC. Autophagy and the immune response to TB. Transbound Emerg Dis. 2009;56:248–254. doi: 10.1111/j.1865-1682.2009.01069.x. [DOI] [PubMed] [Google Scholar]
- 31.Floto RA, Sarkar S, Perlstein EO, Kampmann B, Schreiber SL, Rubinsztein DC. Small molecule enhancers of rapamycin-induced TOR inhibition promote autophagy, reduce toxicity in Huntington’s disease models and enhance killing of mycobacteria by macrophages. Autophagy. 2007;3:620–622. doi: 10.4161/auto.4898. [DOI] [PubMed] [Google Scholar]
- 32.Ni Cheallaigh C, Keane J, Lavelle EC, Hope JC, Harris J. Autophagy in the immune response to tuberculosis: clinical perspectives. Clin Exp Immunol. 2011;164:291–300. doi: 10.1111/j.1365-2249.2011.04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta A, Pant G, Mitra K, Madan J, Chourasia M, Misra A. Inhalable particles containing rapamycin for induction of autophagy in macrophages infected with Mycobacterium tuberculosis. Mol Pharm. 2014;11:1201–1207. doi: 10.1021/mp4006563. [DOI] [PubMed] [Google Scholar]
- 34.Park IH, Yeum CE, Chae GT, Lee SB. Effect of rifampicin to inhibit rapamycin-induced autophagy via the suppression of protein phosphatase 2A activity. Immunopharmacol Immunotoxicol. 2008;30:837–849. doi: 10.1080/08923970802135732. [DOI] [PubMed] [Google Scholar]
- 35.Fleming A, Noda T, Yoshimori T, Rubinsztein DC. Chemical modulators of autophagy as biological probes and potential therapeutics. Nat Chem Biol. 2011;7:9–17. doi: 10.1038/nchembio.500. [DOI] [PubMed] [Google Scholar]
- 36.Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, Fourie PB, Colombo P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis. 2011;91:71–81. doi: 10.1016/j.tube.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Smith LM, May RC. Mechanisms of microbial escape from phagocyte killing. Biochem Soc Trans. 2013;41:475–490. doi: 10.1042/BST20130014. [DOI] [PubMed] [Google Scholar]
- 38.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 39.Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K, Mollenkopf H, Kaufmann SH. Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol. 2006;36:631–647. doi: 10.1002/eji.200535496. [DOI] [PubMed] [Google Scholar]
- 40.Killick KE, Ni Cheallaigh C, O’Farrelly C, Hokamp K, MacHugh DE, Harris J. Receptor-mediated recognition of mycobacterial pathogens. Cell Microbiol. 2013;15:1484–1495. doi: 10.1111/cmi.12161. [DOI] [PubMed] [Google Scholar]
- 41.Koch R. A further communication on a remedy for tuberculosis. Br Med J. 1891;1:125–127. doi: 10.1136/bmj.1.1568.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fitzwalter BE, Thorburn A. Recent insights into cell death and autophagy. FEBS J. 2015;282:4279–4288. doi: 10.1111/febs.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujita N, Itoh T, Omori H, Fukuda M, Noda T, Yoshimori T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol Biol Cell. 2008;19:2092–2100. doi: 10.1091/mbc.E07-12-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang C, Lee JS, Inn KS, Gack MU, Li Q, Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C, Jung JU. Beclin1-binding UVRAG targets the class C Vps complex to coordinate autophagosome maturation and endocytic trafficking. Nat Cell Biol. 2008;10:776–787. doi: 10.1038/ncb1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamasaki M, Shibutani ST, Yoshimori T. Up-to-date membrane biogenesis in the autophagosome formation. Curr Opin Cell Biol. 2013;25:455–460. doi: 10.1016/j.ceb.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Itakura E, Kishi-Itakura C, Mizushima N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell. 2012;151:1256–1269. doi: 10.1016/j.cell.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh V, Jamwal S, Jain R, Verma P, Gokhale R, Rao KV. Mycobacterium tuberculosis-driven targeted recalibration of macrophage lipid homeostasis promotes the foamy phenotype. Cell Host Microbe. 2012;12:669–681. doi: 10.1016/j.chom.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Fujimoto T, Ohsaki Y. Cytoplasmic lipid droplets: rediscovery of an old structure as a unique platform. Ann N Y Acad Sci. 2006;1086:104–115. doi: 10.1196/annals.1377.010. [DOI] [PubMed] [Google Scholar]
- 50.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velikkakath AK, Nishimura T, Oita E, Ishihara N, Mizushima N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol Biol Cell. 2012;23:896–909. doi: 10.1091/mbc.E11-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ganaie AA, Lella RK, Solanki R, Sharma C. Thermostable hexameric form of Eis (Rv2416c) protein of M. tuberculosis plays an important role for enhanced intracellular survival within macrophages. PLoS One. 2011;6:e27590. doi: 10.1371/journal.pone.0027590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shui W, Petzold CJ, Redding A, Liu J, Pitcher A, Sheu L, Hsieh TY, Keasling JD, Bertozzi CR. Organelle membrane proteomics reveals differential influence of mycobacterial lipoglycans on macrophage phagosome maturation and autophagosome accumulation. J Proteome Res. 2011;10:339–348. doi: 10.1021/pr100688h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romagnoli A, Etna MP, Giacomini E, Pardini M, Remoli ME, Corazzari M, Falasca L, Goletti D, Gafa V, Simeone R, Delogu G, Piacentini M, Brosch R, Fimia GM, Coccia EM. ESX-1 dependent impairment of autophagic flux by Mycobacterium tuberculosis in human dendritic cells. Autophagy. 2012;8:1357–1370. doi: 10.4161/auto.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang D, de Souza RF, Anantharaman V, Iyer LM, Aravind L. Polymorphic toxin systems: comprehensive characterization of trafficking modes, processing, mechanisms of action, immunity and ecology using comparative genomics. Biol Direct. 2012;7:18. doi: 10.1186/1745-6150-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation, and immunity. Nat Rev Immunol. 2013 doi: 10.1038/nri3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Levine B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell. 2005;120:159–162. doi: 10.1016/j.cell.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 58.Henault J, Martinez J, Riggs JM, Tian J, Mehta P, Clarke L, Sasai M, Latz E, Brinkmann MM, Iwasaki A, Coyle AJ, Kolbeck R, Green DR, Sanjuan MA. Noncanonical autophagy is required for type I interferon secretion in response to DNA-immune complexes. Immunity. 2012;37:986–997. doi: 10.1016/j.immuni.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanjuan MA, Dillon CP, Tait SW, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- 60.Cemma M, Brumell JH. Interactions of pathogenic bacteria with autophagy systems. Curr Biol. 2012;22:R540–R545. doi: 10.1016/j.cub.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 61.Klionsky DJ, Eskelinen EL, Deretic V. Autophagosomes, phagosomes, autolysosomes, phagolysosomes, autophagolysosomes… Wait, I’m confused. Autophagy. 2014;10:549–551. doi: 10.4161/auto.28448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deretic V. Autophagy in immunity and cell-autonomous defense against intracellular microbes. Immunol Rev. 2011;240:92–104. doi: 10.1111/j.1600-065X.2010.00995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ponpuak M, Deretic V. Autophagy and p62/sequestosome 1 generate neo-antimicrobial peptides (cryptides) from cytosolic proteins. Autophagy. 2011;7:336–337. doi: 10.4161/auto.7.3.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Copley SD. Moonlighting is mainstream: paradigm adjustment required. BioEssays. 2012;34:578–588. doi: 10.1002/bies.201100191. [DOI] [PubMed] [Google Scholar]
- 65.Tal MC, Iwasaki A. Autophagic control of RLR signaling. Autophagy. 2009;5:749–750. doi: 10.4161/auto.5.5.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yu X, Li C, Hong W, Pan W, Xie J. Autophagy during Mycobacterium tuberculosis infection and implications for future tuberculosis medications. Cell Signal. 2013;25:1272–1278. doi: 10.1016/j.cellsig.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Lorenzi PL, Claerhout S, Mills GB, Weinstein JN. A curated census of autophagy-modulating proteins and small molecules: candidate targets for cancer therapy. Autophagy. 2014;10 doi: 10.4161/auto.28773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner D, Maser J, Lai B, Cai Z, Barry CE, Höner zu Bentrup K, Russell DG, Bermudez LE. Elemental analysis of Mycobacterium avium-, Mycobacterium tuberculosis-, and Mycobacterium smegmatis-containing phagosomes indicates pathogen-induced microenvironments within the host cell’s endosomal system. J Immunol. 2005;174:1491–1500. doi: 10.4049/jimmunol.174.3.1491. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Guzman C, Vargas MH, Quinonez F, Bazavilvazo N, Aguilar A. A cholesterol-rich diet accelerates bacteriologic sterilization in pulmonary tuberculosis. Chest. 2005;127:643–651. doi: 10.1378/chest.127.2.643. [DOI] [PubMed] [Google Scholar]
- 70.Bhuyan UN, Ramalingaswami V. Systemic macrophage mobilization and granulomatous response to BCG in the protein-deficient rabbit. Am J Pathol. 1974;76:313–322. [PMC free article] [PubMed] [Google Scholar]
- 71.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vergne I, Roberts E, Elmaoued RA, Tosch V, Delgado MA, Proikas-Cezanne T, Laporte J, Deretic V. Control of autophagy initiation by phosphoinositide 3-phosphatase jumpy. EMBO J. 2009;28:2244–2258. doi: 10.1038/emboj.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nabatov AA, Hatzis P, Rouschop KM, van Diest P, Vooijs M. Hypoxia inducible NOD2 interacts with 3-O-sulfogalactoceramide and regulates vesicular homeostasis. Biochim Biophys Acta. 2013;1830:5277–5286. doi: 10.1016/j.bbagen.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 74.Shin DM, Jeon BY, Lee HM, Jin HS, Yuk JM, Song CH, Lee SH, Lee ZW, Cho SN, Kim JM, Friedman RL, Jo EK. Mycobacterium tuberculosis Eis regulates autophagy, inflammation, and cell death through redox-dependent signaling. PLoS Pathog. 2010;6:e1001230. doi: 10.1371/journal.ppat.1001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim KH, An DR, Song J, Yoon JY, Kim HS, Yoon HJ, Im HN, Kim J, Kim do J, Lee SJ, Lee HM, Kim HJ, Jo EK, Lee JY, Suh SW. Mycobacterium tuberculosis Eis protein initiates suppression of host immune responses by acetylation of DUSP16/MKP-7. Proc Natl Acad Sci U S A. 2012;109:7729–7734. doi: 10.1073/pnas.1120251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011;286:18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Campbell GR, Spector SA. Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog. 2012;8:e1002689. doi: 10.1371/journal.ppat.1002689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0 s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA, Park JH, Nunez G, Schlesinger LS. NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol. 2011;13:402–418. doi: 10.1111/j.1462-5822.2010.01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dall’Armi C, Devereaux KA, Di Paolo G. The role of lipids in the control of autophagy. Curr Biol. 2013;23:R33–R45. doi: 10.1016/j.cub.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw SY, Tran K, Castoreno AB, Peloquin JM, Lassen KG, Khor B, Aldrich LN, Tan PH, Graham DB, Kuballa P, Goel G, Daly MJ, Shamji AF, Schreiber SL, Xavier RJ. Selective modulation of autophagy, innate immunity, and adaptive immunity by small molecules. ACS Chem Biol. 2013;8:2724–2733. doi: 10.1021/cb400352d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333:454–464. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu Q, Yang X, Lin L, Li S, Li Q, Zhong S, Peng J, Cui Z. Genetic ablation of solute carrier family 7a3a leads to hepatic steatosis in zebrafish during fasting. Hepatology. 2014;60:1929–1941. doi: 10.1002/hep.27356. [DOI] [PubMed] [Google Scholar]
- 84.Bento CF, Empadinhas N, Mendes V. Autophagy in the fight against tuberculosis. DNA Cell Biol. 2015;34:228–242. doi: 10.1089/dna.2014.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Law BYK, Chan WK, Xu SW, Wang JR, Bai LP, Liu L, Wong VKW. Natural small-molecule enhancers of autophagy induce autophagic cell death in apoptosis-defective cells. Sci Rep. 2014;4 doi: 10.1038/srep05510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahn M-Y, Ahn S-G, Yoon J-H. Apicidin, a histone deaceylase inhibitor, induces both apoptosis and autophagy in human oral squamous carcinoma cells. Oral Oncol. 2011;47:1032–1038. doi: 10.1016/j.oraloncology.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 87.Ahn MY, Kwon SM, Yoon HE, Ahn JW, Lee J, Yoon JH. IGF-1R inhibition by apicidin induces apoptosis and autophagy in salivary mucoepidermoid carcinoma cells. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;3:e163. [Google Scholar]
- 88.Stellrecht CM, Vangapandu HV, Le XF, Mao W, Shentu S. ATP directed agent, 8-chloro-adenosine, induces AMP activated protein kinase activity, leading to autophagic cell death in breast cancer cells. J Hematol Oncol. 2014;7:23. doi: 10.1186/1756-8722-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shaw SY, Tran K, Castoreno AB, Peloquin JM, Lassen KG, Khor B, Aldrich LN, Tan PH, Graham DB, Kuballa P. Selective modulation of autophagy, innate immunity, and adaptive immunity by small molecules. ACS Chem Biol. 2013;8:2724–2733. doi: 10.1021/cb400352d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shen S, Zhang Y, Wang Z, Zhang R, Gong X. Bufalin induces the interplay between apoptosis and autophagy in glioma cells through endoplasmic reticulum stress. Int J Biol Sci. 2014;10:212. doi: 10.7150/ijbs.8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wen L, Huang Y, Xie X, Huang W, Yin J, Lin W, Jia Q, Zeng W. Anti-inflammatory and antinociceptive activities of bufalin in rodents. Mediat Inflamm. 2014;2014 doi: 10.1155/2014/171839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shin SY, Lee KS, Choi YK, Lim HJ, Lee HG, Lim Y, Lee YH. The antipsychotic agent chlorpromazine induces autophagic cell death by inhibiting the Akt/mTOR pathway in human U-87MG glioma cells. Carcinogenesis. 2013;34:2080–2089. doi: 10.1093/carcin/bgt169. [DOI] [PubMed] [Google Scholar]
- 93.He L, Liao S-Y, Tan C-P, Lu Y-Y, Xu C-X, Ji L-N, Mao Z-W. Cyclometalated iridium (iii)–β-carboline complexes as potent autophagy-inducing agents. Chem Commun. 2014;50:5611–5614. doi: 10.1039/c4cc01461h. [DOI] [PubMed] [Google Scholar]
- 94.Chang CP, Yang MC, Liu HS, Lin YS, Lei HY. Concanavalin A induces autophagy in hepatoma cells and has a therapeutic effect in a murine in situ hepatoma model. Hepatology. 2007;45:286–296. doi: 10.1002/hep.21509. [DOI] [PubMed] [Google Scholar]
- 95.Roy B, Pattanaik AK, Das J, Bhutia SK, Behera B, Singh P, Maiti TK. Role of PI3K/Akt/mTOR and MEK/ERK pathway in Concanavalin A induced autophagy in HeLa cells. Chem Biol Interact. 2014;210:96–102. doi: 10.1016/j.cbi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y, Qiu Q, Shen J-J, Li D-D, Jiang X-J, Si S-Y, Shao R-G, Wang Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int J Biochem Cell Biol. 2012;44:1813–1824. doi: 10.1016/j.biocel.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 97.Bruning A, Brem GJ, Vogel M, Mylonas I. Tetracyclines cause cell stress-dependent ATF4 activation and mTOR inhibition. Exp Cell Res. 2014;320:281–289. doi: 10.1016/j.yexcr.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 98.Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G, Feng Y. Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br J Pharmacol. 2011;164:731–742. doi: 10.1111/j.1476-5381.2011.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tsai KL, Huang PH, Kao CL, Leu HB, Cheng YH, Liao YW, Yang YP, Chien Y, Wang CY, Hsiao CY, Chiou SH, Chen JW, Lin SJ. Aspirin attenuates vinorelbine-induced endothelial inflammation via modulating SIRT1/AMPK axis. Biochem Pharmacol. 2014;88:189–200. doi: 10.1016/j.bcp.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 100.Kim A, Kang K, Kim H, Kim D, Choi Y, Lee S, Hyun J. A ginseng metabolite, compound K, induces autophagy and apoptosis via generation of reactive oxygen species and activation of JNK in human colon cancer cells. Cell Death Dis. 2013;4:e750. doi: 10.1038/cddis.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ueda J-y, Athikomkulchai S, Miyatake R, Saiki I, Esumi H, Awale S. (+)-Grandifloracin, an antiausterity agent, induces autophagic PANC-1 pancreatic cancer cell death. Drug Des Devel Ther. 2014;8:39. doi: 10.2147/DDDT.S52168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bahrami F, Pourgholami MH, Mekkawy AH, Rufener L, Morris DL. Monepantel induces autophagy in human ovarian cancer cells through disruption of the mTOR/p70S6K signalling pathway. Am J Cancer Res. 2014;4:558. [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou H, Shen T, Shang C, Luo Y, Liu L, Yan J, Li Y, Huang S. Ciclopirox induces autophagy through reactive oxygen species-mediated activation of JNK signaling pathway. Oncotarget. 2014;5:10140. doi: 10.18632/oncotarget.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JJ, Lee HM, Shin DM, Kim W, Yuk JM, Jin HS, Lee SH, Cha GH, Kim JM, Lee ZW, Shin SJ, Yoo H, Park YK, Park JB, Chung J, Yoshimori T, Jo EK. Host cell autophagy activated by antibiotics is required for their effective antimycobacterial drug action. Cell Host Microbe. 2012;11:457–468. doi: 10.1016/j.chom.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 105.Lauterbach EC. Neuroprotective effects of psychotropic drugs in Huntington’s disease. Int J Mol Sci. 2013;14:22558–22603. doi: 10.3390/ijms141122558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Williams A, Sarkar S, Cuddon P, Ttofi EK, Saiki S, Siddiqi FH, Jahreiss L, Fleming A, Pask D, Goldsmith P, O’Kane CJ, Floto RA, Rubinsztein DC. Novel targets for Huntington’s disease in an mTOR-independent autophagy pathway. Nat Chem Biol. 2008;4:295–305. doi: 10.1038/nchembio.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hechler B, Magnenat S, Zighetti ML, Kassack MU, Ullmann H, Cazenave J-P, Evans R, Cattaneo M, Gachet C. Inhibition of platelet functions and thrombosis through selective or nonselective inhibition of the platelet P2 receptors with increasing doses of NF449 [4, 4′, 4″, 4‴-(carbonylbis (imino-5, 1, 3-benzenetriylbis-(carbonylimino))) tetrakis-benzene-1, 3-disulfonic acid octasodium salt] J Pharmacol Exp Ther. 2005;314:232–243. doi: 10.1124/jpet.105.084673. [DOI] [PubMed] [Google Scholar]
- 108.Lam KK, Zheng X, Forestieri R, Balgi AD, Nodwell M, Vollett S, Anderson HJ, Andersen RJ, Av-Gay Y, Roberge M. Nitazoxanide stimulates autophagy and inhibits mTORC1 signaling and intracellular proliferation of Mycobacterium tuberculosis. PLoS Pathog. 2012;8:e1002691. doi: 10.1371/journal.ppat.1002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yu H-C, Lin C-S, Tai W-T, Liu C-Y, Shiau C-W, Chen K-F. Nilotinib induces autophagy in hepatocellular carcinoma through AMPK activation. J Biol Chem. 2013;288:18249–18259. doi: 10.1074/jbc.M112.446385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zughaier S, Karna P, Stephens D, Aneja R. Potent anti-inflammatory activity of novel microtubule-modulating brominated noscapine analogs. PLoS One. 2010;5:e9165. doi: 10.1371/journal.pone.0009165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang L, Yu J, Pan H, Hu P, Hao Y, Cai W, Zhu H, Albert DY, Xie X, Ma D. Small molecule regulators of autophagy identified by an image-based high-throughput screen. Proc Natl Acad Sci. 2007;104:19023–19028. doi: 10.1073/pnas.0709695104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hao W, Zhang X, Zhao W, Chen X. Psoralidin induces autophagy through ROS generation which inhibits the proliferation of human lung cancer A549 cells. Peer J. 2014;2:e555. doi: 10.7717/peerj.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schmukler E, Grinboim E, Schokoroy S, Amir A, Wolfson E, Kloog Y, Pinkas-Kramarski R. Ras inhibition enhances autophagy, which partially protects cells from death. Oncotarget. 2013;4:142. doi: 10.18632/oncotarget.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsimberidou AM, Rudek MA, Hong D, Ng CS, Blair J, Goldsweig H, Kurzrock R. Phase 1 first-in-human clinical study of S-trans, trans-farnesylthiosalicylic acid (salirasib) in patients with solid tumors. Cancer Chemother Pharmacol. 2010;65:235–241. doi: 10.1007/s00280-009-1027-4. [DOI] [PubMed] [Google Scholar]
- 115.Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, Maglathlin RL, Webster JA, Lewis TA, O’Kane CJ, Schreiber SL. Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol. 2007;3:331–338. doi: 10.1038/nchembio883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chiao M-T, Cheng W-Y, Yang Y-C, Shen C-C, Ko J-L. Suberoylanilide hydroxamic acid (SAHA) causes tumor growth slowdown and triggers autophagy in glioblastoma stem cells. Autophagy. 2013;9:1509–1526. doi: 10.4161/auto.25664. [DOI] [PubMed] [Google Scholar]
- 117.Liikanen I, Ahtiainen L, Hirvinen ML, Bramante S, Cerullo V, Nokisalmi P, Hemminki O, Diaconu I, Pesonen S, Koski A. Oncolytic adenovirus with temozolomide induces autophagy and antitumor immune responses in cancer patients. Mol Ther. 2013;21:1212–1223. doi: 10.1038/mt.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zou Y, Wang Q, Li B, Xie B, Wang W. Temozolomide induces autophagy via ATM-AMPK-ULK1 pathways in glioma. Mol Med Rep. 2014;10:411–416. doi: 10.3892/mmr.2014.2151. [DOI] [PubMed] [Google Scholar]
- 119.Wang H, Liu T, Li L, Wang Q, Yu C, Liu X, Li W. Tetrandrine is a potent cell autophagy agonist via activated intracellular reactive oxygen species. Cell Biosci. 2015;5:4. doi: 10.1186/2045-3701-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng H, Liang Y, Kuo Y, Chuu C, Lin C, Lee M, Wu A, Yeh C, Chen EI, Whang-Peng J. Identification of thioridazine, an antipsychotic drug, as an antiglioblastoma and anticancer stem cell agent using public gene expression data. Cell Death Dis. 2015;6:e1753. doi: 10.1038/cddis.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niso-Santano M, Malik SA, Pietrocola F, Bravo-San Pedro JM, Mariño G, Cianfanelli V, Ben-Younès A, Troncoso R, Markaki M, Sica V. Unsaturated fatty acids induce non-canonical autophagy. EMBO J. 2015;34:1025–1041. doi: 10.15252/embj.201489363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shintani T, Klionsky DJ. Autophagy in health and disease: a double-edged sword. Science (New York, NY) 2004;306:990–995. doi: 10.1126/science.1099993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sachan M, Srivastava A, Ranjan R, Gupta A, Pandya S, Misra A. Opportunities and Challenges for Host-Directed Therapies in Tuberculosis. Curr Pharm Des. 2016;22 doi: 10.2174/1381612822666160128150636. (in press) [DOI] [PubMed] [Google Scholar]
- 124.Shaw SY, Tran K, Castoreno AB, Peloquin JM, Lassen KG, Khor B, Aldrich LN, Tan PH, Graham DB, Kuballa P, Goel G, Daly MJ, Shamji AF, Schreiber SL, Xavier RJ. Selective modulation of autophagy, innate immunity, and adaptive immunity by small molecules. ACS Chem Biol. 2013;8:2724–2733. doi: 10.1021/cb400352d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hasleton PS. The internal surface area of the adult human lung. J Anat. 1972;112:391–400. [PMC free article] [PubMed] [Google Scholar]
- 126.Sethi T, Agrawal A. Structure and function of the tuberculous lung: considerations for inhaled therapies. Tuberculosis. 2011;91:67–70. doi: 10.1016/j.tube.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 127.Verma RK, Agrawal AK, Singh AK, Mohan M, Gupta A, Gupta P, Gupta UD, Misra A. Inhalable microparticles of nitric oxide donors induce phagosome maturation and kill Mycobacterium tuberculosis. Tuberculosis (Edinb) 2013;93:412–417. doi: 10.1016/j.tube.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 128.Verma RK, Singh AK, Mohan M, Agrawal AK, Verma PR, Gupta A, Misra A. Inhalable microparticles containing nitric oxide donors: saying NO to intracellular Mycobacterium tuberculosis. Mol Pharm. 2012;9:3183–3189. doi: 10.1021/mp300269g. [DOI] [PubMed] [Google Scholar]
- 129.Wallace WA, Gillooly M, Lamb D. Intra-alveolar macrophage numbers in current smokers and non-smokers: a morphometric study of tissue sections. Thorax. 1992;47:437–440. doi: 10.1136/thx.47.6.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kummar S, Rubinstein L, Kinders R, Parchment RE, Gutierrez ME, Murgo AJ, Ji J, Mroczkowski B, Pickeral OK, Simpson M, Hollingshead M, Yang SX, Helman L, Wiltrout R, Collins J, Tomaszewski JE, Doroshow JH. Phase 0 clinical trials: conceptions and misconceptions. Cancer J. 2008;14:133–137. doi: 10.1097/PPO.0b013e318172d6f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Labiris NR, Dolovich MB. Pulmonary drug delivery. Part I: physiological factors affecting therapeutic effectiveness of aerosolized medications. Br J Clin Pharmacol. 2003;56:588–599. doi: 10.1046/j.1365-2125.2003.01892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loira-Pastoriza C, Todoroff J, Vanbever R. Delivery strategies for sustained drug release in the lungs. Adv Drug Deliv Rev. 2014;75:81–91. doi: 10.1016/j.addr.2014.05.017. [DOI] [PubMed] [Google Scholar]
- 133.Oh YK, Swanson JA. Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol. 1996;132:585–593. doi: 10.1083/jcb.132.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Misra A, Hickey AJ, Rossi C, Borchard G, Terada H, Makino K, Fourie PB, Colombo P. Inhaled drug therapy for treatment of tuberculosis. Tuberculosis (Edinb) 2011;91:71–81. doi: 10.1016/j.tube.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 135.Hickey AJ, Misra A, Fourie PB. Dry powder antibiotic aerosol product development: inhaled therapy for tuberculosis. J Pharm Sci. 2013;102:3900–3907. doi: 10.1002/jps.23705. [DOI] [PubMed] [Google Scholar]
- 136.Kaur J, Muttil P, Verma RK, Kumar K, Yadav AB, Sharma R, Misra A. A hand-held apparatus for “nose-only” exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. Eur J Pharm Sci. 2008;34:56–65. doi: 10.1016/j.ejps.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 137.Müller NL, Tomás F, Lee KS. Pulmonary Tuberculosis. In: Silva C, editor. Imaging of Pulmonary Infections. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 56–78. [Google Scholar]
- 138.Hoppentocht M, Hoste C, Hagedoorn P, Frijlink HW, de Boer AH. In vitro evaluation of the DP-4M PennCentury™ insufflator. Eur J Pharm Biopharm. 2014 doi: 10.1016/j.ejpb.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 139.Hoppentocht M, Hagedoorn P, Frijlink HW, de Boer AH. Technological and practical challenges of dry powder inhalers and formulations. Adv Drug Deliv Rev. 2014 doi: 10.1016/j.addr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 140.Doan TV, Gregoire N, Lamarche I, Gobin P, Marchand S, Couet W, Olivier JC. A preclinical pharmacokinetic modeling approach to the biopharmaceutical characterization of immediate and microsphere-based sustained release pulmonary formulations of rifampicin. Eur J Pharm Sci. 2013;48:223–230. doi: 10.1016/j.ejps.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 141.Verma RK, Mukker JK, Singh RS, Kumar K, Verma PR, Misra A. Partial biodistribution and pharmacokinetics of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to rhesus macaques. Mol Pharm. 2012;9:1011–1016. doi: 10.1021/mp300043f. [DOI] [PubMed] [Google Scholar]
- 142.Verma RK, Kaur J, Kumar K, Yadav AB, Misra A. Intracellular time course, pharmacokinetics, and biodistribution of isoniazid and rifabutin following pulmonary delivery of inhalable microparticles to mice. Antimicrob Agents Chemother. 2008;52:3195–3201. doi: 10.1128/AAC.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, de Chastellier C, Altare F. Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wang D, Zhou W, Lu S, Wang Q, Feng Y, Zhu G, Li L, Song Y, Gao Q. Increased density of macrophage migration inhibitory factor (MIF) in tuberculosis granuloma. Exp Mol Pathol. 2012;93:207–212. doi: 10.1016/j.yexmp.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 145.Fenaroli F, Westmoreland D, Benjaminsen J, Kolstad T, Skjeldal FM, Meijer AH, van der Vaart M, Ulanova L, Roos N, Nystrom B, Hildahl J, Griffiths G. Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: direct visualization and treatment. ACS Nano. 2014 doi: 10.1021/nn5019126. [DOI] [PubMed] [Google Scholar]
- 146.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61:235–237. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- 147.Baik J, Stringer KA, Mane G, Rosania GR. Multiscale distribution and bioaccumulation analysis of clofazimine reveals a massive immune system-mediated xenobiotic sequestration response. Antimicrob Agents Chemother. 2013;57:1218–1230. doi: 10.1128/AAC.01731-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Verma RK, Germishuizen WA, Motheo MP, Agrawal AK, Singh AK, Mohan M, Gupta P, Gupta UD, Cholo M, Anderson R, Fourie PB, Misra A. Inhaled microparticles containing clofazimine are efficacious in treatment of experimental tuberculosis in mice. Antimicrob Agents Chemother. 2013;57:1050–1052. doi: 10.1128/AAC.01897-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharma R, Muttil P, Yadav AB, Rath SK, Bajpai VK, Mani U, Misra A. Uptake of inhalable microparticles affects defence responses of macrophages infected with Mycobacterium tuberculosis H37Ra. J Antimicrob Chemother. 2007;59:499–506. doi: 10.1093/jac/dkl533. [DOI] [PubMed] [Google Scholar]
- 150.Sharma R, Yadav AB, Muttil P, Kajal H, Misra A. Inhalable microparticles modify cytokine secretion by lung macrophages of infected mice. Tuberculosis (Edinb) 2011;91:107–110. doi: 10.1016/j.tube.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 151.Verma RK, Misra A. Regulation of cell death by intracellular delivery of nitric oxide to macrophages infected with virulent or avirulent Mycobacterium tuberculosis. Tuberculosis (Edinb) 2015;95:627–628. doi: 10.1016/j.tube.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 152.Yadav AB, Muttil P, Singh AK, Verma RK, Mohan M, Agrawal AK, Verma AS, Sinha SK, Misra A. Microparticles induce variable levels of activation in macrophages infected with Mycobacterium tuberculosis. Tuberculosis (Edinb) 2010;90:188–196. doi: 10.1016/j.tube.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 153.Yadav AB, Sharma R, Muttil P, Singh AK, Verma RK, Mohan M, Patel SK, Misra A. Inhalable microparticles containing isoniazid and rifabutin target macrophages and ‘stimulate the phagocyte’ to achieve high efficacy. Indian J Exp Biol. 2009;47:469–474. [PubMed] [Google Scholar]
- 154.Hamberg H, Edman P. Induced autophagocytosis in macrophages. Origin of the segregating membranes. Acta Pathol Microbiol Immunol Scand A. 1983;91:1–8. doi: 10.1111/j.1699-0463.1983.tb02719.x. [DOI] [PubMed] [Google Scholar]
- 155.Eidi H, Joubert O, Nemos C, Grandemange S, Mograbi B, Foliguet B, Tournebize J, Maincent P, Le Faou A, Aboukhamis I, Rihn BH. Drug delivery by polymeric nanoparticles induces autophagy in macrophages. Int J Pharm. 2012;422:495–503. doi: 10.1016/j.ijpharm.2011.11.020. [DOI] [PubMed] [Google Scholar]