Abstract
Neural stem cells (NSCs) are being investigated as a possible treatment for amyotrophic lateral sclerosis (ALS) through intraspinal transplantation, but no longitudinal imaging studies exist that describe the survival of engrafted cells over time. Allogeneic firefly luciferase-expressing murine NSCs (Luc+-NSCs) were transplanted bilaterally (100,000 cells/2 μl) into the cervical spinal cord parenchyma of pre-symptomatic (63 day-old) SOD1G93A transgenic ALS mice (n=14) and wild-type age-matched littermates (n=14). Six control ALS mice were injected with saline. Mice were immunosuppressed using a combination of tacrolimus+sirolimus (1 mg/kg each, i.p.) daily. Compared to non-transplanted SOD1G39A control mice, a transient improvement (p<0.05) in motor performance (rotarod test) was observed after NSC transplantation only at the early disease stage (weeks 2 and 3 post-transplantation). Compared to day one post-transplantation, there was a significant decline in bioluminescent imaging (BLI) signal in ALS mice at the time of disease onset (71.7±17.9 % at 4 weeks post-transplantation, p<0.05), with a complete loss of BLI signal at endpoint (120 day-old mice). In contrast, BLI signal intensity was observed in wild-type littermates throughout the entire study period, with only a 41.4±8.7% decline at endpoint. In ALS mice, poor cell survival was accompanied by accumulation of mature macrophages and the presence of astrogliosis and microgliosis. We conclude that the disease progression adversely affects the survival of engrafted murine Luc+-NSCs in SOD1G93A transgenic ALS mice as a result of the hostile ALS microenvironment, further emphasizing the challenges that face successful cell therapy of ALS.
Keywords: Neural stem cell, transplantation, amyotrophic lateral sclerosis, cell survival, bioluminescence imaging
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder that is characterized by the progressive loss of motor neurons. The motor neuron degeneration leads to paralysis, respiratory failure, and death within three to five years from disease onset (Chio et al., 2009; Chio et al., 2013). The pathomechanisms of motor neuron degeneration are poorly understood (Boillee et al., 2006), and there is only one FDA-approved drug that marginally extends the life expectancy of ALS patients by three to four months (Bensimon et al., 1994). A long duration between the onset of pathological changes and the manifestation of clinical symptoms in ALS complicates the management of this disease. Considering the complexity of the disease, the development of a therapy that can maintain or restore motor neuron function would provide the most comprehensive approach to treating ALS (Chen et al., 2016).
In the last decade, several preclinical studies showed promising outcomes using stem cells in ALS transgenic animal models (Teng et al., 2012; Xu et al., 2011). In 2009, the FDA approved the first safety clinical trial of direct intraspinal transplantation of human NSCs into patients with ALS (Glass et al., 2012; Riley et al., 2012). One of the major obstacles in successful stem cell therapy is the short-term survival of engrafted cells due to immune rejection (Barker and Widner, 2004). In ALS, several factors could lead to the rejection of transplanted stem cells. For example, disease progression in ALS is accompanied by a multi-phased immune response, and this plays an active role in shaping ALS pathology (Alexianu et al., 2001; Chiu et al., 2009; Yamanaka et al., 2008). An inevitable inflammation due to invasive cell transplantation surgery, and the resultant local trauma, could also influence the survival of engrafted therapeutic cells. In addition to the ability to determine long-term survival and the functionality of transplanted cells, real-time information on the accuracy of cell transplantation and adverse outcomes, such as teratoma formation and migration of cells to inappropriate locations, could also greatly affect the outcome of therapy. The current histological methods to analyze transplanted cells are highly invasive, require euthanasia of the animal, and limit our ability to monitor engrafted cells in real-time over an elongated period of time. In a phase 1/2 open-safety clinical trial, iron-labeled MSCs were injected and tracked in the central nervous system (CNS) by MRI in multiple sclerosis (MS) and ALS patients (Karussis et al., 2010). However, this approach is only suitable for short-term monitoring of immediate cell engraftment, and not for imaging cell survival (Srivastava and Bulte, 2014). Teng et al. (2012) showed the most potent therapeutic effect for a multi-level NSC transplantation approach to escalate cell dose, indicating the importance of administrating sufficient cell numbers into critically functioning spinal cord segments to combat ALS pathology. It is therefore pivotal to develop longitudinal imaging strategies to monitor donor cell fate for devising cell therapies for ALS.
In this study, we used two different imaging modalities, bioluminescence imaging (BLI) and computed tomography (CT), to non-invasively visualize the accuracy of cell transplantation and to longitudinally monitor the survival of intraspinally transplanted murine Luc+-NSCs in a transgenic SOD1G93A mouse model of ALS during disease progression.
Materials and Methods
Isolation of NSCs
Allogeneic Luc+-NSCs were isolated from the brain of a homozygous firefly luciferase transgenic mouse (FVB background, Jackson Laboratories, stock # 008450) at the E13.5 stage (Ferrari et al., 2010). Briefly, cells were maintained in serum-free DMEM-F12 medium supplemented with epidermal growth factor (10 ng/ml) and basic fibroblast growth factor (10 ng/ml). After seven days of culture, cells had grown to free-floating neurospheres. For passaging, spheres were dissociated using 1 ml Accutase (Life Technologies) for 10 min. Single cells were resuspended in the same medium.
BLI of Luc+-NSCs in vitro
Luc+-NSCs were plated into poly L-lysine/laminin-coated 96-well plates at 10,000, 1,000, and 100 cells per well. For bioluminescence measurements, the medium was removed and 30 mg/ml D-luciferin in 10 mM phosphate buffered saline, pH=7.4 (PBS) was added. The luminescence signal was measured using an IVIS SpectrumCT (Perkin Elmer). Images were acquired at one-minute intervals for 10 minutes until peak signal was observed. BLI was quantified by the creation of regions of interest (ROIs) over the wells, and the data were expressed as photon flux (p/sec).
Cell preparation for transplantation
On the day of transplantation, neurospheres were collected, washed with PBS, and then treated with 1 ml Accutase (Life Technologies) for 10 min at room temperature to make a single cell suspension. The cells were then centrifuged at 200 g for five minutes and the supernatant was removed. The cell pellet was resuspended in PBS and the cell number was determined using an automated cell counter (Cellometer Auto 2000, Nexcelom Bioscience).
Immunosuppression
Mice were immunosuppressed by intraperitoneal (i.p.) daily administration of a cocktail of tacrolimus (FK-506) + sirolimus (Rapamycin) (1 mg/kg each; LC Laboratories; Woburn, MA), beginning five days before cell transplantation, and then, continuously until sacrifice.
Cell transplantation
Twenty male SOD1G93A transgenic ALS mice (B6SJL-Tg(SOD1*G93A)1Gur/J: Stock # 002726) (Jacksons Laboratory, Bar Harbor, ME) and 14 male wild-type littermates were used in this study. The animals were divided into three groups: (i) SOD1G93A transgenic ALS mice (n=14) transplanted with Luc+-NSCs; (ii) wild-type littermates (n=14) transplanted with Luc+-NSCs; and (iii) control SOD1G93A transgenic ALS mice (n=6) injected with PBS.
Cells were transplanted in the cervical spinal cord region of 63-day old mice as previously described (Lepore et al., 2011). In brief, animals were anesthetized with 350 mg/kg chloral hydrate i.p. (Sigma). Each mouse received two grafts (bilaterally at the C5 cervical spinal cord region) of 100,000 NSCs/site (in 2 μL saline) into the ventral horn. Control ALS mice were injected with only 2 μL saline/site into the ventral horn. Cells were delivered using a 10 μL Hamilton Gastight syringe with an attached 30-gauge 45° beveled needle (Hamilton; Reno, NV). The injection pipette was secured to a manual micromanipulator (World Precision Instruments; Sarasota, FL) attached to an 80° tilting base. The tip was lowered to a depth of 0.75 mm below the surface of the cord and was held in place for two minutes before and after cell injection to minimize backflow. Cells were delivered under the control of a micro-syringe pump controller (World Precision Instruments) at a rate of 0.5 μl/minute.
Animals were maintained in a normal day-night cycle (12/12) with free access to food and water. All animal procedures were approved and conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of our Institute.
Computed tomography
CT images were acquired using an IVIS SpectrumCT (Perkin Elmer) using 50 kVp x-rays at 1mA of current, 50 ms exposure time, and with an aluminum filter. A total of 720 projections, spaced 0.5° apart, were acquired and the CT volume was reconstructed using Living Image 4.3 software (Perkin Elmer Inc.), which provided a field of view (FOV) of 12.0 × 12.0 × 3.0 cm and an isotropic resolution of 0.15 mm.
BLI of intraspinally engrafted Luc+-NSCs
Bioluminescent images of the animals were acquired using the cooled CCD camera of the same IVIS SpectrumCT instrument. For each animal, anesthesia was induced using 2% isoflurane gas in oxygen, and 150 mg/kg body weight of D-luciferin was injected intraperitoneally (i.p.). Images were acquired 10 min after injection to maximize the bioluminescence signal. To generate 2D bioluminescent images, no emission filter was used during imaging. Images were quantified by drawing ROIs over the spinal cord region and the data were expressed as photon flux (p/sec). To generate 3D bioluminescent images, four spectrally resolved images were acquired using emission filters at 600, 620, 640, and 660 nm, with a bandwidth of 20 nm each. Imaging parameters were an exposure time of 180 s, an aperture of f/1, a FOV = 13 cm, and 2048 × 2048 pixels resolution. Pixel binning was set to an 8×8 bin width for an effective image resolution of 256 × 256 pixels. Imaging parameters were identical for both 2D and 3D imaging.
Three-dimensional micro-CT/BLI reconstruction
Three-dimensional reconstruction of the bioluminescent source and superposition over the CT images was achieved with the diffuse light imaging tomographic (DLIT) algorithm using Living Image 4.3 software. The software predefined the bioluminescent source and tissue absorption spectra for the luciferase reporter and the mouse tissue. The source distribution was visualized in Living Image using a voxel size of 0.31 mm without smoothing.
Disease onset and end-stage assessment
Mice were observed for weight loss and visible signs of weakness in splaying the hind limb to determine disease onset. To determine disease end-stage in a humane manner, the end stage was defined as the inability of mice to right themselves within 30 seconds when placed on their sides.
Rotarod test
For behavioral analysis, the motor performance of animals was evaluated using a Rotarod apparatus (7650 accelerating model, Ugo Basile, Varese, Italy). Mice were placed on the accelerating rod at a starting speed of 4 rpm, reaching a final speed of 40 rpm in three minutes. All mice underwent three trials. The mice were allowed to stay on the rod for a maximum of 120 seconds and the time of their hold on the rod was scored.
Tissue preparation and immunohistochemistry
Animals were anesthetized and perfused intracardially with 4% paraformaldehyde in PBS. Spinal cords were extracted, post-fixed in the same solution overnight at 4°C, and cryopreserved in 30% sucrose. Frozen transverse sections, 30 μm thick, were cut using a Thermo Scientific HM 550 cryostat. For immunohistochemical analysis, non-specific binding was blocked by incubating with a solution consisting of 10% donkey serum and 0.1% Triton X-100-PBS for two hours at room temperature. Sections were incubated overnight at 4°C with the appropriate dilution of primary antibody in blocking solution. The corresponding secondary antibodies (Alexa Fluor-488; Alexa Fluro-546 secondary fluorescent antibodies, Invitrogen, Carlsbad, CA) were added at a ratio of 1:200 in blocking solution for two hours at room temperature. Sections were then rinsed with 0.1 M PBS and placed on coverslips with aqueous non-fluorescent mounting medium (Immu-mount, Thermo Scientific, Pittsburgh, PA). Images were obtained with a Zeiss AX10 fluorescence microscope. Primary antibodies used were: anti-firefly luciferase (1:3000 dilution. GeneTex); anti-GFAP (1:1000 dilution, Santa Cruz, USA); anti-Iba1 (1:1000 dilution, Wako); anti-Olig2 (1:250 dilution, Santa Cruz); anti-NeuN (1:500 dilution, Milipore); and anti-F4/80 (1:250 dilution, Abcam). All images were obtained using the same exposure time.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using GraphPad Prism software (version 6.0f). Linear regression analysis was performed to determine the correlation between two variables. Student’s t-test was used with a significance level set at p<0.05.
Results
Initial validation studies
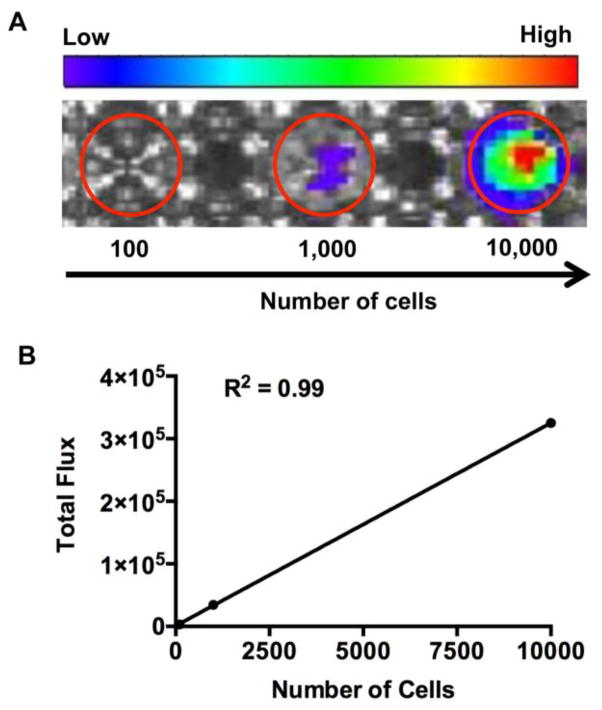
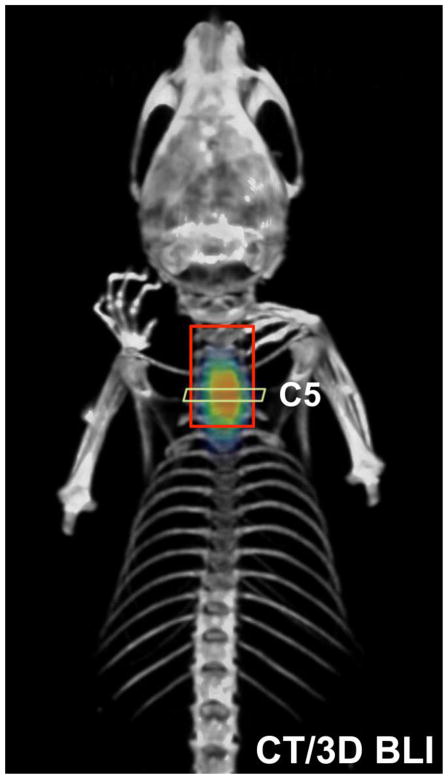
In vitro BLI analysis of Luc+-NSCs plated at different cell densities showed a linear correlation between cell number and BLI signal (R2 = 0.99, Figure 1), validating the use of BLI for quantification of luciferase-expressing NSCs. For validation of accurate placement of cells at the target site, in vivo three-dimensional BLI and CT images were co-registered on 1 day post cell transplantation. An accurate overlay of the cellular BLI signal with the anatomical location of the cervical region (C5) was observed. (Figure 2).
Figure 1.
(A) In vitro BLI of plated Luc+-NSCs GRPs at several cell densities. (B) Linear correlation between the number of luciferase-expressing cells and BLI signal (R2 =0.99).
Figure 2.
Co-registration of CT (gray scale) and BLI (color scale) to confirm the correct placement of cells at the targeted injection site (C5 spinal cord region). Images were obtained at day 1 post-transplantation.
Motor performance and weight loss
ALS mice displayed a typical pattern of disease progression, as evidenced by the presence of neurological deficits in motor behavior and a reduction in body weight. Compared to saline-injected animals, in mice engrafted with NSCs alone, a significant but transient improvement in motor performance was observed only at the early stages of the disease (Figure 3). There was no significance difference in weight loss between the two groups.
Figure 3.
Rotarod test behavioral analysis shows a transient improvement in the motor performance of ALS mice transplanted with Luc+-NSC (n=6) compared to ALS mice injected with saline (n=6, *p<0.05).
Survival of Luc+-NSCs in SOD1G93A ALS vs. wild-type mice during disease progression
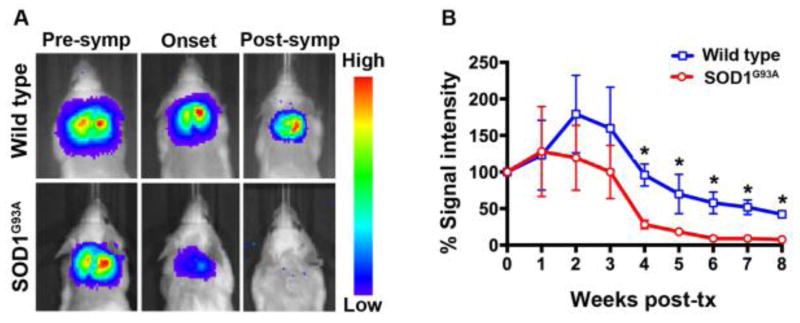
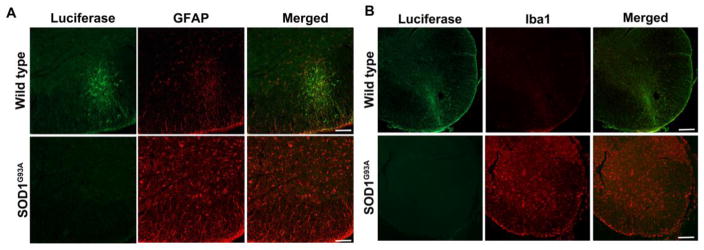
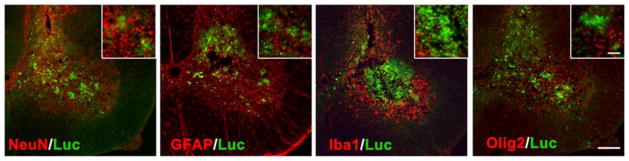
The survival of transplanted allogeneic Luc+-NSCs was monitored by serial BLI during disease progression. In ALS mice, a significant 71.7±17.9% decline in BLI signal was observed at week 4 post-transplantation compared to day 1 post-transplantation (p<0.05). There was a further decline in the BLI signal intensity as the disease progressed, and signal was completely lost at the post-symptomatic stage of the disease (8 weeks post-transplantation). In contrast, in wild-type littermates, the BLI signal was still detectable (42.14±8.7%), indicating that almost half of the transplanted cells were able to survive in wild-type mice (Figure 4). Prolonged survival of grafted cells in wild-type mice was further confirmed by histology (Figure 5). Luc+-NSCs were predominantly detected in the ventral horn of the spinal cord, with transplanted cells migrating to the surrounding white matter. No Luc+ cells were observed in the spinal cord of ALS mice at the end-stage of the disease. Two weeks post-transplantation, spinal cord tissue sections stained for GFAP (astrocytes), Iba1 (microglia), NeuN (differentiating neurons), Olig2 (oligodendrocytes), and luciferase revealed that engrafted cells did not express any markers for neuronal or glial lineages (Figure 6).
Figure 4.
(A) Representative BL images of Luc+-NSCs engrafted SOD1G39A mice at the pre-symptomatic, onset, and post-symptomatic (end) stage of the disease. Wild-type mice were engrafted at the same time points. (B) Percentage loss of BLI signal compared to day 1 post-transplantation (set as 100%) (n=10, *=p<0.05).
Figure 5.
(A) Astrogliosis in the spinal cord of SOD1G39A mice. Representative micrographs of anti-GFAP (red) and anti-luciferase (green) staining demonstrate activated astrocytes at the site of transplantation in ALS spinal cord at the post-symptomatic stage of the disease (8 weeks after transplantation). Wild-type littermates exhibit less expression of GFAP around the injection site of luciferase-positive cells at the same time point. Scale bar =100 μm. (b) Microgliosis in the spinal cord of SOD1G39A mice. Representative micrographs of anti-Iba1 (red) and anti-luciferase (green) staining demonstrate activated microglia at the site of transplantation in the ALS spinal cord at the post-symptomatic stage of the disease (8 weeks after transplantation). Wild-type littermates exhibit less expression of Iba1 around the injection site of luciferase-positive cells at the same time point. Scale bar =100 μm.
Figure 6.
Luc+-NSCs 2 weeks do not express differentiation markers at 2 weeks post-transplantation in SOD1G39A mice spinal cord. Shown are stainings for anti-NeuN (red), anti-GFAP (red), anti-Iba1 (red), anti-Olig2 (red), and anti-luciferase (green). Scale bar = 100 μm, inset scale bar = 50 μm.
Host immune environment
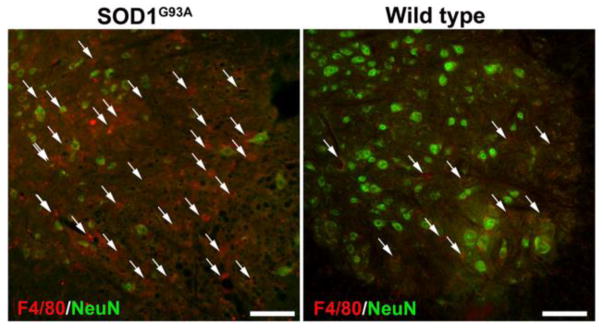
Activated glial cells and immune cells could be detected in the spinal cord parenchyma around the site of cell transplantation. A higher degree of microgliosis and astrogliosis were observed in the spinal cord of ALS mice compared to wild-type littermates at the post-symptomatic stage of the disease (Figure 5). A high expression of F4/80, a marker for mature mouse macrophages, was observed in the ventral horn of the cervical spinal cord of ALS mice, whereas wild-type littermates had much fewer of these resident macrophages (Figure 7).
Figure 7.
Accumulation of mature macrophages in the spinal cord of SOD1G39A mice. Shown are stainings for anti-F4/80 (red) and anti-NeuN (green) at 8 weeks post-transplantation demonstrate accumulation of mature macrophages at the site of transplantation in the ALS spinal cord. Wild-type littermates demonstrate far fewer F4/80+ cells. Scale bar=50 μm.
Discussion
ALS is an incurable and fatal disease, characterized by the rapid and selective death of both upper and lower motor neurons. Therapeutic grafting of NSCs has proven to be one of the most promising approaches to replace and/or protect dying neurons (Fischer, 2000). NSC engraftment not only results in the replacement of neurons and glia cells, but may also improve functional outcomes through bystander mechanisms, such as neurotrophism (Barnabe-Heider and Miller, 2003; Lu et al., 2003). However, one of the major challenges in the therapeutic use of NSCs is potential graft rejection (Hefferan et al., 2011; Jablonska et al., 2013; Kim et al., 2006; Li et al., 2005; Tambuyzer et al., 2009; Yan et al., 2006). Graft rejection could result either by pathogenic immune signaling that results from the disease process itself (Chen and Palmer, 2008), or by an adaptive host immune response immediately after cell engraftment (Zhao et al., 2011).
To the best of our knowledge, there have been no systematic in vivo studies on the survival of transplanted cells as related to the progression of ALS disease symptoms. In this study, we non-invasively imaged engrafted allogeneic murine NSCs in the spinal cords of SOD1G93A transgenic ALS mice, and found that the survival of cells was greatly reduced with disease progression. The high sensitivity, low background signal, and non-invasive nature of BLI allowed us to quantitatively assess the viability of engrafted cells in animals at various time points during the course of the disease, without the need for animal euthanasia and tissue harvesting at multiple time points (Srivastava et al., 2016). As visualized by co-registered BLI/CT imaging, we were able to accurately reproduce cell injection at the targeted cervical spinal cord region site without spillover to distal regions.
In this study, we used allogeneic murine NSCs as allogeneic cells offer the most practical source for cell therapy of neurodegenerative disorders in a clinical setting (Glass et al., 2012; Mazzini et al., 2015). We observed that, after engraftment, Luc+-NSCs did not express any markers for neuronal or glial lineages in the SOD1G39A mouse, confirming that they did not reconstitute the cellular composition of the ALS spinal cord. This was in contrast to WT mice, where Luc+/NeuN+, GFAP+, and Olig2+ cells were present by week 2 post-transplantation (data not shown). This finding is in agreement with a previous study by Tadesse et al., in which the authors reported that human NSCs engrafted in the spinal cord of ALS patients maintained their stem cell phenotypes even after 2.5 years of transplantation (Tadesse et al., 2014). The reason for this lack of differentiation is unclear, and may be a function of complexities intrinsic to the ALS spinal cord microenvironment.
After a transient early increase at the onset of disease, we observed a dramatic loss of engrafted murine NSCs with disease progression. This reduction in engrafted cell survival must be caused by the non-conducive microenvironment in the ALS spinal cord, as identical experiments in wild-type mice did not show such cell loss, ruling out the host anti-graft immune rejection as primary mechanism. Li et al. (2005) have reported a failure of immortalized human neuronal progenitor xenografts in the spinal cord of Sprague-Dawley rats despite immunosuppression. The failure of these grafts was ascribed to apoptotic cell death and the presence of an innate immune response. In this study, we observed infiltration of mature macrophages in the gray matter of ALS mice at the end-stage of the disease. We also observed excessive microgliosis and astrogliosis in the same region. In ALS, neuronal death is associated with a robust multi-phased response by CNS immune cells, microglia, and astrocytes (Hall et al., 1998), and these responses become more severe as the disease progresses (Alexianu et al., 2001; Chiu et al., 2009; Yamanaka et al., 2008). This progressive hostile ALS spinal cord microenvironment suggests that careful selection of an immunosuppression strategy that maximizes cell survival will be important for use of engrafted NSCs for motor neuron repair and regeneration. Whether other neural progenitor cell types respond similarly requires further investigation (Corti et al., 2007; Haidet-Phillips et al., 2015).
We observed a poor graft survival of grafted NSCs in ALS mice despite the fact that these cells are known to be immunoprivileged and exhibit a low expression of major histocompatibility complex (MHC) antigens ((Hori et al., 2003). However, exposure of NSCs to pro-inflammatory cytokines including interferon γ (IFNγ) may result in upregulation of the expression of MHC antigens (Hori et al., 2003), making them a target for the host immune response. In ALS, interferon signaling in astrocytes is triggered by pathologic changes in motor neurons (Wang et al., 2011) that can lead to higher levels of IFNγ in the spinal cord (Aebischer et al., 2012). Similarly, the blood-spinal cord barrier in ALS becomes impaired at an early stage of the disease and worsens with disease progression (Garbuzova-Davis et al., 2007; Zhong et al., 2008). This barrier disruption may potentially expose engrafted NSCs to the host immune system and lead to cell death.
For evaluating functional outcome, a limitation of our study was the use of the rotarod test as the only behavioral test for cervical spinal cord function. Further studies should be performed using a more comprehensive battery of behavioral tests, combined with longitudinal imaging of cells transplanted at multiple levels of the spinal cord. In summary, our present findings that the ALS disease progression severely affects NSC survival calls for additional approaches to protect engrafted cells from the hostile ALS microenvironment.
Highlights.
Bioluminescence imaging is well suited to monitor the survival of intraspinally transplanted neural stem cells over time.
The survival of transplanted allogeneic neural stem cells was adversely affected by the ALS disease progression.
The poor transplanted cell survival was due to the hostile spinal cord microenvironment in ALS.
Acknowledgments
This study was supported by grants 2RO1 NS045062, S10 OD010744, R01 NS076573, and ALSA 16-IIP-252. The authors thank Anka Jablonska, Irina Shats, and Mary McAllister for technical and editorial assistance.
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aebischer J, Moumen A, Sazdovitch V, Seilhean D, Meininger V, Raoul C. Elevated levels of IFNgamma and LIGHT in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. European journal of neurology : the official journal of the European Federation of Neurological Societies. 2012;19:752–759. e745–756. doi: 10.1111/j.1468-1331.2011.03623.x. [DOI] [PubMed] [Google Scholar]
- Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnabe-Heider F, Miller FD. Endogenously produced neurotrophins regulate survival and differentiation of cortical progenitors via distinct signaling pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5149–5160. doi: 10.1523/JNEUROSCI.23-12-05149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. The New England journal of medicine. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Chen KS, Sakowski SA, Feldman EL. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Annals of neurology. 2016;79:342–353. doi: 10.1002/ana.24584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Cellular repair of CNS disorders: an immunological perspective. Hum Mol Genet. 2008;17:R84–92. doi: 10.1093/hmg/ddn104. [DOI] [PubMed] [Google Scholar]
- Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, Traynor BG, Eurals C. Prognostic factors in ALS: A critical review. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases. 2009;10:310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chio A, Logroscino G, Traynor BJ, Collins J, Simeone JC, Goldstein LA, White LA. Global epidemiology of amyotrophic lateral sclerosis: a systematic review of the published literature. Neuroepidemiology. 2013;41:118–130. doi: 10.1159/000351153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu IM, Phatnani H, Kuligowski M, Tapia JC, Carrasco MA, Zhang M, Maniatis T, Carroll MC. Activation of innate and humoral immunity in the peripheral nervous system of ALS transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20960–20965. doi: 10.1073/pnas.0911405106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti S, Locatelli F, Papadimitriou D, Del Bo R, Nizzardo M, Nardini M, Donadoni C, Salani S, Fortunato F, Strazzer S, Bresolin N, Comi GP. Neural stem cells LewisX+ CXCR4+ modify disease progression in an amyotrophic lateral sclerosis model. Brain. 2007;130:1289–1305. doi: 10.1093/brain/awm043. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Binda E, De Filippis L, Vescovi AL. Isolation of neural stem cells from neural tissues using the neurosphere technique. Curr Protoc Stem Cell Biol. 2010;Chapter 2(Unit2D):6. doi: 10.1002/9780470151808.sc02d06s15. [DOI] [PubMed] [Google Scholar]
- Fischer I. Candidate cells for transplantation into the injured CNS. Progress in brain research. 2000;128:253–257. doi: 10.1016/S0079-6123(00)28022-9. [DOI] [PubMed] [Google Scholar]
- Garbuzova-Davis S, Saporta S, Haller E, Kolomey I, Bennett SP, Potter H, Sanberg PR. Evidence of compromised blood-spinal cord barrier in early and late symptomatic SOD1 mice modeling ALS. PloS one. 2007;2:e1205. doi: 10.1371/journal.pone.0001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells. 2012;30:1144–1151. doi: 10.1002/stem.1079. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Doreswamy A, Gross SK, Tang X, Campanelli JT, Maragakis NJ. Human glial progenitor engraftment and gene expression is independent of the ALS environment. Experimental neurology. 2015;264:188–199. doi: 10.1016/j.expneurol.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Hefferan MP, Johe K, Hazel T, Feldman EL, Lunn JS, Marsala M. Optimization of immunosuppressive therapy for spinal grafting of human spinal stem cells in a rat model of ALS. Cell transplantation. 2011;20:1153–1161. doi: 10.3727/096368910X564553. [DOI] [PubMed] [Google Scholar]
- Hori J, Ng TF, Shatos M, Klassen H, Streilein JW, Young MJ. Neural progenitor cells lack immunogenicity and resist destruction as allografts. Stem Cells. 2003;21:405–416. doi: 10.1634/stemcells.21-4-405. [DOI] [PubMed] [Google Scholar]
- Jablonska A, Janowski M, Lukomska B. Different methods of immunosuppresion do not prolong the survival of human cord blood-derived neural stem cells transplanted into focal brain-injured immunocompetent rats. Acta neurobiologiae experimentalis. 2013;73:88–101. doi: 10.55782/ane-2013-1924. [DOI] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, Slavin S. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of neurology. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DE, Tsuji K, Kim YR, Mueller FJ, Eom HS, Snyder EY, Lo EH, Weissleder R, Schellingerhout D. Neural stem cell transplant survival in brains of mice: assessing the effect of immunity and ischemia by using real-time bioluminescent imaging. Radiology. 2006;241:822–830. doi: 10.1148/radiol.2413050466. [DOI] [PubMed] [Google Scholar]
- Lepore AC, O’Donnell J, Kim AS, Williams T, Tuteja A, Rao MS, Kelley LL, Campanelli JT, Maragakis NJ. Human glial-restricted progenitor transplantation into cervical spinal cord of the SOD1 mouse model of ALS. PloS one. 2011;6:e25968. doi: 10.1371/journal.pone.0025968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Tessler A, Han SS, Fischer I, Rao MS, Selzer ME. Fate of immortalized human neuronal progenitor cells transplanted in rat spinal cord. Archives of neurology. 2005;62:223–229. doi: 10.1001/archneur.62.2.223. [DOI] [PubMed] [Google Scholar]
- Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Experimental neurology. 2003;181:115–129. doi: 10.1016/s0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- Mazzini L, Gelati M, Profico DC, Sgaravizzi G, Projetti Pensi M, Muzi G, Ricciolini C, Rota Nodari L, Carletti S, Giorgi C, Spera C, Domenico F, Bersano E, Petruzzelli F, Cisari C, Maglione A, Sarnelli MF, Stecco A, Querin G, Masiero S, Cantello R, Ferrari D, Zalfa C, Binda E, Visioli A, Trombetta D, Novelli A, Torres B, Bernardini L, Carriero A, Prandi P, Servo S, Cerino A, Cima V, Gaiani A, Nasuelli N, Massara M, Glass J, Soraru G, Boulis NM, Vescovi AL. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. doi: 10.1186/s12967-014-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, Taub J, Kesner V, Feldman EL, Boulis NM. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery. 2012;71:405–416. doi: 10.1227/NEU.0b013e31825ca05f. discussion 416. [DOI] [PubMed] [Google Scholar]
- Srivastava AK, Bulte CA, Shats I, Walczak P, Bulte JW. Co-transplantation of syngeneic mesenchymal stem cells improves survival of allogeneic glial-restricted precursors in mouse brain. Experimental neurology. 2016;275(Pt 1):154–161. doi: 10.1016/j.expneurol.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava AK, Bulte JW. Seeing stem cells at work in vivo. Stem cell reviews. 2014;10:127–144. doi: 10.1007/s12015-013-9468-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadesse T, Gearing M, Senitzer D, Saxe D, Brat DJ, Bray R, Gebel H, Hill C, Boulis N, Riley J, Feldman E, Johe K, Hazel T, Polak M, Bordeau J, Federici T, Glass JD. Analysis of graft survival in a trial of stem cell transplant in ALS. Annals of clinical and translational neurology. 2014;1:900–908. doi: 10.1002/acn3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambuyzer BR, Bergwerf I, De Vocht N, Reekmans K, Daans J, Jorens PG, Goossens H, Ysebaert DK, Chatterjee S, Van Marck E, Berneman ZN, Ponsaerts P. Allogeneic stromal cell implantation in brain tissue leads to robust microglial activation. Immunol Cell Biol. 2009;87:267–273. doi: 10.1038/icb.2009.12. [DOI] [PubMed] [Google Scholar]
- Teng YD, Benn SC, Kalkanis SN, Shefner JM, Onario RC, Cheng B, Lachyankar MB, Marconi M, Li J, Yu D, Han I, Maragakis NJ, Llado J, Erkmen K, Redmond DE, Jr, Sidman RL, Przedborski S, Rothstein JD, Brown RH, Jr, Snyder EY. Multimodal actions of neural stem cells in a mouse model of ALS: a meta-analysis. Science translational medicine. 2012;4:165ra164. doi: 10.1126/scitranslmed.3004579. [DOI] [PubMed] [Google Scholar]
- Wang R, Yang B, Zhang D. Activation of interferon signaling pathways in spinal cord astrocytes from an ALS mouse model. Glia. 2011;59:946–958. doi: 10.1002/glia.21167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Shen P, Hazel T, Johe K, Koliatsos VE. Dual transplantation of human neural stem cells into cervical and lumbar cord ameliorates motor neuron disease in SOD1 transgenic rats. Neurosci Lett. 2011;494:222–226. doi: 10.1016/j.neulet.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Chun SJ, Boillee S, Fujimori-Tonou N, Yamashita H, Gutmann DH, Takahashi R, Misawa H, Cleveland DW. Astrocytes as determinants of disease progression in inherited amyotrophic lateral sclerosis. Nature neuroscience. 2008;11:251–253. doi: 10.1038/nn2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Xu L, Welsh AM, Chen D, Hazel T, Johe K, Koliatsos VE. Combined immunosuppressive agents or CD4 antibodies prolong survival of human neural stem cell grafts and improve disease outcomes in amyotrophic lateral sclerosis transgenic mice. Stem Cells. 2006;24:1976–1985. doi: 10.1634/stemcells.2005-0518. [DOI] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. doi: 10.1038/nature10135. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV. ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nature neuroscience. 2008;11:420–422. doi: 10.1038/nn2073. [DOI] [PMC free article] [PubMed] [Google Scholar]