Abstract
Purpose
In the study of microbial keratitis, in vivo animal models often require a large number of animals, and in vitro monolayer cell culture does not maintain the three-dimensional structure of the tissues or cell-to-cell communication of in vivo models. Here, we propose reproducible ex vivo models of single- and dual-infection keratitis as an alternative to in vivo and in vitro models.
Methods
Excised rabbit and human corneoscleral rims maintained in organ culture were infected using 108 cells of Staphylococcus aureus, Pseudomonas aeruginosa, Candida albicans or Fusarium solani. The infection was introduced by wounding with a scalpel and exposing corneas to the microbial suspension or by intrastromal injection. Post-inoculation, corneas were maintained for 24 and 48 h at 37 °C. After incubation, corneas were either homogenised to determine colony-forming units (CFU)/cornea or processed for histological examination using routine staining methods. Single- and mixed-species infections were compared.
Results
We observed a significant increase in CFU after 48 h compared to 24 h with S. aureus and P. aeruginosa. However, no such increase was observed in corneas infected with C. albicans or F. solani. The injection method yielded an approximately two- to 100-fold increase (p < 0.05) in the majority of organisms from infected corneas. Histology of the scalpel-wounded and injection models indicated extensive infiltration of P. aeruginosa throughout the entire cornea, with less infiltration observed for S. aureus, C. albicans and F. solani. The models also supported dual infections.
Conclusions
Both scalpel wounding and injection methods are suitable for inducing infection of ex vivo rabbit and human cornea models. These simple and reproducible models will be useful as an alternative to in vitro and in vivo models for investigating the detection and treatment of microbial keratitis, particularly when this might be due to two infective organisms.
Keywords: Ex vivo cornea, Microbial keratitis, Colony-forming units, Corneal model
Introduction
Microbial keratitis is a major problem worldwide and is an important cause of vision loss and blindness. In vivo animal models, in vitro cell culture and ex vivo models have been used for investigating different aspects of this disease, including pathogenicity and treatment strategies [1–7].
In vivo studies require the use of a large number of animals to answer a research question. The welfare of these animals has become an important ethical issue [8], leading to the promotion of the philosophy of ‘replacement, reduction and refinement’ in the use of animals in research [9].
One way to overcome these issues is to use in vitro monolayer cultures of cells, including immortalised [5] or primary [4] corneal epithelial cells. However, these are not representative of the in vivo situation. They lack a three-dimensional (3D) structure and cross-talk between different epithelial cells, limbal cells and keratocytes. Consequently, advances have been made in 3D multi-layered tissue-engineered corneal constructs, and a few of these (EpiOcular™ from Mattek Corporation, Ashland, MA, USA, and HCE/corneal epithelium (SkinEthic) from Episkin, Lyon, France) are commercially available [10]. These have been used to study corneal pathogenesis [6, 11]. Whilst they possess the 3D architecture of their in vivo counterparts, these models often use immortalised cell lines and lack intrinsic innate immune molecules which occur in vivo.
Recently, there has been some interest in the use of ex vivo corneal models to study keratitis [12, 13]. Although these models lack immune elements, the 3D architecture remains, as do the intracellular innate immune molecules and cellular–stromal components. These models have been used for studying wound healing [14], microbial adherence [12] and molecular microbial pathogenicity [13]. In our laboratory, we have used ex-vivo corneal models to study corneal epithelial regeneration [15, 16] and to develop models of inflammation. We have also shown that by gently rocking media over the corneas, they can be maintained in culture for at least 4 weeks [17].
To the best of our knowledge, a comparison of bacterial and fungal infections and of mixed infections has not been undertaken in ex vivo corneal models. We report a comparison of single- and mixed-species infections in both rabbit and human corneas to better understand the use of these models in microbial keratitis.
Materials and methods
Materials
We used corneas from two types of rabbits—wild brown rabbits (Blackface Meat Company, Dumfries, Scotland) and New Zealand rabbits (University of Sheffield, from rabbits sacrificed at the end of a licenced study). There was no difference in the performance of corneas from these two types of rabbits. Cadaveric human corneas unsuitable for transplant were acquired from the Ramayamma International Eye Bank, L V Prasad Eye Institute, Hyderabad, India. All corneas were obtained following procedures approved by the institutional review board for the protection of human subjects.
Dispase II was obtained from Roche Diagnostics (Burgess Hill, UK), and Videne® antiseptic solution was purchased from Ecolab (St. Paul, MN, USA). Mouse 3T3 fibroblasts (used in India) were from the American Type Culture Collection (ATCC; Manassas, VA, USA), and those used in the UK were an established J2 3T3 cell line originally from Professor Howard Green, USA. Epidermal growth factor was obtained from Invitrogen (Paisley, UK). For the culture of microorganisms, brain-heart infusion (BHI) agar and broth were purchased from Oxoid (Hampshire, UK) or HiMedia (Mumbai, India). All other reagents were obtained from Sigma-Aldrich (Dorset, UK) unless otherwise stated. Calcofluor-white was obtained from Sigma-Aldrich (Dorset, UK) and from HiMedia (Mumbai, India).
Isolation of rabbit corneas
Corneas with sclera rims were dissected using a standard procedure including decontamination with povidone iodine, and were immediately placed into phosphate-buffered saline (PBS) [15].
Ex vivo corneal organ culture
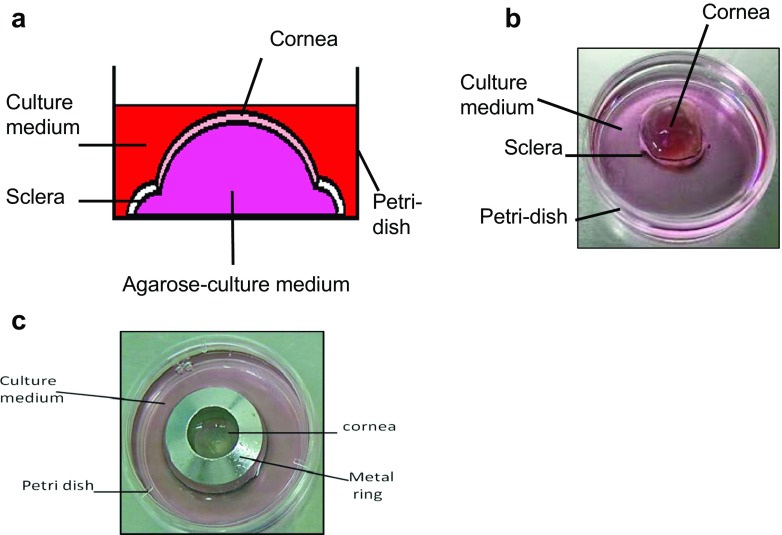
Organ cultures were as previously described [15, 18]. Rabbit and human corneoscleral buttons were placed epithelial side down in 35-mm petri dishes, and 500 μl Dulbecco’s modified eagle’s medium (DMEM)–agarose (0.5 % w/v) solution was pipetted into the endothelial side of the cornea. The solution was allowed to solidify, and the buttons were then inverted so that the epithelium was facing up (Fig. 1a and b). Culture medium (DMEM: Ham’s F12 [1:1] supplemented with 10 % fetal calf serum [FCS], 100 U ml−1 penicillin and 100 U ml−1 streptomycin, 2.5 μg ml−1 amphotericin B, 5 μg ml−1 insulin and 10 ng ml−1 epidermal growth factor [EGF]) was added to submerge the ex vivo corneas. Prior to infection, corneas were washed three times with PBS and incubated in antibiotic- and antifungal-free medium for at least 24 h to remove residual antimicrobials.
Fig. 1.
Schematic representation of a cross section (a) and a top-down image (b) of a corneoscleral button in organ culture. To infect corneas, a metal ring was placed on the corneoscleral button after wounding to form a seal, and bacteria/fungi were added to the surface of the cornea (c)
All experimental work was performed on rabbit corneas in the UK and on human corneas in India.
Culture of bacteria and fungi
For rabbit corneas, laboratory strains of S. aureus (S-235), P. aeruginosa (SOM-1), C. albicans (SC5314) and F. solani strain (NCPF 2699), purchased from the National Collection of Pathogenic Fungi (UK), were used. For human corneas, ATCC cultures of S. aureus (25923), P. aeruginosa (27853) and C. albicans (90028) were used. All bacterial and fungal strains were cultured on brain-heart infusion (BHI) agar at 37 °C overnight and then maintained at 4 °C. For use in experiments, one colony was sub-cultured from agar into BHI broth and incubated overnight at 37 °C. Stationary-phase microbes were used in rabbit cornea experiments. For human corneal experiments, on the day of corneal inoculation, a fresh broth was inoculated, and exponential-phase bacteria/fungi were used based on predetermined growth curves.
Infection of ex vivo corneas
Corneas were wounded with a scalpel (3 slashes vertically and 3 slashes horizontally), and a metal ring was placed on the corneoscleral button, creating a watertight seal. Into the centre of the ring, 108 S. aureus, P. aeruginosa, C. albicans or F. solani were added (Fig. 1c), or the corneas were injected intrastromally (using a 26-gauge needle; Becton Dickinson, Oxford, UK) with the same number of organisms.
The infected corneas were incubated for 24 or 48 h at 37 °C, and were then homogenised and the resulting suspension serially diluted and spotted onto agar plates for colony enumeration. A set of infected corneas was also processed for histology and sections stained using Gram (bacteria) and periodic acid–Schiff (PAS) stains (fungi). Corneas not exposed to microbes were used as controls. Histological sections were imaged using a BX51 upright microscope and cell3D imaging software (Olympus, Essex, UK) in the UK or the ProgRes CapturePro 2.5 software (Jenoptik) in India.
Imaging of microorganisms on the corneal surface
To visualise bacteria, 108 S. aureus or P. aeruginosa were labelled using 1 mg ml−1 fluorescein isothiocyanate (FITC) for 1 h at 4 °C, followed by four washes with PBS. For fungi, whole corneas were covered with 1:1 calcofluor white in 10 % (v/v) potassium hydroxide for 10 min and washed three times with PBS. Bacteria- and fungi-infected corneas were imaged using fluorescence microscopy as described above.
Statistical analysis
Box-and-whisker plots of colony-forming units (CFU) per cornea were plotted using GraphPad Prism 6 software. All comparisons were analysed using Student’s unpaired two-tailed t test, using Microsoft® Excel (Microsoft® Office, 2010). A p value ≤ 0.05 was considered significant.
Results
Macroscopic view of rabbit and human corneas
Corneas infected with bacteria and fungi showed a visible increase in haze compared with uninfected corneas (Fig. 2). The scratches were visible in all corneas, but were more evident in infected corneas than uninfected corneas (Fig. 2).
Fig. 2.
Fluorescein-stained rabbit and human corneas showing turbidity of infected versus non-infected corneas. Corneas were scalpel-wounded and exposed to S. aureus, P. aeruginosa, C. albicans or F. solani for 24 h. Corneas were briefly washed and stained with 0.5 mg ml−1 of fluorescein isothiocyanate for 30 min, washed again and photographed. Arrows indicate scalpel wounds
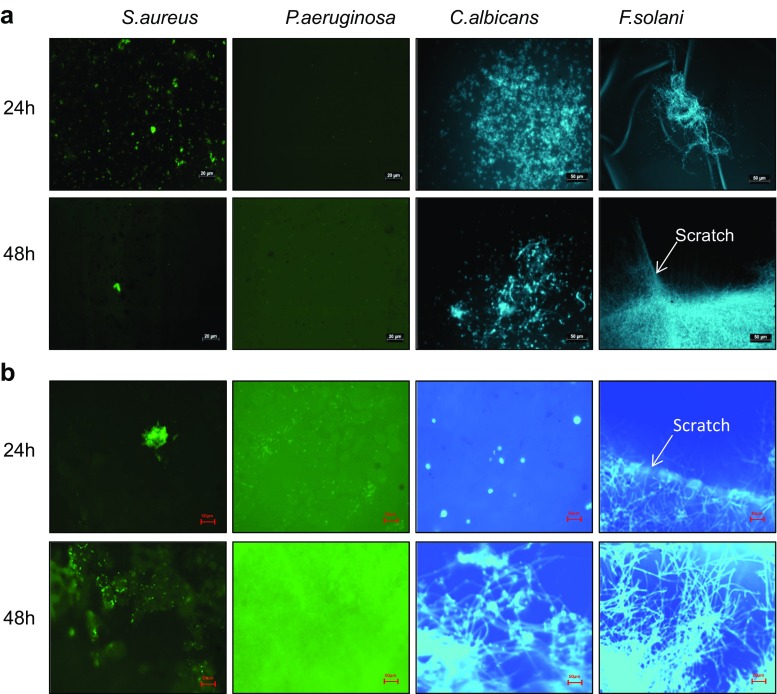
FITC-labelled bacteria within infected rabbit and human corneas are shown in Fig. 3. It was observed that S. aureus cells covered the surface of the cornea at 24 h and 48 h, and at certain locations, clumps of bacteria ranging from 5 to 25 μm in diameter were detected. On the other hand P. aeruginosa-infected corneas showed fewer clumps than observed with S. aureus, after 24 and 48 h. A microscopic view of the surface of calcofluor white-stained C. albicans-infected corneas showed a more uniform spread of yeast and a few hyphal forms on the surface of the cornea after 24 h, which increased in both the distribution of individual yeast cells and the spread of hyphae by 48 h (Fig. 3). The distribution of F. solani at 24 h was more punctuated with hyphae at distinct places in both rabbit and human corneas (Fig. 3). After 48 h, the surface of the cornea was covered with a mat of fungi, where the hyphae could be observed extending into the scratch and in all directions, away from the fungal bulk (Fig. 3). The coverage of bacteria and fungi over the corneal surface was similar between rabbit and human corneas.
Fig. 3.
FITC-labelled S. aureus or P. aeruginosa were incubated with scalpel wounded rabbit (a) and human (b) corneas for 24 and 48 h, washed and imaged using a fluorescent microscope. Unlabelled C. albicans and F. solani were incubated with rabbit and human corneas for 24 and 48 h, washed, the model stained with Calcofluor White and imaged using a fluorescent microscope. The distribution of bacteria and fungi over the surface of the cornea and located within the scratch wound can be observed
Single-species infection of rabbit and human corneas
After 24 h, CFU recovered per cornea for S. aureus, P. aeruginosa, C. albicans and F. solani were as follows (Fig. 4): 5.1 ± 1.0 × 105, 1.9 ± 0.3 × 107, 3.0 ± 0.6 × 105 and 2.5 ± 0.9 × 105 CFU/rabbit cornea, respectively, and 3.8 ± 0.8 × 106, 4.4 ± 0.6 × 108, 1.9 ± 0.3 × 105 and 1.8 ± 0.1 × 103 CFU/human cornea, respectively. A significantly higher number of S. aureus and P. aeruginosa were recovered after 48 h of incubation in both rabbit (1.7 ± 0.3 × 106 (p = 0.00005), 4.4 ± 0.7 × 107 (p = 0.0009) and human corneas (1.5 ± 0.4 × 107 (p = 0.0004), 6.5 ± 3.0 × 108 (p = 0.0057), respectively, compared to yields at 24 h. There was no significant difference in the recovery of C. albicans or F. solani after 48 h, with 5.1 ± 1.5 × 105 (p = 0.159) and 1.6 ± 0.7 × 106 (p = 0.090) CFU/rabbit cornea, respectively, and 5.3 ± 1.6 × 105(p = 0.108) and 2.1 ± 0.1 × 103(p = 0.081) CFU/human cornea, respectively. In addition, there was approximately tenfold greater recovery of bacteria from human corneas than from rabbit corneas after both 24 and 48 h.
Fig. 4.
Single-species infection of ex vivo rabbit (a) and human (b) corneas. Ex vivo corneas were scratched six times with a scalpel and exposed to single-species inoculum of S. aureus, P. aeruginosa, C. albicans or F. solani for 24 h at 37 °C. The models were then homogenised, and the resulting suspension serially diluted and plated onto agar plates. The number of colony-forming units per cornea were plotted for each cornea. The box plot shows the minimum and maximum values depicted by the bars; the upper quartile, median and lower quartile are depicted by the top, middle and bottom horizontal lines, respectively
The injection method involved the introduction of bacteria and fungi into the stroma. Compared to the scalpel method, after 24 h, injection of a single-species organism resulted in higher CFU/cornea (p < 0.05) for all organisms, with the exception of C. albicans in human corneas, where no significant difference was observed (p = 0.057).
The histology of single-species-infected corneas after 24 h is shown in Fig. 5a. Here, vast infiltration of P. aeruginosa can be seen covering the epithelium and entire stroma and infiltrating the Descemet’s membrane. This was independent of the method of inoculation (data not shown). The histology of the S. aureus-infected corneas was characterised by the concentration of the majority of organisms within the scratches (Fig. 5a). The number of bacteria within tissue sections correlated with the CFU/cornea data (Fig. 4).
Fig. 5.
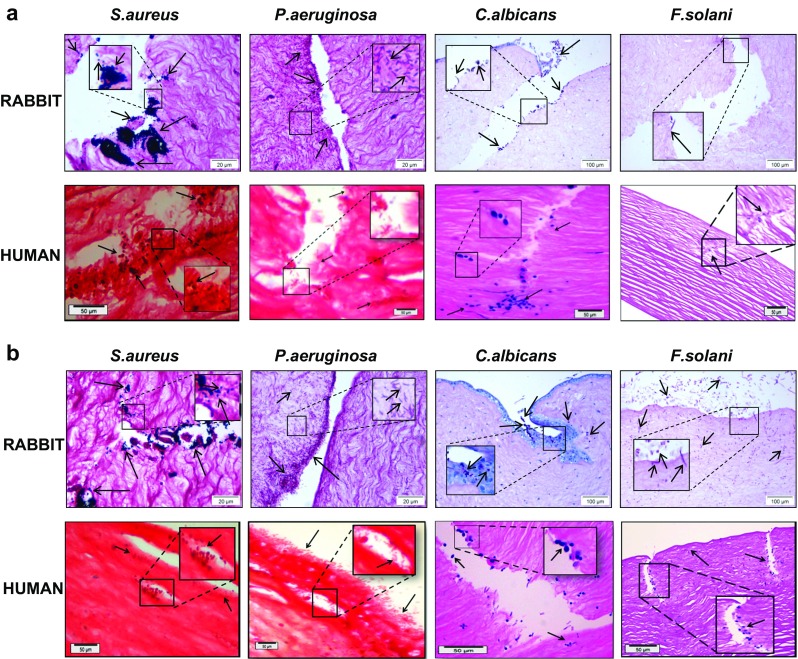
Histology of single-species infection of ex vivo rabbit and human corneas. Ex vivo rabbit and human corneas were scratched six times with a scalpel and exposed to a single-species inoculum of S. aureus, P. aeruginosa, C. albicans or F. solani for 24 h (a) or 48 h (b). Corneas were fixed in 10 % buffered formalin, embedded in paraffin, sectioned and stained using Gram stain (S. aureus and P. aeruginosa) or PAS stain (C. albicans and F. solani). Gram-positive (purple) cocci (S. aureus), Gram-negative (pink) rods (P. aeruginosa), and purple round yeast and hyphae (C. albicans and F. solani) can be observed at the epithelial surface and within the scratch, and are present in the stroma
The distribution of C. albicans within the corneal tissue was similar between the human and rabbit corneas. Yeast cells and hyphal elements were observed close to the scratch site, with no infiltration beyond 150 μm into the stroma (Fig. 5a). The number of hyphae and the infiltration of C. albicans cells into the stroma after 48 h did not differ from that observed after 24 h.
The tissue penetration by F. solani after scalpel wounding was less than that for C. albicans at 24 h, with infiltration not more than 10 μm into the stroma from the site of inoculation (Fig. 5a), which increased after 48 h in both number and depth of penetration.
Two-species infection of corneas
In the two-species infection model, we were able to recover both bacterial species from infected rabbit and human corneas (Table 1). Histological examination confirmed the quantitative colony count data (Fig. 6). As with the single-species model, infiltration of P. aeruginosa cells was found throughout the stroma, including Descemet’s membrane, whereas S. aureus showed little spread beyond the injection site.
Table 1.
Numbers of CFU/cornea recovered from multi-bacterial/fungal infections after 24 h
| A | ||||
| RABBIT | HUMAN | |||
| S. aureus - P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | P. aeruginosa |
| 1.88 ± 0.6 × 106 | 3.09 ± 0.9 × 107 | 5.25 ± 1.6 × 105 | 2.43 ± 3.2 × 108 | |
| C. albicans - P. aeruginosa | C. albicans | P. aeruginosa | C. albicans | P. aeruginosa |
| 4.20 ± 1.6 × 106 | 2.12 ± 0.9 × 108 | 5.15 ± 6.6 × 105 | 1.00 ± 1.2 × 108 | |
| B | ||||
| RABBIT | HUMAN | |||
| S. aureus - P. aeruginosa | S. aureus | P. aeruginosa | S. aureus | P. aeruginosa |
| 9.26 ± 4.2 × 106 | 5.83 ± 2.4 × 108 | 2.50 ± 5.7 × 104 | 3.72 ± 1.08 × 108 | |
| C. albicans - P. aeruginosa | C. albicans | P. aeruginosa | C. albicans | P. aeruginosa |
| 4.25 ± 1.1 × 106 | 6.84 ± 0.6 × 108 | 4.05 ± 9.6 × 105 | 7.65 ± 1.0 × 108 | |
Two multi-pathogen infections were investigated. These were a mixed S. aureus/P. aeruginosa and a mixed C. albicans/P. aeruginosa infection. A: EX vivo rabbit and human corneas were wounded with a scalpel and exposed to a mixture of 108 of both organisms for 24 h B: Ex vivo rabbit and human corneas were intrastromally injected with 108 cells of the first organism at 3–5 distinct locations, and then 108 cells of the second organism were similarly injected at different sites. The corneas were incubated for 24 h, washed, homogenised, serially diluted and plated onto agar plates, and the CFU/cornea calculated. Data is expressed in CFU/cornea ± SEM of at least three independent experiments performed in triplicate
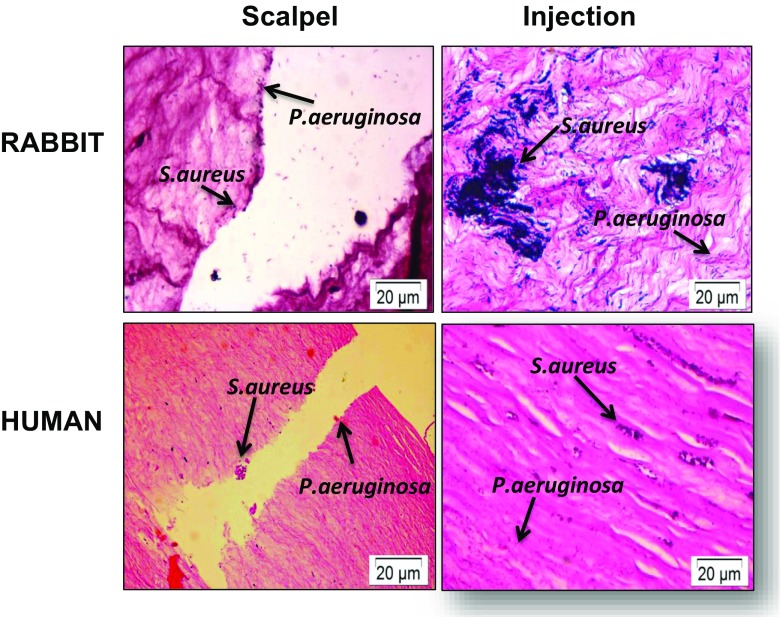
Fig. 6.
Histology of rabbit and human ex vivo models showing a mixed S. aureus and P. aeruginosa infection. At different sites within the same cornea, ex vivo corneas were intrastromally injected with 108 S. aureus and 108 P. aeruginosa and incubated for 24 h (injection). Alternatively, corneas were wounded with a scalpel, and 108 S. aureus and 108 P. aeruginosa were added to the surface of the cornea for 24 h (scalpel). Sections were Gram-stained and imaged to visualise S. aureus and P. aeruginosa within their injection sites at distinct locations within the stroma. P. aeruginosa shows widespread infiltration into the tissue, whereas S. aureus shows less infiltration
A mixed infection involving C. albicans and P. aeruginosa is the most commonly observed clinically [19]. In ex vivo models of this mixed infection, both organisms were recovered after 24 h following scalpel wounding, with P. aeruginosa showing dominance within the tissue by both colony counting (4.20 ± 1.6 × 106 and 2.12 ± 0.9 × 108 CFU/rabbit cornea and 5.15 ± 6.6 × 105 and 1.00 ± 1.2 × 108 CFU/human cornea for C. albicans and P. aeruginosa, respectively) and histology (Table 1 and Fig. 6). The level of recovery of both organisms was approximately the same regardless of the method of introduction of organisms.
Discussion
Ex vivo models have previously been used to study corneal–microbial interactions [12, 13]. However, there have been no direct comparisons of single and mixed bacterial and fungal infections or a comparison of the infection of rabbit and human corneas. Here, we describe the numbers of viable organisms recovered from rabbit and human corneas after 24 and 48 h, showing histological images from scalpel wounding and intrastromal injection as ways of introducing organisms to the cornea.
A variety of methods have been described in the literature for introducing bacteria or fungi to experimental (in vivo, in vitro or ex vivo) corneas. These include the use of bacterial/fungal-inoculated contact lenses [20, 21], blotting paper and ethylene glycol tetraacetic acid (EGTA) [7], and mechanical removal of the epithelial surface [22, 23]. However, the most commonly described methods are corneal scratch [24, 25] and intrastromal injection [26, 27]. Therefore, we chose to scratch the corneas with a scalpel six times, so that the scratch revealed the upper stromal compartment, and also to introduce organisms intrastromally using an injection method. Although the injection method gave a greater yield of organisms than the scalpel method, we observed that the scalpel method mimicked clinical infection in which infection is initiated from an abrasion on the corneal surface [28]. Because it was thought that the infiltrative capacity of P. aeruginosa within the tissue might inhibit or prevent the growth and propagation of S. aureus and/or C. albicans when introduced together through a scalpel wound, we compared this method with intrastromal injection, injecting organisms at separate sites and thus preventing their interaction. However, the scalpel method did not prevent the recovery of S. aureus or C. albicans, suggesting that either method is suitable for establishing a mixed infection model. Therefore, we show that an infection can be induced in the ex vivo corneas for both single and multiple species using either scalpel wounding or intrastromal injection.
The following aspects of this model need further discussion:
A large bacterial/fungal inoculum was used, because this corneal model does not have a blood supply or immune system. Consequently, the damage that the inflammation causes to the local tissue, and which provides additional nutrients via the vasculature for the bacteria/fungi, was not present.
S. aureus was not typically found at the epithelial surface, but rather within the scratches, commonly in clusters, and not migrating into surrounding and deeper cornea. This has been described previously as well [29, 30]. The observed attachment of S. aureus at the stromal surface is also supported by the observations of Rhem et al. [29], who demonstrated that collagen-binding clinical S. aureus isolates expressing the cna collagen-binding gene showed enhanced tissue disruption compared to a cna − isogenic mutant. The cna protein is considered to be a virulence factor mediating bacterial adherence to the epithelial surface and the stroma, and neutrophil recruitment to the infection site. Of the two strains of S. aureus that we used in this study, the ATCC 25923 strain is known to express this gene [31], which could be the reason for the higher level of binding within the scratch than at the surface (it is unknown whether this gene is expressed in the local clinical strain, S235). According to reports in the literature, in vivo corneas infected with S. aureus by intrastromal injection returned bacterial counts of approximately 104–107 CFU/cornea [32, 33], depending on the number of bacteria in the starting inoculum and the length of time the bacteria were incubated with the eye. These values are in line with the recovery we obtained from ex vivo models, suggesting that our model is representative of an in vivo infection in terms of the number of bacteria recovered.
We did not observe any ulceration or corneal edema. This is because ex vivo corneas lack inflammatory cells that are primarily responsible for epithelial ulceration [32], stromal polymorphonuclear neutrophil (PMN) infiltration [34–36], and ulcer formation [37] seen in clinical S. aureus infection.
Compared to the S. aureus model, P. aeruginosa-infected corneas yielded a greater number of CFU/cornea and were seen infiltrating the entire cornea, despite having the same inoculum. This high level of infiltration has been shown to be the result of proteolytic bacterial enzymes, including type III secretion system-associated cytotoxins, exoenzyme U and exoenzyme S [38, 39], alkaline protease and elastase [40], which have been shown to contribute to corneal erosion [41]. In addition, host proteolytic enzymes also contribute to corneal ulceration [26]. We observed a softening of P. aeruginosa-infected corneas and increased opacity compared with control corneas, but no ulceration. As mentioned previously, this was due to the lack of an immune cell component in these ex vivo cultures.
Previous studies have reported an increase in the recovery of P. aeruginosa from corneas compared with the initial inoculum [42, 43]. However, we did not find this increased recovery of P. aeruginosa. Although we have no definitive explanation for this observation, one possible explanation is that only a portion of these organisms actually adhere to the corneal surface and are able to invade/colonise. The data presented suggest that 106–107 CFU/cornea is the maximum number that can be recovered from an ex vivo cornea, and this maximal amount occurs after 24 h.
In contrast to the bacterial infections, single-species infections with C. albicans and F. solani did not show a significant increase in the recovery of organisms after 48 h versus 24 h, and the numbers of organisms recovered were lower, despite the same inoculum. This has been described briefly in the literature, where as many as 109–106 CFU/cornea for C. albicans and 103 CFU/cornea for F. solani were inoculated into in vivo or ex vivo murine, rabbit or rat models, with recovery of as little as 105–103 C. albicans CFU/cornea [12, 44–46], and 103 F. solani CFU/cornea [47], respectively. The reason for this is not fully understood.
From the 24-h histology images presented here, little infiltration of fungi into the corneal tissue is seen, with organisms remaining predominantly at the surface. However, particularly for F. solani, there was vast infiltration of fungal cells throughout the stroma after 48 h. The histology of infected in vivo cultures mimics histological images of clinical infection, with a dense white fungal plaque, corneal opacity, corneal infiltration, oedema, ulcer formation, satellite lesions, corneal neovascularisation and hypopyon [47–51]. Yeast forms of C. albicans and conidia of F. solani are shown to adhere to the stroma, and after a period of time, hyphae form that penetrate the stroma [12, 45, 52] to a depth of approximately 150 μm [44]. This was observed in our ex vivo rabbit and human cornea models.
Differences were observed between rabbit and human corneas primarily in the number of organisms recovered from each cornea, i.e. there was an approximately tenfold increase in the recovery of bacteria from human versus rabbit corneas. This may have been due to the use of different bacterial strains (ATTC strains [human] and local clinical strains [rabbit]), intrinsic differences between the corneas of the two species, including anatomical and molecular differences such as differences in the Bowman’s membrane [44] arrangement of collagen fibres [53], size, thickness [54], secretion of antimicrobial peptides [55], or surface mucin modifications [56]. Furthermore, the difference in bacterial recovery could be due to the use of stationary-phase organisms in rabbit experiments and log-phase bacteria in human corneal experiments. However, in comparing these two types of inocula, we have established that a similar number of bacteria in either phase still results in a clinically relevant level of infection for both single- and multi-species infection in both corneas, showing comparable histology.
The length of time these models were cultured in vitro was short. The acute nature of such an infection limits its use to short-term experiments involving, for example, investigation of treatment strategies [57], innate immune responses [58], detection of organisms [59] and host–microbe interactions [18]. These models were not intended to replicate the clinical outcome of infection that can be observed in vivo, which may develop over several weeks when not treated effectively. In these ex vivo models, there is an obvious lack of a host immune component, and the presence and infiltration of inflammatory cells is thought to contribute to the severity of disease [26]. As such, these ex vivo models do not form corneal ulcers as typically observed clinically [18, 41, 48]. They also lack tear films, which play a defensive role.
In summary, we achieved our aim of establishing a reproducible infection of both human and animal corneas. It is certain that no model system (including animals) is a perfect surrogate for the natural human infection. Nonetheless, useful data can still be obtained. We show that we can establish a reproducible in vitro bacterial and fungal infection, with the final number of recoverable bacteria/fungi comparable to that from natural in vivo experiments. These models are now being used in the evaluation of microbial detection systems.
Compliance with ethical standards
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
The Wellcome Trust provided financial support in the form of a grant through the Affordable Healthcare in India Initiative (no. 0998800/B/12/Z). The sponsor had no role in the design or conduct of this research.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Chandra J, Pearlman E, Ghannoum M. Animal models to investigate fungal biofilm formation. Methods Mol Biol. 2014;1147:141–157. doi: 10.1007/978-1-4939-0467-9_10. [DOI] [PubMed] [Google Scholar]
- 2.Evans DJ, Fleiszig SM. Why does the healthy cornea resist Pseudomonas aeruginosa infection? Am J Ophthalmol. 2013;155:961–970. doi: 10.1016/j.ajo.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang K, Wu M, Li M, Li D, Peng A, Nie X, Sun M, Wang J, Wu Y, Deng Q (2014) miR-155 suppresses bacterial clearance in Pseudomonas aeruginosa keratitis by targeting Rheb. J Infect Dis 89–98. doi: 10.1093/infdis/jiu002 [DOI] [PubMed]
- 4.Zaidi T, Zaidi T, Yoong P, Pier GB. Staphylococcus aureus corneal infections: effect of the Panton-valentine leukocidin (PVL) and antibody to PVL on virulence and pathology. Invest Ophthalmol Vis Sci. 2013;54:4430–4438. doi: 10.1167/iovs.13-11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolar SSN, Luca V, Baidouri H, Mannino G, McDermott AM, Mangoni ML (2014) Esculentin-1a (1-21) NH2: a frog skin-derived peptide for microbial keratitis. Cell Mol Life Sci 1–11. doi: 10.1007/s00018-014-1694-0 [DOI] [PMC free article] [PubMed]
- 6.Alarcon I, Evans DJ, Fleiszig SM. The role of twitching motility in Pseudomonas aeruginosa exit from and translocation of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:2237–2244. doi: 10.1167/iovs.08-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alarcon I, Tam C, Mun JJ, LeDue J, Evans DJ, Fleiszig SM. Factors impacting corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Invest Ophthalmol Vis Sci. 2011;52:1368–1377. doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badyal DK, Desai C. Animal use in pharmacology education and research: the changing scenario. Indian J Pharm. 2014;46:257–265. doi: 10.4103/0253-7613.132153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Editorial (2010) Reduce, refine, replace. Nat Immunol 11:971. doi: 10.1038/ni1110-971 [DOI] [PubMed]
- 10.Shah A, Brugnano J, Sun S, Vase A, Orwin E. The development of a tissue-engineered cornea: biomaterials and culture methods. Pediatr Res. 2008;63:535–544. doi: 10.1203/PDR.0b013e31816bdf54. [DOI] [PubMed] [Google Scholar]
- 11.Augustin DK, Heimer SR, Tam C, Li WY, Le Due JM, Evans DJ, Fleiszig SM. Role of defensins in corneal epithelial barrier function against Pseudomonas aeruginosa traversal. Infect Immun. 2011;79:595–605. doi: 10.1128/IAI.00854-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Q, Chen H, Qu M, Wang Q, Yang L, Xie L. Development of a novel ex vivo model of corneal fungal adherence. Graefes Arch Clin Exp Ophthalmol. 2011;249:693–700. doi: 10.1007/s00417-010-1601-9. [DOI] [PubMed] [Google Scholar]
- 13.Hua X, Yuan X, Di Pietro A, Wilhelmus KR. The molecular pathogenicity of Fusarium keratitis: a fungal transcriptional regulator promotes hyphal penetration of the cornea. Cornea. 2010;29:1440–1444. doi: 10.1097/ICO.0b013e3181d8383a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castro-Combs J, Noguera G, Cano M, Yew M, Gehlbach PL, Palmer J, Behrens A. Corneal wound healing is modulated by topical application of amniotic fluid in an ex vivo organ culture model. Exp Eye Res. 2008;87:56–63. doi: 10.1016/j.exer.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 15.Deshpande P, Notara M, Bullett N, Daniels JT, Haddow DB, MacNeil S. Development of a surface-modified contact lens for the transfer of cultured limbal epithelial cells to the cornea for ocular surface diseases. Tissue Eng A. 2009;15:2889–2902. doi: 10.1089/ten.tea.2008.0528. [DOI] [PubMed] [Google Scholar]
- 16.Ortega Í, Deshpande P, Gill AA, MacNeil S, Claeyssens F. Development of a microfabricated artificial limbus with micropockets for cell delivery to the cornea. Biofabrication. 2013;5:025008. doi: 10.1088/1758-5082/5/2/025008. [DOI] [PubMed] [Google Scholar]
- 17.Deshpande P, Ortega Í, Sefat F, Sangwan VS, Green N, Claeyssens F, MacNeil S. Rocking media over ex vivo corneas improves this model and allows the study of the effect of proinflammatory cytokines on wound healing. Invest Ophthalmol Vis Sci. 2015;56:1553–1561. doi: 10.1167/iovs.14-15308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alekseev O, Tran AH, Azizkhan-Clifford J (2012) Ex vivo organotypic corneal model of acute epithelial herpes simplex virus type I infection. J Vis Exp e3631. doi: 10.3791/3631 [DOI] [PMC free article] [PubMed]
- 19.Ray M, Nigel LC, Tan AM. Triple infection keratitis. Eye Contact Lens. 2014;40:123–126. doi: 10.1097/ICL.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 20.Lawin-Brussel CA, Refojo MF, Leong FL, Hanninen L, Kenyon KR. Effect of Pseudomonas aeruginosa concentration in experimental contact lens-related microbial keratitis. Cornea. 1993;12:10–18. doi: 10.1097/00003226-199301000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Cole N, Hume EB, Vijay AK, Sankaridurg P, Kumar N, Willcox MD. In vivo performance of melimine as an antimicrobial coating for contact lenses in models of CLARE and CLPU. Invest Ophthalmol Vis Sci. 2010;51:390–395. doi: 10.1167/iovs.09-4068. [DOI] [PubMed] [Google Scholar]
- 22.Stangogiannis-Druya E, Stangogiannis-Druya C, Naranjo-Tackman R, Vanzzini V, Villar-Kuri J. Bacterial corneal ulcer treated with intrastromal antibiotic. Experimental model in vivo. Arch Soc Esp Oftalmol. 2009;84:123–132. doi: 10.4321/S0365-66912009000300004. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–5332. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaylock WK, Yue BY, Robin JB. The use of concanavalin A to competitively inhibit Pseudomonas aeruginosa adherence to rabbit corneal epithelium. CLAO J. 1990;16:223–227. [PubMed] [Google Scholar]
- 25.Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM. Human tear fluid protects against Pseudomonas aeruginosa keratitis in a murine experimental model. Infect Immun. 2007;75:2325–2332. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kessler E, Mondino BJ, Brown SI. The corneal response to Pseudomonas aeruginosa: histopathological and enzymatic characterization. Invest Ophthalmol Vis Sci. 1977;16:116–125. [PubMed] [Google Scholar]
- 27.Barequet IS, Bourla N, Pessach YN, Safrin M, Yankovich D, Ohman DE, Rosner M, Kessler E. Staphylolysin is an effective therapeutic agent for Staphylococcus aureus experimental keratitis. Graefes Arch Clin Exp Ophthalmol. 2012;250:223–229. doi: 10.1007/s00417-011-1822-6. [DOI] [PubMed] [Google Scholar]
- 28.Deorukhkar S, Katiyar R, Saini S. Epidemiological features and laboratory results of bacterial and fungal keratitis: a five-year study at a rural tertiary-care hospital in western Maharashtra, India. Singapore Med J. 2012;53:264–267. [PubMed] [Google Scholar]
- 29.Rhem MN, Lech EM, Patti JM, McDevitt D, Hook M, Jones DB, Wilhelmus KR. The collagen-binding adhesin is a virulence factor in Staphylococcus aureus keratitis. Infect Immun. 2000;68:3776–3779. doi: 10.1128/IAI.68.6.3776-3779.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hume EB, Dajcs JJ, Moreau JM, Sloop GD, Willcox MD, O’Callaghan RJ. Staphylococcus corneal virulence in a new topical model of infection. Invest Ophthalmol Vis Sci. 2001;42:2904–2908. [PubMed] [Google Scholar]
- 31.Almeida LM, de Almeida MZP, Mendonça CL, Mamizuka EM. Comparative analysis of agr groups and virulence genes among subclinical and clinical mastitis Staphylococcus aureus isolates from sheep flocks of the Northeast of Brazil. Braz J Microbiol. 2013;44:493–498. doi: 10.1590/S1517-83822013000200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oguz H, Ozbilge H, Oguz E, Gurkan T. Effectiveness of topical taurolidine versus ciprofloxacin, ofloxacin, and fortified cefazolin in a rabbit Staphylococcus aureus keratitis model. Curr Eye Res. 2005;30:155–161. doi: 10.1080/02713680490908733. [DOI] [PubMed] [Google Scholar]
- 33.Barequet IS, Ben Simon GJ, Safrin M, Ohman DE, Kessler E. Pseudomonas aeruginosa LasA protease in treatment of experimental staphylococcal keratitis. Antimicrob Agents Chemother. 2004;48:1681–1687. doi: 10.1128/AAC.48.5.1681-1687.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hume EB, Cole N, Khan S, Garthwaite LL, Aliwarga Y, Schubert TL, Willcox MD. A Staphylococcus aureus mouse keratitis topical infection model: cytokine balance in different strains of mice. Immunol Cell Biol. 2005;83:294–300. doi: 10.1111/j.1440-1711.2005.01326.x. [DOI] [PubMed] [Google Scholar]
- 35.Hsiao CH, Ong SJ, Chuang CC, Ma DH, Huang YC. A comparison of clinical features between community-associated and healthcare-associated methicillin-resistant Staphylococcus aureus keratitis. J Ophthalmol. 2015;2015:923941. doi: 10.1155/2015/923941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shetty R, Kaweri L, Nuijts RM, Nagaraja H, Arora V, Kumar RS. Profile of microbial keratitis after corneal collagen cross-linking. Biomed Res Int. 2014;2014:340509. doi: 10.1155/2014/340509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sueke H, Shankar J, Neal T, Winstanley C, Tuft S, Coates R, Horsburgh MJ, Kaye S. lukSF-PV in Staphylococcus aureus keratitis isolates and association with clinical outcome. Invest Ophthalmol Vis Sci. 2013;54:3410–3416. doi: 10.1167/iovs.12-11276. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Conibear TC, Bandara R, Aliwarga Y, Stapleton F, Willcox MD. Type III secretion system-associated toxins, proteases, serotypes, and antibiotic resistance of Pseudomonas aeruginosa isolates associated with keratitis. Curr Eye Res. 2006;31:297–306. doi: 10.1080/02713680500536746. [DOI] [PubMed] [Google Scholar]
- 39.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect Immun. 2001;69:6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thibodeaux BA, Caballero AR, Marquart ME, Tommassen J, O’Callaghan RJ. Corneal virulence of Pseudomonas aeruginosa elastase B and alkaline protease produced by Pseudomonas putida. Curr Eye Res. 2007;32:373–386. doi: 10.1080/02713680701244181. [DOI] [PubMed] [Google Scholar]
- 42.Zaidi TS, Zaidi T, Pier GB, Priebe GP. Topical neutralization of interleukin-17 during experimental Pseudomonas aeruginosa corneal infection promotes bacterial clearance and reduces pathology. Infect Immun. 2012;80:3706–3712. doi: 10.1128/IAI.00249-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tajima K, Miyake T, Koike N, Hattori T, Kumakura S, Yamaguchi T, Matsumoto T, Fujita K, Kuroda M, Ito N, Goto H. In vivo challenging of polymyxins and levofloxacin eye drop against multidrug-resistant Pseudomonas aeruginosa keratitis. J Infect Chemother. 2014;20:343–349. doi: 10.1016/j.jiac.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Goldblum D, Frueh BE, Sarra GM, Katsoulis K, Zimmerli S. Topical caspofungin for treatment of keratitis caused by Candida albicans in a rabbit model. Antimicrob Agents Chemother. 2005;49:1359–1363. doi: 10.1128/AAC.49.4.1359-1363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jackson BE, Mitchell BM, Wilhelmus KR. Corneal virulence of Candida albicans strains deficient in Tup1-regulated genes. Invest Ophthalmol Vis Sci. 2007;48:2535–2539. doi: 10.1167/iovs.06-0909. [DOI] [PubMed] [Google Scholar]
- 46.Yuan X, Hua X, Wilhelmus KR. The corneal expression of antimicrobial peptides during experimental fungal keratitis. Curr Eye Res. 2010;35:872–879. doi: 10.3109/02713683.2010.495812. [DOI] [PubMed] [Google Scholar]
- 47.Yavas GF, Ozturk F, Kusbeci T, Cetinkaya Z, Ermis SS, Kiraz N, Inan UU. Antifungal efficacy of voriconazole, itraconazole and amphotericin b in experimental Fusarium solani keratitis. Graefes Arch Clin Exp Ophthalmol. 2008;246:275–279. doi: 10.1007/s00417-007-0687-1. [DOI] [PubMed] [Google Scholar]
- 48.Das S, Sharma S, Mahapatra S, Sahu SK. Fusarium keratitis at a tertiary eye care centre in India. Int Ophthalmol. 2015;35:387–393. doi: 10.1007/s10792-014-9961-5. [DOI] [PubMed] [Google Scholar]
- 49.Ledbetter EC, Norman ML, Starr JK. In vivo confocal microscopy for the detection of canine fungal keratitis and monitoring of therapeutic response. Vet Ophthalmol. 2015 doi: 10.1111/vop.12287. [DOI] [PubMed] [Google Scholar]
- 50.Thomas PA, Kaliamurthy J. Mycotic keratitis: epidemiology, diagnosis and management. Clin Microbiol Infect. 2013;19:210–220. doi: 10.1111/1469-0691.12126. [DOI] [PubMed] [Google Scholar]
- 51.Zhu JL, Gao XR, Cui HP, Lang LL, Li Q, Liao X. Experimental model of Fusarium solani keratitis in rats. Int J Ophthalmol. 2011;4:371–376. doi: 10.3980/j.issn.2222-3959.2011.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan X, Wang Y, Zhou Q, Chen P, Xu Y, Chen H, Xie L. Activation of focal adhesion kinase enhances the adhesion of Fusarium solani to human corneal epithelial cells via the tyrosine-specific protein kinase signaling pathway. Mol Vis. 2011;17:638–646. [PMC free article] [PubMed] [Google Scholar]
- 53.Ojeda JL, Ventosa JA, Piedra S. The three-dimensional microanatomy of the rabbit and human cornea. A chemical and mechanical microdissection-SEM approach. J Anat. 2001;199:567–576. doi: 10.1046/j.1469-7580.2001.19950567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marquart ME. Animal models of bacterial keratitis. J Biomed Biotechnol. 2011;2011:680642. doi: 10.1155/2011/680642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Durr UH, Sudheendra US, Ramamoorthy A. LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1408–1425. doi: 10.1016/j.bbamem.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 56.Royle L, Matthews E, Corfield A, Berry M, Rudd PM, Dwek RA, Carrington SD. Glycan structures of ocular surface mucins in man, rabbit and dog display species differences. Glycoconj J. 2008;25:763–773. doi: 10.1007/s10719-008-9136-6. [DOI] [PubMed] [Google Scholar]
- 57.Sriram S, Gibson DJ, Robinson P, Pi L, Tuli S, Lewin AS, Schultz G. Assessment of anti-scarring therapies in ex vivo organ cultured rabbit corneas. Exp Eye Res. 2014;125:173–182. doi: 10.1016/j.exer.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marino A, Pergolizzi S, Lauriano ER, Santoro G, Spataro F, Cimino F, Speciale A, Nostro A, Bisignano G. TLR2 activation in corneal stromal cells by Staphylococcus aureus-induced keratitis. Acta Pathol Microbiol Immunol Scand. 2015;123:163–168. doi: 10.1111/apm.12333. [DOI] [PubMed] [Google Scholar]
- 59.Posch LC, Zhu M, Robertson DM. Multipurpose care solution-induced corneal surface disruption and Pseudomonas aeruginosa internalization in the rabbit corneal epithelium. Invest Ophthalmol Vis Sci. 2014;55:4229–4237. doi: 10.1167/iovs.14-14513. [DOI] [PMC free article] [PubMed] [Google Scholar]