Abstract
Background & Aims
Surveillance of patients with cirrhosis increases early detection of hepatocellular carcinoma (HCC) and prolongs survival. However, its effectiveness is limited by underuse, particularly among racial/ethnic minorities and individuals of low socioeconomic status. We compared effectiveness of mailed outreach strategies, with and without patient navigation, in increasing the numbers of patients with cirrhosis undergoing surveillance for HCC in a racially diverse and socioeconomically disadvantaged cohort.
Methods
We performed a prospective study of patients with documented or suspected cirrhosis at a large safety-net health system from December 2014 through March 2016. Patients were randomly assigned (1:1:1) to groups that received mailed invitations for an ultrasound screening examination (n=600), mailed invitations for an ultrasound screening examination and patient navigation (barrier assessment and motivational education for patients who declined screening; n=600), or usual care (visit-based screening; n=600). Patients who did not respond to outreach invitations within 2 weeks received up to 3 reminder telephone calls. The primary outcome was completion of abdominal imaging within 6 months of randomization.
Results
Baseline characteristics were similar among groups. Cirrhosis was documented, based on ICD-9 codes, for 79.6% of patients and suspected, based on non-invasive markers of fibrosis, for 20.4%. In an intent to treat analysis, significantly greater proportions of patients who received the mailed invitation and navigation (47.2%) or the mailed invitation alone (44.5%) underwent HCC screening than patients who received usual care (24.3%) (P<0.001 for both comparisons). However, screening rates did not significantly differ between outreach the outreach groups (P=0.25). The effects of the outreach program were consistent in all subgroups, including Caucasian vs. non-Caucasian race, documented vs. suspected cirrhosis, Child Pugh A vs. B cirrhosis, and receipt of gastroenterology care.
Conclusions
In a prospective study, we found outreach strategies to double the percentage of patients with cirrhosis who underwent ultrasound screening for HCC. However, adding patient navigation to telephone reminders provided no significant additional benefit. ClinicalTrials.gov no: NCT02312817
Keywords: Liver cancer, randomized controlled trial, prevention, intervention
BACKGROUND
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and a leading cause of death in patients with cirrhosis.1 HCC incidence in the U.S. is anticipated to increase over the next 20 years due to the growing burden of advanced hepatitis C virus (HCV) and/or non-alcoholic steatohepatitis (NASH) and projected to surpass breast and colorectal cancer (CRC) to become the 3rd leading cause of cancer-related death by 2030.2 The Annual Report to the Nation on the Status of Cancer highlighted HCC mortality rates are increasing by greater than 2% per year in the U.S.3
HCC surveillance can potentially improve early tumor detection and overall survival in patients with cirrhosis. A randomized trial with >18,000 patients demonstrated HCC surveillance lowered mortality by 37% (mortality rate ratio 0.63) among hepatitis B-infected patients.4 Similarly, cohort studies have demonstrated cirrhosis patients undergoing surveillance have earlier tumor stage and improved survival, after adjusting for lead-time bias, than those not undergoing surveillance.5 Given these data, the National Comprehensive Cancer Network, Department of Veterans Affairs, and American Association for Study of Liver Diseases (AASLD) recommend HCC surveillance with abdominal ultrasound every 6 months.6, 7
However, fewer than 20% of cirrhosis patients undergo HCC surveillance, with lower rates among non-Caucasians and those of low socioeconomic status.8, 9 As with breast and CRC screening, providers offer opportunistic HCC surveillance during face-to-face visits.10 However, providers report barriers to performing HCC surveillance including clinic time constraints, inadequate knowledge, and uncertainty if responsibility lies with primary care or specialists.11 Population management programs that systematically invite patients for screening, i.e. outreach programs, and patient navigation interventions effectively increase screening rates for other cancers, whether implemented within a healthcare organizational or community,12-16 although it is unclear if these interventions would be effective for HCC surveillance given unique challenges. Identifying patients eligible for HCC surveillance is challenging compared to breast or CRC screening, as providers must recognize the presence of liver disease and transition to cirrhosis, which can occur without clinical symptoms.17, 18 Further, cirrhosis patients have higher rates of medical illness due to varying severity of liver dysfunction and lower socioeconomic status, likely increasing barriers to preventive care.19, 20
We performed a pragmatic, randomized comparative effectiveness trial of a mailed outreach strategy for screening ultrasound, screening ultrasound outreach plus patient navigation, and usual care for increasing HCC screening participation among a racially diverse and socioeconomically disadvantaged population served by a large safety-net health system. The primary aim of our trial is to evaluate the effectiveness of the interventions to increase repeat surveillance rates over a two-year period. Herein, we report a pre-planned interim analysis comparing one-time HCC screening participation rates across the groups.
METHODS
Study Population
The trial was conducted at Parkland Health and Hospital System (PHHS) from December 2014 to March 2016. PHHS, the sole safety-net provider for Dallas County, is a publically funded integrated health system that includes a 900-bed hospital, 12 community-based primary care clinics, specialty clinics, and radiology suites. PHHS offers a sliding fee scale program, Parkland Financial Assistance (PFA), which provides access to primary and subspecialty medical care, including HCC surveillance, at low cost for uninsured Dallas County residents.
The study population included patients with documented or suspected cirrhosis with at least one outpatient clinic visit in the year preceding randomization. Patients with suspected cirrhosis were included given the high proportion of HCC patients who fail to undergo surveillance due to previously unrecognized cirrhosis.17 “Documented cirrhosis” was defined using ICD-9 codes for cirrhosis or cirrhosis-related complications.21 “Suspected cirrhosis” was defined as AST to platelet ratio index (APRI) ≥1.0 in the presence of liver disease (Supplemental material).22 The APRI cut-off was increased to 1.5 in January 2015 to increase its positive predictive value for cirrhosis. Given this was a pragmatic trial, cirrhosis was determined using electronic medical record (EMR) data and not confirmed by chart review. Patients with Child C cirrhosis who were not transplant candidates and those with significant comorbid conditions (e.g. extra-hepatic malignancy) were excluded given limited benefit of HCC surveillance in those subgroups. Additional exclusion criteria included no address or phone number on file and primary language other than English or Spanish. Inclusion and exclusion criteria were applied using Parkland EMR data. We obtained waiver of informed consent to avoid volunteer bias, in which patients interested in screening are selectively included. The study was approved by the IRB at UT Southwestern Medical Center and registered at ClinicalTrials.gov. The trial protocol is available on clinicaltrials.gov (NCT02312817).
HCC Screening Interventions
Eligible patients were randomly assigned to receive usual care with opportunistic, visit-based screening (Arm 1), mailed outreach invitations for screening ultrasound (Arm 2), or mailed screening outreach plus patient navigation (Arm 3) in a 1:1:1 ratio using a computer-generated randomization sequence. Research staff stratified randomization based on documented vs. suspected cirrhosis because intervention effect could differ between the subgroups.
Research staff conducted all mailings and reminder telephone calls; thus, patients, primary and specialty care providers were blinded to the presence of other intervention groups. We conducted the study as a pragmatic trial whereby patients in each arm could receive visit-based HCC screening as recommended by primary or specialty care providers.
Outreach interventions (Arms 2 and 3) were initiated with one-page letters with basic information regarding HCC risk and recommendation to undergo HCC screening (Supplemental material). Mailings, provided in English and Spanish, were written at a low-literacy level with assistance from health communication experts and underwent cognitive testing with English and Spanish speakers.23 Patients who did not respond to mailed invitations within two weeks, including those with returned mail, received telephone reminder calls to participate. Trained research staff conducted telephone calls in English or Spanish, based on patients’ preferred language of communication, using standardized scripts (Supplemental material). Attempts at telephone contact were stopped for patients with non-working phone numbers and those who could not be contacted after three attempts. For Arm 3 patients who declined screening participation during reminder telephone calls, research staff explored potential barriers using standardized phone scripts (Supplemental material) and used motivational education to encourage screening participation. Telephone scripts were tailored to study arm assignment, so barrier assessment and motivational education were only delivered to Arm 3 patients who declined screening participation. Research staff did not deliver motivational education to patients in Arm 2 who declined screening participation.
The Parkland Radiology Department makes automated reminder phone calls to patients scheduled for ultrasounds 2 days prior to the appointment. Research staff called patients randomized to patient navigation 5-7 days prior to ultrasound appointments to remind them of the appointment, address any concerns, and reschedule the appointment if needed.
Primary and Secondary Outcomes
The primary outcome – one-time screening participation – was defined as completion of abdominal imaging within 6 months of randomization. For patients in outreach arms, we included tests completed through outreach and usual care, visit-based screening. To ascertain screening participation for all patients, research staff members who did not deliver interventions and were blinded to intervention status queried the EMR for completed ultrasounds, contrast-enhanced CT, or contrast-enhanced MRI. Although contrast-enhanced CT and MRI are not recommended for HCC surveillance, their completion precludes the need for screening ultrasound. Therefore, we included contrast-enhanced CT and MRI, independent of screening intent, in our outcome of screening completion; however, non-contrast CT or MRI was not included. Although patients were invited to complete alpha fetoprotein (AFP) testing at time of the ultrasound, it was not required for the outcome of screening participation given its removal from the 2010 AASLD guidelines.6, 24, 25
A secondary outcome was time-to-response to outreach invitations. We evaluated time-to-response to define the intensity of outreach efforts (invitations alone vs. invitations and telephone reminder calls) that would most efficiently generate the greatest response. Time-to-response was defined as number of days between the outreach invitation date and date when an ultrasound order was requested. In contrast to our primary outcome, this outcome included all patients who responded to outreach invitations and scheduled an ultrasound, regardless of completion status. We categorized time-to-response into three categories: early responders, late responders, and non-responders. “Early responders” requested a screening ultrasound prior to reminder calls. “Late responders” responded to outreach invitations after reminder calls but prior to repeat outreach invitations (~3 months after initial invitation). “Non-responders” never requested HCC screening through the outreach program. Patients who responded after repeat invitation were considered non-responders because it is unlikely screening was triggered by the initial invitation.
Statistical Analysis
We used Pearson Chi-Square and Wilcoxon Rank-Sum tests to compare patients and confirm randomization worked. Patient characteristics included age, gender, race/ethnicity, liver disease etiology, Child Pugh score, Charlson comorbidity index, primary care contact, and receipt of gastroenterology care. We used a validated measure to calculate Child Pugh score using Parkland EMR data.26 Primary care contact was defined as number of primary care visits in year before randomization, and receipt of gastroenterology care was defined as ≥1 visit in the gastroenterology/liver clinic in year before randomization.
For our primary outcome, we used Pearson Chi-Square to compare one-time screening rates across the arms. We evaluated intervention effect across pre-defined subgroups including Caucasian vs. non-Caucasian race, documented vs. suspected cirrhosis, Child Pugh A vs. B cirrhosis, and receipt of gastroenterology care. We then performed an interaction analysis to examine whether intervention effect differed by variables of a priori interest: gender, race/ethnicity, receipt of gastroenterology care, and documented vs. suspected cirrhosis. Finally, we performed multivariable logistic regression analysis to identify additional independent predictors of screening completion. The multivariable model included variables of a priori importance (e.g., outreach receipt, race/ethnicity, documented cirrhosis, and primary care contact) and factors with p<0.05 in univariate analysis. For our secondary outcome, we used Pearson Chi-Square to compare early vs. late responders between intervention arms.
With 600 patients randomly assigned to each arm, we had 90% power to detect a difference of at least 9.3% in one-time screening completion rates between the arms, assuming baseline screening rates of 20% and pre-specified alpha of 0.017 (=0.05/3 accounting for Bonferroni correction). Power analysis was conducted with two-sided Z-test with continuity correction and pooled variance using PASS 14 sample size software. Authors had access to all study data and reviewed and approved the final manuscript. We used intent-to-screen principle to guide analyses. Analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Study Population
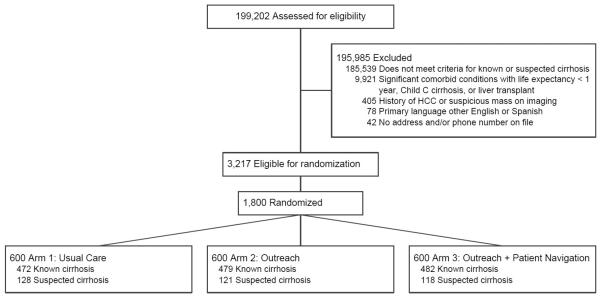
A total of 1800 patients were selected for randomization and included in intent-to-treat analyses (Figure 1). Baseline characteristics across the three arms were similar (Table 1). Mean age was 55.3 (range 21.2-89.6) years and 59.4% were men. The cohort was racially/ethnically diverse with 37.8% Hispanic, 32.1% Black, and 28.3% White. Most (79.6%) patients had documented cirrhosis using ICD-9 codes, with 20.4% having suspected cirrhosis using non-invasive markers of fibrosis. Cirrhosis was due to HCV in 51.0%, alcohol 17.6%, NASH 16.6%, and HBV in 3.4% of patients. Most patients had compensated cirrhosis, with 28.2% having ascites and 12.7% having hepatic encephalopathy. Although >90% of patients had a primary care visit in the year preceding randomization, only 25.7% had received gastroenterology care. Patients had prior abdominal imaging within 6 months before randomization in 31.6% of cases.
Figure 1.
Study Consort Diagram
Table 1.
Characteristics of patients with cirrhosis enrolled in a pragmatic randomized controlled trial promoting HCC screening in safety-net system, overall and by study arm, December 2014 to March 2016, N = 1,800.
| Usual Care n=600 |
Outreach Alone n=600 |
Outreach and Patient Navigation n=600 |
Total N=1,800 |
|
|---|---|---|---|---|
|
| ||||
| Age (years) | ||||
| 21-50 | 183 (30.5) | 174 (29.0) | 158 (26.3) | 515 (28.6) |
| 51-60 | 259 (43.2) | 272 (45.3) | 269 (44.8) | 800 (44.4) |
| 61-90 | 158 (26.3) | 154 (25.7) | 173 (28.8) | 485 (26.9) |
|
| ||||
| Male sex (%) | 350 (58.3) | 361 (60.2) | 358 (59.7) | 1,069 (59.4) |
|
| ||||
| Race/Ethnicity (%) | ||||
| Non-Hispanic White | 182 (30.3) | 165 (27.5) | 163 (27.2) | 510 (28.3) |
| Hispanic | 217 (36.2) | 230 (38.3) | 234 (39.0) | 681 (37.8) |
| Non-Hispanic Black | 186 (31.0) | 197 (32.8) | 195 (32.5) | 578 (32.1) |
| Other/Unknown | 15 (2.5) | 8 (1.3) | 8 (1.3) | 31 (1.7) |
|
| ||||
| Etiology of Liver Disease (%)a | ||||
| Hepatitis C | 320 (53.3) | 285 (47.5) | 313 (52.2) | 918 (51.0) |
| Alcohol | 98 (16.3) | 115 (19.2) | 104 (17.3) | 317 (17.6) |
| NASH | 104 (17.3) | 101 (16.8) | 94 (15.7) | 299 (16.6) |
| Hepatitis B | 21 (3.5) | 27 (4.5) | 14 (2.3) | 62 (3.4) |
| Other | 57 (9.5) | 72 (12.0) | 75 (12.5) | 204 (11.3) |
|
| ||||
| Presence of documented cirrhosis (%)c | 472 (78.7) | 479 (79.8) | 482 (80.3) | 1,433 (79.6) |
|
| ||||
| Hepatic decompensation, No (%)a | 181 (30.2) | 192 (32.0) | 201 (33.5) | 574 (31.9) |
|
| ||||
| Child Pugh A (%)a | 432 (72.0) | 435 (72.5) | 424 (70.7) | 1,291 (71.7) |
|
| ||||
| Charlson Comorbidity Index (%)b | ||||
| 0 | 76 (12.7) | 95 (15.8) | 79 (13.2) | 250 (13.9) |
| 1 | 140 (23.3) | 143 (23.8) | 149 (24.8) | 432 (24.0) |
| 2 | 103 (17.2) | 99 (16.5) | 84 (14.0) | 286 (15.9) |
| 3+ | 281 (46.8) | 263 (43.8) | 288 (48.0) | 832 (46.2) |
|
| ||||
| Number of primary care visitsb | 4 (IQR 2-7) | 3 (IQR 2-7) | 4 (IQR 2-7) | 4 (IQR 2-7) |
|
| ||||
| Receipt of gastroenterology careb | 153 (25.5) | 153 (25.5) | 157 (26.2) | 463 (25.7) |
NASH = Nonalcoholic steatohepatitis; IQR – interquartile range
Prior to randomization
Within one year preceding randomization
Defined using ICD-9 codes for cirrhosis or cirrhosis-related complications
One-time Screening Participation
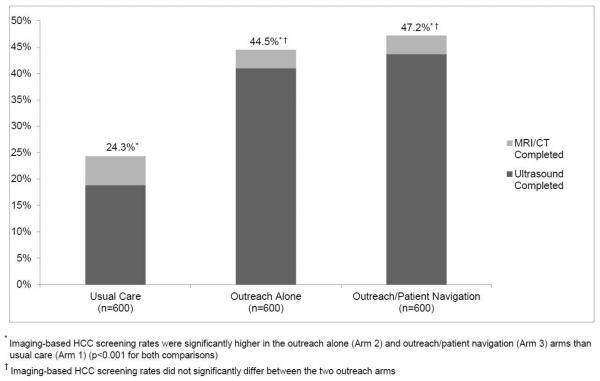
Imaging-based screening participation rates were 24.3% (146/600) for usual care patients, 44.5% (267/600) for outreach-only patients, and 47.2% (283/600) for outreach/navigation patients (Figure 2). Imaging-based screening rates were significantly higher in outreach alone and outreach/patient navigation arms than usual care (p<0.001 for both); however, screening rates did not differ between the outreach arms (p=0.25). Numbers needed to invite were 4.96 and 4.38 for outreach only and outreach/navigation arms, respectively. Intervention effect was consistent across pre-defined subgroups including Caucasian vs. non-Caucasian race, documented vs. suspected cirrhosis, Child Pugh A vs. B cirrhosis, and receipt of gastroenterology care (Figure 3).
Figure 2.
Screening Completion Rates by Study Arm
Figure 3.
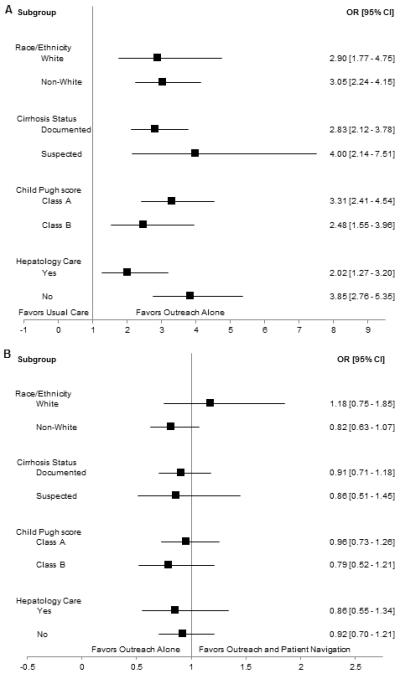
Intervention Effect in Selected Subgroups
A. Usual Care (Arm 1) vs. Outreach Alone (Arm 2)
B. Outreach Alone (Arm 2) vs. Outreach and Patient Navigation (Arm 3)
An additional 4.5%, 16.0%, and 17.2% of patients in the usual care, outreach alone, and outreach/navigation arms, respectively, scheduled an ultrasound but missed or cancelled the appointment (Supplemental Figure). Ultrasound completion was a direct result of outreach efforts in 55.7% (n=137) of outreach-only patients and 58.8% (n=154) of outreach/navigation patients. Among non-responders, 130 (54.9%) outreach-only patients and 115 (53.7%) outreach/navigation patients could not be contacted for reminder calls.
Predictors of Screening Participation
In univariate analysis, predictors of screening participation included randomization to outreach, older age, female sex, Hispanic ethnicity, primary language, Child Pugh score, Charlson comorbidity index, primary care contact, and gastroenterology care. Documented cirrhosis was of borderline significance in univariate analysis (p=0.07) but included in multivariable analysis given a priori importance. Primary language was not included in the multivariable model given collinearity with patient ethnicity. In multivariable analysis, screening participation was positively associated with randomization to outreach, female gender, older age, Hispanic ethnicity, more primary care contact, and gastroenterology care (Table 2). On interaction analysis, intervention effect did not differ by gender, race, receipt of gastroenterology care, or documented vs. suspected cirrhosis.
Table 2.
Predictors of screening participation among patients with cirrhosis enrolled in a pragmatic randomized controlled intervention trial, N = 1800.
| Variable | Univariable Models OR (95% CI) |
Multivariable Model AOR (95% CI) |
|---|---|---|
| Outreach Strategy Usual Care Outreach Alone Outreach and Patient Navigation |
Ref. 3.02 (2.32 – 3.92) 3.34 (2.57 – 4.34) |
Ref. 3.18 (2.43 – 4.16) 3.47 (2.65 – 4.53) |
| Age (years) 21-50 51-60 61-90 |
Ref. 1.52 (1.20 – 1.93) 1.39 (1.06 – 1.81) |
Ref. 1.42 (1.09 – 1.84) 1.15 (0.86 – 1.55) |
| Male sex | 0.77 (0.64 – 0.94) | 0.80 (0.65 – 0.99) |
| Race/Ethnicity Non-Hispanic White Hispanic Non-Hispanic Black Other/Unknown |
Ref. 1.52 (1.19 – 1.95) 1.21 (0.94 – 1.57) 1.13 (0.52 – 2.46) |
Ref. 1.56 (1.20 – 2.02) 1.14 (0.87 – 1.50) 1.23 (0.54 – 2.80) |
| Charlson Comorbidity Index 0 1 2 3+ |
Ref. 1.49 (1.04 – 2.11) 1.85 (1.27 – 2.69) 1.88 (1.36 – 2.59) |
Ref. 1.31 (0.91 – 1.91) 1.60 (1.06 – 2.41) 1.41 (0.97 – 2.04) |
| Documented vs. suspected cirrhosis Documented cirrhosis Suspected cirrhosis |
Ref. 1.26 (0.98 – 1.61) |
Ref. 0.82 (0.62 – 1.09) |
| Child Pugh score Child Pugh A Child Pugh B |
Ref. 1.32 (1.07 – 1.63) |
Ref. 1.04 (0.79 – 1.38) |
| Hepatic decompensation No Yes |
Ref. 1.41 (1.15 – 1.73) |
Ref. 1.14 (0.87 – 1.50) |
| Etiology of Liver Disease Hepatitis C Hepatitis B Alcohol-induced Nonalcoholic steatohepatitis Other |
Ref. 0.64 (0.36 – 1.16) 1.0 (0.76 – 1.30) 1.16 (0.88 – 1.52) 0.74 (0.53 – 1.03) |
NS |
| Number of primary care visitsa | 1.07 (1.05 – 1.09) | 1.05 (1.03 – 1.08) |
| Receipt of gastroenterology carea | 2.01 (1.62 – 2.49) | 1.74 (1.36 – 2.21) |
OR = Odds Ratio; AOR = Adjusted Odds Ratio; CI = Confidence Interval
Within one year preceding randomization
Time-to-Response
Among responders in both outreach arms, median time-to-response was 26 days. Among 585 patients who responded to outreach, 27.5% were “early responders” (median 8 days) and 72.5% were “late responders” (median 32 days). There was no difference in early vs. late response rates between intervention arms.
DISCUSSION
In this prospective pragmatic, randomized controlled trial among a large cohort of patients with cirrhosis, mailed outreach doubled HCC screening rates compared with usual care; however, adding patient navigation provided no significant benefit. Intervention effect did not differ by patient gender, race/ethnicity, receipt of gastroenterology care, or presence of documented vs. suspected cirrhosis. However, HCC screening rates in both outreach arms remained below 50%, highlighting the need for more intensive interventions.
To the best of our knowledge, only 3 quasi-experimental studies have evaluated interventions to increase HCC screening rates. Two studies assessing chronic disease management programs, including nursing-based protocols and automated reminders, demonstrated increased one-time HCC screening rates from 74% and 89% at baseline to 93% and 100%, respectively.27, 28 However, both studies were conducted among patients followed by hepatologists and required patient consent, introducing a selection bias. In a subsequent quasi-experimental study, point-of-care clinical reminders targeting primary care providers increased screening rates from 18.2% to 27.6% (p<0.001);29 however, intervention benefit was limited to patients with documented cirrhosis and those engaged in clinic. Our pragmatic randomized trial adds to this literature, demonstrating success to reach patients with documented or suspected cirrhosis as well as those not engaged in routine clinical care.
It is unknown what intensity of outreach efforts most efficiently generates the greatest population-level response. In our study, reminder calls after outreach invitations accounted for over half of responders; however, patient navigation provided minimal additional benefit with similar rates of screening participation and cancelled ultrasound appointments. Our results are consistent with data demonstrating high patient acceptance and adherence rates for HCC surveillance.30 However, patient navigation in our study only consisted of barrier assessment, motivational education, and assistance with ultrasound rescheduling. More intensive navigation efforts to overcome patient barriers, such as scheduling and transportation assistance, may be effective.14, 15, 31
Independent of the outreach strategy, primary care contact and gastroenterology care were associated with increased screening rates. This finding is consistent with studies demonstrating higher HCC screening rates among patients followed by hepatologists and highlights the continued importance of visit-based screening in addition to outreach interventions.8 Similarly, patients with more primary care visits had higher rates of CRC screening in health systems with aggressive population health outreach programs.25 Provider connectedness may increase patients’ likelihood of responding to outreach and offers opportunities to discuss screening in clinic. However, system-level approaches, such as our outreach intervention, can decrease dependence on regular primary care contact or gastroenterology subspecialty care. Therefore population management interventions may be particularly beneficial in large integrated systems with limited subspecialty capacity.
Our study had limitations that must be considered when interpreting the results. First, the study was conducted in a safety-net health system and results may not be generalized to other health systems. However, racially diverse, socioeconomically disadvantaged patients represent a difficult-to-reach population and are an important population to study given they experience health disparities, including higher HCC incidence and mortality rates19, 33, 34 and lower HCC surveillance rates.35-37 Outreach interventions would likely be equally, if not more, successful in other patient populations. Second, patients may have received HCC screening tests at outside institutions, although this is unlikely because many patients did not have insurance and would have out-of-pocket costs for outside testing. Third, we could not differentiate between imaging performed for screening vs. diagnostic purposes; however, imaging for non-screening intent would preclude the need for imaging for screening purposes. Finally, although our primary outcome was one-time screening completion, effective screening depends on completion of the entire screening process, including repeat screening in patients with normal tests and follow-up of those with abnormal results. Many patients who complete one-time screening fail to undergo repeat screening, patients with suspicious ultrasound findings fail to undergo timely diagnostic evaluation, and those with HCC fail to undergo guideline-concordant treatment.37-39 Our study’s second phase will compare effectiveness of outreach and navigation strategies to increase completion of the entire screening process. We feel strengths of this study, including its large size, its racially and socioeconomically diverse cohort, and its novelty as the first randomized trial to evaluate interventions to increase HCC screening rates, outweigh any weaknesses.
In summary, our large, pragmatic randomized trial demonstrates mailed outreach invitations are effective for promoting HCC screening completion among patients with cirrhosis. Although half of patients in screening outreach arms did not complete HCC screening, screening rates were significantly higher than those observed in usual care. We found this strategy was effective among all subgroups of patients including Caucasians or non-Caucasians, documented or suspected cirrhosis, and those receiving or not receiving gastroenterology care. Given the pervasive nature of HCC screening underuse among at-risk patients, our study presents a model to improve HCC screening rates in large health systems.
Supplementary Material
Supplemental Table. Criteria for inclusion of participants
Supplemental Figure 1: Study Outcomes Flow Diagram for Usual Care (Arm 1)
Supplemental Figure 2: Study Outcomes Flow Diagram for Outreach Alone (Arm 2)
Supplemental Figure 3: Study Outcomes Flow Diagram for Outreach and Patient Navigation (Arm 3)
Acknowledgements
Adrianne Wilson Liver Cancer Association
Financial Support: This study was conducted as part of the Center for Patient-Centered Outcomes Research with support from AHRQ Grant R24 HS022418 and NIH/NCI Cancer Center Support Grant P30 CA142543. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or AHRQ. The funding agency had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Dr. Singal is on the speaker bureau for Bayer Pharmaceutical and received grant funding from Gilead pharmceuticals.
Abbreviations
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NASH
nonalcoholic steatohepatitis
- CRC
colorectal cancer
- AASLD
American Association for the Study of Liver Diseases
- PHHS
Parkland Health and Hospital System
- APRI
AST to platelet ratio index
- EMR
electronic medical record
- BCLC
Barcelona Clinic Liver Cancer
Footnotes
Author contributions: Dr. Singal had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design (Singal, Tiro, and Halm); Acquisition, analysis and interpretation of the data (Singal, Tiro, Marrero, McCallister, Mejias, Adamson, Bishop, Santini, and Halm); Drafting of the manuscript (Singal); Critical revision of the manuscript for important intellectual content (Singal, Tiro, Marrero, McCallister, Mejias, Adamson, Bishop, Santini, and Halm); Obtained funding (Halm); Administrative, technical, and material support (Singal and Halm); and Study supervision (Singal and Halm)
Conflicts of Interest: None of the other authors have any relevant conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007 Jun;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014 Jun 1;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016 Mar 9; doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004 Jul;130(7):417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AG, Pillai A, Tiro J. Early Detection, Curative Treatment, and Survival Rates for Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Meta-analysis. PLoS Med. 2014 Apr;11(4):e1001624. doi: 10.1371/journal.pmed.1001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma.: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001 Sep;35(3):421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 8.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of Hepatocellular Carcinoma Surveillance Among American Patients: A Systematic Review. J Gen Intern Med. 2012 Jan 4;:861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singal AG, X L, Tiro J, et al. Racial, Social, and Clinical Determinants of Hepatocellular Carcinoma Surveillance. Am J Med. 2014 doi: 10.1016/j.amjmed.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breen N, Meissner HI. Toward a system of cancer screening in the United States: trends and opportunities. Annu Rev Public Health. 2005;26:561–582. doi: 10.1146/annurev.publhealth.26.021304.144703. [DOI] [PubMed] [Google Scholar]
- 11.Dalton-Fitzgerald E, Tiro J, Kandunoori P, Halm EA, Yopp A, Singal AG. Practice Patterns and Attitudes of Primary Care Providers and Barriers to Surveillance of Hepatocellular Carcinoma in Patients with Cirrhosis. Clin Gastroenterol Hepatol. 2014 Jul 11; doi: 10.1016/j.cgh.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Intern Med. 2013 Oct 14;173(18):1725–1732. doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Sussman DA, Doubeni CA, et al. Challenges and possible solutions to colorectal cancer screening for the underserved. J Natl Cancer Inst. 2014 Apr;106(4):dju032. doi: 10.1093/jnci/dju032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genoff MC, Zaballa A, Gany F, et al. Navigating Language Barriers: A Systematic Review of Patient Navigators' Impact on Cancer Screening for Limited English Proficient Patients. J Gen Intern Med. 2016 Apr;31(4):426–434. doi: 10.1007/s11606-015-3572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krok-Schoen JL, Oliveri JM, Paskett ED. Cancer Care Delivery and Women's Health: The Role of Patient Navigation. Front Oncol. 2016;6:2. doi: 10.3389/fonc.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muliira JK, D'Souza MS. Effectiveness of patient navigator interventions on uptake of colorectal cancer screening in primary care settings. Jpn J Nurs Sci. 2016 Apr;13(2):205–219. doi: 10.1111/jjns.12102. [DOI] [PubMed] [Google Scholar]
- 17.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol. 2013 May;11(5):472–477. doi: 10.1016/j.cgh.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singal AG, Yopp AC, Gupta S, et al. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 2012 Aug 7;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shebl FM, Capo-Ramos DE, Graubard BI, McGlynn KA, Altekruse SF. Socioeconomic status and hepatocellular carcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2012 Aug;21(8):1330–1335. doi: 10.1158/1055-9965.EPI-12-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jepsen P, Vilstrup H, Andersen PK, Lash TL, Sorensen HT. Comorbidity and survival of Danish cirrhosis patients: a nationwide population-based cohort study. Hepatology. 2008 Jul;48(1):214–220. doi: 10.1002/hep.22341. [DOI] [PubMed] [Google Scholar]
- 21.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013 May-Jun;47(5):e50–54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd El, Rihim AY, Omar RF, Fathalah W, El Attar I, Hafez HA, Ibrahim W. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab J Gastroenterol. 2013 Jun;14(2):44–50. doi: 10.1016/j.ajg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Willis GB. Cognitive Interviewing: A "How To" Guide: Research Triangle Institute. 1999.
- 24.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009 Jul;30(1):37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singal AG, Conjeevaram HS, Volk ML, et al. Effectiveness of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis. Cancer Epidemiol Biomarkers Prev. 2012 Mar 30;21(5):793–799. doi: 10.1158/1055-9965.EPI-11-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan DE, Dai F, Aytaman A, et al. Development and Performance of an Algorithm to Estimate the Child-Turcotte-Pugh Score From a National Electronic Healthcare Database. Clin Gastroenterol Hepatol. 2015 Dec;13(13):2333–2341. e2331–2336. doi: 10.1016/j.cgh.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wigg AJ, McCormick R, Wundke R, Woodman RJ. Efficacy of a chronic disease management model for patients with chronic liver failure. Clin Gastroenterol Hepatol. 2013 Jul;11(7):850–858. e851–854. doi: 10.1016/j.cgh.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Aberra FB, Essenmacher M, Fisher N, Volk ML. Quality improvement measures lead to higher surveillance rates for hepatocellular carcinoma in patients with cirrhosis. Dig Dis Sci. 2013 Apr;58(4):1157–1160. doi: 10.1007/s10620-012-2461-4. [DOI] [PubMed] [Google Scholar]
- 29.Beste LA, Ioannou GN, Yang Y, Chang MF, Ross D, Dominitz JA. Improved Surveillance for Hepatocellular Carcinoma With a Primary Care-Oriented Clinical Reminder. Clin Gastroenterol Hepatol. 2014 May 6; doi: 10.1016/j.cgh.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 30.Singal A, Volk M, Rakoski M, et al. Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol. 2011;45(8):727–732. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 31.Percac-Lima S, Ashburner JM, Zai AH, et al. Patient Navigation for Comprehensive Cancer Screening in High-Risk Patients Using a Population-Based Health Information Technology System: A Randomized Clinical Trial. JAMA Intern Med. 2016 Jun 6; doi: 10.1001/jamainternmed.2016.0841. [DOI] [PubMed] [Google Scholar]
- 32.Trevisani F, De NS, Rapaccini G, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience) Am J Gastroenterol. 2002 Mar;97(3):734–744. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 33.Clark PJ, Stuart KA, Leggett BA, et al. Remoteness, race and social disadvantage: disparities in hepatocellular carcinoma incidence and survival in Queensland, Australia. Liver Int. 2015 Dec;35(12):2584–2594. doi: 10.1111/liv.12853. [DOI] [PubMed] [Google Scholar]
- 34.Ha J, Yan M, Aguilar M, et al. Race/Ethnicity-Specific Disparities in Cancer Incidence, Burden of Disease, and Overall Survival among Patients with Hepatocellular Carcinoma in the U.S. Cancer. 2016;122 doi: 10.1002/cncr.30103. [DOI] [PubMed] [Google Scholar]
- 35.Ha J, Yan M, Aguilar M, et al. Race/Ethnicity-specific Disparities in Hepatocellular Carcinoma Stage at Diagnosis and its Impact on Receipt of Curative Therapies. J Clin Gastroenterol. 2016 May-Jun;50(5):423–430. doi: 10.1097/MCG.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 36.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012 Jul;27(7):861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013 Oct;38(7):703–712. doi: 10.1111/apt.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel N, Yopp AC, Singal AG. Diagnostic Delays are Common Among Patients wtih Hepatocellular Carcinoma. J Natl Compr Canc Netw. 2014 doi: 10.6004/jnccn.2015.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singal AG, Waljee AK, Patel N, et al. Therapeutic delays lead to worse survival among patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2013 Sep 1;11(9):1101–1108. doi: 10.6004/jnccn.2013.0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table. Criteria for inclusion of participants
Supplemental Figure 1: Study Outcomes Flow Diagram for Usual Care (Arm 1)
Supplemental Figure 2: Study Outcomes Flow Diagram for Outreach Alone (Arm 2)
Supplemental Figure 3: Study Outcomes Flow Diagram for Outreach and Patient Navigation (Arm 3)