Abstract
BACKGROUND & AIMS
Prostaglandin E2 (PGE2) is mediator of inflammation that regulates tissue regeneration, but its continual activation has been associated with carcinogenesis. Little is known about factors in the PGE2 signaling pathway that contribute to tumor formation. We investigated whether yes associated protein 1 (YAP1), a transcriptional co-activator in the Hippo signaling pathway, mediates PGE2 function.
METHODS
DLD-1 and SW480 colon cancer cell lines were transfected with vectors expressing transgenes or small hairpin RNAs and incubated with recombinant PGE2, with or without pharmacologic inhibitors of signaling proteins, and analyzed by immunoblot, immunofluorescence, quantitative reverse transcription PCR, transcriptional reporter, and proliferation assays. Dextran sodium sulfate (DSS) was given to induce colitis in C57/BL6 (control) mice, as well as in mice with disruption of the hydroxyprostaglandin dehydrogenase 15 gene (15-PGDH-knockout mice), Yap1 gene (YAP-knockout mice), and double knockout mice. Some mice were also given indomethacin to block PGE2 synthesis. 15-PGDH knockout mice were crossed with mice with intestine-specific disruption of the Salvador family WW domain containing 1 gene (Sav1), which encodes an activator of Hippo signaling. We performed immunohistochemical analyses of colon biopsy samples from 26 patients with colitis-associated cancer and 51 age- and sex-matched patients with colorectal cancer (without colitis).
RESULTS
Incubation of colon cancer cell lines with PGE2 led to phosphorylation of cAMP responsive element binding protein 1 (CREB1) and increased levels of YAP1 mRNA and protein and YAP1’s transcriptional activity. This led to increased transcription of the prostaglandin-endoperoxide synthase 2 gene (PTGS2 or COX2) and prostaglandin E receptor 4 gene (PTGER4 or EP4). Incubation with PGE2 promoted proliferation of colon cancer cell lines, but not cells with knockdown of YAP1. Control mice developed colitis after administration of DSS, but injection of PGE2 led to colon regeneration in these mice. However, YAP-knockout mice did not regenerate colon tissues and died soon after administration of DSS. 15-PGDH-knockout mice regenerated colon tissues more rapidly than control mice after withdrawal of DSS, and had faster recovery of body weight, colon length, and colitis histology scores. These effects were reversed by injection of indomethacin. SAV1 -knockout or 15-PDGH-knockout mice did not develop spontaneous tumors following colitis induction, but SAV1/15-PDGH double knockout mice developed polyps that eventually progressed to carcinoma in situ. Administration of indomethacin to these mice prevented spontaneous tumor formation. Levels of PGE2 correlated with those of YAP levels in human sporadic colorectal tumors and colitis-associated tumors.
Conclusion
PGE2 signaling increases expression and transcriptional activities of YAP1, leading to increased expression of COX2 and EP4 to activate a positive signaling loop. This pathway promotes proliferation of colon cancer cell lines and colon tissue regeneration in mice with colitis. Constitutive activation of this pathway led to formation of polyps and colon tumors in mice.
Keywords: inflammation, tumorigenesis, villus regeneration, mouse model
INTRODUCTION
Prostaglandin E2 (PGE2), a G protein-coupled receptor (GPCR) family ligand, is synthesized from arachidonic acid by the action of cyclooxygenase 2 (COX-2) enzyme1 and functions as a crucial inflammatory cytokine. In the colon, PGE2 is well-known to play an important role in regeneration and tumorigenesis. Thus, PGE2 is an important drug target for regenerative medicine and cancer prevention1,2. For example, recently, a 15-PGDH inhibitor (SW033291) was shown to promote tissue regeneration by increasing PGE2 levels3. On the other hand, prolonged use of COX inhibitors, which decrease PGE2, lowers the risk of colorectal cancer development2 but inhibits colon regeneration after colitis by reducing PGE2 levels. Similarly, an EP4 (PGE2 receptor) antagonist was found to exacerbate DSS-induced colitis but to prevent colon tumorigenesis4. However, the downstream effectors that regulate these PGE2-mediated effects remain poorly understood.
Yes-associated protein (YAP), a downstream oncogene of the Hippo pathway, is also implicated in the regulation of tissue regeneration, proliferation of adult stem cells, and cancer development5–8. Particularly, YAP is essential for tissue regeneration of the colon after DSS-mediated injury9 and hyper-activation YAP leads to intestinal cancer9,10, highly resembling PGE2 phenotypes. YAP activity is regulated by diverse upstream inputs including changes in cell polarity, cell-cell contact, cellular metabolites5 and the actin cytoskeleton11. Importantly, studies performed in cultured cells showed that YAP is also regulated by GPCR ligands12. This, together with their similar roles in colonic stem cell compartment, raised the possibility that YAP might mediate the function of PGE2.
Colorectal cancer (CRC) is one of the most frequently diagnosed cancers with high incidence rates worldwide13. PGE2 and YAP are known to be highly associated with CRC formation; PGE2 and YAP are frequently up-regulated during tumor formation1,2,5,14. However, despite their importance, the relationship between PGE2 and YAP is yet to be elucidated. Here, our in vitro and in vivo studies have revealed the operation of a positive feedback loop between PGE2 signaling and YAP that contributes to tissue regeneration after colitis and to colorectal tumorigenesis. These findings have important implications both biologically and clinically.
RESULTS
PGE2 positively regulates YAP in cultured cell lines and in mice
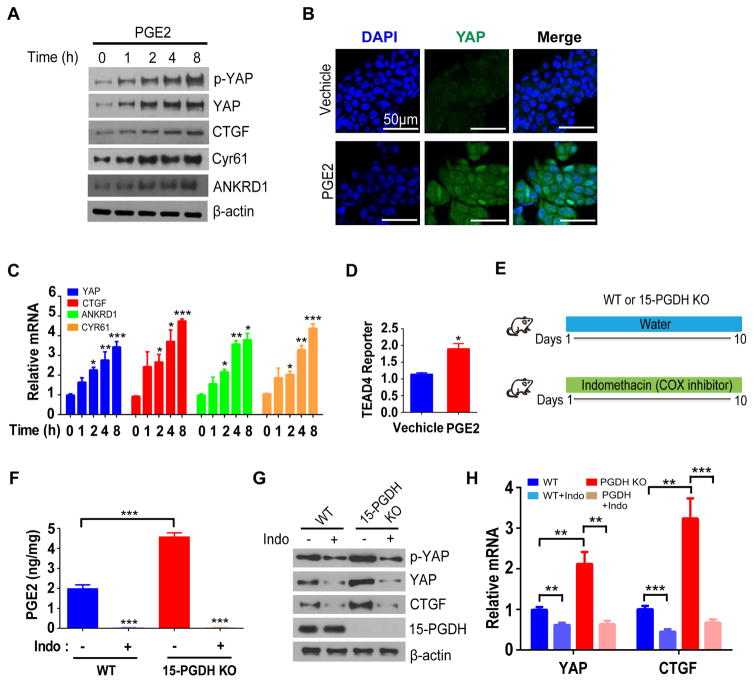
We first examined whether PGE2 regulates YAP in DLD-1 human colon cancer cell line. Exposure of serum starved DLD-1 cells to PGE2 markedly increased the abundance of YAP protein (Figure. 1A and 1B). In contrast, level of TAZ (YAP homolog) was unaffected by PGE2 (Data not shown). LATS kinase is the major negative regulator of YAP, which sequesters YAP in the cytoplasm by phosphorating at Ser127 residue5. However, PGE2 did not affect the ratio of Ser127-phosphorylated YAP to total YAP (Figure. 1A), suggesting that the activity of the LATS kinase was unaltered by PGE2. Despite the proportional increment of YAP phosphorylation, the transcriptional activity of YAP was elevated by PGE2 as indicated by the increased mRNA and protein levels of putative YAP target genes CTGF, CYR61 and ANKRD1 (Figure. 1A and 1C). Since regulation of YAP by PGE2 was unlikely to be due to altered phosphorylation, we then examined if YAP is regulated at the transcriptional level. Indeed, the mRNA level of YAP was increased following PGE2 treatment (Figure. 1C). We also directly measured YAP activity by using YAP reporter construct, which the transcription of the luciferase gene is controlled by upstream tandem TEAD transcription factor (key partner of YAP) binding sequences. As a result, PGE2 increased the YAP-TEAD reporter activity (Figure. 1D). To generalize above findings, we further examined another human colon cancer cell line SW480, and obtained similar results as in DLD-1 (Supplementary Figure. 1).
Figure 1. PGE2 positively regulates YAP expression in human colon cancer cells and mouse colon.
(A) Immunoblot analysis of DLD-1 cells that had been deprived of serum for 24 h and then exposed to PGE2 (10 μM) for the indicated times.
(B) Immunofluorescence staining of YAP in serum-deprived DLD-1 cells exposed to PGE2 (+) or vehicle (−) for 18 h. Nuclei were stained with 4,6-diamidino-2-phenylindole (DAPI). Scale bars, 50 μm.
(C) Reverse transcription (RT) and quantitative polymerase chain reaction (qPCR) analyses of samples from A.
(D) Relative TEAD4 transactivation activity in DLD-1 cells transfected with a luciferase reporter construct containing eight tandem TEAD binding sites, deprived of serum, and then exposed to PGE2 (+) or vehicle (−) for 24h.
(E) Experimental protocol for manipulation of PGE2 levels in the mouse colon by 15-PGDH knockout and indomethacin (Indo) administration.
(F) PGE2 content as determined by mass spectrometry in the colon of WT, WT + Indo, 15-PGDH-KO, and 15-PGDH-KO + Indo mice.
(G, H) Immunoblot (G) and RT-qPCR (H) analyses of the colonic mucosal epithelium and crypts of the indicated groups.
All quantitative data are means ± s.e.m. (n = 5, 7, 5, and 5 in C, D, F and H respectively). *P< 0.05, **P< 0.01, ***p< 0.001 versus the indicated comparisons (two-tailed Student’s t test).
To compare the transcriptional effect on YAP by PGE2 to phosphorylation-mediated effect on YAP, we knocked-out LATS1 and LATS2 by lentivirus-mediated CRISPR-Cas9. As expected, ablation of LATS kinases abolished YAP Sep127 phosphorylation, accompanied by induction of YAP target genes (Supplementary Figure. 2A). Treatment of PGE2 on control cells induced YAP target genes to comparable level as seen in LATS kinase deleted cells. PGE2 further increased YAP target gene levels in LATS1/2 ablated cells (Supplementary Figure. 2A). Using TEAD reporter, we also obtained a similar result (Supplementary Figure. 2B).
To test whether PGE2 also regulates YAP in vivo, we examined: 1) mice deficient in the PGE2-degrading enzyme 15-prostaglandin dehydrogenase (15-PGDH)3,15,16, and 2) both WT and 15-PGDH-KO mice that were treated with the COX inhibitor indomethacin to block PGE2 synthesis (Figure. 1E). Indeed, PGE2 concentration was elevated by 2.5-fold in colonic tissues of 15-PGDH-KO mice compared with those in WT mice, while 10 days of indomethacin feeding reduced PGE2 to an almost undetectable level in both WT and 15-PGDH-KO mice (Figure. 1F; Supplementary Figure. 1E). Accordingly, the abundance of YAP as well as YAP target genes were increased in the colon of 15-PGDH-KO mice compared with those of WT mice, whereas they were reduced by indomethacin treatment (Figure. 1G and 1H). These data thus indicate that PGE2 regulates YAP expression in vivo.
EP4-PKA-CREB signaling mediates PGE2 induced YAP transcription
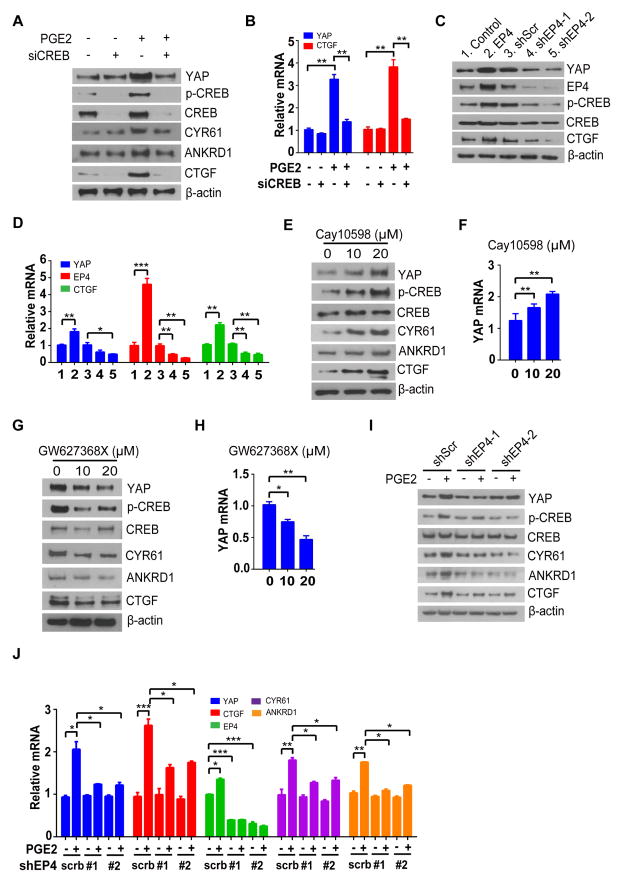
We next investigated the mechanism by which PGE2 up-regulates YAP mRNA. Binding of PGE2 to the GPCR EP4 activates protein kinase A (PKA) and cAMP response element binding protein (CREB) pathway and consequent CREB-mediated transcriptional regulation17,18. Given that CREB was recently shown to promote YAP gene transcription19–21, we examined whether it might also mediate PGE2-induced YAP expression. Indeed, PGE2 failed to up-regulate both protein and mRNA levels of YAP in CREB-depleted DLD-1 and SW480 cells (Figure. 2A and 2B; Supplementary Figure. 3A and 3B), suggesting that the effect of PGE2 on YAP expression is indeed mediated by CREB. We then tested whether EP4 mediates the effect of PGE2 on YAP. Forced expression of EP4 induced CREB phosphorylation at Ser133 as well as YAP expression in both DLD-1 and SW480 cells, whereas depletion of EP4 with two independent shRNAs had opposite effects (Figure. 2C and 2D; Supplementary Figure. 3C and 3D). Furthermore, given the known roles of EP4 agonists and antagonists in regulation CREB phosphorylation, we wondered whether such treatments will also regulate the transcription of YAP. The EP4 agonist CAY10598 increased both CREB phosphorylation and YAP expression (Figure. 2E and 2F; Supplementary Figure. 3E and 3F), whereas the EP4 antagonist GW627368X had the opposite effects (Figure. 2G and 2H; Supplementary Figure. 3G and 3H). Most importantly, depletion of EP4 diminished the levels of phosphorylated CREB, expression of YAP and YAP target genes induced by PGE2 (Figure. 2I and 2J). Together, these results indicated that EP4 and CREB mediate the transcriptional induction of YAP by PGE2.
Figure 2. Prostaglandin E2 (PGE2) promotes phosphorylation of CREB (Ser133) through EP4 receptor, which positively regulates transcription of YAP.
(A, B) Immunoblot (A) and RT-qPCR (B) analyses of DLD-1 cells transfected with CREB (siCREB) or scrambled siRNAs, deprived of serum for 24 h, and exposed to PGE2 (10 μM) or vehicle for 12 h.
(C, D) Immunoblot (C) and RT-qPCR (D) analyses of DLD-1 cells infected with a retrovirus encoding EP4 (EP4) or an empty virus (Control), or with lentiviruses encoding EP4 (shEP4-1 or shEP4-5) or scrambled (Scr) shRNA.
(E, F) Immunoblot (E) and RT-qPCR (F) analyses of DLD-1 cells deprived of serum for 24 h and then exposed to the indicated concentrations of CAY10598 (EP4 agonist) or vehicle for 16 h.
(G, H) Immunoblot (G) and RT-qPCR (H) analyses of DLD-1 cells treated with GW627368X (EP4 antagonist) for 16 h in serum-containing medium.
(I, J) Immunoblot (I) and RT-qPCR (J) analyses of DLD-1 cells treated as indicated.
All quantitative data are means ± s.e.m. (n = 5 for all experiments). *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student’s t-test) versus the indicated comparisons.
Regulation of YAP by PGE2 is independent of ERK, AKT and NF-κB pathways
Previous studies have linked stimulation by PGE2 to activation of Ras-MAPK, PIK3CA-Akt and NF-κB pathways in colorectal cancer cells22–24. Therefore, we sought to examine if any of these pathways contribute to induction of YAP by PGE2. To do this, we blocked each pathways using pharmacological inhibitors: Selumetinib and FR-180204 (Ras-MAPK inhibitor), LY204002 (PIK3CA inhibitor), and IKK-16 (NF-κB inhibitors). We treated cells with PGE2 only or together with the indicated inhibitors and analyzed YAP levels. Consistent with previous reports, Ras-MAPK or PIK3CA inhibitors alone down-regulated YAP protein level without affecting YAP transcript level 22–24, and we found a similar phenomenon with NF-κB inhibitor as well (Supplementary Figure 4A and 4B). Importantly, YAP was still induced in the presence of these inhibitors (Supplementary Figure 4A and 4B). These data suggest that Ras-MAPK, PIK3CA, and NF-κB pathways do not contribute to YAP mRNA induction by PGE2. Reciprocally, we then examined if YAP is required for activation of these pathways by PGE2. To this end, we treated control or YAP shRNA expressing DLD-1 cells with PGE2 and examined pathway activation by Western blotting. While we could not see significant changes in ERK phosphorylation by PGE2, we observed a reproducible increase of AKT T308 phosphorylation and IkB phosphorylation. However, neither of these was affected by YAP depletion (Supplementary Figure 4C). Thus, PIK3CA-AKT, NF-κB and YAP are likely independent downstream pathways of PGE2 signaling.
YAP forms positive feedback loop by up-regulating EP4 and COX-2
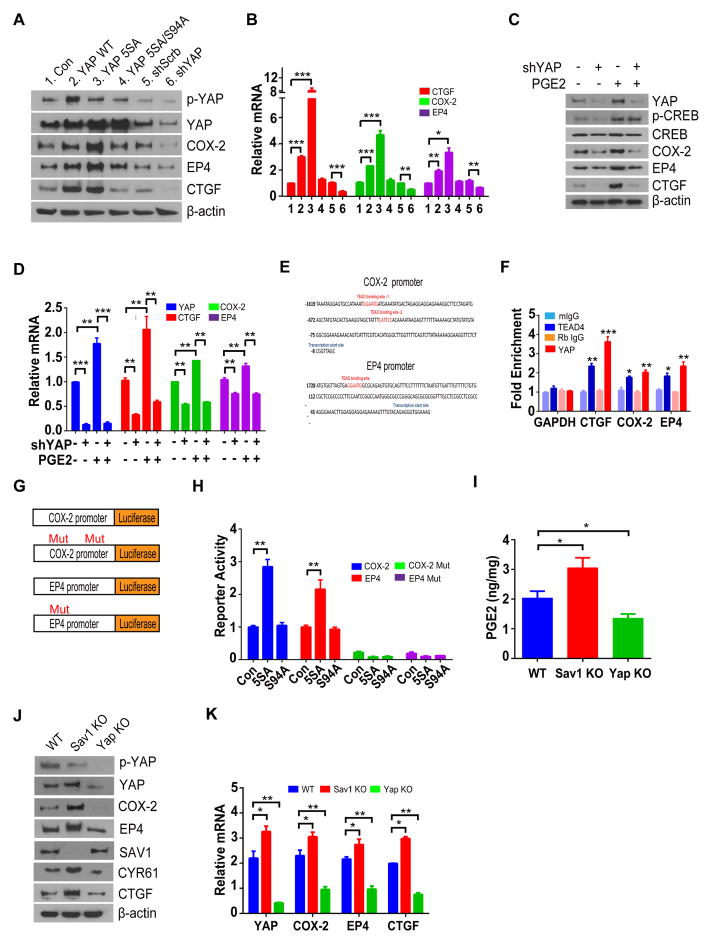
Previous transcriptome analysis showed up-regulation of PTGS2 (encodes COX-2, a key enzyme for PGE2 synthesis) and PTGER4 (encodes EP4) by YAP activation25–27, prompting us to hypothesize that YAP may also trigger PGE2 pathway. We therefore examined whether YAP up-regulates PGE2 signaling in colon cancer cells. Expression of WT or constitutively active mutant (5SA) of YAP increased the abundance of COX-2 and EP4 proteins and mRNAs in DLD-1 and SW480 cells, whereas the 5SA-S94A mutant of YAP, which does not interact with TEAD transcription factors, had no such effects (Figure. 3A and 3B; Supplementary Figures 5A and 5B). Treating cells with Verteporfin (VP), which is known to abolish YAP-TEAD interaction28, also decreased COX-2 and EP4 mRNA levels (Supplementary Figure 5C). In contrast to YAP activation, depletion of YAP by shRNA resulted in reduction of COX-2 and EP4 (Figure. 3A and 3B; Supplementary Figures 5A and 5B). Furthermore, PGE2 failed to induce COX-2 and EP4 in cells depleted of YAP (Figure 3C and 3D).
Figure 3. YAP reinforces PGE2 signaling by forming a positive feedback loop.
(A, B) Immunoblot (A) and RT-qPCR (B) analyses of DLD-1 cells infected with retroviruses encoding YAP (WT), YAP(5SA), YAP(5SA-S94A), scrambled or shRNA against YAP.
(C, D) Immunoblot (C) and RT-qPCR (D) analyses of DLD-1 cells infected with scrambled or YAP shRNA treated with PGE2.
(E) Putative TEAD binding sites (red) in the promoter regions of human PTGS2 and PTGER4.
(F) ChIP-qPCR analyses of PTGS2 and PTGER4 promoter regions performed with rabbit antibodies to YAP, mouse antibodies to TEAD4, and corresponding control immunoglobulin G (IgG) in DLD-1 cells growing in full-growth medium. Data are expressed as fold enrichment relative to input DNA. CTGF and GAPDH were examined as positive and negative controls, respectively.
(G) Luciferase reporter constructs used.
(H) Luciferase reporter activity was measured in cells expressing indicated plasmids.
(I) PGE2 content of the colon in WT, Sav1-KO, and YAP-KO mice.
(J, K) Immunoblot (J) and RT-qPCR (K) analyses of the colonic mucosal epithelium and crypts of the indicated groups.
All quantitative data are means ± s.e.m. (n = 5 for all experiments). *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student’s t-test) versus the indicated comparisons.
Loss of YAP did not affect CREB phosphorylation, confirming that YAP is downstream of CREB activation.
Analysis of COX-2 and EP4 promoter sequences revealed two and one putative TEAD binding sequences in each gene (Figure. 3E). By chromatin immunoprecipitation (ChIP), we confirmed the binding of YAP and TEAD4 at putative TEAD binding sites in the promoter regions of the COX-2 and EP4 both in full-growth media condition and in PGE2 treated cells after serum starvation (Figure. 3F; Supplementary Figures 5D and 5E). To further prove that COX-2 and EP4 are direct targets of YAP-TEAD, we constructed reporter plasmids, of which the expression of luciferase gene is driven by either COX-2 or EP4 promoters (Figure 3G). The reporters were highly activated by YAP 5SA, but not by YAP 5SA/S94A (Figure 3H). Most importantly, the reporters of which the TEAD binding sites were mutated, failed to be activated by YAP 5SA (Figure 3H).
We next examined the regulation of Ptgs2 and Ptger4 by YAP in vivo using colon-specific YAP-KO mice29 and Sav1-KO mice30 (in which YAP is activated as a result of loss of the upstream regulator Sav1) generated by crossing of corresponding floxed mice with Villin-Cre transgenic mice (in which Cre recombinase is expressed under the control of the Villin gene promoter). The abundance of COX-2 and EP4 protein and mRNA levels, as well as the PGE2 content, of the colon were reduced in the YAP-KO mice but increased in the Sav1-KO mice (Figure. 3I–K). We also confirmed that the level of COX-2 and EP4 were elevated in colonic tissue of 15-PGDH KO mice (Supplementary Figure. 6). Collectively, these data suggest that YAP amplifies PGE2 signaling by up-regulating COX-2 and EP4 expression, thus completing a positive feedback loop.
YAP controls PGE2-mediated colon regeneration in vivo
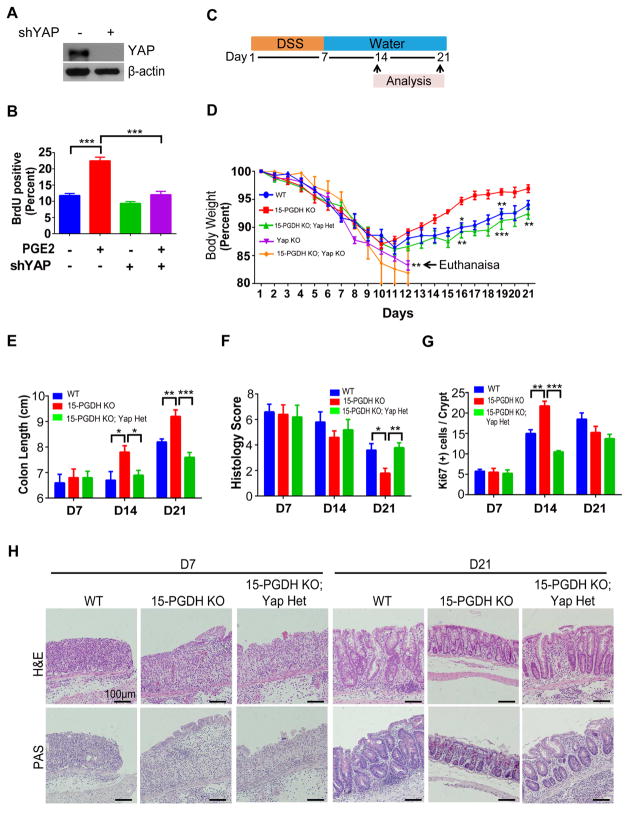
Having dissected the signaling circuit between PGE2 and YAP, the next important question was if YAP is important for the biological functions of PGE2. PGE2 is a well-established mitogen in cultured colon cancer cells1. To examine whether YAP is required for the mitogenic effect of PGE2, we depleted YAP in DLD-1 cells (Figure. 4A). We then serum starved control and YAP depleted cells, followed by stimulation with PGE2. The proportion of proliferating control cells was increased by PGE2 treatment, whereas YAP-depleted cells showed no such response (Figure. 4B). Similar results were observed in SW480 cells (Supplementary Figures 7A and 7B). YAP and PGE2 are thought to be key mediators of colon regeneration following colitis3,9,31. Importantly, YAP and PGE2 are both abruptly up-regulated following tissue injury3,9,32. We therefore examined whether PGE2 might promote colon regeneration by up-regulating YAP expression in mice. For this, we generated mice with varying expression levels of 15-PGDH and YAP, and challenged these mice with dextran sodium sulfate (DSS) (Figure. 4C). Consistent with recent observations3, we found that the rate of colon regeneration after the induction of colitis with DSS was increased in 15-PGDH-KO mice compared with WT mice. 15-PGDH-KO mice thus showed faster recovery of body weight, colon length, and colitis histology score (Figure. 4D–4F). The expressions of Ki-67, COX-2, YAP, EP4, and CTGF were significantly increased in 15-PGDH-KO mice compared with WT mice at D14 but returned to a similar level by D21, because WT was still undergoing regeneration whereas 15-PGDH-KO had almost completely regenerated by this time (Figure. 4G; Supplementary Figure. 8). Strikingly, deletion of one copy of YAP gene (YAP-Hetero) in 15-PGDH-KO mice rendered a similar colon regeneration pattern to that of WT mice (Figure. 4D–4G). Periodic acid–Schiff (PAS) staining of goblet cells, which represents the level of villus regeneration, also revealed that villus regeneration was enhanced in 15-PGDH-KO mice, which was normalized by YAP heterozygosity (Figure. 4H). Consistent with previous findings9, colon-specific YAP-KO mice failed to regenerate the colon after DSS-induced injury. Importantly, 15-PGDH-KO; YAP-KO mice also failed to regenerate the colon and died soon after DSS challenge (Figure 4D and Supplementary Figure S9). Treating 15-PGDH-KO mice with indomethacin either at day 7 or day 14 after start of DSS-treatment (Supplementary Figure. 10A) resulted in an immediate deterioration of the colon as reflected by reduced colon length, increased colitis histology score, and decreased expressions of Ki-67, COX-2, YAP, EP4, and CTGF (Supplementary Figure. S10), confirming that the increased regenerative phenotype of 15-PGDH KO mice depends on PGE2. Together, these data suggest that YAP is an essential effector of PGE2-mediated colon regeneration following DSS-induced colitis.
Figure 4. The PGE2-YAP positive feedback loop promotes colon regeneration.
(A) Immunoblot verification of DLD-1 cells infected with retroviruses encoding shRNA against YAP or scrambled seqeunce.
(B) Assay of BrdU incorporation in DLD-1 cells expressing YAP or scrambled shRNAs, deprived of serum, and exposed to PGE2 or vehicle for 24 h.
(C) Experimental protocol for panels. Mice were provided with drinking water containing 3% DSS for 7 days followed by normal drinking water for 14 days
(D) Change in body weight for the indicated groups of mice. Statistical comparisons are for WT versus 15-PGDH-KO, YAP-Hetero versus 15-PGDH-KO; YAP-Hetero, and YAP-KO versus 15-PGDH-KO; YAP-KO.
(E) Colon length for the indicated groups of mice.
(F) Colitis histology score for the indicated groups of mice.
(G) Histological data of Ki-67 were quantitated from the indicated groups of mice at 7, 14, and 21 days after the induction of colitis by DSS administration.
(H) Representative H&E and PAS staining were shown for serial sections of the colon from the indicated groups of mice at 7 and 21 days after the induction of colitis by DSS administration. Scale bars, 100 μm.
All quantitative data are means ± s.e.m. (n = 5 for all experiments) *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student’s t test) versus the indicated comparisons.
Increased PGE2 and dysregulation of Hippo pathway cooperates to induce colon cancer development
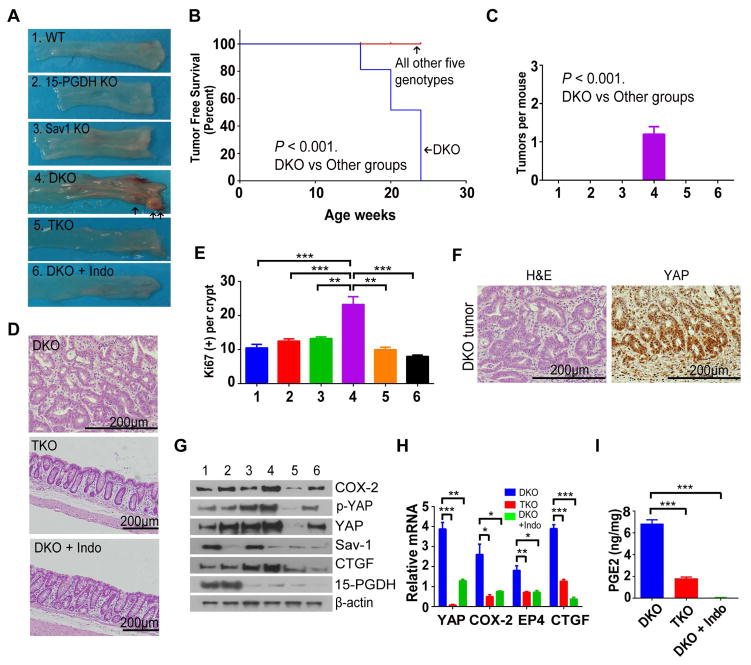
15-PGDH-KO mice, despite their elevated PGE2 levels, do not form spontaneous tumors15. Colon-specific Sav1-KO mice also rarely develop colon tumors spontaneously9 (H.-B.K. & D.-S.L., unpublished observation), however, they do develop sessile serrated polyps at an advanced age after chronic DSS-induced injury9. Therefore, YAP activation by dysregulation of Hippo pathway could synergize with colitis to induce tumor formation. Since we have shown that PGE2 signaling and YAP form a positive feedback loop, we examined whether defective regulation of YAP in Sav1-KO mice and increased PGE2 levels in 15-PGDH-KO mice might synergize to induce tumor formation. We thus generated 15-PGDH-KO; Sav1-KO (DKO) mice to observe tumor formation. We also crossed colon-specific YAP-KO mice with the DKO mice to generate 15-PGDH-KO; Sav1-KO; YAP-KO (TKO) mice, or treated the DKO mice with indomethacin in order to assess the roles of YAP and PGE2, respectively, in tumor formation in DKO mice. Whereas Sav1-KO or 15-PDGH-KO mice did not develop spontaneous tumors up to 1 year of age, the DKO mice started to form polyps from 12 to 16 weeks of age that eventually progressed to carcinoma in situ by 6 months of age (Figure. 5A–5C). Strikingly, the colon of TKO mice or of DKO mice treated with indomethacin did not manifest spontaneous tumor formation up to 6 months of age (Figure. 5A–5D). The expressions of Ki-67, YAP, COX-2, EP4, and CTGF were significantly increased in the colon of DKO mice compared to TKO or indomethacin-treated DKO mice (Figure. 5E and H). In addition, the elevated PGE2 level in the colon of DKO mice was significantly reduced by either YAP ablation or indomethacin treatment (Figure. 5I). Collectively, these data provide compelling evidence that a positive feedback loop between PGE2 and YAP drives tumorigenesis in mouse colon.
Figure 5. Dysregulation of Hippo pathway and increased PGE2 induce spontaneous colon tumorigenesis.
(A) Gross appearance of the colon from indicated groups at 24 weeks of age. TKO, 15-PGDH-KO; Sav1-KO; YAP-KO. Indo, indomethacin.
(B) Curves for 15-PGDH-KO, Sav1-KO, TKO, and DKO + Indo groups were identical to that for WT mice. The age corresponding to a tumor-free-survival rate of 50% was 20 weeks for DKO mice.
(C) Numbers of colon palpable tumors per mouse in WT, 15-PGDH-KO, Sav1-KO, TKO, and DKO + Indo groups.
(D) Representative H&E staining of the colon from DKO, 15-PGDH-KO;Sav1-KO;YAP-KO (TKO), and DKO + Indo mice at 24 weeks of age. Polyps formed in DKO mice progressed to carcinoma in situ. Scale bars, 200 μm.
(E) Histological data of Ki-67 were quantitated from indicated mice group at 24 weeks after the induction of colitis by DSS administration.
(F) Representative hematoxylin-eosin (H&E) staining and immunohistochemical analysis of YAP in carcinoma in situ. Scale bars, 200 μm.
(G, H) Immunoblot (G) and RT-qPCR (H) analyses of the colonic mucosal epithelium and crypts of 24-week-old mice of the indicated groups.
(I) PGE2 content of the colon in DKO, TKO, and DKO + Indo mice.
All quantitative data are means ± s.e.m. (n = 5 for all experiments) *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student’s t test) versus the indicated comparisons
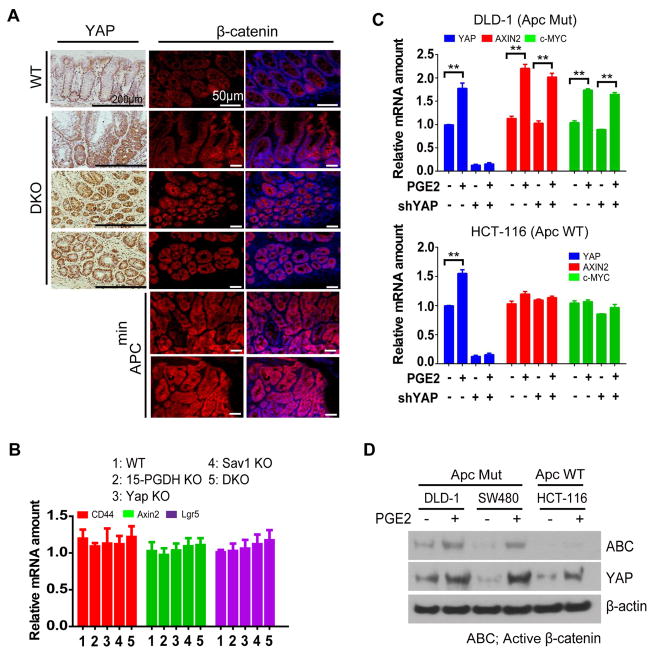
β-catenin is not involved in PGE2-YAP driven tumorigenesis
PGE2 was proposed to promote colon cancer growth via Axin–β-catenin pathway in APC (adenomatous polyposis coli) mutant background33. In addition, previous reports suggested that YAP is part of β-catenin circuit34 and cooperate with β-catenin in colon cancer35. Therefore, the effect of PGE2 could be mediated by both YAP and β-catenin. However, to this end, we could not obtain results supporting the contribution of β-catenin in PGE2-YAP pathway. While all carcinoma in situ developed in DKO mice manifested strong nucleus localization of YAP (Figure. 5F and Figure. 6A), β-catenin showed cytoplasmic and membranous localization (Figure. 6A). In contrast, we observed strong nuclear localization of β-catenin in Apcmin tissue samples (Figure 6A). We further examined β-catenin activity in DKO mice by analyzing several β-catenin target gene levels; CD44, Axin2 and Lgr5. Consistent with no obvious nuclear localization of β-catenin in DKO cancers, none of these genes were elevated in DKO colon tissue (Figure 6B). Consistent with previous reports, PGE2 activated β-catenin in APC mutant colon cancer cell lines DLD-1 and SW480 (Figure. 6C and Supplementary Figure 11A) as judged by induction of Axin2 and Myc, but not in APC WT colon cancer cell line HCT-116 (Figure 6C). However, depletion of YAP did not affect β-catenin target gene induction in DLD-1, HCT-116 and SW480 cells (Figure 6C and Supplementary Figure S11A). Furthermore, PGE2 could up-regulate YAP in both APC wild-type and mutant colorectal cancer cell lines (Figures 6C and 6D; Supplementary Figure S11). These results suggest that PGE2-mediated YAP up-regulation is independent of APC status, and that it is sufficient to induce colorectal cancer without noticeable activation of β-catenin. YAP was recently shown to be required for the development of APC-deficient adenoma in mice14,36. Interestingly, the abundance of PGE2 was found to be increased in human colorectal carcinoma as well as in adenomas of patients with familial adenomatous polyposis37,38 and of ApcMin/+ mice38, which could potentially function by further increasing YAP.
Figure 6. β-catenin is not involved in tumor development in DKO mice.
(A) Representative H&E staining as well as immunohistochemical of YAP and immunofluorescence analysis of β-catenin were shown for serial sections of the colon from DKO mice progressed to carcinoma in situ. Results from section of Apcmin mice were also presented as positive control of nuclear β-catenin. Scale bars, 200 μm (immunohistochemical staining) or 50 μm (immunofluorescence staining).
(B) RT-qPCR analyses of the colonic mucosal epithelium and crypts of 24-week-old mice of the indicated groups.
(C) RT-qPCR analysis of DLD-1 and HCT-116 cells treated as indicated.
(D) Immunoblot analysis of DLD-1, SW480 and HCT-116 cells treated as indicated.
All quantitative data are means ± s.e.m. (n = 5 for all experiments) *P < 0.05, **P < 0.01, ***P < 0.001 (two-tailed Student’s t test) versus the indicated comparisons.
YAP and PGE2 status are highly correlated with human colitis-associated cancers and colon cancers
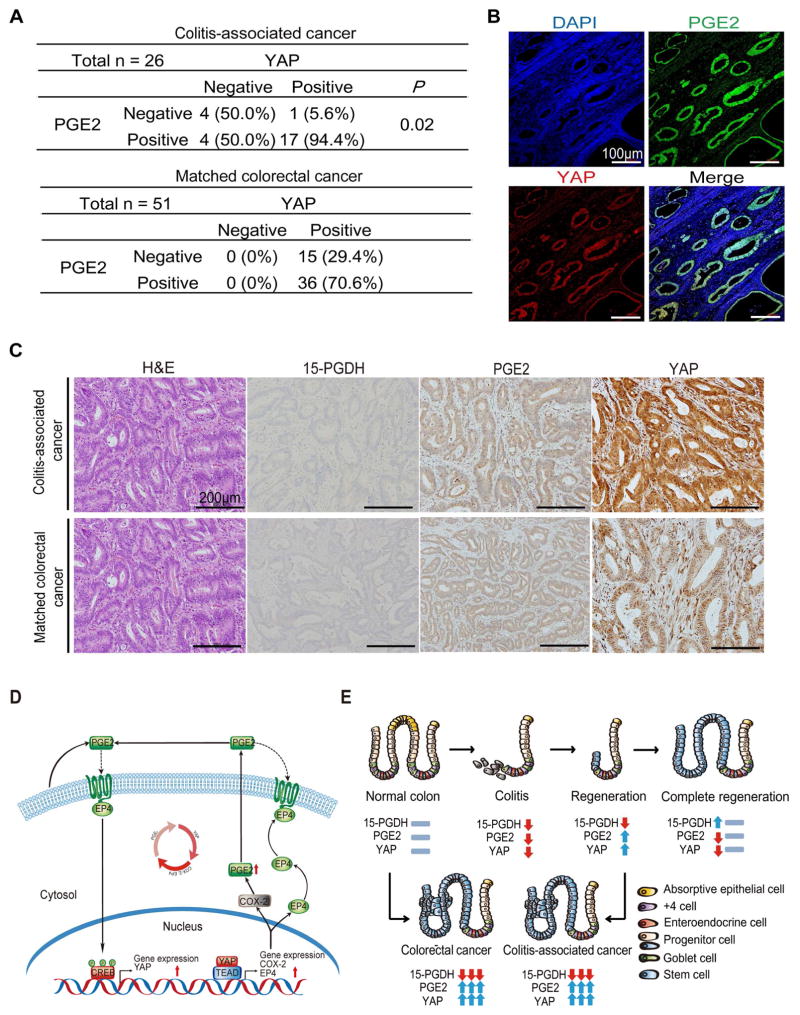
To extend our findings to human patients, we collected 26 colitis-associated cancer and 51 age- and sex-matched colorectal cancer biopsy specimens. Immunohistochemical staining of YAP and PGE2 in the colitis-associated cancer specimens revealed that 94.4% (17/18) of YAP-positive cases were also positive for PGE2, whereas only 5.6% (1/18) of YAP-positive cases were negative for PGE2 (Figure. 7A). Similarly, 70.6% (36/51) of matched colorectal cancer specimens were also positive for both PGE2 and YAP. The expression of YAP was detected in both the nucleus and cytoplasm, and it was mostly co-localized with PGE2 (Figure. 7B and 7C). Consistent with previous findings13,39, we did not detect 15-PGDH expression in any of the 77 cancer biopsy specimens (Supplementary Table. 1). Together, these results suggest that the PGE2-YAP positive feedback loop may also contribute to human colitis-associated cancer and colorectal cancer (Figure. 7D and 7E).
Figure 7. PGE2-YAP pathway in human colorectal cancer specimens.
Relation between PGE2 abundance and YAP expression in human colitis-associated cancer and matched colorectal cancer specimens.
(A) Categorization of immunohistochemical staining patterns for YAP and PGE2 in serial sections of 26 colitis-associated cancer and 51 matched colorectal cancer specimens. 15-PGDH expression was negative in all 77 cancer specimens.
(B) Representative immunofluorescence images showing staining of both PGE2 (green) and YAP (red) in colitis-associated cancer. Nuclei were stained with DAPI (blue). Scale bar, 100 μm.
(C) Representative H&E staining as well as immunohistochemical staining of 15-PGDH, PGE2, and YAP in colitis-associated cancer and matched colorectal cancer specimens. Scale bar, 200 μm.
(D) The model depicts the regulation of YAP gene expression by PGE2 and the regulation of COX-2 and EP4 gene expression by YAP.
(E) An illustration depicting the role of the PGE2-YAP positive feedback loop in colon regeneration and tumorigenesis.
DISCUSSION
Here, our results clearly show a new positive feedback mechanism involving PGE2 and YAP in the colon (Figure. 7D), which plays a critical role during regeneration following colitis and tumorigenesis (Figure. 7E). In summary, we discovered that PGE2 regulates colon regeneration and carcinogenesis by up-regulating YAP. We showed that PGE2 increases YAP transcription both in vivo and in vitro, and identified CREB phosphorylation as the underlying mechanism. While identification of YAP targets in colon awaits further analysis, we found that key components of the PGE2 pathway (COX-2 and EP4) are themselves YAP targets. We speculate that this positive feedback loop enables quick signal amplification after tissue injury. We provided key in vivo evidences that YAP is required for PGE2 function for colitis-induced regeneration as well as spontaneous tumorigenesis. Finally, our findings were also relevant in human colon cancers. These results provide a significant insight into the mechanism of colon regeneration and tumorigenesis.
YAP is implicated in various cancers7 and is often over-expressed in more aggressive cancers. Regulation of YAP phosphorylation (or localization) has been extensively studied; however, dysregulation of this mechanism is unlikely to explain YAP activation in cancers40–42. Except for NF2, mutations of upstream Hippo components are rarely found in cancers. In addition, no single case of YAP mutation alone has been reported. Although YAP locus (11q22) is amplified in a subset of epithelial cancers, this accounts for only a small portion. Importantly, mRNA level of YAP (and TAZ) sufficiently correlates with target gene expression and well-associates with cancer progression10,43. Moreover, most of the immunohistochemistry and microarray data in cancer specimens show increased total YAP abundance rather than focused nuclear localization of YAP10,43. To our knowledge, this is the first report to show the physiological importance of YAP regulation by PGE2 in vivo. In addition, our study underscores the importance of transcriptional regulation of YAP. While much of previous studies focused on mutations or locations of YAP expression, our data supports the recently proposed idea that the excessive expression of YAP is actually a more important factor in tumorigenesis. Therefore, our findings provide a clinically relevant mechanism by which YAP mRNA is increased in human colon cancers.
Importantly, our results suggest diversification of signaling mechanisms downstream of GPCRs in regulating YAP. It has been shown that GPCRs differentially affect YAP/TAZ activity12. Especially, Gαs-linked GPCRs were shown to inhibit YAP/TAZ activity through PKA44–46. Our results are contradictory to these previous studies as EP4, a Gαs-linked GPCR, actually activates YAP. We do not yet understand the reason behind this. Differential effect of GPCRs on YAP/TAZ activity might reflect the use of different cell types. It is also important to note that even in the original work on GPCR-Hippo signaling, the authors observed diverse consequence of different Gαs-linked GPCRs (e.g. EP2 decreases YAP phosphorylation, while Glucagon increases YAP phosphorylation). Our results suggest that the exact signaling mechanism might differ for each GPCRs, and underscores the importance of studying them in physiologically relevant situations.
Recently, a 15-PGDH inhibitor (SW033291) was shown to promote tissue regeneration by increasing PGE2 levels3. However, chronic down-regulation of 15-PGDH promotes the development of colorectal cancer due to the maintained high PGE2 level15. Previous studies have demonstrated that prolonged use of COX inhibitors prevents the risk of developing colorectal cancer and inhibits colon regeneration during colitis by down-regulating PGE22,4. However, the exact mechanism as to how COX inhibitors exhibit these effects was poorly understood. Here, we possibly explain this phenomenon by demonstrating the positive feedback loop between PGE2 and YAP and that COX-2 acts as a bridge that completes this loop. In support of these ideas, administration of an EP4 receptor antagonist is known to deteriorate DSS-mediated colitis 4 but prevent colon tumorigenesis.
Our study has demonstrated that the positive feedback loop between PGE2 and YAP plays a central role during colon regeneration and colorectal tumorigenesis. Notably, the Cancer Research UK and the National Institute for Health Research has recently funded the Add-Aspirin phase III trial, the largest clinical trial to have ever taken place. Since Aspirin is also a COX inhibitor, the mechanism elucidated in our study might open new avenues and possible interpretations that will greatly benefit this clinical trial. Inhibitors of YAP may be also synergistic with COX inhibitors both in colon cancer prevention and possibly inhibition of tumor growth by blocking this positive feedback loop.
Materials and Methods
Cell culture
DLD-1, SW480, HCT-116, and RPE-1 cells were maintained in Dulbecco’s modified Eagle’s medium (Bio-west) supplemented with 10% fetal bovine serum (Gibco) and an antibiotic-antimycotic mixture (Gibco). Cells at 70 to 80% confluence were treated with Compounds or dimethyl sulfoxide vehicle. PGE2, CAY10598, GW627368X, and AZD6244(Selumetinib) were from Cayman Chemical. FR-180204 was from TOCRIS Bioscience. LY294002, IKK16, and Verteporfin were purchased from Sigma Aldrich.
Isolation of colon epithelial cells
Colons at 1 cm way from the anus were cut longitudinally and then into three sections (Proximal, middle, distal). Colons were rinsed in DPBS And distal pieces (0.5 ~ 0.7 cm) of colon were incubated in DPBS with 5 mM EDTA for 20 min at 37°C with strong shaking to release colon epithelial cells. To extract RNA and protein from colon epithelial cells, the supernatant containing colon epithelial cells was carefully collected and centrifuged. The colon epithelial cell pellets were washed in DPBS and subjected to RNA extraction or protein extraction.
ChIP-qPCR analysis
ChIP was performed with the use of a Pierce Magnetic ChIP Kit (Thermo Fisher Scientific). In brief, cells (1 × 106) were fixed with 1% formaldehyde for 10 min at room temperature, supplied with glycine solution to terminate the reaction, and chromatin was digested with micrococcal nuclease to yield DNA fragments with an average size of 100 to 300 bp. The chromatin fragments were subjected to immunoprecipitation overnight at 4°C with antibodies to TEAD4 (Abcam, ab58310) or ChIP-grade mouse IgG (Abcam, ab18413) as a negative control, or with antibodies to YAP (generated in-house) or ChIP-grade rabbit IgG (Abcam, ab37415) as a negative control. After the addition of ChIP-grade protein A/G magnetic beads, the mixtures were incubated for 2 h at 4°C and the protein-DNA complexes were washed before elution and then treated with proteinase. The purified DNA was subjected to qPCR analysis with gene-specific primers (Supplementary Table S1). The amount of ChIP DNA was expressed as fold enrichment relative to input.
Histological scoring of DSS-induced colitis
Histological assessment of colitis was based on hematoxylin-eosin staining of tissue sections. The number and area of ulcers and erosions were determined. The extent of mucosal crypt damage (cryptitis score) was graded from 0 to 4: Grade 0, no damage; Grade 1, damage in one-third of basal layer; Grade 2, damage in two-third of basal layer; Grade 3, crypt lost and surface epithelium present; Grade 4, loss of the entire crypt and surface epithelial layer. We measured the relevent areas at the same time and scored them from 0 to 4 according to their extent. Final score was calculated by multiplication of grade of damage by area involved in the crypt of the whole colon.
Human specimens and pathological analysis
Human colitis-associated cancer and spontaneous colorectal cancer tissue specimens were collected with the approval of the Asan Internal Review Board and written informed consent were obtained from adult patients undergoing surgical resection at the Department of Gastroenterology, Asan Hospital, between 1998 and 2010. Normal colon tissue present at the periphery of resected tumors was studied as a control. Immunohistochemical staining score was calculated based on the percentage of positively stained cells and the immunostaining intensity. Positive specimens were defined as those that passed a cutoff value that was determined for all samples. Negative specimens were defined as those with no detectable immunoreactivity (with normal colon tissue as a negative control) or those that did not pass the cutoff value.
LATS1/2 sgRNA
sgRNAs targeting either human LATS1 and LATS2 were designed using the online E-CRISP tool, then cloned into pLentiCRISPR_V2 puro vector. Following lentivirus production in 293T cells using psPAX2 and pMD2.G packaging vectors, the supernatant containing lentiviral particles for sgLATS1 or sgLATS2 were simultaneously infected into target cells using polybrene. Infected cells were then re-seeded as single cells in 96-well plates, and resulting clones were assessed for knockout efficiency by Western blot analysis followed by genomic DNA sequencing. The sgRNA sequences are as follows:
sgCAG: 5′-GTTCCGCGTTACATAACTTA-3′
sgLATS1: 5′-GCAACCTAACATACCAGTG-3′
sgLATS2: 5′-GTAGGACGCAAACGAATCG-3′
Generation of ANKRD1 antibody
The anti-human ANKRD1 polyclonal antibody was raised against GST-ANKRD1 (1-320 amino acids) fusion protein by immunizing the purified protein with Freund’s Adjuvant (Sigma-Aldrich) into New Zealand white rabbits. The anti-sera was then enriched for ANKRD1-binding IgG via affinity chromatography, following flow-through in GST-conjugated columns to remove GST-binding IgG. Supplementary Figure 12 shows the verification of ANKRD1 antibody. We note that this antibody only reacts with human ANKRD1, but not with mouse.
Statistical analysis
Quantitative data are presented as mean ± s.e.m and were analyzed with two-tailed Student’s t test, log-rank test for Kaplan-Meier curves or the chi-square test. Statistical analysis was performed with the use of IBM SPSS 19 software. A P value of < 0.05 was considered statistically significant. The experiments were not randomized. Biological experiments were performed at least three independent times, and tissue staining results are representative of at least three independent determinations. No animals or samples were excluded from analysis. Graphs were drawn with the use of Grahpad Prism 6.
Supplementary Material
Acknowledgments
We thank Eun-Ji Lee for preparation of Figure 7D and 7E; Hye-Jin Park for technical assistance; Dae-Hee Hwang for editing of the manuscript; Eric N. Olson for providing YAPflox/flox mice; and Min-Seon Kim for providing Villin-Cre mice.
Funding
This work was supported by grants from the National Creative Research Program (2012-0001228), Science Research Center Program (NRF-2016R1A5A1010764), the Bio & Medical Technology Development Program (2011-0019632), the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (HI06C0868), Asan Institute for the Life Sciences (2013-261), and NIH grants (P50 CA150964).
Footnotes
Author contributions
H.-B.K., M.-C.K., D.-S.L., and S.-J.M. conceived the project and wrote the manuscript. H.-B.K. performed experiments. H.-B.K. and M.-C.K. analyzed the data. Y.-S.P. performed immunohistochemical and histological staining and interpretation. M.-C.K., I.-.P., T.K., and S.D.M. discussed the data. S.-Y.Y. discussed the colitis model. C.J.C. performed the analysis of human cancer specimens. J.-H.J. S.W.H, and D.H.H performed mouse experiments. H.-B.K. and S.W.H. performed statistical analysis. M.-C.K., D.-S.L, and S.-J.M. directed the study.
Disclosure The authors disclose no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information includes Supplemental Experimental Procedures, six Figures, and one table and can be found with this article online
References
- 1.Wang D, Dubois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Desai A, Yang SY, et al. Tissue Regeneration. Inhibition of the prostaglandin-degrading enzyme 15-PGDH potentiates tissue regeneration. Science. 2015;348:aaa2340. doi: 10.1126/science.aaa2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabashima K, Saji T, Murata T, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. Journal of Clinical Investigation. 2002;109:883–893. doi: 10.1172/JCI14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–28. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13:63–79. doi: 10.1038/nrd4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer. 2013;13:246–57. doi: 10.1038/nrc3458. [DOI] [PubMed] [Google Scholar]
- 8.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 9.Cai J, Zhang N, Zheng Y, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–8. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou D, Zhang Y, Wu H, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 12.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gregorieff A, Liu Y, Inanlou MR, et al. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–8. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 15.Myung SJ, Rerko RM, Yan M, et al. 15-Hydroxyprostaglandin dehydrogenase is an in vivo suppressor of colon tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:12098–102. doi: 10.1073/pnas.0603235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coggins KG, Latour A, Nguyen MS, et al. Metabolism of PGE2 by prostaglandin dehydrogenase is essential for remodeling the ductus arteriosus. Nat Med. 2002;8:91–2. doi: 10.1038/nm0202-91. [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–7. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama U, Iwatsubo K, Umemura M, et al. The prostanoid EP4 receptor and its signaling pathway. Pharmacol Rev. 2013;65:1010–52. doi: 10.1124/pr.112.007195. [DOI] [PubMed] [Google Scholar]
- 19.Xia W, Liu Y, Jiao J. GRM7 regulates embryonic neurogenesis via CREB and YAP. Stem Cell Reports. 2015;4:795–810. doi: 10.1016/j.stemcr.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Ma L, Weng W, et al. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology. 2013;58:1011–20. doi: 10.1002/hep.26420. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Zhang J, You X, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–9. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 22.Choi HJ, Zhang H, Park H, et al. Yes-associated protein regulates endothelial cell contact-mediated expression of angiopoietin-2. Nat Commun. 2015;6:6943. doi: 10.1038/ncomms7943. [DOI] [PubMed] [Google Scholar]
- 23.Lin L, Sabnis AJ, Chan E, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015;47:250–6. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.You B, Yang YL, Xu Z, et al. Inhibition of ERK1/2 down-regulates the Hippo/YAP signaling pathway in human NSCLC cells. Oncotarget. 2015;6:4357–68. doi: 10.18632/oncotarget.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorrentino G, Ruggeri N, Specchia V, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–66. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- 26.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim T, Yang SJ, Hwang D, et al. A basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemness. Nat Commun. 2015;6:10186. doi: 10.1038/ncomms10186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xin M, Kim Y, Sutherland LB, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–45. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi K, Wu LW, Grivennikov SI, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castellone MD, Teramoto H, Williams BO, et al. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-beta-catenin signaling axis. Science. 2005;310:1504–10. doi: 10.1126/science.1116221. [DOI] [PubMed] [Google Scholar]
- 34.Azzolin L, Panciera T, Soligo S, et al. YAP/TAZ incorporation in the beta-catenin destruction complex orchestrates the Wnt response. Cell. 2014;158:157–70. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbluh J, Wang X, Hahn WC. Genomic insights into WNT/beta-catenin signaling. Trends Pharmacol Sci. 2014;35:103–9. doi: 10.1016/j.tips.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cai J, Maitra A, Anders RA, et al. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang VW, Shields JM, Hamilton SR, et al. Size-dependent increase in prostanoid levels in adenomas of patients with familial adenomatous polyposis. Cancer Research. 1998;58:1750–1753. [PubMed] [Google Scholar]
- 39.Fink SP, Yamauchi M, Nishihara R, et al. Aspirin and the risk of colorectal cancer in relation to the expression of 15-hydroxyprostaglandin dehydrogenase (HPGD) Sci Transl Med. 2014;6:233re2. doi: 10.1126/scitranslmed.3008481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan SW, Lim CJ, Chen L, et al. The Hippo pathway in biological control and cancer development. J Cell Physiol. 2011;226:928–39. doi: 10.1002/jcp.22435. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Qin F, Deng X, et al. Hippo pathway in intestinal homeostasis and tumorigenesis. Protein Cell. 2012;3:305–10. doi: 10.1007/s13238-012-2913-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–92. doi: 10.1016/j.ccr.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 44.Kim M, Kim M, Lee S, et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–55. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu FX, Zhang Y, Park HW, et al. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Genes Dev. 2013;27:1223–32. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iglesias-Bartolome R, Torres D, Marone R, et al. Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.