Abstract
Objective
To examine the role of psychosocial factors in mediating the relationship between African American (AA) race and both increased pain sensitivity and blunted stress reactivity.
Methods
Participants included 133 AA and non-Hispanic White (nHW) individuals (mean (SD) age = 37 (9)) matched for age, sex and socioeconomic status. Participants underwent mental stress testing (Trier Social Stress Test) while cardiovascular, hemodynamic, and neuroendocrine reactivity were measured. Participants completed questionnaires assessing potential sources of psychosocial stress and were tested for pain responses to cold pain and the temporal summation of heat pulses. Mediation analyses were used to determine the extent to which exposure to psychosocial stress accounted for the observed racial differences in stress reactivity and pain.
Results
Chronic stress exposure and reactivity to mental stress was largely similar among AAs and nHWs; however, AAs exhibited heightened pain to both cold (p = .012) and heat (p = .004). Racial differences in the relationship between stress reactivity and pain were also observed: while greater stress reactivity was associated with decreased pain among nHWs, reactivity was either unrelated to or even positively associated with pain among AAs (e.g. r = −.21 among nHWs and r = .41 among AAs for stroke volume reactivity and cold pressor intensity). Adjusting for minor racial differences in chronic psychosocial stress did not change these findings.
Conclusion
Accounting for psychosocial factors eliminated racial differences in stress reactivity but not racial differences in sensitivity to experimental pain tasks. Increased exposure to chronic stress may not explain AAs’ increased pain sensitivity in laboratory settings.
Keywords: African Americans, pain sensitivity, social factors, stress reactivity, blood pressure, norepinephrine, cortisol
INTRODUCTION
African Americans (AAs) experience more chronic pain, report more pain associated with chronic medical conditions and have poorer pain-related quality of life than Caucasians (see (1) for review); AAs also have lower pain tolerance levels in response to experimental pain tests involving a variety of noxious stimuli (1). Prior work from our laboratory suggests that at least two mechanisms may help explain these racial differences. With regards to the first mechanism, it is known that in both humans and animals, higher blood pressure (BP) is related to reduced pain sensitivity, mediated via BP stimulation of arterial baroreceptors, which, along with reducing BP, also produces antinociceptive effects through the release of endogenous opioids (2) and direct projections to central regions involved in pain regulation (3). Thus, BP levels are inversely related to pain sensitivity in animals (4, 5) and humans (6, 7). Our laboratory was the first to examine racial differences in the relationship between BP and pain sensitivity (8) and find that while Caucasians exhibited the expected positive relationship between BP and pain sensitivity, all relationships involving BP and pain tolerance were low and non-significant in the AAs. We later replicated these findings in a study of AAs and non-Hispanic Whites (nHWs) (9).
These results led us to propose an etiologic model of racial differences in pain sensitivity (8) by which AAs’ greater chronic psychosocial stress exposure may contribute to frequent increases in BP, activation of baroreceptors and, over time, a ‘wear and tear’ on the system (10); such a process would lead to a blunting of the cardiovascular stress response, a desensitization of the baroreflex pathway and an uncoupling of the BP-pain relationship such that the anti-nociceptive effects of baroreceptor stimulation are lessened in AAs. This model would be consistent with the fact that, on average, AAs have lower individual and family socioeconomic status (SES) (11, 12) and are more likely to be the victims of violence (13) and discrimination (14) than nHWs; furthermore, chronic stress is predictive of both chronic pain development and hyperalgesia to acute pain tasks (see (15) for review). The current study aimed to directly test this model of pain sensitivity in AAs by assessing a comprehensive array of psychosocial factors which may modify both cardiovascular stress reactivity and pain sensitivity.
A second mechanism that may explain racial differences in pain sensitivity involves a phenomenon called central sensitization of pain processing, which describes the increase in excitability of central nervous system nociceptive neurons triggered by nociceptive input, leading to a reduction in the stimulation threshold (16). The progressive increase in magnitude of the neuronal response to repetitive stimulation is referred to as temporal summation (17) or ‘wind-up’ and has been interpreted as a process by which input from peripheral nociceptors are amplified in the spinal cord (18). Enhanced temporal summation has been observed in chronic pain patients (19, 20) and pain-free individuals at greater risk for clinical pain (21). We conducted the first study examining racial differences in temporal summation to heat pain and found that AAs exhibited greater temporal summation than nHWs (9). A recent study has since replicated this finding (22). Although one recent study found no racial differences in temporal summation to heat pain among youth ages 10–17 (23), this is consistent with the hypothesis that long-term exposure to chronic stress may explain AAs’ increased pain sensitivity.
The current study matched AAs and nHWs on income, education and occupation, thus removing SES as a potential confound in the relationship between race and pain sensitivity, and assessed multiple indicators of chronic stress and/or stress buffers whose frequency might differ by race. Participants underwent mental stress testing as well as pain testing. We then sought to answer the following questions: 1) Are there racial differences in chronic stress, stress reactivity, pain sensitivity and the stress reactivity-pain relationships? 2) To the extent that racial differences in indicators of chronic stress exist, are these indicators associated with alterations in stress reactivity, pain sensitivity and the relationship between stress reactivity and pain sensitivity? 3) Does adjusting for these indicators of life stress eliminate racial differences in stress reactivity, pain sensitivity and the stress reactivity-pain sensitivity relationship?
Methods
Participants
While a total of 215 participants were recruited for this study, only the 133 participants with complete stress testing and pain testing data were retained for the current analyses. However, participants with incomplete stress or pain testing data did not differ from the others with regards to the baseline characteristics reported in Table 1 (ps = .220–.747).
Table 1.
Participant demographics; mean (SE) or N (%).
| African American | Non-Hispanic White | |
|---|---|---|
| N | 58 | 75 |
| Age (years) | 35.9 (1.1) | 38.6 (1.1) |
| Female | 30 (51.7%) | 32 (42.7%) |
| Education* (1–8; 6 = some college) | 6.8 (0.2) | 6.0 (0.2) |
| Occupation (1–9; 5 = clerical/sales) | 5.7 (0.3) | 5.1 (0.2) |
| Income (1–10; 6 = $35–39.9K) | 5.8 (0.3) | 6.0 (0.4) |
| BDI score | 3.8 (0.5) | 5.8 (0.6) |
| STAI Anxiety Score | 33.1 (1.1) | 34.4 (1.1) |
p<.01 based on a t-test
Based on self-identification, 58 of these participants (52% female) were Black of African descent, and 75 participants (43% female) self-identified as nHW. In order to decrease the likelihood of irregular menstrual cycles, only participants 18 – 50 years of age were enrolled. Women had to have intact ovaries, report regular menstrual cycles (25 – 32 days), and not be pregnant or nursing. All participants were medically healthy, not taking any prescription medication, were not taking over-the-counter medications (e.g., nonsteroidal anti-inflammatory) on a regular basis (> 3 times/month) and were non-smokers (< 10 cigarettes in lifetime). Also excluded were any participants with a chronic pain disorder (e.g. arthritis, fibromyalgia), any cardiovascular disorder, including high BP (≥160 SBP and/or ≥ 90 DBP), with a history of seizure disorders, with hepatic or renal impairment, neuroendocrine disorders (including thyroid), respiratory disorders or gastrointestinal disorders. Participants with Beck Depression Inventory scores > 20 were excluded but were provided with referral information. Special recruitment efforts were initiated to match AAs and nHWs in terms of demographic and socioeconomic variables. As summarized in Table 1, this goal was achieved for age, sex, income and occupation. However, AAs were slightly more educated than nHWs.
Procedure
Screening
After an initial phone screen interview, each participant attended an in-person screening session. During the screening session, informed consent was obtained and questionnaires assessing medical history, psychosocial stress and mental health were administered. Participants were then scheduled for a subsequent laboratory visit. For women, the laboratory test session was scheduled in their follicular phase of the menstrual cycle (days 2 – 10) while men were matched for number of days between screening and testing.
Test Session Overview
Data collection took place from October 2009 to March 2011. In order to control for diurnal effects on stress testing measures, all laboratory testing began at 3:00 p.m. Participants were asked to refrain from all over-the-counter medications for 24 hours and from caffeine for 7 hours prior to testing. AA participants were tested by an AA experimenter while nHW participants were tested by a nHW experimenter. The sequence of laboratory events was as follows: 1) temporal summation procedure (5 min); 2) rest (5 min); 3) CP task (5 min); 4) rest (5 min) 5) BP instrumentation; 6) intravenous setup; 7) venipuncture recovery (20 min); 8) baseline rest (10 min); 9) Trier Social Stress Test (TSST) (25 min); 10) stress recovery (20 min). Order of pain vs. stress testing was fully counterbalanced within each ethnic and gender group in order to control for any possible carry-over effect that one category of procedures (pain versus mental stress testing) may have on responses to the other. However, no effect of testing order on pain sensitivity or stress reactivity was observed. The study protocol was approved by the University of North Carolina’s Institutional Review Board.
Pain Testing
Temporal summation procedure
The temporal summation procedure assesses sensitization to repeated exposure to painful stimuli. The protocol described by Maixner and his team (21, 24), which involves a total of ten 53° C heat pulses were applied to the ventral surface of the right hand at the base of the index finger with the use of a 1-cm contact thermode, was followed. Participants were instructed to rate the intensity of each thermal pulse using a 0 to 100 numerical scale with ‘0’ representing ‘no sensation’, ‘20’ representing ‘just painful’ and ‘100’ representing ‘the most intense pain imaginable’. The procedure was terminated when participants reported a value of ‘100’ or when ten trails elapsed.
Hand cold pressor procedure
The CP test was administered following the standard procedure, described elsewhere (9). Participants were instructed to indicate to the experimenter when the sensations in their hand first became painful (pain threshold) and to also indicate when they were no longer willing or able to tolerate the pain by saying “stop” (pain tolerance). After the participants indicated their pain tolerance but before removing their hand from the ice water bath, participants indicated their pain intensity and unpleasantness ratings out of 100. A maximum time limit of 5 minutes was imposed, though participants were not informed of this limit (25).
Stress Testing
Baseline
Immediately following the IV setup and recovery (20 min), 10 min of quiet rest ensued. BP was measured at minutes 1, 3, 5, 7, and 9 of the rest period and then averaged to constitute baseline levels. Blood was sampled at minute 10 for baseline concentrations of plasma norepinephrine (NE) and cortisol.
The Trier Social Stress Test (TSST)
A modified version of the TSST, which reliably induces large and consistent hypothalamic-pituitary-adrenal (HPA) axis, cardiovascular and NE responses (26, 27), was used. Modifications from the standard procedure included the use of the Paced Auditory Serial Addition Test (PASAT) rather than the traditional subtraction and the immobilization of participants to allow for blood draws. The exact procedure followed is described elsewhere (9) – the only deviation from this description is that recovery was 20 minutes long in the current study rather than 10 minutes as described.
Cardiovascular and Neuroendocrine Sampling during the TSST
The Suntech Exercise BP monitor, Model 4240 (SunTech Medical Instruments, Inc., Raleigh, NC) provided automated measurement of BP during the sessions. Prior to initiating the baseline rest period, five standard stethoscopic BPs were taken simultaneously with the automated pressures in order to ensure correct microphone placement and cuff positioning.
Impedance cardiography was used to noninvasively monitor cardiovascular activity, including cardiac output (CO), stroke volume (SV), total vascular resistance (VR), pre-ejection period (PEP) and heart rate (HR). The exact procedure and equipment used are described elsewhere (28). CO, SV and VR were adjusted for individual variations in body size by using body surface areas to derive cardiac index (CI), stroke volume index (SVI) and vascular resistance index (VRI).
Cardiovascular measures were taken at minutes 1, 3, 5, 7 and 9 of the Speech Preparation Period, minutes 1, 3 and 5 of Speech, and minutes 1,3 and 5 of the PASAT and averaged to yield task levels. NE was sampled at the end of minute 2 of Speech delivery and at the end of minute 2 of serial addition since catecholamines peak within the first minutes of stress and have a very short half-life (3 min) (29). Cortisol was sampled immediately after and at 10 and 20 minutes following the end of the stress since peak cortisol is reliably found 10 – 30 min after cessation of the TSST (26). β-endorphin was sampled immediately after the TSST, at which time we would expect levels to peak (30).
Neuroendocrine Assays
Plasma Norepinephrine (NE)
NE was determined using RIA in the UNC Endocrine Assay Lab of the Psychiatry department. The lower limit of quantification with this system is 10 pg/ml, and the intra- and interday coefficients of variation are less than 10%.
Plasma cortisol
Cortisol was determined using radioimmunoassay (RIA) techniques commercially available from ICN Biomedical, Inc. Sensitivity of the assay is excellent at 0.07 ug/dL, and the specificity high, showing 0.05 – 2.2% cross-reactivity with similar compounds, except predinisolone, where 94% cross-reactivity is obtained.
Plasma β-endorphins
β-endorphins in EDTA plasma was determined following extraction by RIA using a kit from INCSTAR Corporation (Stillwater, Minnesota). The intra- and inter-assay coefficients of variation from the assay are approximately 10% and 15%, respectively, and the assay sensitivity is 3 pmol/L.
Psychosocial Measures
Personal and family health history
This questionnaire assessed the participant’s personal and family health history, including information on cardiovascular and other diseases, illnesses and medication.
Socioeconomic status (SES)
Three indicators of family SES were used: education, gross household income (GHI), and occupation. Education was scored on a scale from 1 to 8, with 1 corresponding to 0–4 grades and 8 corresponding to post-graduate work at a university. GHI was based on total household income (e.g. from earnings, unemployment or workers compensation, Social Security, alimony, child support, etc.) during the preceding calendar year. For occupational status, we relied on the Hollingshead Codes where job categories are ranked from 0 to 9 (31).
Depressive symptoms
The Beck depression inventory (BDI) (32) was used to measure depressive symptoms (33).
Trait anxiety
The 20-item self-evaluation questionnaire STAI form Y-2 was used to measure how anxious a participant generally feels (34).
Perceived ethnic discrimination
The Lifetime Exposure Scale of the Perceived Ethnic Discrimination Questionnaire-Community Version (PEDQ-CV) (35) was used to assess perceived discrimination, though it was modified to ask about perceived discrimination in the past two years in order to make these responses temporally contiguous with the other measures of chronic stress.
Lifetime abuse
Participants were asked about sexual and physical abuse histories using a modified version of a validated interview developed by Dr. Jane Leserman (36–38). The details of what constitutes sexual and physical abuse are described elsewhere (39). Physical abuse was only counted if the incident occurred separately from sexual abuse.
Stressful life events
Recent stressful life events were assessed by interview using a modified Life Events Survey (LES) (40) that assessed the presence of stressful events during the 6 months before the baseline assessment. The list had been modified to include only those events that are considered moderately to severely stressful based on previous studies with interviewer-based objectively rated stresses (41–43). In addition, a single “total negative life events” score was calculated by multiplying the total number of stressful life events and the sum of perceived stressfulness ratings, which are on a 5-point scale from “not stressful” to “extremely stressful”.
Social support
The Interpersonal Social Evaluation List (ISEL) (44) was used to measure social support, which assesses the perceived availability of four separate domains of social support: tangible (perceived availability of material aid); appraisal (the perceived availability of someone to talk to); belonging (the perceived availability of people one can do things with); and self-esteem (the perceived availability of a positive comparison when comparing one’s self to others).
Religious Involvement
The following dimension of religious involvement were measured using the multidimensional measure of religious involvement for African Americans developed by Chatters and her colleagues, (45, 46): the following dimensions the frequency of attendance at religious service, frequency of prayer, perceived importance of faith or spiritual beliefs and perceived help received from the members of their place of worship.
Coping
To assess coping styles, the COPE Inventory (47) was used, which includes fourteen subscales (of four items each): active coping, planning, suppression of competing activities, restraint coping, seeking of instrumental social support, seeking of emotional support, positive reinterpretation, acceptance, denial, turning to religion, focus on and venting emotions, behavioral disengagement, mental disengagement and alcohol-drug disengagement.
Trait Anger
Four subscales from the Siegel Multidimensional Anger Inventory (48) were used to measure trait anger: anger in, anger out, anger-arousal and range of anger-eliciting situations.
Statistical Analyses
Data were analyzed using analysis of variance and multivariate regression analysis. To test whether racial differences in stress reactivity and pain sensitivity were mediated by racial differences in exposure to chronic stress, we used the methods proposed by Baron & Kenny (49) to answer three primary questions (see Supplemental Digital Content 1 for details): 1) Are there racial differences in chronic stress, stress reactivity, pain sensitivity and the stress reactivity-pain relationship?; 2) To the extent that there are racial differences in indicators of chronic stress, are these indicators associated with alterations in stress reactivity and pain sensitivity? and 3) Does adjusting for indicators of life stress eliminate racial differences in stress reactivity, pain sensitivity and the stress reactivity-pain sensitivity relationship?
RESULTS
Racial Differences in Chronic Stress
As summarized in Table 2, AAs reported more perceived discrimination, more childhood sexual abuse (but less childhood physical abuse), and less social support. AAs reported more restraint and turning to religion as coping strategies as well as higher levels of church membership and church support; however, nHWs reported higher frequencies of church attendance and prayer and a greater importance of faith. AAs and nHWs did not differ with regards to recent stressful life events or anger management. Upon adjustment of p-values within each of the seven groups of variables presented, the relationships between race and appraisal social support and restraint coping disappeared; however, all relationships with discrimination, abuse history, and religious involvement remained significant with p < 0.05.
Table 2.
Racial differences in psychosocial stress-related factor as determined using multiple regression, adjusting for education; mean (SE) or %.
| African American | Non-Hispanice White | p | |
|---|---|---|---|
| Recent Stressful Life Events | |||
| Total number of events | 2.3 (0.3) | 2.0 (0.2) | .345 |
| Total Number X severity | 8.2 (1.0) | 7.1 (0.9) | .432 |
| Discrimination (1–5) | |||
| Ethnic exclusion | 1.76 (0.08) | 1.38 (0.07) | <.001 |
| Stigmatization | 1.67 (0.08) | 1.25 (0.07) | <.001 |
| Workplace discrimination | 1.70 (0.10) | 1.24 (0.08) | <.001 |
| Threat | 1.25 (0.06) | 1.15 (0.05) | .208 |
| Mean discrimination score | 1.65 (0.07) | 1.29 (0.06) | <.001 |
| Social Support (0–30) | |||
| Appraisal | 22.0 (0.6) | 24.0 (0.5) | .019 |
| Tangible | 23.0 (0.7) | 25.6 (0.6) | .005 |
| Self-esteem | 21.6 (0.5) | 21.9 (0.4) | .635 |
| Belonging | 24.0 (0.6) | 23.3 (0.5) | .426 |
| Abuse History (%) | |||
| Child physical abuse | 7.5% | 22.8% | .003 |
| Child sexual abuse | 25.5% | 10.5% | .005 |
| Adult physical abuse | 21.1% | 31.5% | .086 |
| Adult sexual abuse | 17.1% | 12.8% | .386 |
| Coping (1–4) | |||
| Active coping | 3.1 (0.1) | 3.1 (0.1) | .707 |
| Planning | 3.4 (0.1) | 3.3 (0.1) | .641 |
| Suppression of competing activities | 2.7 (0.1) | 2.5 (0.1) | .071 |
| Restraint coping | 2.7 (0.1) | 2.5 (0.1) | .016 |
| Instrumental social support | 3.0 (0.1) | 3.0 (0.1) | .887 |
| Emotional social support | 2.8 (0.1) | 2.9 (0.1) | .902 |
| Positive reinterpretation | 3.4 (0.1) | 3.2 (0.1) | .069 |
| Acceptance | 2.8 (0.1) | 2.8 (0.1) | .969 |
| Turning to religion | 3.4 (0.1) | 2.2 (0.1) | <.001 |
| Venting | 2.2 (0.1) | 2.3 (0.1) | .340 |
| Denial | 1.3 (0.1) | 1.2 (0.1) | .221 |
| Behavioral disengagement | 1.6 (0.1) | 1.4 (0.1) | .060 |
| Mental disengagement | 2.2 (0.1) | 2.1 (0.1) | .605 |
| Alcohol-Drug Disengagement | 1.1 (0.1) | 1.2 (0.1) | .568 |
| Religious Involvement | |||
| Frequency of church attendance | 2.9 (0.1) | 4.0 (0.1) | <.001 |
| Frequency of prayer | 1.8 (0.2) | 3.7 (0.2) | <.001 |
| Importance of faith | 1.2 (0.1) | 2.1 (0.1) | <.001 |
| Church membership | 0.6 (0.1) | 0.3 (0.1) | <.001 |
| Church support | 2.1 (0.2) | 1.2 (0.2) | .006 |
| Anger Management | |||
| Anger-arousal (12–60) | 25.0 (1.1) | 25.6 (0.9) | .694 |
| Range of anger-eliciting situations (9–45) | 25.2 (1.0) | 23.5 (0.9) | .220 |
| Anger-Out (4–20) | 12.5 (0.3) | 12.7 (0.2) | .571 |
| Anger-In (6–30) | 12.1 (0.6) | 12.8 (0.5) | .378 |
Racial Differences in Stress Reactivity
Systolic BP, Diastolic BP and HR all significantly increased in response to the mental stressors (ps<.001) as did plasma NE (p < .001), CI and SVI (ps<.001). There was a significant decrease in VRI (p<.001) but no effect of the mental stress on plasma cortisol (p = .884).
As summarized in Table 3, adjusting for education, AAs had higher baseline systolic BP (p = .022), lower baseline cortisol levels (p = .044) and lower cortisol AUCg (p = .019). Adjusting for education and baseline HR, AAs also had lower HR reactivity (p = .040). However, upon p-value adjustment for multiple testing, only the racial difference in cortisol AUCg remained significant. There were no statistically significant racial differences in any of the other reactivity measures.
Table 3.
Racial differences in mean (SE) stress reactivity and baseline cardiovascular and neuroendocrine measures as determined by multiple regression adjusting for education and baseline cardiovascular or neuroendocrine measure.
| African American | Non-Hispanic White | p | |
|---|---|---|---|
| SBP (mmHg) | |||
| Baseline | 122.4 (1.7) | 117.2 (1.5) | .022 |
| Reactivity | 21.4 (1.7) | 24.0 (1.4) | .257 |
| DBP (mmHg) | |||
| Baseline | 70.0 (1.2) | 71.3 (1.1) | .418 |
| Reactivity | 14.6 (1.1) | 14.5 (0.9) | .960 |
| HR (bpm) | |||
| Baseline | 68.0 (1.4) | 66.4 (1.3) | .412 |
| Reactivity | 12.4 (1.1) | 15.4 (0.9) | .040 |
| CI (l/min) | |||
| Baseline | 3.4 (0.1) | 3.2 (0.1) | .283 |
| Reactivity | 0.8 (0.1) | 1.0 (0.1) | .127 |
| SVI (ml/beat per M2) | |||
| Baseline | 50.5 (2.1) | 48.4 (1.8) | .478 |
| Reactivity | 2.6 (0.9) | 3.1 (0.8) | .693 |
| VRI (dyne s cm−5 M2) | |||
| Baseline | 2277.7 (112.0) | 2365.0 (98.0) | .566 |
| Reactivity | −139.9 (50.4) | −210.4 (44.1) | .305 |
| Norepinephrine (pg/ml) | |||
| Baseline | 424.0 (22.8) | 446.4 (20.0) | .469 |
| Reactivity | 40.9 (15.9) | 53.3 (13.9) | .565 |
| AUCg (pg/ml*min) | 7636.2 (370.4) | 8091.3 (324.1) | .366 |
| Cortisol (pg/ml) | |||
| Baseline | 6.9 (0.6) | 8.4 (0.5) | .044 |
| Reactivity | −0.4 (0.3) | 0.3 (0.3) | .145 |
| AUCg (pg/ml*min) | 262.5 (22.4) | 335.0 (19.6) | .019 |
| Beta-endorphins (pmol/L) | 0.1 (0.0) | 0.1 (0.0) | .489 |
Racial Differences in Pain Sensitivity
Cold Pressor
Adjusting for education, AAs exhibited lower pain tolerance during the CP (p = .009; M = 37.9±9.0 vs. 70.4±7.9 sec.) and rated the pain as being more intense (p = .012; M = 63.8±2.7 vs. 54.3±2.4). However, neither pain threshold (onset) (p = .929) nor unpleasantness (p = .148) differed by race.
Temporal Summation
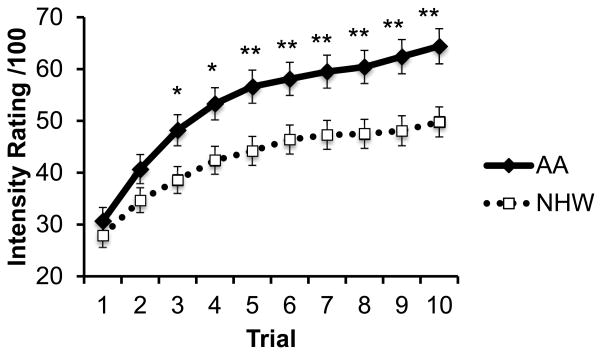
Adjusting for education, AAs reported greater pain in response to the Temporal Summation pain task. Repeated measures analyses revealed an overall effect of race on pain ratings throughout the ten trials (p = .008) as well as a significant time by race effect (p = .022) such that the effect of race progressively increased across the trials (Figure 1). In line with this, there was a larger difference between the minimum and maximum pain ratings (i.e. ‘wind-up’) among AAs than nHWs (p = .004).
Figure 1.
Racial differences in intensity ratings during temporal summation procedure, adjusting for education. Standard error bars represent ±1 standard error from the mean. *p<.05; **p<.01
Racial Differences in the Stress Reactivity-Pain Relationship
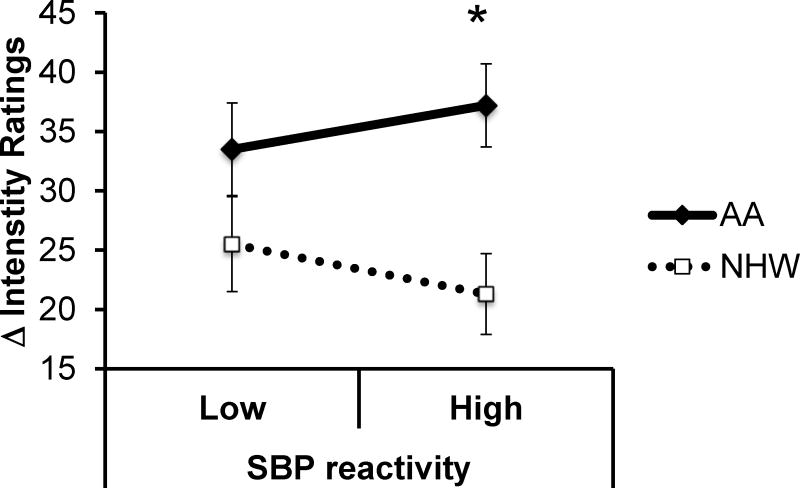
There were nine significant racial differences in the association between indicators of stress reactivity and pain sensitivity (seen bolded in Table 4). Relationships between NE AUCg and CP threshold, SVI and CP intensity, VRI and CP intensity, and SBP and wind-up remained significant (p<0.05) following correction for multiple testing within each of the five outcomes. All associations (NE AUCg and CP threshold (p = .004), CI and CP intensity (p = .001), SVI and CP intensity (p <.001), VRI and CP intensity (p = .002), SBP and wind-up (p = .006), CI and wind-up (p = .034), SVI and wind-up (p = .038), PEP reactivity and wind-up (p = .032) and NE AUCg and wind-up (p = .031)), suggest that the expected negative relationship between reactivity and pain sensitivity can be observed among nHWs; however, increased reactivity is either unrelated to pain or even positively related to pain sensitivity among AAs. Figure 2 illustrates the association between SBP reactivity and wind-up to heat pain as a function of race while Figure 3 illustrates the association between CI reactivity and CP intensity by race. It should be noted that in both cases, stress reactivity and pain sensitivity were analyzed as continuous variables but have been split at the median for illustration purposes.
Table 4.
Racial differences in the relationship between stress reactivity and pain sensitivity as determined by partial correlations adjusting for education.
| CP Tolerance | CP threshold | CP intensity | CP unpleasantness | Central sensitization | ||
|---|---|---|---|---|---|---|
| SBP reactivity | AA | −.04 | −.06 | −.03 | −.02 | .18 |
| nHW | .15 | .24* | −.07 | −.17 | −.30** | |
| DBP reactivity | AA | −.06 | −.08 | −.09 | .05 | .11 |
| nHW | .26* | .22 | −.13 | −.23* | −.21 | |
| HR reactivity | AA | −.05 | −.01 | .08 | −.13 | .14 |
| nHW | −.01 | .23* | −.09 | −.08 | −.07 | |
| CI reactivity | AA | −.10 | −.01 | .36** | −.06 | .30* |
| nHW | .20 | .20 | −.20 | −.24* | −.07 | |
| SVI reactivity | AA | −.10 | −.01 | .41** | .01 | .32* |
| nHW | .17 | .11 | −.21 | −.18 | −.04 | |
| VRI reactivity | AA | .09 | −.01 | −.34* | .05 | −.18 |
| nHW | −.01 | −.10 | .18 | .17 | −.10 | |
| PEP reactivity | AA | .03 | .06 | −.08 | .10 | −.21 |
| nHW | −.07 | −.24* | .16 | .13 | .17 | |
| Cortisol AUCg | AA | .16 | .33* | −.25 | −.21 | .00 |
| nHW | .03 | .15 | .05 | −.06 | −.12 | |
| NE AUCg | AA | .07 | −.13 | −.13 | −.04 | .13 |
| nHW | .08 | .37* | −.16 | −.20 | −.25* | |
| Beta endorphins | AA | .10 | .08 | −.23 | −.21 | −.17 |
| nHW | .14 | .05 | −.18 | −.24* | −.26* |
Bolded coefficients indicate significant racial differences.
p<.05;
p<.01
Figure 2.
Central sensitization by race and SBP reactivity, adjusting for education and baseline SBP. Standard error bars represent ±1 standard error from the mean. *p<.05
Figure 3.
Cold pressor intensity by race and CI reactivity, adjusting for education and baseline CI. Standard error bars represent ±1 standard error from the mean. **p<.001
Chronic Stress Indicators and Associations With Alterations in Stress Reactivity and Pain Sensitivity
Stepwise regression models predicting stress reactivity and pain sensitivity in the whole sample (collapsing across race) included the following predictor variables: education, childhood sexual abuse, childhood physical abuse, total perceived discrimination score, appraisal social support, tangible social support, coping strategies found to differ by race (restraint coping and turning to religion) and all five aspects of religious involvement (frequency of church attendance, frequency of prayer, importance of faith, church membership and church support). In addition, age and sex were forced into all models to ensure that any effect of psychosocial stressors was not confounded by its relationship with age and/or sex, both of which are associated with stress reactivity and pain sensitivity.
Stress Reactivity
As shown in Table S1, Supplemental Digital Content 1, education, tangible social support and importance of faith were all associated with at least one indicator of increased reactivity to stress. In contrast, perceived discrimination, restraint coping, which involves coping passively by holding back coping attempts until they can be useful, turning to religion as a coping strategy, church attendance and church support were all associated with decreased reactivity (Table S1).
Pain Sensitivity
Discrimination was associated with decreased CP pain tolerance while childhood sexual abuse, church attendance and church support were associated with increased CP pain intensity. Importance of faith was negatively associated with wind-up.
Adjustments for Life Stress Indicators—Impact on Racial Differences in Stress Reactivity, Pain Sensitivity, and the Relationship between Stress Reactivity and Pain Sensitivity
Stress Reactivity
Adjusting for education and the one variable associated with cortisol AUCg and found to differ by race – turning to religion – the previously observed racial difference in AUCg was no longer significant (p = .219). However, turning to religion was not a significant predictor of cortisol AUCg (p = .104) when included in the same model as race, thus providing evidence against mediation. Similarly, adjusting for education and the one variable associated with HR reactivity and found to differ by race – turning to religion – the previously observed racial difference in HR reactivity was no longer significant (p = .367). However, turning to religion was not a significant predictor of HR reactivity (p = .079) when included in the same model as race, thus providing evidence against mediation.
Pain Sensitivity
Adjusting for education and the first variable associated with both race and pain intensity – childhood sexual abuse – AAs continued to report increased pain intensity (p = .028) and childhood sexual abuse continued to be significantly associated with pain intensity (p = .028), suggestive of partial mediation. Adjusting for childhood sexual abuse reduced the β associated with race from 9.4 to 8.2. Adjusting for education and the second variable associated with CP intensity and found to differ by race – church attendance – the previously-observed racial difference in AUCg was no longer significant (p = .117). However, turning to religion was not a significant predictor of cortisol CP pain intensity (p = .115) when included in the same model as race, thus providing evidence against mediation. Adjusting for education and the third variable associated with race and pain intensity – church support – AAs continued to report higher pain intensity than nHWs (p = .004). Church support did not continue to be a significant predictor of CP pain intensity (p = .077), providing evidence against even partial mediation.
Adjusting for education and the one variable differing by race and associated with CP tolerance – perceived discrimination – AAs continued to exhibit decreased pain tolerance (p = .041). Discrimination was not associated with tolerance (p = .119) when included in the same model as race, thus ruling out partial mediation. The other indicators of pain sensitivity were not examined because they were either not associated with race (CP threshold) or were not associated with any psychosocial stressors (CP unpleasantness).
Additional sensitivity analyses were conducted to determine whether cortisol AUCg or HR reactivity might mediate the relationship between race and CP intensity. It was found that when both race and cortisol AUCg were included in the same regression model predicting CP intensity, race remained a significant predictor (p = .020) but cortisol did not (p = .517). Thus, cortisol AUCg as a mediator in the relationship between race and CP intensity is not supported. Similarly, when both race and HR reactivity were included in the same regression model predicting CP intensity, race remained a significant predictor (p = .017) but HR reactivity did not (p = .899).
Stress Reactivity – Pain Sensitivity Relationship
Where significant correlations existed between stress reactivity and pain sensitivity relationships in either race group, the correlation between each pair of variables was re-calculated while adjusting for education and any psychosocial stressor found to be associated with either variable in the pair. With the exception of the relationship between CI reactivity and central sensitization, which was no longer significant when adjusting for perceived discrimination and tangible social support (p = .159), all pairs of correlation coefficients that had been found to significantly differ by race (bolded in Table 4) continued to significantly differ.
Discussion
The current study examined the role of chronic stress in explaining racial differences in pain sensitivity. More specifically, it tested the validity of a proposed etiologic model of racial differences in pain sensitivity in which the chronic psychosocial stress to which AAs are disproportionately exposed, over time, results in blunted stress reactivity and an uncoupling of the BP-pain relationship; the model proposes that because of this, AAs benefit less from the anti-nociceptive effects of BP stimulation of the arterial baroreceptors.
Our results support portions of this proposed etiologic model. First, in line with our model, despite equivalency in SES, AAs reported somewhat more psychosocial stress than nHWs. However, this difference was admittedly less than expected and mainly specific to discrimination and lack of tangible social support. Second, we found that in both AAs and nHWs, chronic psychosocial stressors such as discrimination were, in fact, associated with blunted stress reactivity. Third, stress reactivity was similar among AAs and nHWs, suggesting that most racial differences in stress reactivity are eliminated when controlling for racial differences in income, occupation and education. Thus, we might conclude that chronic stress, mostly resulting from low SES, may help explain why previous research has observed blunted cardiovascular and norepinephrine stress reactivity among AAs (50). Such a mechanism would be consistent with an allostatic load model of adaptation to chronic or traumatic stress (51) and previous studies linking chronic (39) or traumatic (52) stress exposure to blunted stress reactivity
Also in line with our previous research and our proposed model, we found AAs to exhibit decreased pain tolerance, but not a lower pain threshold, during the CP task (8, 9). Furthermore, we replicated our previous observation that AAs exhibit greater temporal summation of heat pain intensity (9), suggesting there may be ethnic differences in the temporal integration of painful stimuli by the central nervous system. Also consistent with our proposed model and previous research (8), in the current study we observed racial differences in the degree to which stress reactivity and pain sensitivity are related. Among nHWs, the expected analgesic effect of greater cardiovascular stress reactivity was observed. However, among AAs, stress reactivity and pain sensitivity are either unrelated or even positively associated. This is consistent with the observation that AAs may differ in BP regulatory mechanisms (53–55) and other research showing decreased baroreceptor function during sleep in AAs (56) and abnormal BP responses to postural challenge, indicative of alterations in baroreceptor function (57), relative to Caucasians. However, the mechanisms underlying this altered relationship between cardiovascular stress reactivity and pain perception in AAs is unknown.
Consistent with our proposed model, childhood sexual abuse was a partial mediator in the relationship between race and CP pain intensity. This finding is consistent with previous literature linking childhood adversity with clinical pain syndromes (58). However, stress-induced blunting of the stress response, leading to an uncoupling of the BP-pain relationship, does not appear to be the mechanism underlying the link between childhood sexual abuse and pain sensitivity, suggesting another mechanism may be at play. One possibility may be related to epigenetic changes in other pain regulatory processes. To our knowledge, epigenetic mechanisms have yet to be investigated in linking childhood abuse and chronic pain. However, preliminary evidence from animal models suggests that epigenetic mechanisms may influence pain sensitivity through inflammatory pathways and cortical pain processing (59); furthermore, early life adversity is associated with epigenetic changes in relation to other syndromes, namely depression (60). However, the effect size associated with race’s effect on CP pain intensity changed relatively little when childhood sexual abuse was included in the statistical model, suggesting its role in explaining racial differences in pain sensitivity is relatively small.
Since, for the most part, racial differences in chronic psychosocial stress were eliminated by matching AAs and nHWs in terms of SES and adjusting for the few remaining racial differences did not appear to account for racial differences in pain sensitivity or the stress reactivity-pain relationship, there may be a role for genetic influences. Some studies suggest that genetic polymorphisms coding for mu opioid receptors (61, 62), which have the highest affinity for β-endorphins, vary by race; ethnicity may also interact with mu opioid receptor genotype such that the G allele of the A118G SNP is associated with decreased pain sensitivity among nHWs but not AAs (63). However, we found no racial differences in the association between β-endorphin levels and pain perception, though this was based on a single stress measure. Other endogenous opioids such as enkephalins or dynorphins may also, through mu opioid receptor activation, influence racial differences in pain sensitivity. Also of relevance is one study finding that European American women with the Val585 allele of the vanilloid receptor subtype 1 gene, whose function is to detect and regulate body temperature, exhibited significantly higher tolerance to the CP compared to other polymorphisms. However, the Val585 allele was not associated with increased pain tolerance among AA women (64). Thus, genetic polymorphisms involved in pain perception may differ in their effect among AAs and nHWs and thus help explain racial differences in pain sensitivity. Further research exploring genetic differences that may influence pain perception is clearly needed.
The current study should be interpreted in light of its limitations. First, because we did not assess subjective mood during the TSST, we could not assess the effects of subjective responses to stress on physiologic stress reactivity. Second, we did not assess physical fitness levels, which can influence both pain sensitivity (65) and stress reactivity (66, 67). Third, future research should include other ethnic minorities such as Hispanics, who exhibit similar pain responses as AAs (68). Finally, due to the large number of comparisons and, therefore, an increased risk of Type 1 error, caution should be exercised in interpreting the current study’s findings. When a stepwise Bonferroni correction was applied to minimize the experiment-wide error rate, several findings were no longer significant: nHWs and AAs no longer differed in terms of baseline SBP, baseline cortisol or HR reactivity, appraisal social support and their endorsement of various coping strategies. Furthermore, several instances in which the relationship between stress reactivity and pain sensitivity differed between nHWs and AAs were no longer significant. Nonetheless, the study’s overall conclusions remain unchanged.
In conclusion, the current study suggests that, consistent with previous research, AAs exhibit decreased pain tolerance and report greater pain intensity during the CP task as well as increased central sensitization to heat pain. Furthermore, AAs exhibit an altered relationship between cardiovascular stress reactivity and pain. However, contrary to our predictions, increased exposure to psychosocial stress does not appear to account for these ethnic differences. Thus, future research investigating possible genetic differences that may help account for racial differences in pain sensitivity are warranted.
Supplementary Material
Acknowledgments
Source of Funding: This research was supported by NIDA grants R01 DA013705 and F31 DA023009. Dr. Gordon is also the recipient of a Postdoctoral Fellowship of the Fonds de la Recherche du Québec – Santé (FRQS).
Abbreviations used
- AA
African American
- nHW
Non-Hispanic White
- CP
cold pressor
- SES
socioeconomic status
- TSST
Trier Social Stress Test
- HR
heart rate
- BP
blood pressure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- PEP
pre-ejection period
- CO
cardiac output
- CI
cardiac index
- SV
stroke volume
- SVI
stroke volume index
- VR
vascular resistance
- VRI
vascular resistance index
- AUCg
area under the curve with respect to ground
Footnotes
Conflicts of Interest: There are no conflicts of interest to disclose.
References
- 1.Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94:133–7. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- 2.France CR, Ditto B. Risk for high blood pressure and decreased pain perception. Current Directions in Psychological Science. 1996:120–5. [Google Scholar]
- 3.Bruehl S, Chung OY. Interactions between the cardiovascular and pain regulatory systems: an updated review of mechanisms and possible alterations in chronic pain. Neuroscience & Biobehavioral Reviews. 2004;28:395–414. doi: 10.1016/j.neubiorev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Maixner W, Touw KB, Brody MJ, Gebhart GF, Long JP. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res. 1982;237:137–45. doi: 10.1016/0006-8993(82)90562-5. [DOI] [PubMed] [Google Scholar]
- 5.Dworkin BR, Filewich RJ, Miller NE, Craigmyle N, Pickering TG. Baroreceptor activation reduces reactivity to noxious stimulation: implications for hypertension. Science. 1979;205:1299–301. doi: 10.1126/science.472749. [DOI] [PubMed] [Google Scholar]
- 6.Bruehl S, Carlson CR, McCubbin JA. The relationship between pain sensitivity and blood pressure in normotensives. Pain. 1992;48:463–7. doi: 10.1016/0304-3959(92)90099-W. [DOI] [PubMed] [Google Scholar]
- 7.Ghione S. Hypertension-Associated Hypalgesia evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 8.Mechlin MB, Maixner W, Light KC, Fisher JM, Girdler SS. African Americans show alterations in endogenous pain regulatory mechanisms and reduced pain tolerance to experimental pain procedures. Psychosomatic medicine. 2005;67:948–56. doi: 10.1097/01.psy.0000188466.14546.68. [DOI] [PubMed] [Google Scholar]
- 9.Mechlin B, Heymen S, Edwards CL, Girdler SS. Ethnic differences in cardiovascular-somatosensory interactions and in the central processing of noxious stimuli. Psychophysiology. 2011;48:762–73. doi: 10.1111/j.1469-8986.2010.01140.x. [DOI] [PubMed] [Google Scholar]
- 10.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress: elaborating and testing the concepts of allostasis and allostatic load. Annals of the New York Academy of Sciences. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 11.Anderson NB, McNeilly M, Myers H. Toward understanding race differences in autonomic reactivity: A proposed contextual model. In: Turner JR, Sherwood A, Light KC, Turner JR, Sherwood A, Light KC, editors. Individual Differences in Cardiovascular Response to Stress. New York, NY, US: Plenum Press; 1991. pp. 125–45. [Google Scholar]
- 12.Troxel WM, Matthews KA, Bromberger JT, Sutton-Tyrrell K. Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychology. 2003;22:300. doi: 10.1037/0278-6133.22.3.300. [DOI] [PubMed] [Google Scholar]
- 13.Crouch JL, Hanson RF, Saunders BE, Kilpatrick DG, Resnick HS. Income, race/ethnicity, and exposure to violence in youth: Results from the national survey of adolescents. Journal of Community Psychology. 2000;28:625–41. [Google Scholar]
- 14.Schulz A, Williams D, Israel B, Becker A, Parker E, James SA, Jackson J. Unfair treatment, neighborhood effects, and mental health in the Detroit metropolitan area. Journal of Health and Social Behavior. 2000:314–32. [PubMed]
- 15.Jennings EM, Okine BN, Roche M, Finn DP. Stress-induced hyperalgesia. Progress in neurobiology. 2014;121:1–18. doi: 10.1016/j.pneurobio.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983 doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 17.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3:57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 18.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Progress in neurobiology. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 19.Lluch E, Torres R, Nijs J, Van Oosterwijck J. Evidence for central sensitization in patients with osteoarthritis pain: a systematic literature review. European Journal of Pain. 2014;18:1367–75. doi: 10.1002/j.1532-2149.2014.499.x. [DOI] [PubMed] [Google Scholar]
- 20.English B. Neural and Psychosocial Mechanisms of Pain Sensitivity in Fibromyalgia. Pain Management Nursing. 2014;15:530–8. doi: 10.1016/j.pmn.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–7. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 22.Goodin BR, Bulls HW, Herbert MS, Schmidt J, King CD, Glover TL, Sotolongo A, Sibille KT, Cruz-Almeida Y, Staud R. Temporal summation of pain as a prospective predictor of clinical pain severity in adults aged 45 years and above with knee osteoarthritis: ethnic differences. Psychosomatic medicine. 2014;76:302. doi: 10.1097/PSY.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris MC, Walker L, Bruehl S, Hellman N, Sherman AL, Rao U. Race effects on temporal summation to heat pain in youth. Pain. 2015;156:917–22. doi: 10.1097/j.pain.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maixner W, Fillingim R, Sigurdsson A, Kincaid S, Silva S. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain: evidence for altered temporal summation of pain. Pain. 1998;76:71–81. doi: 10.1016/s0304-3959(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 25.Klatzkin RR, Mechlin B, Bunevicius R, Girdler SS. Race and histories of mood disorders modulate experimental pain tolerance in women. The Journal of Pain. 2007;8:861–8. doi: 10.1016/j.jpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirschbaum C, Pirke K-M, Hellhammer DH. The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- 27.Kirschbaum C, Strasburger C, Langkrär J. Attenuated cortisol response to psychological stress but not to CRH or ergometry in young habitual smokers. Pharmacology Biochemistry and Behavior. 1993;44:527–31. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- 28.Gordon JL, Girdler SS. Mechanisms underlying hemodynamic and neuroendocrine stress reactivity at different phases of the menstrual cycle. Psychophysiology. 2014;51:309–18. doi: 10.1111/psyp.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dimsdale J, Ziegler M. What do plasma and urinary measures of catecholamines tell us about human response to stressors? Circulation. 1991;83:II36–42. [PubMed] [Google Scholar]
- 30.Young EA, Lopez JF, Murphy-Weinberg V, Watson SJ, Akil H. Hormonal evidence for altered responsiveness to social stress in major depression. Neuropsychopharmacology. 2000;23:411–8. doi: 10.1016/S0893-133X(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 31.Deonandan R, Campbell K, Ostbye T, Tummon I, Robertson J. A comparison of methods for measuring socio-economic status by occupation or postal area. Chronic Dis Can. 2000;21:114–8. [PubMed] [Google Scholar]
- 32.Beck AT, Beamesderfer A. Assessment of depression: the depression inventory. S. Karger; 1974. [DOI] [PubMed] [Google Scholar]
- 33.Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical psychology review. 1988;8:77–100. [Google Scholar]
- 34.Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): comparison to the State-Trait Anxiety Inventory (STAI) Psychological assessment. 2007;19:369. doi: 10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- 35.Brondolo E, Kelly KP, Coakley V, Gordon T, Thompson S, Levy E, Cassells A, Tobin JN, Sweeney M, Contrada RJ. The perceived ethnic discrimination questionnaire: Development and preliminary validation of a community version. Journal of Applied Social Psychology. 2005;25:335–65. [Google Scholar]
- 36.Leserman J, Drossman DA, Li Z. The reliability and validity of a sexual and physical abuse history questionnaire in female patients with gastrointestinal disorders. Behav Med. 1995;21:141–50. doi: 10.1080/08964289.1995.9933752. [DOI] [PubMed] [Google Scholar]
- 37.Leserman J, Drossman DA, Li Z, Toomey TC, Nachman G, Glogau L. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med. 1996;58:4–15. doi: 10.1097/00006842-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Leserman J, Li Z, Drossman DA, Toomey TC, Nachman G, Glogau L. Impact of sexual and physical abuse dimensions on health status: development of an abuse severity measure. Psychosom Med. 1997;59:152–60. doi: 10.1097/00006842-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Girdler SS, Leserman J, Bunevicius R, Klatzkin R, Pedersen CA, Light KC. Persistent alterations in biological profiles in women with abuse histories: influence of premenstrual dysphoric disorder. Health psychology. 2007;26:201. doi: 10.1037/0278-6133.26.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. Journal of consulting and clinical psychology. 1978;46:932. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 41.Leserman J, Petitto J, Gu H, Gaynes B, Barroso J, Golden R, Perkins D, Folds J, Evans D. Progression to AIDS, a clinical AIDS condition and mortality: psychosocial and physiological predictors. Psychological medicine. 2002;32:1059–73. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 42.Leserman J, Petitto JM, Perkins DO, Folds JD, Golden RN, Evans DL. Severe Stress, Depressive Symptoms, and Changes in Lymphocyte Subsets in Human Immunodeficiency Virus—Infected Men: A 2-Year Follow-up Study. Archives of General Psychiatry. 1997;54:279–85. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]
- 43.Leserman J, Ironson G, O'Cleirigh C, Fordiani JM, Balbin E. Stressful l events and adherence in HIV. AIDS patient care and STDs. 2008;22:403–11. doi: 10.1089/apc.2007.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen S, Hoberman HM. Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology. 1983;13:99–125. [Google Scholar]
- 45.Olphen J, Schulz A, Israel B, Chatters L, Klem L, Parker E, Williams D. Religious Involvement, Social Support, and Health Among African-American Women on the East Side of Detroit. Journal of General Internal Medicine. 2003;18:549–57. doi: 10.1046/j.1525-1497.2003.21031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levin JS, Taylor RJ, Chatters LM. A multidimensional measure of religious involvement for African Americans. The Sociological Quarterly. 1995;36:157–73. [Google Scholar]
- 47.Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: a theoretically based approach. Journal of personality and social psychology. 1989;56:267. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- 48.Siegel JM. The multidimensional anger inventory. Journal of personality and social psychology. 1986;51:191. doi: 10.1037/0022-3514.51.1.191. [DOI] [PubMed] [Google Scholar]
- 49.Baron RM, Kenny DA. The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of personality and social psychology. 1986;51:1173. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 50.Anderson NB. Racial differences in stress-induced cardiovascular reactivity and hypertension: Current status and substantive issues. Psychological bulletin. 1989;105:89. doi: 10.1037/0033-2909.105.1.89. [DOI] [PubMed] [Google Scholar]
- 51.McEwen BS. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–24. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 52.Evans GW, Kim P. Childhood poverty and health cumulative risk exposure and stress dysregulation. Psychological Science. 2007;18:953–7. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 53.Girdler SS, Hinderliter AL, Light KC. Peripheral adrenergic receptor contributions to cardiovascular reactivity: influence of race and gender. J Psychosom Res. 1993;37:177–93. doi: 10.1016/0022-3999(93)90085-t. [DOI] [PubMed] [Google Scholar]
- 54.Light KC, Turner JR, Hinderliter AL, Girdler SS, Sherwood A. Comparison of cardiac versus vascular reactors and ethnic groups in plasma epinephrine and norepinephrine responses to stress. Int J Behav Med. 1994;1:229–46. doi: 10.1207/s15327558ijbm0103_4. [DOI] [PubMed] [Google Scholar]
- 55.Sherwood A, May CW, Siegel WC, Blumenthal JA. Ethnic differences in hemodynamic responses to stress in hypertensive men and women. Am J Hypertens. 1995;8:552–7. doi: 10.1016/0895-7061(95)00036-O. [DOI] [PubMed] [Google Scholar]
- 56.Crisostomo I, Zayyad A, Carley DW, Abubaker J, Onal E, Stepanski EJ, Lopata M, Basner RC. Chemo- and barorespnses differ in African-Americans and Caucasians in sleep. J Appl Physiol. 1998;85:1413–20. doi: 10.1152/jappl.1998.85.4.1413. [DOI] [PubMed] [Google Scholar]
- 57.Goldstein IB, Shapiro D. The cardiovascular response to postural change as a function of race. Biol Psychol. 1995;39:173–86. doi: 10.1016/0301-0511(94)00958-Z. [DOI] [PubMed] [Google Scholar]
- 58.Davis DA, Luecken LJ, Zautra AJ. Are reports of childhood abuse related to the experience of chronic pain in adulthood?: a meta-analytic review of the literature. The Clinical journal of pain. 2005;21:398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- 59.Denk F, McMahon SB. Chronic pain: emerging evidence for the involvement of epigenetics. Neuron. 2012;73:435–44. doi: 10.1016/j.neuron.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nemeroff CB, Binder E. The preeminent role of childhood abuse and neglect in vulnerability to major psychiatric disorders: toward elucidating the underlying neurobiological mechanisms. J Am Acad Child Adolesc Psychiatry. 2014;53:395–7. doi: 10.1016/j.jaac.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: population studies, and allele frequencies in alcohol-and drug-dependent subjects. Molecular psychiatry. 1999;4:476–83. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 62.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM. Single-nucleotide polymorphism in the human mu opioid receptor gene alters β-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences. 1998;95:9608–13. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hastie BA, Riley JL, Kaplan L, Herrera DG, Campbell CM, Virtusio K, Mogil JS, Wallace MR, Fillingim RB. Ethnicity interacts with the OPRM1 gene in experimental pain sensitivity. PAIN®. 2012;153:1610–9. doi: 10.1016/j.pain.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain. 2004;109:488–96. doi: 10.1016/j.pain.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 65.Forcier K, Stroud LR, Papandonatos GD, Hitsman B, Reiches M, Krishnamoorthy J, Niaura R. Links between physical fitness and cardiovascular reactivity and recovery to psychological stressors: A meta-analysis. Health Psychology. 2006;25:723. doi: 10.1037/0278-6133.25.6.723. [DOI] [PubMed] [Google Scholar]
- 66.Alderman BL, Arent SM, Landers DM, Rogers TJ. Aerobic exercise intensity and time of stressor administration influence cardiovascular responses to psychological stress. Psychophysiology. 2007;44:759–66. doi: 10.1111/j.1469-8986.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 67.de Geus E, Van Doornen L, Orlebeke J. Regular exercise and aerobic fitness in relation to psychological make-up and physiological stress reactivity. Psychosomatic Medicine. 1993;55:347–63. doi: 10.1097/00006842-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Rahim-Williams FB, Riley JL, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129:177–84. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.