Abstract
Glutamatergic neurotransmission entails a tonic loss of glutamate from nerve endings into the synapse. Replacement of neuronal glutamate is essential in order to avoid depletion of the internal pool. In brain this occurs primarily via the glutamate-glutamine cycle, which invokes astrocytic synthesis of glutamine and hydrolysis of this amino acid via neuronal phosphate-dependent glutaminase. This cycle maintains constancy of internal pools, but it does not provide a mechanism for inevitable losses of glutamate N from brain. Importation of glutamine or glutamate from blood does not occur to any appreciable extent. However, the branched-chain amino acids (BCAA) cross the blood-brain barrier swiftly. The brain possesses abundant branched-chain amino acid transaminase activity which replenishes brain glutamate and also generates branched-chain ketoacids. It seems probable that the branched-chain amino acids and ketoacids participate in a “glutamate-BCAA cycle” which involves shuttling of branched-chain amino acids and ketoacids between astrocytes and neurons. This mechanism not only supports the synthesis of glutamate, it also may constitute a mechanism by which high (and potentially toxic) concentrations of glutamate can be avoided by the re-amination of branched-chain ketoacids.
1. Brain Handling of Glutamate: The Glutamate-Glutamine Cycle
The overriding goal of intermediary metabolism is to assure constancy of energy supply and metabolite concentrations. To this end, a dense skein of membrane transporters, enzyme systems and regulatory factors work in a coordinated manner to maintain thermodynamic equilibrium and to avoid potentially toxic excursions of metabolite levels.
Maintaining a constant biochemical milieu poses special challenges for the brain, a metabolically intense system which is characterized by continual alterations of membrane potential and ionic gradients, the abrupt release (and reuptake) of neurotransmitters and dramatic fluctuations in regional blood flow. This dynamic system must articulate with the external circulation in a tightly controlled manner. Thus, the blood-brain barrier admits a limited repertoire of compounds in order to minimize toxic exposures and to assure that energy is expended solely for import of essential nutrients. Conversely, brain conserves those essential resources – neurotransmitters such as glutamate are an example – which the system needs to transfer information between neurons. Brain concentrates these compounds within a series of intracellular compartments in order to maximize the signal-to-noise ratio upon their release into the synapse and to avoid the neurotoxicity which accompanies an untoward rise in the extracellular concentration.
Our current understanding of glutamate as a neurotransmitter recapitulates each of these themes. We have long known not only that glutamate depolarizes neurons (Hayashi, 1952; Curtis and Watkins, 1960), but that it is, indeed, the primary excitatory neurotransmitter (Meldrum, 2000; Cooper and Jeitner, 2016). Furthermore, since the seminal studies of Berl et al (1962) it has been evident that brain glutamate handling is not enacted in a single, metabolically homogeneous pool, but in a multitude of discrete and inter-connected compartments that remain functionally isolated. Insight into the anatomy of this multi-compartmental system came with the finding that glutamine synthetase, the microsomal enzyme which converts glutamate to glutamine, is restricted to astrocytes (Martinez-Hernandez, 1977; Norenberg and Martinez-Hernandez, 1979). Over the past 50 years the concept of a “glutamate-glutamine cycle” has emerged as the central figuration in our understanding of brain amino acid metabolism (Westergaard et al, 1995; McKenna, 1996; 2007; Albrecht et al, 2007; Rothman et al, 2012; Walls et al, 2015; Cooper and Jeitner, 2016). This model posits neuronal release of glutamate into the synapse, uptake into astrocytes via high-affinity transporters (Robinson and Jackson, 2016), glial amidation of glutamate to glutamine, export of the latter to neurons via specific transport systems (Albrecht et al, 2007; Jenstad et al, 2013) and subsequent uptake of glutamine into neurons, where phosphate-dependent glutaminase regenerates glutamate in mitochondria.
2. Limitations of the Glutamate-Glutamine Cycle: The External Sources of Glutamate N
The essential elements of the glutamate-glutamine cycle have been integrated into a generally accepted conceptualization of brain amino acid handling. However, this model never was intended to be an exhaustive rendering of brain metabolism. Like all models, the glutamate-glutamine cycle is a deliberate simplification which highlights key elements of a complex system which become susceptible to experimental verification.
One limitation of the model is that it posits a stoichiometric relationship between glutamate released into the synapse, glutamine formed in astrocytes, and glutamate regenerated in neurons. However, McKenna (2007) has emphasized that both neurons and astrocytes include multiple subcellular compartments of glutamate metabolism and that glutamate carbon derives from various sources, including glucose, lactate and 3-hydroxybutyrate. In addition, it is clear that astrocytes not only synthesize glutamine, they also oxidize this amino acid (Yudkoff et al, 1996; Kvamme et al, 1982; Westergaard et al, 1995; Zielke et al, 1989; Cardona et al, 2015). Nor is all glutamine formed in glia restored to the neuronal compartment: transport of large neutral amino acids from blood-to-brain involves exchange with endogenously formed brain glutamine (James et al, 1978; Cangiano et al, 1983; Grill et al, 1992; Lee et al, 1998).
A related conceptual problem of the glutamate-glutamine cycle is that it describes only intra-cerebral glutamate handling and ignores the crucial question of the precursors to glutamate in the peripheral circulation. Clearly, no organ system can function in isolation from blood. Oxidative metabolism inevitably give rise to end-products - primarily CO2, H2O and NH3 – which must be eliminated from the system. It also is probable that brain supports a net efflux of lactate (Shulman et al, 2001; Dienel 2012), especially in response to heightened neuronal activity. As noted above, a tonic export of glutamine from brain is essential to the uptake of neutral amino acids from blood (James et al, 1978; Cangiano et al, 1983; Grill et al, 1992; Lee et al, 1998).
These losses from brain must be compensated. For individuals on a regular diet (that is, a non-ketogenic diet) the uptake of glucose from blood enables the replenishment of virtually all glutamate carbon (Dienel 2012; Shen, 2013), but the precursors to glutamate N are less obvious. Potential donors include blood-borne glutamate, NH3 or amino acids which donate an α-amino group via transamination. Direct importation of blood glutamate (usual concentration 20–50 µmoles/l) seems an infelicitous strategy, since trafficking of large amounts of glutamate through cerebral extracellular fluid invites the risk of evoking unbridled neuronal depolarization. Studies with 13NH3, a positron-emitting species, demonstrate virtually no incorporation of ammonia N into glutamate via reductive amination of α-ketoglutarate (Cooper et al, 1979). Hence, transamination of amino acids transported from blood constitutes the primary mechanism for synthesis of glutamate. The transport studies of Smith et al (1987) showed that the brain uptake system for neutral amino acids is near saturation at physiologic blood concentrations and is approximately 50% occupied by the sum of phenylalanine and leucine. It should be emphasized that this system is minimally (5%) occupied by glutamine. Furthermore, at physiologic plasma concentrations, the predicted uptake of leucine exceeds that of any other amino acid (Smith et al, 1987).
3. Branched-Chain Amino Acids as a Source of Brain Glutamate N
BCAA Are Major N Donor in Peripheral Tissues
A large body of evidence highlights the role of the branched-chain amino acids as nitrogen donors in peripheral tissues, particularly skeletal muscle, a major site for BCAA transamination (Miller, 1961; Manchester, 1965; Harper et al, 1984; Hutson, 1988; Bonfils et al, 2000). Transamination of muscle leucine, derived both from degradation of skeletal muscle protein and from insulin-enhanced uptake of blood leucine, supports formation of glutamate, which, in turn, is transaminated to yield alanine:
| (Reaction 1) |
| (Reaction 2) |
The alanine is released to liver, where the carbon becomes a gluconeogenic substrate and the nitrogen is converted to urea (Snell and Duff, 1981). These reactions assume heightened adaptive significance during starvation (Felig, 1975), when as much as 30% of alanine N is derived from the branched-chain amino acids (Haymond et al, 1978).
Uptake of BCAA Into the CNS
Amino acid uptake into brain is an intense and active process (Oldendorf, 1971) which obliges the coordinated action of several different transport systems. Influx of leucine into parietal cortex is faster (10 µmol/s/g wet weight) than that of any other amino acid (Smith et al, 1971). At physiologic blood concentrations of leucine (~ 50–150 µmol/l) transport is almost always saturated (Km 29 µmol/l) (Smith et al, 1981). Amino acid uptake must be closely linked to transamination of these compounds, since the neutral amino acids are transported in exchange for intra-cerebral glutamine (Cangiano et al, 1983; Grill et al, 1992).
After exiting blood, brain-bound metabolites enter astrocytes, which interpose between brain and vasculature (Brightman and Cheng-Tao, 1988; Abbot et al, 2006). Uptake of neutral amino acids into cultured astrocytes is largely sodium-independent and enhanced by addition of glutamine to the medium (Brookes 1992; 1993); glial leucine transport is marked augmented (80%) by glutamine (Brookes, 1992). The likely Km for astrocyte leucine transport is 80–119 µmol/liter.
Neurons also transport branched-chain amino acids via low- and high-affinity systems (Tan et al, 1987; 1988; Rao and Murthy, 1994; Rao et al, 1995; Yudkoff et al, 1996) which demonstrate marked sodium dependence (Yudkoff et al, 1996). Transport into synaptosomes is very rapid: Vmax of the low-affinity system is 62 nmol/min per mg protein and 118 nmol/min per mg protein leucine and valine, respectively.
Handling of the Branched-Chain Amino Acids in the CNS
Metabolism of these amino acids within brain proceeds in 3 distinct steps, which are exemplified by the example of leucine:
Transamination: Leucine + α-ketoglutarate ↔ α-ketoisocaproate + glutamate
Conversion of leucine-C into a form that enters the tricarboxylic acid cycle: α-ketoisocaproate → → acetoacetyl-CoA → acetyl-CoA
Oxidation of leucine-C in the tricarboxylic acid cycle: acetyl-CoA →→ CO2.
Complete oxidation of branched-chain carbon to CO2 occurs in both brain slices and synaptosomes (Shambaugh and Koehler 1981; 1983; Chaplin et al, 1976), but the rate of transamination greatly exceeds the rate of oxidation. Most branched-chain ketoacid formed in brain is converted back to the parent amino acid – in the case of α-ketoisocaproate to leucine (Shambaugh and Koehler, 1983). This “reverse” reaction was at least twice the rate of α-ketoisocaproate oxidation in brain slices from young rats. Brand (1981) demonstrated that in heart and kidney branched-chain ketoacids are primarily decarboxylated, but in brain these species are largely transaminated, in the process consuming an equimolar amount of glutamate.
It must be emphasized that many organs – and even cells within organs – do not completely oxidize branched-chain amino acids to completion. Instead, an intermediary metabolite may be released and then oxidized in another site. Indeed, as noted above, a fundamental motif of whole body amino acid metabolism involves release of branched-chain ketoacids from skeletal muscle and their subsequent oxidation in liver (Felig, 1975; Harris et al, 2001). This pattern even prevails within the brain. Thus, astrocytes can produce and release ketone bodies which they derive from the metabolism of leucine and fatty acids (Auestad et al, 1991; Bixel and Hamprecht, 1994; Hamprecht et al, 1995).
Transamination of Branched-Chain Amino Acids
Production of glutamate from BCAA obviously entails the transaminase (Reaction 1; BCAT) noted above. Transamination of all 3 branched-chain amino acids occurs in both mitochondria and cytosol, with distinct enzymes identified in either compartment. The mitochondrial isozyme is a 41 kDA protein (Hutson et al, 1992) while that in the cytosol is a 50 kDA species which likely forms a homodimer of apparent mass 91 kDa (Hall et al, 1993). Distribution of the two transaminases, which share a ~ 58% homology (Davoodi et al, 1998), varies in a tissue-specific manner. Most tissues possess a mitochondrial activity, but in brain nearly 70% of activity is cytosolic (Sweatt et al, 2004a; 2004b; Garcia-Espinosa et al, 2004; Hull et al, 2012). Activity may vary broadly, even within a single organ: in rat kidney activity of the transaminase is 40-fold greater in the thick ascending limb of Henle than it is in the proximal convoluted tubule (Burch et al, 1985).
In rat brain the cytosolic transaminase is restricted to neurons (Sweatt et al 2004a; 2004b). In many glutamatergic neurons this activity occurs in axons and nerve terminals, but in some GABA-ergic neurons activity predominates in the cell body. Autonomic neurons of the gut and axons of the sciatic nerve also contain primarily cytosolic activity. Astrocytes manifest primarily mitochondrial activity, but in developing oligodendroglia transamination also proceeds in the cytosol (Bixel et al, 1997). Hull et al (2012) found that in human brain the cytosolic enzyme is neuronal, but the mitochondrial variant is confined primarily to cells of the vascular endothelium. Both human glutamatergic and GABA-ergic neurons manifested the cytosolic enzyme in both cell bodies and axons.
The kinetic constants for the mitochondrial and cytosolic species are nearly identical (Hall et al, 1993). Michaelis constants for the amino acids (1–5 mmol/l) exceed the estimated level of these compounds in brain (0.1–0.2 µmol/g wet weight) (Siesjo, 1978). Similarly, the Km for α-ketoglutarate (0.6 mmol/l) is greater than the brain level of this metabolite (0.2 µmmol/g wet weight) (Erecinska et al, 1984).
Studies of the kinetics, molecular expression or distribution of the transaminase are not a surrogate for direct measurements of flux from branched-chain amino acid N to the amino groups of glutamate and glutamine. The latter information obliges the use of an isotopic tracer. The positron-emitting species, 13N, has been utilized productively to assay short-term transfer of nitrogen from ammonia to the amide and amino groups of glutamine (Cooper et al, 2016), but the very brief half-life of the tracer (~ 10 minutes) precludes longer-term experiments or measurements at steady-state. To this end, we deployed [15N]leucine (0.1 mmol/l) as a tracer in cultured astrocytes (Yudkoff et al, 1994a). We utilized gas chromatography-mass spectrometry to assay the appearance of the label in [15N]glutamate and [2-15N]glutamine. In addition to the labeled leucine, the medium included 15 unlabelled amino acids and 15NH4Cl, thereby providing the cells with a panoply of alternative sources of N for glutamate synthesis from α-ketoglutarate. At steady-state the ratio of label of [15N]glutamate/[15N]leucine was 0.21, indicating that about one-fifth of glutamate N had been derived from leucine alone. This finding was congruent with an earlier study (Yudkoff et al, 1983) in cerebellar explants in which we found that leucine and valine together contributed at least one-third of the amino groups utilized to form glutamate. A virtually identical result was obtained in cultures of GABA-ergic neurons (Yudkoff et al, 1990).
Similar results have been obtained by investigators working in other systems. Thus, Zielke et al (1995) used microdialysis to perfuse leucine into the extracellular fluid of awake, freely-moving rats. They observed a significant increase in the concentration of interstitial large, neutral amino acids, suggesting that these compounds competed with leucine for uptake into brain cells via a common carrier. They also reported a significant increase in the level of interstitial glutamine, a finding consistent with the notion (Brookes 1992; 1993) that: (a) transport of leucine and other neutral amino acids is achieved via exchange for internal glutamine and (b) that leucine N had been used for the purpose of glutamate synthesis. Kanamori et al (1998) used magnetic resonance spectroscopy to measure N transfer from leucine to glutamate in rats that received an intravenous infusion of [15N]leucine. The authors estimated, assuming a Km of brain branched-chain transaminase of 1.2 mM and a physiologic brain leucine concentration of 0.11 µmol/g, that leucine alone provided ~ 25% of glutamate N.
Compartmentation of BCAAMetabolism in Brain: Possibility of A BCAA-Leucine Shuttle
It is the nature of glutamatergic (and GABA-ergic) neurotransmission to be at odds with the need to maintain metabolic stability of overall brain metabolism. Release of glutamate (or GABA) from nerve endings obliges the stoichiometric restoration of that which was lost. The straightforward importation of fresh glutamate, conceivably from peripheral blood, would not be feasible, since this strategy would tend to elevate the external glutamate concentration, thereby rendering the system vulnerable to additional depolarization and both jeopardizing maintenance of an optimal signal-to-noise ratio and increasing the risk of excitotoxicity.
The glutamate-glutamine cycle (above) restores glutamate to neurons as glutamine, a non-neuroactive species which is present in abundance in brain extracellular fluid and which does not invite the risk of depolarization. This cycle is the essential figuration in our current understanding of brain amino acid handling, but, as would be true of any model, it is a simplified and incomplete rendering of a more complex biochemical and physiologic reality.
Several lines of evidence suggest that branched-chain amino acids and ketoacids also could be participants in cycle(s) which facilitate movement of N between astrocytes and neurons: (a) These compounds are swiftly transported into brain, thereby establishing a route of efficient articulation between brain and the peripheral circulation; (b) As noted above, the enzymes mediating transamination of branched-chain amino acids/ketoacids display a rigorous anatomic and subcellular (mitochondrial vs cytosolic) distribution in neurons and astrocytes, suggesting the possibility of differential function in either cell type and/or organelle; (c) Neither branched-chain amino acids nor the cognate ketoacids evoke neuronal depolarization, indicating that these compounds can be safely “trafficked” within the CNS; (d) Transport systems for both these amino acids and the ketoacids are present on the surface of both neurons and astrocytes (Brookes et al, 1992; 1993; Broer et al, 1994; Tan et al, 1987; 1988; Rao and Murthy, 1994; Rao et al, 1995; Yudkoff et al, 1996; Mac et al, 2000).
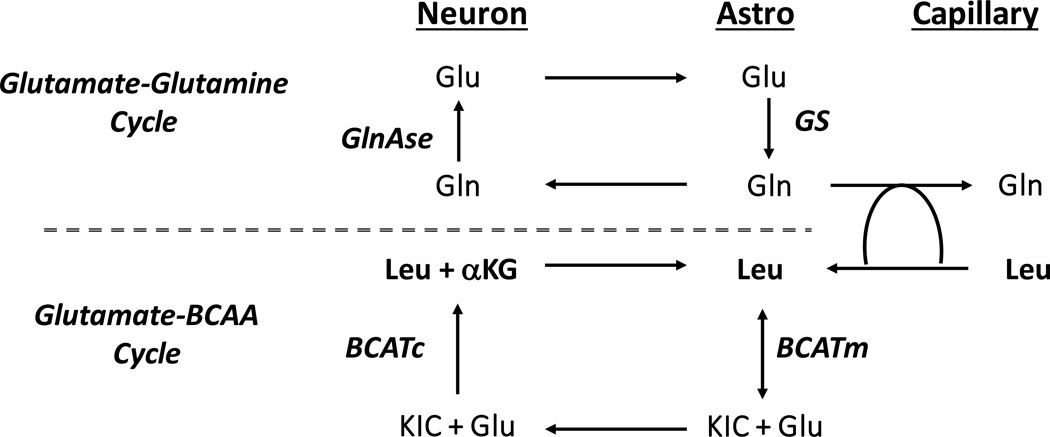
A structure for the proposed cycle is given in Figure 1 (adapted from Yudkoff et al, 1996; Daikhin and Yudkoff, 2000; Hutson et al, 2001; Lieth et al, 2001; Yudkoff et al, 2005). This model, which represents leucine as an exemplar of all 3 branched-chain amino acids, posits this compound entering brain in exchange for glutamine. Metabolism is initiated in astrocytes, where mitochondrial branched-chain amino acid transaminase (BCATm) forms α-ketoisocaproate (KIC) and glutamate. The ketoacid is released to neurons, which transport this compound (Mac et al, 2000) and readily convert it back to glutamate (Yudkoff et al, 1996), in the process consuming the latter compound. The leucine formed by “reverse” transamination then can be restored to astrocytes, thereby completing the cycle. The putative “glutamate-BCAA cycle” would articulate with the “glutamine-glutamine cycle”, with the latter furnishing the glutamine which supports brain entry of leucine via a counter-exchange mechanism (Figure 1).
Figure 1.
Proposed schematic of glutamate-BCAA cycle. Leucine illustrated to typify metabolism of isoleucine and valine. Leucine enters brain in exchange for glutamine, formed in astrocytes. In the astrocyte mitochondrial branched-chain transaminase (BCATm) forms glutamate and α-ketoisocaproate (KIC), the cognate ketoacid. The latter is transferred from astrocyte to neuron, which reaminates KIC via cytosolic branched-chain amino acid transaminase (BCATc), in the process consuming glutamate. The leucine so formed is restored to astrocytes, thereby completing the cycle.
Coupling of these two cycles would accomplish several purposes: (a) A mechanism for the importation of branched-chain amino acids into brain; (b) A possible mechanism for the “buffering” of neuronal glutamate when the concentration of the latter rises. Indeed, our prior work (Yudkoff et al, 1996) suggested that in synaptosomes the “reverse” transamination pathway which results in formation of leucine is more active than the “forward” pathway which leads to the production of α-ketoisocaproate. This process might assume heightened physiologic significance during periods of relatively intense glutamate release since heightened neural activity recruits greater regional blood flow (Petzold and Murthy, 2011), in the process making available relatively more branched-chain amino acid to a set of neurons which must dispose swiftly of a relatively greater synaptic “load” of neuroactive glutamate. The putative glutamate-BCAA cycle (Figure 1) would afford a complementary mechanism that complements the action of the glutamate-glutamine cycle in momentarily “disposing” of synaptic glutamate.
We should, of course, avoid a facile extrapolation from the model implicit in Figure 1 to physiologic reality. Brain is a heterogeneous organ in which metabolism may varying dramatically depending upon anatomic location and cell type. Thus, Bak et al (2012) found in cultured glutamatergic cerebellar neurons that valine – rather than leucine or isoleucine – was capable of maintaining neurotransmitter pools of glutamate in response to repeated depolarization.
Additional research will be needed to validate or refute this model. However, many features of this conceptualization are supported our current understanding of brain chemistry and physiology: (a) Leucine and the other branched-chain amino acids have long been known to function as N donors in peripheral tissues (Felig, 1975). It would be unsurprising if a similar pattern exists in the CNS. (b) Brain actively transports these compounds: leucine enters brain more quickly than any other amino acid (Smith et al, 1987). (c) Brain has abundant capacity for the transamination of branched-chain amino acids. Indeed, the brain is virtually unique among body organs in possessing both mitochondrial and cytosolic forms of branched-chain amino acid transaminase. (d) The rate of brain transamination of branched-chain amino acids greatly exceeds the rate of their oxidation (Shambaugh and Koehler, 1983), indicating that brain has ready access to a steady supply of the ketocids. (e) Neurons are capable of transporting the ketoacids (Mac et al, 2000). (f) Transamination can proceed in either the “forward” or “reverse” direction, depending upon the relative concentration of the reactants. However, some evidence suggests (Yudkoff et al, 1996) that in neurons the equilibrium favors reamination of the ketoacid to yield the parent amino acid. It seems unsurprising that reamination should prevail, given the fact that the intracellular concentration of the brain metabolic pool of glutamate is as high as 10–13 mmol/l (Hädel et al, 2013), particularly in the neuronal compartment. Studies with a microdialysis system in the non-anesthetized rat demonstrated that infusion of α-ketoisocaproate into the extracellular fluid enhanced the oxidation of 14C-glutamate and 14C-glutamine (Zielke et al, 1998), thus indicating that transamination of the ketoacid converted glutamate to α-ketoglutarate, which then entered the tricarboxylic acid cycle and was combusted to CO2. (g) Studies with magnetic resonance spectroscopy suggest that carriage of leucine from neuron-to-astrocyte might suffice to replenish glial nitrogen pools (Rothman et al, 2012), although this methodology may not render with adequate temporal or anatomic sensitivity the dynamic and fine-scale metabolic adjustments that undoubtedly occur in response to altered neuronal activity.
Brain Handling of Branched-Chain Amino Acids: Clinical Considerations
The foregoing review explored the role of the branched-chain amino acids in modulating brain handling of glutamic acid, an assignment which is pivotal to all cerebral metabolism. We encountered an extremely intricate system which obliges stringent anatomic segregation of enzymes into distinct cell types and even subcellular organelles, the polarization of transport processes to enable vectorality of metabolite transfer and, above all, the elaboration of control mechanisms which assure reasonable constancy of glutamate metabolism - even in a system characterized by episodic release of this neurotransmitter and the requirement to swiftly restore it to a neuronal compartment.
Clearly, an inherited mutation which affects the function of this tightly controlled system would be expected to have unwelcome and potentially severe consequences for brain metabolism. This hypothesis has been lamentably proven accurate by our experience with maple syrup urine disease, or a deficiency of branched-chain ketoacid dehydrogenase (reaction 2, below):
| (Reaction 1) |
| (Reaction 2) |
An inherited deficiency of branched-chain ketoacid dehydrogenase gives rise to maple syrup urine disease (MSUD) (Burrage et al, 2014; Zinnanti and Lazovic, 2012). The defect in this enzyme, which belongs to the same protein “family” as α-ketoglutarate dehydrogenase and pyruvate dehydrogenase, results in extreme accumulation in blood and tissues of branched-chain ketoacids as well as the parent amino acids, the latter generated via re-amination. Effective treatment includes a special diet which is purposefully low in the offending amino acids. If therapy can be instituted quickly – and all states today screen neonates for this disorder – severe brain damage may be avoidable. Unfortunately, metabolic decompensation can occur at any time, especially in association with the catabolic stress of acute infection. The affected child manifests lethargy, confusion, coma and, in severe cases, frank brain swelling (Riviello and Rezvani, 1991). Liver transplantation may ward off acute metabolic decompensation, but long-range followup of patients indicates that even such drastic intervention will not prevent eventual neurologic and psychiatric deterioration (Muelly et al, 2013).
The cause of brain damage in MSUD is multi-factorial, but evidence suggests that the very factors which have made branched-chain amino acids vital contributors to healthy neurologic function become destructive and toxic agents if these amino acids and ketoacids are present in excessive amount. Thus, as noted above, the branched-chain amino acids, which brain so avidly transports (Smith et al, 1981), in high concentration deplete levels of other large neutral amino acids (Prensky and Moser, 1966; Dodd et al, 1991) by efficiently excluding these metabolites from binding to the relevant transporter(s). The result is a failure of both brain protein synthesis and of neurotransmitter synthesis, since the latter – notably serotonin and dopamine – are formed from tryptophan and tyrosine.
Similarly, during a MSUD-induced crisis the brain concentrations of glutamate, glutamine and aspartate are very low – and correlated with the intensity of branched-chain amino acid accumulation. The cause appears to be a relative intensification of glutamate consumption via “reversal” of branched-chain amino acid transamination in response to an extravagant increase of the cognate ketoacids (Prensky and Moser, 1966; Dodd et al, 1991). It is thought that depletion of brain glutamate and aspartate impairs function of the malate-aspartate shuttle, the main mechanism in brain for the transfer of glycolytically-derived reducing equivalents from cytosolic to mitochondrion (Llorenti-Folch et al, 2013). These pathologic events result in an increased level of both brain lactate and branched-chain amino acids – phenomena which have been observed with magnetic resonance spectroscopy during acute metabolic decompensation (Jan et al, 2003).
The foregoing analysis involves a profound – and often life-threatening - biochemical derangement which accompanies a near-complete inherited deficiency of branched-chain ketoacid dehydrogenase. In recent years, however, considerable research has focused on the possibility that less onerous increases of branched-chain amino acids and ketoacids might prove beneficial in preventing brain injury, possibly by subtly modulating intra-cerebral levels of glutamate and/or glutamine. To this end, investigators have scrutinized the therapeutic potential of dietary branched-chain amino acid supplementation in diverse pathologic conditions, among them hepatic encephalopathy (Dam et al, 2013; Bak et al, 2013), fatigue (Newsholme and Blomstrand, 2006; Choi et al, 2013; Gluud et al, 2013), traumatic brain injury (Cole et al, 2010); appetite and energy sensing (Schwartz, 2013), obesity (Drgonova, 2013) and epilepsy (Evangeliou et al, 2009). It is probable that future investigation will broaden this repertoire still more.
Conclusions
Branched-chain amino acids have emerged as “key players” in brain biochemistry. They serve as an important external source of amino groups for the synthesis of brain glutamate and, as branched-chain ketoacids, they may provide a mechanism for the “buffering” of internal levels of glutamate should the latter rise to potentially toxic concentration. The ketoacids may seem unlikely candidates for the latter role, since brain glutamate normally is present at a concentration (10–13 mmol/l) which exceeds that of branched-chain amino acids and ketoacids is lower by orders of magnitude. However, it is important to emphasize that the glutamate pool which obliges “buffering” is not the internal metabolic pool of neurons but neuroactive glutamate which is resident in the synapse. Obviously, intra-synaptic levels of glutamate – even after depolarization and release from nerve endings - are far lower than the internal concentration in neurons or astrocytes. Indeed, it is probable that the intra-synaptic concentration is comparable that of the branched-chain amino acids and ketoacids, which then could serve as “transamination partners” for glutamate.
It should be emphasized that branched-chain amino acids and/or ketoacids could prove therapeutically beneficial through mechanisms that do not involve their direct involvement in metabolic pathways. Leucine functions as a regulator of metabolism through its role as an activator of the mTOR signaling pathway (Murin and Hamprecht, 2008; Li et al, 2011; Jewel et al, 2013). Indeed, it may be that tissue damage in inborn errors of metabolism can result from derangements of leucine concentration (Boneh, 2015). Nonetheless, much evidence points to a salient role for branched-chain amino acids and ketoacids as key players in the metabolism of the nervous system. Such a role would accord well with the well-established function of these compounds as essential pivotal carriers and exchangers in the nitrogen economy of the periphery.
Acknowledgments
Supported by NICHD grant U54HD086984
References
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006 Jan;7(1):41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Sonnewald U, Waagepetersen HS, Schousboe A. Glutamine in the central nervous system: function and dysfunction. Front Biosci. 2007;12:332–343. doi: 10.2741/2067. [DOI] [PubMed] [Google Scholar]
- Auestad N, Korsak RA, Morrow JW, Edmond J. Fatty acid oxidation and ketogenesis by astrocytes in primary culture. J. Neurochem. 1991;56:1376–1386. doi: 10.1111/j.1471-4159.1991.tb11435.x. [DOI] [PubMed] [Google Scholar]
- Bak LK, Johansen ML, Schousboe A, Waagepetersen Valine but not leucine or isoleucine supports neurotransmitter glutamate synthesis during synaptic activity in cultured cerebellar neurons. J Neurosci Res. 2012;90:1768–1775. doi: 10.1002/jnr.23072. [DOI] [PubMed] [Google Scholar]
- Bak LK, Waagepetersen HS, Sørensen M, Ott P, Vilstrup H, Keiding S, Schousboe A. Role of branched chain amino acids in cerebral ammonia homeostasis related to hepatic encephalopathy. Metab Brain Disabilities. 2013;28:209–215. doi: 10.1007/s11011-013-9381-7. [DOI] [PubMed] [Google Scholar]
- Berl S, Takagaki G, Clarke DD, Waelsch H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J BiolChem. 1962;237:2562–2569. [PubMed] [Google Scholar]
- Bixel MG, Hamprecht B. Metabolism of branched-chain amino acids in astroglial-rich primary culture. J. Neurochem. 1994;63:S62A. [Google Scholar]
- Bixel M, Hutson S, Hamprecht B. Cellular Distribution of Branched chain amino acid aminotransferase isoenzymes among rat brain glial cells in culture. J Histochem Cytochem. 1997;45:685–694. doi: 10.1177/002215549704500506. [DOI] [PubMed] [Google Scholar]
- Boneh A. Signal transduction in inherited metabolic disorders: a model for a possible pathogenetic mechanism. J Inherit Metab Disabilities. 2015;38:729–740. doi: 10.1007/s10545-015-9820-1. [DOI] [PubMed] [Google Scholar]
- Bonfils J, Faure M, Gibrat JF, Glomot F, Papet I. Sheep cytosolic branched-chain amino acid aminotransferase: cDNA cloning, primary structure and molecular modelling and its unique expression in muscles. Biochim Biophys Acta. 2000;1494:129–136. doi: 10.1016/s0167-4781(00)00227-x. [DOI] [PubMed] [Google Scholar]
- Brand K. Metabolism of 2-oxoacid analogues of leucine, valine and phenylalanine by heart muscle, brain and kidney of the rat. Biochim Biophys. Acta. 1981;677:126–132. doi: 10.1016/0304-4165(81)90153-7. [DOI] [PubMed] [Google Scholar]
- Broer S, Broer A, Hamprecht Expression of Na+-independent isoleucine transport activity from rat brain in Xenopus laevis oocytes. Biochim. Biophys. Acta. 1994;1192:95–100. doi: 10.1016/0005-2736(94)90147-3. [DOI] [PubMed] [Google Scholar]
- Brookes N. Effect of intracellular glutamine on the uptake of large neutral amino acids in astrocyes: concentrative Na(+)-independent transport. J. Neurochem. 1992;59:227–235. doi: 10.1111/j.1471-4159.1992.tb08895.x. [DOI] [PubMed] [Google Scholar]
- Brookes N. Interaction between the glutamine cycle and the uptake of large neutral amino acids in astrocytes. J. Neurochem. 1993;60:1923–1928. doi: 10.1111/j.1471-4159.1993.tb13421.x. [DOI] [PubMed] [Google Scholar]
- Burch HB, Cambon N, Lowry OH. Branched-chain amino acid aminotransferase along the rabbit and rat nephron. Kidney Int. 1985;28:114–117. doi: 10.1038/ki.1985.129. [DOI] [PubMed] [Google Scholar]
- Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet. 2014;23:R1–R8. doi: 10.1093/hmg/ddu123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangiano C, Cardelli-Cangiano P, James J, Rossi Fanelli F, Patrizi MA, Brackett KA, Strom R, Fischer JE. Brain microvessels take up large neutral amino acids in exchange for glutamine. J. Biol. Chem. 1983;258:8949–8954. [PubMed] [Google Scholar]
- Cardona C, Sánchez-Mejías E, Dávila JC, Martín-Rufián M, Campos-Sandoval JA, Vitorica J, Alonso FJ, Matés JM, Segura JA, Norenberg MD, Rama Rao KV, Jayakumar AR, Gutiérrez A, Márquez J. Expression of Gls and Gls2 glutaminase isoforms in astrocytes. Glia. 2015;63:365–382. doi: 10.1002/glia.22758. [DOI] [PubMed] [Google Scholar]
- Chaplin ER, Goldberg AL, Diamond I. Leucine oxidation in brain slices and nerve endings. J. Neurochem. 1976;26:701–707. doi: 10.1111/j.1471-4159.1976.tb04440.x. [DOI] [PubMed] [Google Scholar]
- Choi S, Disilvio B, Fernstrom MH, Fernstrom JD. Oral branched-chain amino acid supplements that reduce brain serotonin during exercise in rats also lower brain catecholamines. Amino Acids. 2013;45:1133–1142. doi: 10.1007/s00726-013-1566-1. [DOI] [PubMed] [Google Scholar]
- Cole JT, Mitala CM, Kundu S, Verma A, Elkind JA, Nissim I, Cohen AS. Dietary branched chain amino acids ameliorate injury-induced cognitive impairment. Proc Natl Acad Sci U S A. 2010;107:366–371. doi: 10.1073/pnas.0910280107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJL, McDonald JM, Gelbard AS, Gledhill RF, Duffy TE. The metabolic fate of 13N-labelled ammonia in rat brain. J. Biol. Chem. 1979;254:4982–4992. [PubMed] [Google Scholar]
- Cooper AJL, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules. 2016;6 doi: 10.3390/biom6020016. pii: E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J. Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S–1031S. doi: 10.1093/jn/130.4.1026S. [DOI] [PubMed] [Google Scholar]
- Dam G, Ott P, Aagaard NK, Vilstrup H. Branched-chain amino acids and muscle ammonia detoxification in cirrhosis. Metab Brain Disabilities. 2013;28:217–220. doi: 10.1007/s11011-013-9377-3. [DOI] [PubMed] [Google Scholar]
- Davoodi J, Drown PM, Bledsoe RK, Wallin R, Reinhart GD, Hutson SM. Overexpression and characterisation of the human mitochondrial and cytosolic branched-chain aminotransferases. J Biol Chem. 1998;273:4982–4989. doi: 10.1074/jbc.273.9.4982. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd PR, Williams SH, Gundlach AL, Harper PA, Healy PJ, Dennis JA, Johnston GA. Glutamate and gamma-aminobutyric acid neurotransmitter systems in the acute phase of maple syrup urine disease and citrullinemia encephalopathies in newborn calves. J Neurochem. 1992;59:582–590. doi: 10.1111/j.1471-4159.1992.tb09409.x. [DOI] [PubMed] [Google Scholar]
- Drgonova J, Jacobsson JA, Han JC, Yanovski JA, Fredriksson R, Marcus C, Schiöth HB, Uhl GR. Involvement of the neutral amino acid transporter SLC6A15 and leucine in obesity-related phenotypes. PLoS One. 2013;8:e68245. doi: 10.1371/journal.pone.0068245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecinska M, Nelson D, Wilson DF, Silver IA. Neurotransmitter amino acids in the CNS. I. Regional changes in amino acid levels in rat brain during ischemia and reperfusion. Brain Res. 1984;304:9–22. doi: 10.1016/0006-8993(84)90857-6. [DOI] [PubMed] [Google Scholar]
- Evangeliou A, Spilioti M, Doulioglou V, Kalaidopoulou P, Ilias A, Skarpalezou A, Katsanika I, Kalamitsou S, Vasilaki K, Chatziioanidis I, Garganis K, Pavlou E, Varlamis S, Nikolaidis N. Branched chain amino acids as adjunctive therapy to ketogenic diet in epilepsy: pilot study and hypothesis. J Child Neurol. 2009;24:1268–1272. doi: 10.1177/0883073809336295. [DOI] [PubMed] [Google Scholar]
- Felig P. Amino acid metabolism in man. Annu. Rev. Biochem. 1975;44:933–955. doi: 10.1146/annurev.bi.44.070175.004441. [DOI] [PubMed] [Google Scholar]
- García-Espinosa MA, Wallin R, Hutson SM, Sweatt AJ. Widespread neuronal expression of branched-chain aminotransferase in the CNS: implications for leucine/glutamate metabolism and for signaling by amino acids. J Neurochem. 2007;100:1458–1468. doi: 10.1111/j.1471-4159.2006.04332.x. [DOI] [PubMed] [Google Scholar]
- Gluud LL, Dam G, Borre M, Les I, Cordoba J, Marchesini G, Aagaard NK, Risum N, Vilstrup H. Oral branched-chain amino acids have a beneficial effect on manifestations of hepatic encephalopathy in a systematic review with meta-analyses of randomized controlled trials. J Nutr. 2013;143:1263–1268. doi: 10.3945/jn.113.174375. [DOI] [PubMed] [Google Scholar]
- Grill V, Björkhem M, Gutniak M, Lindqvist M. Brain uptake and release of amino acids in nondiabetic and insulin-dependent diabetic subjects: important role of glutamine release for nitrogen balance. Metabolism. 1992;41:28–32. doi: 10.1016/0026-0495(92)90186-e. [DOI] [PubMed] [Google Scholar]
- Hädel S, Wirth C, Rapp M, Gallinat J, Schubert F. Effects of age and sex on the concentrations of glutamate and glutamine in the human brain. J Magn Res Imag. 2013;38:1480–1487. doi: 10.1002/jmri.24123. [DOI] [PubMed] [Google Scholar]
- Hall TR, Wallin R, Reinhart GD, Hutson SM. Branched chain aminotransferase isoenzymes. Purification and characterization of the rat brain isoenzyme. J. Biol. Chem. 1993;268:3092–3098. [PubMed] [Google Scholar]
- Hamprecht B, Schmoll D, Cesar M, Bixel B, Vogel R, Kurz G, Wiesinger H. Metabolism of glucogenic and ketogenic amino acids and energy metabolism in astroglial cells. J. Neurochem. 1995;64:S110A. [Google Scholar]
- Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Ann. Rev. Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Harris RA, Kobayashi R, Murakami T, Shimomura Y. Regulation of branched-chain alpha-keto acid dehydrogenase kinase expression in rat liver. J Nutr. 2001;131:841S–845S. doi: 10.1093/jn/131.3.841S. [DOI] [PubMed] [Google Scholar]
- Hayashi T. A physiological study of epileptic seizures following cortical stimulation in animals and its application to human clinics. Jpn. J. Physiol. 1952;3:46–64. doi: 10.2170/jjphysiol.3.46. [DOI] [PubMed] [Google Scholar]
- Haymond MW, Ben-Galim E, Strobel KE. Glucose and alanine metabolism in children with maple syrup urine disease. J. Clin. Invest. 1978;78:398–405. doi: 10.1172/JCI109141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull J, Hindy ME, Kehoe PG, Chalmers K, Love S, Conway ME. Distribution of the branched chain aminotransferase proteins in the human brain and their role in glutamate regulation. J Neurochem. 2012;123:997–1009. doi: 10.1111/jnc.12044. [DOI] [PubMed] [Google Scholar]
- Hutson SM. Subcellular distribution of branched-chain aminotransferase activity in rat tissues. J Nutr. 1988;118:1475–1481. doi: 10.1093/jn/118.12.1475. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues. J. Biol. Chem. 1992;267:15681–15686. [PubMed] [Google Scholar]
- Hutson SM, Berkich D, Drown P, Xu B, Aschner M, LaNoue KF. Role of branched-chain aminotransferase isoenzymes and gabapentin in neurotransmitter metabolism. J Neurochem. 1998;71:863–874. doi: 10.1046/j.1471-4159.1998.71020863.x. [DOI] [PubMed] [Google Scholar]
- Hutson SM, Lieth E, LaNoue KF. Function of leucine in excitatory neurotransmitter metabolism in the central nervous system. J Nutr. 2001;131:846S–850S. doi: 10.1093/jn/131.3.846S. [DOI] [PubMed] [Google Scholar]
- James JH, Escourrou J, Fischer JE. Blood brain neutral amino acid transport activity is increased after portacaval anastomosis. Science. 1978;200:1395–1397. doi: 10.1126/science.663619. [DOI] [PubMed] [Google Scholar]
- Jan W, Zimmerman RA, Wang ZJ, Berry GT, Kaplan PB, Kaye EM. MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology. 2003;45:393–399. doi: 10.1007/s00234-003-0955-7. [DOI] [PubMed] [Google Scholar]
- Jenstad Monica, Chaudhry Farrukh Abbas. The amino acid transporters of the glutamate/GABA-glutamine cycle and their impact on insulin and glucagon secretion. Frontiers in Endocrinology ISSN. 2013:1664–2392. doi: 10.3389/fendo.2013.00199. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. 2013;14:133–139. doi: 10.1038/nrm3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori K, Ross BD, Kondrat RW. Rate of glutamate synthesis from leucine in rat brain measured in vivo by 15N NMR. J Neurochem. 1998 Mar;70(3):1304–1315. doi: 10.1046/j.1471-4159.1998.70031304.x. [DOI] [PubMed] [Google Scholar]
- Kvamme E, Svenneby G, Hertz L, Schousboe A. Properties of phosphate activated glutaminase in astrocytes cultured from mouse brain. Neurochem Research. 1982;7:761–770. doi: 10.1007/BF00965528. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Hawkins RA, Viña JR, Peterson DR. Glutamine transport by the blood-brain barrier: a possible mechanism for nitrogen removal. Am J Physiol. 1998;274:C1101–C1107. doi: 10.1152/ajpcell.1998.274.4.C1101. [DOI] [PubMed] [Google Scholar]
- Li F, Yin Y, Tan B, Kong X, Wu G. Leucine nutrition in animals and humans: mTOR signaling and beyond. Amino Acids. 2011;41:1185–1193. doi: 10.1007/s00726-011-0983-2. [DOI] [PubMed] [Google Scholar]
- Lieth E, LaNoue KF, Berkich DA, Xu B, Ratz M, Taylor C, Hutson SM. Nitrogen shuttling between neurons and glial cells during glutamate synthesis. J Neurochem. 2001;76:1712–1723. doi: 10.1046/j.1471-4159.2001.00156.x. [DOI] [PubMed] [Google Scholar]
- Llorente-Folch I, Rueda CB, Amigo I, del Arco A, Saheki T, Pardo B, Satrústegui J. Calcium-regulation of mitochondrial respiration maintains ATP homeostasis and requires ARALAR/AGC1-malate aspartate shuttle in intact cortical neurons. J Neurosci. 2013;33:13957–13971. doi: 10.1523/JNEUROSCI.0929-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac M, Nehlig A, Nałecz MJ, Nałecz KA. Transport of alpha-ketoisocaproate in rat cerebral cortical neurons. Arch Biochem Biophys. 2000;376:347–353. doi: 10.1006/abbi.2000.1724. [DOI] [PubMed] [Google Scholar]
- Manchester KL. Biochim. Biophys. Acta. 1965;100:295–298. doi: 10.1016/0304-4165(65)90457-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Meldrum BS. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000;130:1007S–1015S. doi: 10.1093/jn/130.4.1007S. [DOI] [PubMed] [Google Scholar]
- Miller LL. In: Amino Acid Pools. Holden JT, editor. Amsterdam: Elsevier; 1961. pp. 708–721. [Google Scholar]
- McKenna MC. Introduction: metabolic trafficking comes of age in the decade of the brain. Dev Neurosci. 1996;18:333–335. doi: 10.1159/000111425. [DOI] [PubMed] [Google Scholar]
- McKenna MC. The glutamate-glutamine cycle is not stoichiometric: fates of glutamate in brain. J Neurosci Res. 2007;85:3347–3358. doi: 10.1002/jnr.21444. [DOI] [PubMed] [Google Scholar]
- Muelly ER, Moore GJ, Bunce SC, Mack J, Bigler DC, Morton DH, Strauss KA. Biochemical correlates of neuropsychiatric illness in maple syrup urine disease. J Clin Invest. 2013;123:1809–1820. doi: 10.1172/JCI67217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murín R, Hamprecht B. Metabolic and regulatory roles of leucine in neural cells. Neurochem Res. 2008;33:279–284. doi: 10.1007/s11064-007-9444-4. [DOI] [PubMed] [Google Scholar]
- Newsholme EA, Blomstrand E. Branched-chain amino acids and central fatigue. J Nutr. 2006;136:274S–276S. doi: 10.1093/jn/136.1.274S. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res (1979) 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acids, amines and hexoses after arterial injection. Am. J. Physiol. 1971;221:1629–1635. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Murthy VN. Role of astrocytes in neurovascular coupling. Neuron. 2011;71:782–797. doi: 10.1016/j.neuron.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Prensky AL, Moser HW. Brain lipids, proteolipids, and free amino acids in maple syrup urine disease. J Neurochem. 1966;13:863–874. doi: 10.1111/j.1471-4159.1966.tb05882.x. [DOI] [PubMed] [Google Scholar]
- Riviello JJ, Jr, Rezvani I, et al. Cerebral edema causing death in children with maple syrup urine disease. J Pediatr. 1991;119:42–45. doi: 10.1016/s0022-3476(05)81036-4. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Jackson JG. Astroglial glutamate transporters coordinate excitatory signaling and brain energetics. Neurochem Int. 2016 doi: 10.1016/j.neuint.2016.03.014. pii: S0197-0186(16)30040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, DeFeyter HM, Maciejewski PK, Behar KL. Is there in vivo evidence for amino acid shuttles carrying ammonia from neurons to astrocytes? Neurochem Res. 2012;37:2597–2612. doi: 10.1007/s11064-012-0898-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ. Central leucine sensing in the control of energy homeostasis. Endocrinol Metab Clin North Am. 2013;42:81–87. doi: 10.1016/j.ecl.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shambaugh GE, III, Koehler RA. Fetal fuels. IV. Regulation of branched-chain amino and keto acid metabolism in fetal brain. Am. J. Physiol. 1981;241:E200–E207. doi: 10.1152/ajpendo.1981.241.3.E200. [DOI] [PubMed] [Google Scholar]
- Shambaugh GE, Koehler RA. Fetal fuels VI. Metabolism of a-ketoisocaproic acid in fetal rat brain. Metabolism. 1983;32:421–427. doi: 10.1016/0026-0495(83)90001-x. [DOI] [PubMed] [Google Scholar]
- Shen J. Modeling the glutamate–glutamine neurotransmitter cycle. Front. Neuroenerget. 2013 Jan 28;5:1. doi: 10.3389/fnene.2013.00001. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman RG, Hyder F, Rothman DL. Lactate efflux and the neuroenergetic basis of brain function. NMR Biomed. 2001;14:389–396. doi: 10.1002/nbm.741. [DOI] [PubMed] [Google Scholar]
- Siesjö BK. Brain Energy Metabolism. New York, New York: John Wiley and Sons; 1978. [Google Scholar]
- Smith QR, Momma S, Aoyagi M, Rapoport SI. Kinetics of neutral amino acid transport across the blood-brain barrier. J. Neurochem. 1987;49:1651–1658. doi: 10.1111/j.1471-4159.1987.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Snell K, Duff DA. In: Metabolism and Clinical Implications of Branched Chain Amino and Ketoacids. Walser M, Williamson JR, editors. New York: Elsevier/North-Holland; 1981. pp. 251–256. [Google Scholar]
- Sweatt A, Garcia-Espinosa M, Wallin R, Hutson S. Branched-chain amino acids and neurotransmitter metabolism: Expression of cytosolic brainched chain aminotransferase (BCATc) in the cerebellum and hippocampus. J Compar Neurol. 2004a;477:360–370. doi: 10.1002/cne.20200. [DOI] [PubMed] [Google Scholar]
- Sweatt A, Wood M, Suryawan A, Wallin R, Willingham M, Hutson S. Branched-chain amino acid catabolism: Unique segregation of pathway enzymes in organ systems and peripheral nerves. Amer J Phys Endocr Metab. 2004b;286:E64–E76. doi: 10.1152/ajpendo.00276.2003. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015;40(2):402–409. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- Westergaard N, Sonnewald U, Schousboe A. Metabolic trafficking between neurons and astrocytes: the glutamate/glutamine cycle revisited. Dev Neurosci. 1995;17:203–211. doi: 10.1159/000111288. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Kim SU, Pleasure D, Hummeler K, Segal S. [15N]Leucine as a source of [15N] glutamate in organotypic cerebellar explants. Biochem. Biophys. Res. Commun. 1983;115:l74–l79. doi: 10.1016/0006-291x(83)90985-3. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Nissim I, Hertz L. Precursors of glutamic acid nitrogen in primary neuronal cultures: studies with 15N. Neurochem. Res. 1990;15:1191–1196. doi: 10.1007/BF01208579. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Lin Z-P, Nissim Ilana, Stern J, Pleasure D, Nissim I. Inter-relationships of leucine and glutamate metabolism in cultured astrocytes. J. Neurochem. 1994a;62:1192–1202. doi: 10.1046/j.1471-4159.1994.62031192.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nelson D, Nissim I, Erecińska M. Neuronal metabolism of branched-chain amino acids: flux through the aminotransferase pathway in synaptosomes. J Neurochem. 1996;66:2136–2145. doi: 10.1046/j.1471-4159.1996.66052136.x. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, Nissim I. Brain amino acid requirements and toxicity: the example of leucine. J Nutr. 2005;135:1531S–1538S. doi: 10.1093/jn/135.6.1531S. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Tildon JT, Zielke CL, Baab PJ, Landry ME. Functional intracellular glutaminase activity in intact astrocytes. Neurochem Research. 1989;14:327–332. doi: 10.1007/BF01000035. [DOI] [PubMed] [Google Scholar]
- Zielke HR, Huang Y, Zielke CL, Baab PJ, Tildon JT. a-Ketoisocaproate and leucine infusion into the brain alters the amino acid levels in the interstitital space. J. Neurochem. 1995;64:S56A. [Google Scholar]
- Zielke HR, Huang Y, Baab PJ, Collins RM, Jr, Zielke CL, Tildon JT. Effect of alpha-ketoisocaproate and leucine on the in vivo oxidation of glutamate and glutamine in the rat brain. Neurochem Research. 1998;22:1159–1164. doi: 10.1023/a:1027325620983. [DOI] [PubMed] [Google Scholar]
- Zinnanti WJ, Lazovic J. Interrupting the mechanisms of brain injury in a model of maple syrup urine disease encephalopathy. J Inherit Metab Dis. 2012;35:71–79. doi: 10.1007/s10545-011-9333-5. [DOI] [PubMed] [Google Scholar]