Abstract
Background
Little is known about what factors modify the effect of long-term exposure to PM2.5 on mortality, in part because in most previous studies certain groups such as rural residents and individuals with lower socioeconomic status (SES) are under-represented.
Methods
We studied 13.1 million Medicare beneficiaries (age ≥65) residing in seven southeastern US states during 2000–2013 with 95 million person-years of follow-up. We predicted annual average of PM2.5 in each zip code tabulation area (ZCTA) using a hybrid spatiotemporal model. We fit Cox proportional hazards models to estimate the association between long-term PM2.5 and mortality. We tested effect modification by individual-level covariates (race, sex, eligibility for both Medicare and Medicaid, and medical history), neighborhood-level covariates (urbanicity, percentage below poverty level, lower education, median income, and median home value), mean summer temperature, and mass fraction of 11 PM2.5 components.
Results
The hazard ratio (HR) for death was 1.021 (95% confidence interval: 1.019–1.022) per one μg m-3 increase in annual PM2.5. The HR decreased with age. It was higher among males, non-whites, dual-eligible individuals, and beneficiaries with previous hospital admissions. It was higher in neighborhoods with lower SES or higher urbanicity. The HR increased with mean summer temperature. The risk associated with PM2.5 increased with relative concentration of elemental carbon, vanadium, copper, calcium, and iron and decreased with nitrate, organic carbon, and sulfate.
Conclusions
Associations between long-term PM2.5 exposure and death were modified by individual-level, neighborhood-level variables, temperature, and chemical compositions.
Keywords: long-term PM2.5, mortality, older adults, effect modification
Introduction
The association between long-term exposure to PM2.5 and increased risk of mortality has been well documented over the past two decades.1-6 The US Environmental Protection Agency (EPA) has revised the National Ambient Air Quality Standards (NAAQS) for the annual PM2.5 to 12 μg m-3 which helps reduce health risks related to the chronic effects of PM2.5.7 A number of studies have suggested that the effect estimate for long-term exposure to PM2.5 was heterogeneous across populations,6,8-10 to which individual susceptibility or differences in PM2.5 composition could potentially contribute. But there is still a lack of understanding of what factors modify the association. Identifying effect modifiers could add knowledge to future risk assessment studies and future revision of air quality emission standards to protect sensitive populations.
Although some studies have shown that individual-level and neighborhood-level covariates may change individual vulnerability to long-term PM2.5 exposure such as age, sex, and socio-economic status (SES),2,3,6,11-14 the results were mixed.8 First, to make use of PM2.5 data, studies often restricted the study population to residents who live close to an air pollution monitoring site. Rural residents were often under-represented. Second, studies such as the Nurses' Health Study13 and the American Cancer Society2 recruited primarily individuals with high socio-economic status (SES), making it difficult to identify the difference in effect between high- and low-SES individuals by design. The exclusion of a large proportion of the general population reduced the statistical power to detect effect modification and limited the generalizability of the study. Additionally, there are also some individual characteristics that very few studies have tested. For example, individual medical history such as previous hospital admissions reflected the healthiness of an individual, which may also change individual sensitivity to air pollution but which was not clearly understood.
Moreover, PM2.5, coming from a variety of sources including soil, road dust, oil combustion, traffic emission, and power plants, has a range of chemical compositions and toxicity.16 Although there are some studies looking at effect modification by chemical components for short-term PM2.5 exposure,17-19 there are few studies that examine the interaction of chemical components with long-term PM2.5 exposure.6,20 In addition, both mortality among the elderly and PM2.5 composition could be affected by climate.21-24 Little is known about the interaction between long-term temperature and long-term PM2.5 exposure on mortality.
Average PM2.5 concentrations from a nearby monitoring have been widely used to estimate the exposure in early studies.1,4 Residents living far away from monitoring sites were often excluded due to lack of exposure data. Another popular approach is land-use regression, which has been used, for example, in the European Study of Cohorts for Air Pollution Effects (ESCAPE) study and the Nurses' Health Study in the US.25,26 Land-use regression relies on extensive monitoring of pollutants in only a few years. The temporal resolution for the land-use regression was also often limited. Simulations have shown that point estimates and standard errors of the health effects may be biased if the exposure predictions relied solely on land use variables.27 Recently, remote sensing data (aerosol optical depth) was combined with land-use variables to predict PM2.5 concentrations in the US, which substantially improved the prediction ability. For example, Beckerman et al. (2013) used a hybrid approach to predicting monthly PM2.5 in the US.28 This model has also been used in the American Cancer Society's Cancer Prevention Study II examining the associations between long-term exposure to PM2.5 and cardiovascular deaths.29 Kloog et al. (2014) and Lee et al. (2015) developed a hybrid approach combining land use regression and satellite remote sensing on aerosol optical depth to predict daily ground-level PM2.5 with high spatial resolution (1 × 1 km).30,31 Both models showed excellent prediction ability. The model for the northeast has been applied to estimate the causal effects of long-term PM2.5 exposure on mortality in New Jersey during 2004-2009.32 The present study made use of the hybrid PM2.5 predictions for the southeastern US to estimate the association between long-term exposure to PM2.5 and mortality among a large cohort of older adults (age ≥65) and test a wide range of effect modifiers including individual-level variables, neighborhood-level variables, seasonal temperature, and PM2.5 components.
Method
Study population
The study population comprised fee-for-service Medicare beneficiaries (aged 65 or above) from January 1st, 2000 to December 31st, 2013 residing in seven states in the southeastern U.S. (Alabama, Florida, Georgia, Mississippi, North Carolina, South Carolina, and Tennessee). From the enrollment record for each year, we extracted age, race, sex, zip code of residence, and dual eligibility (an individual that was eligible for both Medicare and Medicaid; dual eligible beneficiaries generally had a lower SES). From the Medicare Provider and Analysis Review (MedPAR) files, we obtained the number of days staying in coronary care unit (CCU) or intensive care unit (ICU) for each of the eligible beneficiaries as individual-level risk factors. We also used the International Classification of Diseases, Ninth Revision (ICD-9) code at discharge to extract data on if a Medicare beneficiary had ever been admitted, since the start of follow-up, due to primary admissions of congestive heart failure (CHF, ICD-9 code 428), primary admissions of myocardial infarction (MI, ICD-9 code 410), primary admissions of chronic obstructive pulmonary disease (COPD, ICD-9 code 490 – 492, 494 – 496), and primary and secondary admissions of diabetes (ICD code 250). Each individual was assigned to a zip code tabulation area (ZCTA) according to the location of the post office of each zip code and ZCTA 2010. The protocol of this study was approved by the Harvard T.H. Chan School of Public Health Human Subjects Committee.
PM2.5 exposure
We applied a three-stage hybrid model to predict daily PM2.5 concentration with a 1 × 1 km spatial resolution in the southeast of the US during 2000–2013. Briefly, at the first stage, we calibrated a mixed-effects model using daily ground-level PM2.5 monitoring data. The predictors of the model included the Moderate Resolution Imaging Spectroradiometer (MODIS) aerosol optical depth data with a 1 × 1 km spatial resolution, meteorological variables, normalized difference vegetation index, mixing height, and land-use covariates. The mean out-of-sample R2 using ten-fold cross-validation was 0.70-0.81. At the second stage, we used the model to predict PM2.5 in grid cell on days when aerosol optical depth data were available. At the third stage, we built a model regressing PM2.5 on days when aerosol optical depth was available against nearby monitoring data and used that model to predict PM2.5 when aerosol optical depth was missing.31 We obtained the annual average of PM2.5 for each of the 1 × 1 km grids, took the arithmetic mean of the annual average PM2.5 across all grids in each of the ZCTAs, and assigned that exposure to each subject in that ZCTA for that year.
Temperature
Air temperature was estimated for each 1 × 1 km grid cell in the southeastern US by incorporating satellite remote sensing and land use variables. More details have been described elsewhere.33 The predicted air temperature was calibrated using the temperature measurements from weather stations. The model was unbiased and had excellent predictive performance. The yearly mean summer temperatures (over June, July, and August) in each year for each ZCTA were calculated and assigned to each individual.
Mass fraction of PM2.5 species
We obtained PM2.5 mass concentration and PM2.5 component data for the seven southeastern states and the surrounding states from the Environmental Protection Agency (EPA) and the Interagency Monitoring of Protected Visual Environment (IMPROVE) monitoring sites. We considered 11 chemical components of PM2.5: elemental carbon (EC), organic carbon (OC), sulfate, nitrate, aluminum, calcium, copper, iron, nickel, vanadium, and zinc. We calculated the mass fraction of each component to the total PM2.5 and took the annual average. Each ZCTA was assigned a mass fraction using Voronoi Tesselation according to the Euclidean distances between the centroids of that ZCTA and the monitoring sites.
Census variables and behavioral variables
We obtained the percentage of people below the poverty level, percentage of less educated people, median income, and median home value for each ZCTA from the US Census Bureau 2000 Census Summary File 334 and the American Community Survey (ACS) five-year estimates of 2009-2013.35 To account for the time-varying nature of these variables, we assigned the Census 2000 variables to observations from 2000-2006 and assigned the ACS estimates to observations from 2007-2013. The rural areas were defined as areas with a population density below the first tertile of the population density in the seven states (51 per square mile) according to the Census 2000 data. We also obtained age adjusted yearly prevalence estimates of percentage of smokers and percentage of obesity from CDC Behavioral Risk Factor Surveillance System (BRFSS).36
Statistical methods
We conducted an open cohort study. Each of the eligible beneficiaries was followed up from January 1st of the year when the individual entered Medicare. We constructed a counting process survival dataset following the scheme proposed by Andersen and Gill.37 We fit Cox proportional hazards models to estimate the hazard ratio of annual average PM2.5 on mortality of older adults. The model was stratified by age groups (65-74, 75-84, and >84), sex, and race (white, black, and others), and adjusted for dummy variables for each year, any previous admission due to CHF, COPD, MI, and diabetes, number of days spent in ICU and CCU, dual eligibility, dummy variables for each state, ZCTA-level percentage of the less educated, percentage below the poverty level, median income, median home value, urbanicity, mean summer temperature, and county-level percentage of smokers, and percentage of obesity.
We tested univariate effect modification of the association by age, sex, race, previous hospital admissions, number of days in ICU or CCU, dual eligibility, mean summer temperature, urbanicity, percentage of less educated, percentage of below poverty, median income, and median home value by adding into the model a cross-product term between the modifier and the exposure. In addition, we tested if the predicted mass fractions of PM2.5 components modified the effects.
We subset the data to each of the sex and race group combinations, and fit simultaneous interaction models of PM2.5 with dual eligibility, hospital admissions, urbanicity, and mean summer temperature. The result of these models could be further used in risk assessments of long-term exposure to PM2.5 and identification of susceptible populations.
We also conducted an analysis to examine the effect of long-term PM2.5 at low concentration by restricting the follow-up to person-years with PM2.5 level below the current EPA standard for the annual average (12 μg m-3).38 If an individual's exposure exceeded the current standard, this follow-up year will be excluded from the analysis.
Sensitivity Analyses
We relaxed the proportionality assumptions for dual eligibility and dummy variables for each state and fitted a Cox model additionally stratified by state and dual eligibility. Moreover, we conducted a sensitivity analysis by restricting the study population to ZCTAs within 35 km from the monitoring sites.
Results
Characteristics of the study population and exposure
Within the modeling area of PM2.5, we studied 13.1 million older adults during the period of 2000 – 2013 residing in seven southeastern US states (4185 ZCTAs) with 95.1 million person-years. 4.7 million (35%) beneficiaries died. This cohort represented the majority of adults 65 or older, compared to the older population in the seven states.39 The characteristics of the study population in year 2000, 2005, and 2010 are presented in Table 1. The number of beneficiaries increased over time with over 50% in age 65-74 and over 30% in age 75-84, over 80% whites and 13% blacks, 57%-59% females, and over 15% beneficiaries eligible for both Medicare and Medicaid. Around 5% of beneficiaries died in each year. The annual PM2.5 concentrations had a median of 10.7 μg m-3 and an interquartile range width of 3.8 μg m-3 (Table 2). The PM2.5 concentration had a decreasing trend from 2000 to 2013, and was lower in Florida than other states. All ZCTAs were included either in whole or in part in the restricted analysis at low concentration.
Table 1.
Characteristics of the study population in year 2000, 2005, and 2010.
| Variable | Subgroup | 2000 | 2005 | 2010 |
|---|---|---|---|---|
| Number of beneficiaries (million) | 6.0 | 6.5 | 7.3 | |
| Age group (% of total) | 65-74 years | 53.7 | 53.1 | 55.5 |
| 75-84 years | 34.6 | 34.9 | 32.0 | |
| >84 years | 11.7 | 12.0 | 12.5 | |
| Race (% of total) | Whites | 84.3 | 83.6 | 82.7 |
| Blacks | 13.1 | 13.4 | 13.7 | |
| Others | 2.6 | 3.0 | 3.6 | |
| Sex (% of total) | Females | 59.0 | 58.0 | 56.8 |
| Males | 41.0 | 42.0 | 43.2 | |
| Dual eligibility (% of total) | Yes | 15.0 | 16.1 | 15.4 |
| No | 85.0 | 83.9 | 84.6 | |
| Death (% of total) | Yes | 5.2 | 5.1 | 4.7 |
| No | 94.8 | 94.9 | 95.3 |
Table 2.
Spatial and temporal variability of annual PM2.5 exposure (μg/m3).
| Min | 5th | 25th | 50th | 75th | 95th | Max | |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| 6.0 | 7.3 | 9.1 | 10.7 | 12.9 | 15.1 | 20.6 | |
| By Year | |||||||
| 2000 | 10.4 | 11.1 | 12.4 | 14.8 | 16.0 | 17.8 | 20.6 |
| 2001 | 9.1 | 9.4 | 11.4 | 13.2 | 14.5 | 15.7 | 18.7 |
| 2002 | 7.9 | 8.6 | 10.2 | 12.3 | 13.4 | 14.6 | 16.7 |
| 2003 | 8.2 | 8.7 | 10.0 | 12.2 | 13.3 | 14.5 | 16.5 |
| 2004 | 8.6 | 9.3 | 10.7 | 12.4 | 13.6 | 14.7 | 17.1 |
| 2005 | 8.7 | 9.6 | 11.2 | 13.4 | 14.3 | 15.4 | 18.6 |
| 2006 | 8.3 | 9.4 | 10.6 | 12.3 | 13.5 | 14.7 | 17.0 |
| 2007 | 7.8 | 8.6 | 10.2 | 12.1 | 13.2 | 14.4 | 18.3 |
| 2008 | 7.1 | 7.8 | 8.8 | 10.7 | 11.8 | 12.4 | 15.7 |
| 2009 | 6.5 | 6.9 | 7.9 | 9.6 | 10.1 | 10.7 | 11.8 |
| 2010 | 6.8 | 7.3 | 8.4 | 10.3 | 10.9 | 11.6 | 13.6 |
| 2011 | 6.7 | 7.3 | 8.5 | 10.1 | 10.8 | 11.4 | 13.8 |
| 2012 | 6.8 | 7.1 | 7.9 | 9.0 | 9.5 | 10.0 | 12.1 |
| 2013 | 6.0 | 6.4 | 6.9 | 8.4 | 8.7 | 9.2 | 10.7 |
| By State (Number of ZCTAs) | |||||||
| Alabama (590) | 7.5 | 8.8 | 10.3 | 12.0 | 13.8 | 16.0 | 20.6 |
| Florida (863) | 6.0 | 6.8 | 8.0 | 9.2 | 10.6 | 12.1 | 15.4 |
| Georgia (675) | 7.2 | 8.9 | 10.5 | 12.2 | 14.3 | 15.8 | 19.4 |
| Mississippi (377) | 7.2 | 8.6 | 10.0 | 11.5 | 12.6 | 14.2 | 18.5 |
| North Carolina (715) | 7.3 | 8.5 | 10.0 | 12.3 | 13.7 | 15.2 | 17.9 |
| South Carolina (373) | 7.7 | 8.6 | 10.3 | 12.0 | 13.8 | 15.3 | 17.8 |
| Tennessee (592) | 6.5 | 8.7 | 10.0 | 12.2 | 13.6 | 15.8 | 20.2 |
Associations between long-term PM2.5 and mortality among older adults
After simultaneous adjustment for a variety of individual risk factors, neighborhood-level variables, county-level variables, and dummy variables for each state, the overall hazard ratio (HR) of mortality for each one μg m-3 increase in annual PM2.5 was 1.021 (95% confidence interval: 1.019–1.022). We found a higher HR, 1.033 (1.031 – 1.035), when the concentration was below current EPA standard (<12 μg m-3). A sensitivity analysis stratifying dual eligibility and dummy variable for each state showed a close HR, 1.025 (95% confidence interval: 1.024–1.026), to the main analysis.
Effect modification by individual-level risk factors
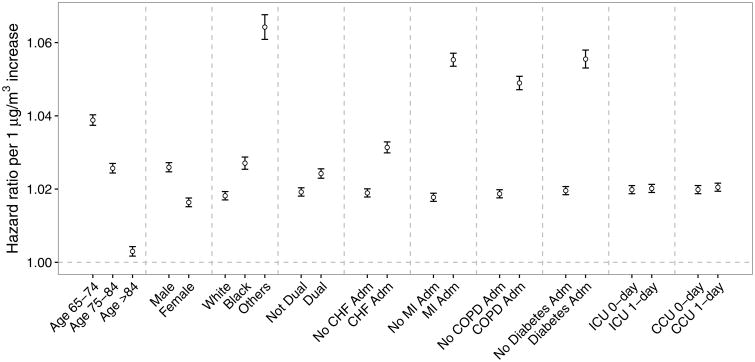
The association of long-term PM2.5 exposure with mortality was modified by individual-level variables (Figure 1). The HR was smaller for older adults. Females were less susceptible than males. The blacks and other race groups had higher HRs than the whites. A higher HR was found among beneficiaries that are eligible for both Medicare and Medicaid. Moreover, people with any previous admissions of CHF, MI, COPD, or diabetes, or ever staying in ICU or CCU were more susceptible to an increase in long-term PM2.5 exposure.
Figure 1.
Effect modification of the association of long-term PM2.5 exposure (hazard ratios for each one μg m-3 increase) with mortality among older adults using a univariate interaction model by individual-level covariates including age group, sex, race, dual eligibility, previous hospitalizations due to congestive heart failure (CHF), myocardial infarction (MI), chronic obstructive pulmonary disease (COPD), and diabetes, and number of days staying in intensive care unit (ICU) and coronary care unit (CCU) (one day versus zero day).
Effect modifications by neighborhood-level variables
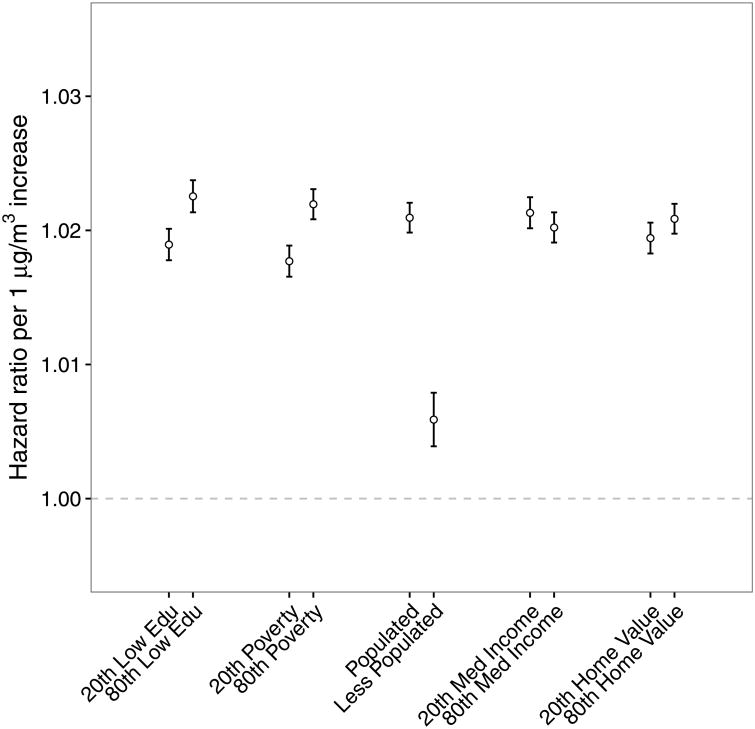
The association of PM2.5 with mortality was also modified by neighborhood-level variables (Figure 2). Individuals living in a neighborhood with higher percentage of less educated people, higher percentage of people living below poverty level, higher urbanicity, lower median income, or higher home value were more susceptible to PM2.5.
Figure 2.
Effect modification of the association of long-term PM2.5 exposure (hazard ratios for each one μg m-3 increase) with mortality among older adults by neighborhood-level covariates including percentage of less educated (20th percentile versus 80th percentile), percentage below the poverty level (20th percentile versus 80th percentile), urbanicity, median income, and median home value.
Effect modification by mean summer temperature
The HR for long-term PM2.5 and mortality increased by 0.60% (0.57%-0.63%) for each 1°C increase in temperature.
Simultaneous interaction models with individual-level variables, urbanicity, and mean summer temperature
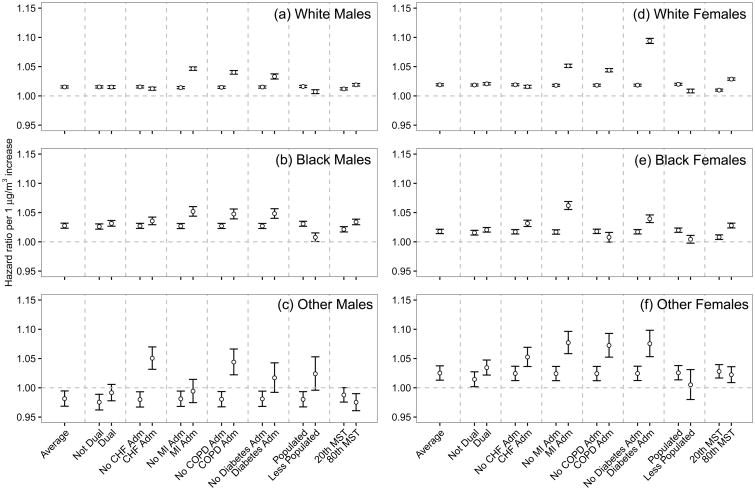
Figure 3 shows the simultaneous interaction models with individual-level variables, urbanicity, and mean summer temperature for each of the sex and race combinations.
Figure 3.
Effect modification of the association of long-term PM2.5 exposure (HRs per one μg m-3 increase) with mortality among older adults using simultaneous interaction models. The dataset was restricted to each of the sex and race combinations. Modifiers include individual-level covariates (dual eligibility, previous hospitalizations due to congestive heart failure (CHF), myocardial infarction (MI), chronic obstructive pulmonary disease (COPD), and diabetes), urbanicity, and mean summer temperature. The estimate labeled “average” shows the HR when all modifiers were set at their means. The hazard ratio (HR) for each modifier set that modifier at the corresponding value and set the remaining modifiers at their means.
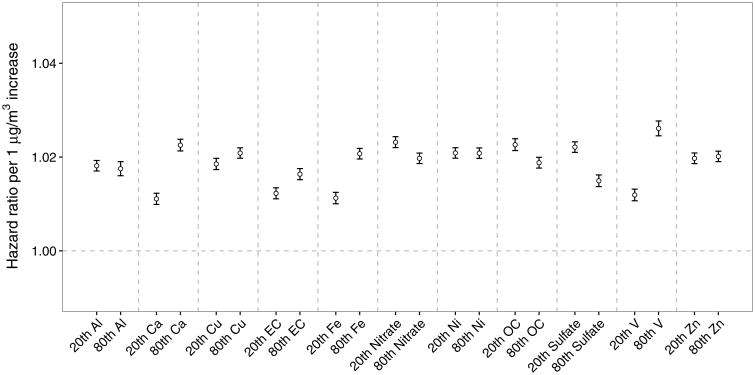
Effect modification by mass fractions of PM2.5 components
PM2.5 with a higher concentration of sulfate, nitrate, and OC had a lower HR. An increase in Al, Ca, Cu, EC, Fe, or V was associated with an increased HR. Ni and Zn did not substantially change the HR (Figure 3). A sensitivity analysis restricting the observations to ZCTAs within 35 km from the monitoring sites (Supplementary eFigure 1) shows that the directions for each elemental ratio remained unchanged.
Discussion
The present study examined a large cohort of 13.1 million older adults with 95.1 million person-years residing in seven southeastern US states in 2000-2013. We found that long-term exposure to PM2.5 was associated with an increased risk of mortality. The HR was higher when PM2.5 concentration was below the current EPA standard for annual average PM2.5. The large number of follow-up years and deaths provided large statistical power which is partly reflected in the narrow confidence intervals. For example, by comparison, the NHS study recruited 0.066 million individuals with a follow-up of up to 10 years.26 More importantly, with sufficient power, we found that a variety of covariates modified the association of long-term PM2.5 with survival including individual-level risk factors, neighborhood-level census covariates, temperature, and PM2.5 components. Based on a general population, the results are more generalizable.
We found a higher HR for mortality in urban areas than in rural areas. The spatially- and temporally-resolved PM2.5 model produced PM2.5 estimates in those regions, and our population-based samples made it feasible and provided substantial power to detect the difference.
Association between PM2.5 and mortality increased if beneficiaries had previously been hospitalized due to COPD, MI, CHF, or diabetes, or had ever stayed in ICU or CCU. Some studies examined CHF, MI, and COPD as outcomes of PM2.5 exposure.12,13,40 Their role as an effect modifier was not well understood. A medical history of being admitted due to MI or diabetes had a larger impact on the effect of PM2.5 among all four previous hospitalizations than COPD and CHF. In the simultaneous interaction models (Figure 3), the effect modifications by MI and diabetes also had a consistent and larger impact than other modifiers. Consistently, there have been several studies showing increased apparent susceptibility among diabetic individuals and PM2.5 was associated with increased risk of hospitalizations due to diabetes.19,41,42 The number of days staying in ICU or CCU had a smaller effect, possibly because ICU or CCU stays are more related to the acute condition in the hospital and not a chronic increase in susceptibility following discharge.
We found that blacks and other non-white groups had a higher risk of death overall than whites. In the subgroup analyses, we also see that blacks had a stronger association between PM2.5 and mortality than whites. Whether other racial groups had a larger association was dependent upon sex. However, the confidence intervals became much larger in the subgroup analysis because there were few observations in the non-white, non-black subgroup. By comparison, a study of older adults in 207 US cities reported a higher HR of long-term PM2.5 as the percentage of black residents increased.11 A study in New Jersey also estimated that the effect increased with the percentage of black residents in each census tract.32 However, they did not analyze race on an individual level and instead used the percentage of black residents as an ecologic surrogate.
In addition, the results suggested that people with lower SES showed a stronger association between PM2.5 and mortality. People with lower SES included dual-eligible beneficiaries (for both Medicare and Medicaid) or those who lived in a neighborhood with lower median income, more less educated individuals, or higher percentage of people living below poverty. A possible explanation is that people with a lower income had poorer baseline health or poorer access to health care services.11 Consistently, a recent study in New Jersey also found that the risks are higher among individuals living in a census tract with lower median income.32
We observed that a higher mean summer temperature was associated with an increased HR. The relationship still holds in most of the subgroups. The results are consistent with a previous study on residents in US cities11 and a recent study in New Jersey.32 There is even less evidence regarding the potential mechanisms. An increase in temperature may potentially alter the chemical composition of PM2.5 and its toxicity by changing (1) the emission of precursor gases,43 (2) the rate of atmospheric reactions and the partitioning of semi-volatile organic compounds between gas and particle phase,24 and (3) the removal of aerosols.22
We found that the HR decreased with age. One possible explanation would be hazard ratio measures the association on multiplicative scale instead of additive scale. The baseline hazard is likely to increase substantially with age. Although the association may increase with age on additive scale, the hazard ratios may not be able to show this pattern due to the changes in baseline with age. Future studies estimating the additive effect directly would be helpful to address this issue. By comparison, the effect modification by age found in the ACS study was not substantial.2 The overall HR of males was greater than that for females. In the subgroup analysis, this relationship is dependent on race. This might be related to differences in the distribution of other covariates between males and females. By comparison, the ACS study reported a slightly lower risk for women.2
The basic message from the subgroup analysis is the same as the individual interactions, with some new features. An MI or diabetes admission was consistently associated with an increased HR among all race and sex groups. COPD admission was consistent in most subgroups. A CHF admission increased risk in non-whites, possibly suggesting that access to adequate medical care for heart failure, which requires careful monitoring, is an issue. The association among dual-eligible people is consistent and robust. We found a larger difference among non-whites than whites between dual-eligible enrollees and non-dual-eligible enrollees. We also observed a higher HR among blacks than whites in the subgroup analysis. However, two variables are unlikely to capture all differences in susceptibility between race groups. Some studies have suggested psychosocial stress is an important modifier, which is higher in the black community, but not measured in our study. The interactions with urbanicity were consistent among whites and blacks. These simultaneous interaction models suggested that the difference between urban and rural areas could not be completely explained by race, dual eligibility, sex, or medical records. Other variables that explained the difference between urban and rural areas, such as access to greenness, might be able to explain the difference. The results from these models could be used for further identification and protection of susceptible populations in future revisions of air quality standard.
For PM2.5 components, first, we found that an increase in the proportion of sulfate, OC, or nitrate was associated with a decreased HR for mortality. This result indicates that the secondary inorganic species were less toxic than others, although they represent an important but declining fraction over time of the mass concentration. This is consistent with the result that the HR was lower in rural areas. Second, calcium, from soil or road dust, increased associations with PM2.5, whereas we found little interaction with aluminum, which comes from similar sources. Third, nickel and vanadium are primarily generated from oil combustion. Vanadium was associated with an increased association with PM2.5, whereas there was little interaction with nickel. Fourth, elements from industrial emissions (but also road dust due to tire and brake wear) such as copper and iron added toxicity of PM2.5. Fifth, we found that EC, which comes from traffic emissions, elevated toxicity. By comparison, a previous study in the US cities found null or negative interactions for crustal elements and positive interactions for nickel, vanadium, and EC,6 again suggesting traffic and oil combustion particles are more toxic. A European study did not find associations between long-term exposure to eight elemental components and cardiovascular mortality among 0.3 million participants.20 In the present study, the measurement error for the mass fractions of PM2.5 components certainly limits the interpretations of our results on the interactions with the mass fractions. However, the sensitivity suggests the robustness of the directions of the interactions. The reason why the sensitivity analyses generally had a smaller HR is partly because the monitoring sites might not locate at representative positions for the whole southeast.
The HR of PM2.5 lower than 12 μg m-3, 1.033 (1.031 – 1.035), was larger than the average, suggesting that the concentration-response relationship was not linear with a larger slope at low concentration and a smaller slope at high concentration. Consistently, a study for the northeastern US also found that PM2.5 below the current US EPA standard was associated with increased mortality.38 Both results suggest the existence of effect below the current standard. If the dose–response relationship is steeper below concentrations of 12 μg m-3, this indicates that the health benefit of reducing PM2.5 concentrations, if shown to be causal, goes up as concentrations go down.
We acknowledge that the study has limitations. First, hospital admissions over the period of follow-up may not reflect the actual comorbidity or whether one was admitted for these diseases before enrollment. Second, the dataset does not provide individual-level behavioral data such as smoking, obesity, diet, and alcohol consumption. These individual behavioral variables were not adjusted for in the model. Third, the exposure assessment may be less precise in rural areas than urban areas as there were fewer monitoring sites available in rural areas than urban areas to calibrate the prediction model. Fourth, although the 1 km satellite-based exposure model substantially improved exposure assessment in air pollution epidemiology, future studies could consider to further improve the spatial resolution to capture the local variability of PM2.5.30
In conclusion, we observed increased risk of mortality for long-term PM2.5 exposure among older adults in the southeastern US. Sex, race, dual eligibility, medical history, and neighborhood-level variables modified the association between PM2.5 exposure and mortality. Oil combustion, traffic emission, and some crustal elements were associated with an increased HR. We found larger HRs at pollutant concentrations below current EPA standard for annual average PM2.5.
Supplementary Material
Figure 4.
Effect modification of the association of long-term PM2.5 exposure with mortality among older adults by species-to-PM2.5 ratios covariates including aluminum (Al), calcium (Ca), copper (Cu), elemental carbon (EC), iron (Fe), nitrate, nickel (Ni), organic carbon (OC), sulfate, vanadium (V), and zinc (Zn). The hazard ratios (HR) for each one μg m-3 increase at the 20th and 80th percentile of the modifiers were compared.
Acknowledgments
This publication was made possible by USEPA grant RD-83587201. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication. This publication was also made possible by National Institutes of Environmental Health Sciences (NIEHS) Grant ES-000002, R01 ES024332-01, R21 ES024012, and P50 MD010428-01.
Footnotes
Conflict of interest: None declared.
Data and Code Availability for Replication: Because the Medicare dataset was used under a Data User Agreement, the dataset and code are not available for replication.
References
- 1.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Jr, Speizer FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329(24):1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 2.Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA. 2002;287(9):1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pope CA, 3rd, Ezzati M, Dockery DW. Fine-particulate air pollution and life expectancy in the United States. N Engl J Med. 2009;360(4):376–86. doi: 10.1056/NEJMsa0805646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope CA, 3rd, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW., Jr Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151(3 Pt 1):669–74. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 5.Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA, 3rd, Thurston G, Calle EE, Thun MJ, Beckerman B, DeLuca P, Finkelstein N, Ito K, Moore DK, Newbold KB, Ramsay T, Ross Z, Shin H, Tempalski B. Res Rep Health Eff Inst. 140. 2009. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality; pp. 5–114. discussion 115-36. [PubMed] [Google Scholar]
- 6.Kioumourtzoglou MA, Austin E, Koutrakis P, Dominici F, Schwartz J, Zanobetti A. PM2.5 and survival among older adults: effect modification by particulate composition. Epidemiology. 2015;26(3):321–7. doi: 10.1097/EDE.0000000000000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.USEPA. National Ambient Air Quality Standards for particulate matter. Final rule. Fed Reg. 2013;78:3086–3287. 40 CFR Parts 50, 51, 52 53 and 58. [Google Scholar]
- 8.Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long-term air pollution exposure and cardio- respiratory mortality: a review. Environ Health. 2013;12(1):43. doi: 10.1186/1476-069X-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000-2005) Environ Health Perspect. 2008;116(12):1614–9. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pelucchi C, Negri E, Gallus S, Boffetta P, Tramacere I, La Vecchia C. Long-term particulate matter exposure and mortality: a review of European epidemiological studies. BMC Public Health. 2009;9:453. doi: 10.1186/1471-2458-9-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kioumourtzoglou MA, Schwartz J, James P, Dominici F, Zanobetti A. PM2.5 and mortality in 207 US cities: Modification by temperature and city characteristics. Epidemiology. 2015 doi: 10.1097/EDE.0000000000000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356(5):447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 13.Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. Effect Modification of Long-Term Air Pollution Exposures and the Risk of Incident Cardiovascular Disease in US Women. J Am Heart Assoc. 2015;4(12) doi: 10.1161/JAHA.115.002301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen LH, Knutsen SF, Shavlik D, Beeson WL, Petersen F, Ghamsary M, Abbey D. The association between fatal coronary heart disease and ambient particulate air pollution: Are females at greater risk? Environ Health Perspect. 2005;113(12):1723–9. doi: 10.1289/ehp.8190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD. Long- and short-term exposure to PM2.5 and mortality: using novel exposure models. Epidemiology. 2013;24(4):555–61. doi: 10.1097/EDE.0b013e318294beaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippmann M, Chen LC, Gordon T, Ito K, Thurston GD. National Particle Component Toxicity (NPACT) Initiative: integrated epidemiologic and toxicologic studies of the health effects of particulate matter components. Res Rep Health Eff Inst. 2013;(177):5–13. [PubMed] [Google Scholar]
- 17.Franklin M, Koutrakis P, Schwartz P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology. 2008;19(5):680–9. doi: 10.1097/ede.0b013e3181812bb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai L, Zanobetti A, Koutrakis P, Schwartz JD. Associations of fine particulate matter species with mortality in the United States: a multicity time-series analysis. Environ Health Perspect. 2014;122(8):837–42. doi: 10.1289/ehp.1307568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zanobetti A, Franklin M, Koutrakis P, Schwartz J. Fine particulate air pollution and its components in association with cause-specific emergency admissions. Environ Health. 2009;8:58. doi: 10.1186/1476-069X-8-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang M, Beelen R, Stafoggia M, Raaschou-Nielsen O, Andersen ZJ, Hoffmann B, Fischer P, Houthuijs D, Nieuwenhuijsen M, Weinmayr G, Vineis P, Xun WW, Dimakopoulou K, Samoli E, Laatikainen T, Lanki T, Turunen AW, Oftedal B, Schwarze P, Aamodt G, Penell J, De Faire U, Korek M, Leander K, Pershagen G, Pedersen NL, Ostenson CG, Fratiglioni L, Eriksen KT, Sorensen M, Tjonneland A, Bueno-de-Mesquita B, Eeftens M, Bots ML, Meliefste K, Kramer U, Heinrich J, Sugiri D, Key T, de Hoogh K, Wolf K, Peters A, Cyrys J, Jaensch A, Concin H, Nagel G, Tsai MY, Phuleria H, Ineichen A, Kunzli N, Probst-Hensch N, Schaffner E, Vilier A, Clavel-Chapelon F, Declerq C, Ricceri F, Sacerdote C, Marcon A, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Katsoulis M, Trichopoulou A, Keuken M, Jedynska A, Kooter IM, Kukkonen J, Sokhi RS, Brunekreef B, Katsouyanni K, Hoek G. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects. Environ Int. 2014;66:97–106. doi: 10.1016/j.envint.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Shi L, Kloog I, Zanobetti A, Liu P, Schwartz JD. Impacts of Temperature and its Variability on Mortality in New England. Nat Clim Chang. 2015;5:988–991. doi: 10.1038/nclimate2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenfeld D, Andreae MO, Asmi A, Chin M, de Leeuw G, Donovan DP, Kahn R, Kinne S, Kivekäs N, Kulmala M, Lau W, Schmidt KS, Suni T, Wagner T, Wild M, Quaas J. Global observations of aerosol-cloud-precipitation-climate interactions. Reviews of Geophysics. 2014;52(4):750–808. [Google Scholar]
- 23.Zanobetti A, O'Neill MS, Gronlund CJ, Schwartz JD. Summer temperature variability and long-term survival among elderly people with chronic disease. Proc Natl Acad Sci U S A. 2012;109(17):6608–13. doi: 10.1073/pnas.1113070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takekawa H, Minoura H, Yamazaki S. Temperature dependence of secondary organic aerosol formation by photo-oxidation of hydrocarbons. Atmospheric Environment. 2003;37(24):3413–3424. [Google Scholar]
- 25.Beelen R, Raaschou-Nielsen O, Stafoggia M, Andersen ZJ, Weinmayr G, Hoffmann B, Wolf K, Samoli E, Fischer P, Nieuwenhuijsen M, Vineis P, Xun WW, Katsouyanni K, Dimakopoulou K, Oudin A, Forsberg B, Modig L, Havulinna AS, Lanki T, Turunen A, Oftedal B, Nystad W, Nafstad P, De Faire U, Pedersen NL, Ostenson CG, Fratiglioni L, Penell J, Korek M, Pershagen G, Eriksen KT, Overvad K, Ellermann T, Eeftens M, Peeters PH, Meliefste K, Wang M, Bueno-de-Mesquita B, Sugiri D, Kramer U, Heinrich J, de Hoogh K, Key T, Peters A, Hampel R, Concin H, Nagel G, Ineichen A, Schaffner E, Probst-Hensch N, Kunzli N, Schindler C, Schikowski T, Adam M, Phuleria H, Vilier A, Clavel-Chapelon F, Declercq C, Grioni S, Krogh V, Tsai MY, Ricceri F, Sacerdote C, Galassi C, Migliore E, Ranzi A, Cesaroni G, Badaloni C, Forastiere F, Tamayo I, Amiano P, Dorronsoro M, Katsoulis M, Trichopoulou A, Brunekreef B, Hoek G. Effects of long-term exposure to air pollution on natural-cause mortality: an analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet. 2014;383(9919):785–95. doi: 10.1016/S0140-6736(13)62158-3. [DOI] [PubMed] [Google Scholar]
- 26.Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, Speizer FE, Laden F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect. 2009;117(11):1697–701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexeeff SE, Schwartz J, Kloog I, Chudnovsky A, Koutrakis P, Coull BA. Consequences of kriging and land use regression for PM2.5 predictions in epidemiologic analyses: insights into spatial variability using high-resolution satellite data. J Expo Sci Environ Epidemiol. 2015;25(2):138–44. doi: 10.1038/jes.2014.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beckerman BS, Jerrett M, Serre M, Martin RV, Lee SJ, van Donkelaar A, Ross Z, Su J, Burnett RT. A Hybrid Approach to Estimating National Scale Spatiotemporal Variability of PM2.5 in the Contiguous United States. Environmental Science & Technology. 2013;47(13):7233–7241. doi: 10.1021/es400039u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pope CA, 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116(1):108–15. doi: 10.1161/CIRCRESAHA.116.305060. [DOI] [PubMed] [Google Scholar]
- 30.Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA, Lyapustin A, Wang Y, Schwartz J. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmospheric Environment. 2014;95:581–590. doi: 10.1016/j.atmosenv.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M, Kloog I, Chudnovsky A, Lyapustin A, Wang Y, Melly S, Coull B, Koutrakis P, Schwartz J. Spatiotemporal prediction of fine particulate matter using high-resolution satellite images in the Southeastern US 2003-2011. J Expo Sci Environ Epidemiol. 2015 doi: 10.1038/jes.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Kloog I, Coull BA, Kosheleva A, Zanobetti A, Schwartz JD. Estimating Causal Effects of Long-Term PM Exposure on Mortality in New Jersey. Environ Health Perspect. 2016 doi: 10.1289/ehp.1409671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi L, Liu P, Kloog I, Lee M, Kosheleva A, Schwartz J. Estimating daily air temperature across the Southeastern United States using high-resolution satellite data: A statistical modeling study. Environ Res. 2015;146:51–58. doi: 10.1016/j.envres.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Census Bureau. Summary File 3 (SF 3) 2000 [Google Scholar]
- 35.U.S. Census Bureau. American Community Survey Five-year Estimates 2009-2013. 2013 [Google Scholar]
- 36.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. BRFSS 2013 Survey Data and Documentation. 2013 [Google Scholar]
- 37.Andersen PK, Gill RD. Cox's Regression Model for Counting Processes: A Large Sample Study. The Annals of Statistics. 1982;10(4):1100–1120. [Google Scholar]
- 38.Shi L, Zanobetti A, Kloog I, Coull BA, Koutrakis P, Melly SJ, Schwartz JD. Low-Concentration PM2.5 and Mortality: Estimating Acute and Chronic Effects in a Population-Based Study. Environ Health Perspect. 2016;124(1):46–52. doi: 10.1289/ehp.1409111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.U.S. Census Bureau. The older population: 2010. 2011 [Google Scholar]
- 40.Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, Xu JF. Short term exposure to ambient fine particulate matter (pm2.5) increases hospitalizations and mortality of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Chest. 2015 doi: 10.1378/chest.15-0513. [DOI] [PubMed] [Google Scholar]
- 41.Zanobetti A, Dominici F, Wang Y, Schwartz JD. A national case-crossover analysis of the short-term effect of PM2.5 on hospitalizations and mortality in subjects with diabetes and neurological disorders. Environ Health. 2014;13(1):38. doi: 10.1186/1476-069X-13-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O'Neill MS, Veves A, Sarnat JA, Zanobetti A, Gold DR, Economides PA, Horton ES, Schwartz J. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64(6):373–9. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geron CD, Nie D, Arnts RR, Sharkey TD, Singsaas EL, Vanderveer PJ, Guenther A, Sickles JE, Kleindienst TE. Biogenic isoprene emission: Model evaluation in a southeastern United States bottomland deciduous forest. Journal of Geophysical Research: Atmospheres. 1997;102(D15):18889–18901. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.