Abstract
Introduction
Alpha particles are radiation of high energy and short range, properties that can lead to radiolysis-mediated complications in labeling chemistry at the high radioactivity levels required for clinical application. In previous papers in this series, we have shown that radiation dose has a profound effect on the astatine species that are present in the labeling reaction and their suitability for the synthesis of N-succinimidyl 3-[211At]astatobenzoate. The purpose of this study was to evaluate the effects of adding N-chlorosuccinimide (NCS) to the methanol solution used for initial isolation of 211At after distillation, a process referred to as 211At stabilization, on 211At chemistry after exposure to high radiation doses.
Methods
High performance liquid chromatography was used to evaluate the distribution of 211At species present in methanol in the 500–65,000 Gy radiation dose range and the synthesis of SAB from N-succinimidyl 3-(tri-n-butylstanny)benzoate in the 500–120,000 Gy radiation dose range using different 211At time activity combinations under conditions with/without 211At stabilization.
Results
In the absence of NCS stabilization, a reduced form of astatine, At(2), increased with increasing radiation dose, accounting for about half the total activity by about 15,000 Gy, while with stabilization, At(2) accounted for <10% of 211At activity even at doses >60,000 Gy. SAB yields without stabilization rapidly declined with increasing dose, falling to ~20% at about 5,000 Gy while with stabilization, yields >80% were obtained with 211At solutions stored for more than 23 h and receiving radiation doses >100,000 Gy.
Conclusions
Adding NCS to the methanol solution used for initial isolation of 211At is a promising strategy for countering the deleterious effects of radiolysis on 211At chemistry.
Advances in Knowledge and Implications for Patient Care
This strategy could facilitate the ability to perform 211At labeling at sites remote from its production and at the high activity levels required for clinical applications.
Keywords: 211At, targeted radiotherapy, radiolysis, α-particles, radionuclide therapy
1. Introduction
Alpha-particle emitting radionuclides are attractive for molecularly targeted radiotherapy because they deposit a large amount of energy within a few cell diameters [1]. One of the most attractive α-emitters for this purpose, 211At, has a half-life of 7.2 h, and has two features distinguishing it from other α-emitters of potential clinical interest: absence of potentially confounding long-lived α-emitting daughters and halogenic rather than metallic character [2]. As a consequence of the later, labeling methods for 211At are different than those for α-emitting radiometals, with electrophilic demetallation of stannyl or silyl precursors being the most widely used approach for labeling biomolecules with 211At [3,4]. This labeling strategy has been successfully utilized at 211At activity levels of the order of 37 MBq with a variety of compounds. In addition, synthesis of N-succinimidyl 3-[211At]astatobenzoate (SAB) from an N-succinimdyl 3-(tri-n-butylstannyl)benzoate (BuSTB) precursor followed by SAB coupling to a monoclonal antibody (mAb) could be reliably performed up to 211At-labeled mAb final product activities of up to about 250 MBq [5]. However, further dose escalation became problematic due to dramatically declining yields for SAB synthesis and mAb coupling, as well as compromised immunoreactivity of the labeled mAb [6].
In an attempt to address this problem, we have been investigating the potential effects of increased α-particle radiation dose on various factors that could influence the synthesis of SAB from its tin precursor. Previous publications evaluating the consequences of α-particle radiolysis for 211At labeling reactions demonstrated three important phenomena that could have a profound influence on high dose labeling reactions such as those required for clinical application. First, with increasing radiation dose, the nature of the solvent used in the astatodestannylation reaction had a profound influence on the fate of the tin precursor, and in chloroform, a cold byproduct was generated that interfered with SAB synthesis [7]. Second, radiolysis-mediated processes have a major effect on the nature of the labeled product generated and the yields of SAB as a function of both radiation dose and pH [8]. And third, radiation dose have a profound effect on the nature of the astatine species present before initiation of the labeling reaction [9]. In that study, HPLC analysis revealed two peaks, designated as At(1) and At(2), were present before performing the labeling reaction, with the fraction of 211At present as the At(2) species increasing at higher radiation doses. At(1) was shown to be a neutral species and suitable for electrophilic astatodestannylation reactions, while the presence of At(2) was observed to result in a significant decline in subsequent SAB labeling efficiency. We hypothesized that At(2) was a radiolysis-induced, reduced form of astatine – most likely astatide - for several reasons [9]: a) At(1) was nearly completely converted to At(2) by treatment with the reducing agent, sodium sulfite, b) the retention time of At(2) was almost identical to that of sodium [131I]iodide, and c) At(2) was shown to be an anionic species by electrophoresis, whereas At(1) was shown to be a neutral species.
The goal of the present study was to evaluate a strategy for protecting or stabilizing the 211At from the deleterious consequences of its own radiation field in order to preserve it in a form suitable for use in an electrophilic labeling reaction with SAB serving as the model compound. Based on our previous studies evaluating radiolysis-mediated effects on 211At labeling chemistry [7, 8], methanol was selected as the solvent and the possibility of excluding acetic acid from the reaction mixture was investigated. The current study assessed the effects of adding NCS to the methanol solution used for initial isolation of 211At after distillation (a process referred to as 211At stabilization) on 211At chemistry at high radiation doses. First, the effect of NCS stabilization on astatide production in the reaction media was determined by HPLC by monitoring generation of the At(2) species described above. And second, the effect of NCS stabilization on SAB yields at dose levels where radiolysis-mediated effects have been previously shown to be a major problem [8,9] were determined. Our results demonstrate that with this stabilization approach, SAB can be produced efficiently at high radiation doses, thereby paving the way for reliable 211At labeling at the higher activity levels needed for clinical studies.
2. Materials and methods
Methanol was anhydrous 99.8% grade Sure/Seal with <0.002% water and <0.0003% evaporation residue (Aldrich). All other solvents were reagent grade or better and were used as purchased. HPLC analyses were performed on a Beckman System Gold HPLC equipped with a diode array detector and a radioisotope detector. The analyses were made with a Waters Xterra 4.6 x 250 mm (10 μm) reversed-phase column eluted at a flow rate of 1 mL/min with solvent A [acetonitrile/water/acetic acid (5/95/0.1)] and solvent B [acetonitrile/acetic acid 0.1%] using two different HPLC gradients conditions. Gradient system (I): 72:28 (A/B) held for 15 min to 0:100 (A/B) over 20 min, followed by 100% solvent B for the remainder of the HPLC run. Gradient system (II): 90:10 (A/B) held for 10 min to 72:28 (A/B) over 10 min then held for another 10 min, followed by 0:100 (A/B) over 20 min followed by 100% solvent B for the remainder of the HPLC run. SAB synthesis was performed by astatodestannylation of BuSTB, which was prepared as described previously [10]. The purity of the tin precursor was confirmed before each set of experiments by thin layer chromatography.
2.1 Production of 211At and Radiation Dose Calculations
Astatine-211 was produced at the Duke University Medical Center cyclotron by bombarding natural bismuth metal targets with 28.0-MeV α-particles using the 209Bi(α, 2n)211At reaction [11] and isolated by dry distillation using a PEEK tubing cryotrap as described [9]. At the end of the distillation, the tubing was removed from the apparatus, both ends covered with Parafilm and allowed to warm to room temperature. The 211At captured in the PEEK tubing was eluted just before each experiment into a small volume of methanol (200–850 μL) and the activity, volume and time were recorded for use in the radiation dose calculations.
For the radiation dose calculations, it was assumed that all of the α-particle and α-recoil nuclei decay energy was deposited in these solutions because of the short range of these emissions relative to the dimensions of the reaction mixtures. Uniform distribution of the reactants in the solvent also was assumed. The absorbed dose was calculated as [5]:
where D is expressed in Gy, Ai is the initial activity in MBq, λ is the decay constant for 211At (s−1), t is the exposure time in seconds, m the mass of the solution (g), and Δi is the mean energy emitted per nuclear transition. Considering the dose contributions from α-particles and α-recoil nuclei, a Δi = 1.09 x 10−3 Gy · g/MBq · s was calculated for 211At decay [12]. A density of 0.791 g/mL was used to convert methanol volume to mass.
2.2 Radiolysis and astatine species: Effect of NCS pre-addition
To determine the potential effect of NCS on the nature of the astatine species present with increasing radiation dose, 211At was eluted from the PEEK tubing in methanol and immediately separated into aliquots where one was used as such and to the other, NCS was added (50–2100 μg per 500 μL 211At/methanol). Because preliminary experiments indicated that 100–200 μg NCS was optimal, this level was used in subsequent studies. As a preliminary evaluation of initial dose rate effects, two 211At/methanol solutions were prepared, one containing 165 MBq and the other, 40 MBq, with initial dose rates of 2.51 Gy/s and 0.97 Gy/s, respectively. The solutions were allowed to sit for different time periods such that both received similar total radiation doses (0.97 Gy/s, 16,100 Gy; 2.51 Gy/s, 15,800 Gy), and then diluted with methanol to yield a 200 μL final volume for the SAB labeling reaction.
2.3 Radiolysis and SAB synthesis: Effect of NCS pre-addition
Experiments were performed to evaluate the effect of adding N-chlorosuccinimide (NCS) to the 211At/methanol solution immediately after its elution from the PEEK tubing cryotrap as a strategy for improving 211At labeling chemistry after exposure to high radiation doses, which is referred to hereafter as 211At stabilization. The radiation dose ranges that were studied were selected to encompass those that might be encountered when SAB synthesis would be performed as the first step in the preparation of clinical level doses of 211At-labeled proteins and peptides. The activity levels of 211At used in each run ranged between 40 and 953 MBq, and were measured using a CRC-7 dose calibrator (Capintec). Samples for HPLC analysis were taken at various times and selected based on the radiation dose level of interest in each experiment. The radiation dose deposited in the reaction vessel ranged between <1,000 and ~125,000 Gy that were achieved with exposure periods between minutes and about 24 h. Within this range, a dose of 4,700 Gy served as a practical benchmark because this is the estimated dose to an SAB reaction that would occur with the initial 211At activity needed to produce 370 MBq of 211At-labeled mAb, a dose where labeling irreproducibility was previously encountered [5,9].
Synthesis of SAB was performed as described previously [9] except that if NCS had been added before the reaction to the 211At/methanol PEEK tubing wash, then it was omitted from the astatodestannylation reaction mixture. Briefly, 50 μg (98 nmole) of BuSTB in 50 μL of methanol, 100 μg (0.75 μmole) of NCS dissolved in 50 μL of methanol, and 20 μL of acetic acid were added to 0.4 mL of 211At/methanol in a Reactivial; unless different conditions are specified, the final concentration of acetic acid was 0.67 M. In some cases, acetic acid also was omitted from the reaction mixture. The reaction mixture was shaken for 20 min at room temperature, and 5–40 μL aliquots were periodically removed for HPLC analysis. The areas of all astatinated species were integrated and results were expressed as the percentage of the total activity eluted from the column and plotted as a function of radiation dose (Gy) and dose rate (Gy/s). To control for potential deleterious effects of high-dose radiation on the HPLC column, N-succinimidyl 3-iodobenzoate (SIB, 3 μg/3 μL methanol) was co-injected periodically as a retention time internal standard for SAB. In these experiments, SAB showed a retention time of 21.2–22.0 min using gradient system (I) and 46.2–47.5 min with gradient system (II).
2.4 Role of acetic acid in context of NCS pre-addition
To evaluate whether acetic acid was a necessary component of SAB synthesis in the context of this 211At stabilization strategy, four additional reactions were run where the 211At/methanol isolated from the distillation trap was immediately treated with NCS and both acetic acid and NCS were excluded from the SAB synthesis reaction. The reactions were performed at radiation doses of 2,600, 41,200, 45,100 and 50,000 Gy.
2.5 Other oxidants
In these experiments, the oxidant under evaluation was added to the 211At/methanol as soon as possible, and then stored at room temperature to accumulate radiation dose, and then reacted with BuSTB and acetic acid, and analyzed using HPLC gradient (II), all as described in Materials and Methods. Oxidant concentrations were in the same range as studied with NCS (5–125 mM) and radiation doses were in the 32,400–72,800 Gy range.
2.6 Labeling IgG with NCS-stabilized 211At
As a preliminary evaluation of the potential effect of the 211At stabilization approach on the subsequent coupling of SAB to proteins, experiments were performed with human IgG (Sigma). The 211At was eluted from the PEEK tubing distillation trap in 600 μL methanol containing 125 μg NCS and the SAB labeling reaction was performed after 22 min (800 MBq added, 2,600 Gy reaction vessel dose) or after 23 h (590–620 MBq, 41,800–50,000 Gy, n=2) in a Reactivial containing 50 μg of BuSTB in 50 μL of methanol. The SAB was evaporated to dryness with a stream of argon in a glass vial to which 10 mg human IgG (20 mg/mL 0.05 M PBS, pH adjusted to 8.5 with borate buffer), and incubated at room temperature for 15 min. The reaction was terminated and 211At-labeled IgG was purified using a PD-10 size exclusion column (Amersham Biosciences) as described previously [10].
3. Results and discussion
The potential utility of 211At for targeted α-particle radiotherapy has been recognized for more than thirty years [13] and the feasibility of patient studies with 211At-labeled radiopharmaceuticals has been demonstrated [6,14]. Nonetheless, significant barriers remain that impede the clinical translation of 211At. Although the limited number of 211At production facilities makes radionuclide availability problematic, it also means that users at locations distant from production sites will receive 211At shipments that have undergone significant decay during transit, which can compromise chemical tractability [4,9]. This most likely is the result of αparticle mediated radiolytic effects, which also are responsible for the problems encountered when attempting to prepare high doses of 211At-labeled compounds such as SAB for clinical trials [5].
Herein, we evaluate a potential strategy for minimizing the deleterious effects of radiolysis on 211At labeling chemistry involving the immediate addition of NCS to the 211At (referred to as astatine stabilization) as it is washed from the PEEK tubing distillation trap used to isolate 211At from the cyclotron target. In a previous study, HPLC analysis was utilized to demonstrate that at radiation doses <1,000 Gy, more than 90% of the activity was present in methanol as a single species, designated as At(1) [9]. However, as radiation dose increased, At(1) declined and a second peak, At(2) emerged, and at about 3,000 Gy, became the predominant species. This second peak was identified to be a reduced form of astatine, most likely astatide, and not suitable for electrophilic labeling reactions.
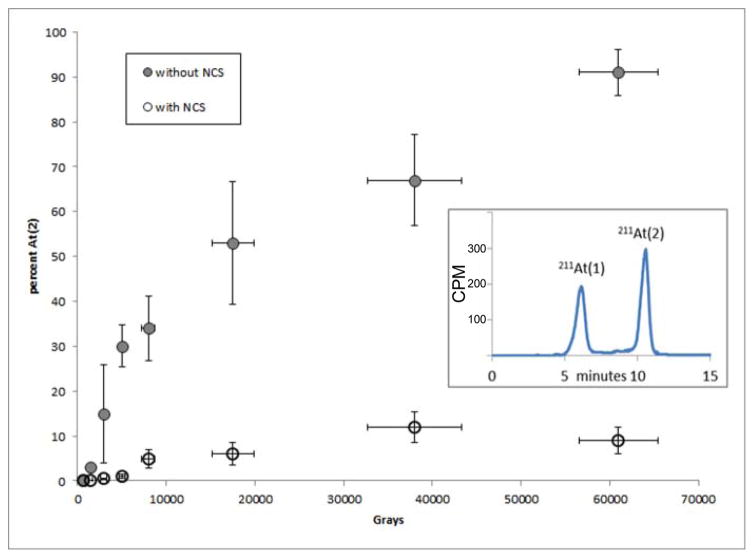
In the current study, the percentage of the activity eluted as At(2) as function of radiation dose with and without addition of NCS to the 211At/methanol solution is shown in Fig. 1. For reasons discussed later, only experiments performed at a dose rate less than 1 Gy/s (0.15–0.96 Gy/s) were included in this figure. As observed previously [9], in the absence of NCS, more than 70% of the activity in 211At/methanol was present as the At(2) species when radiation doses exceeded about 4,000 Gy. In contrast, when NCS was added to the 211At/methanol (typically, within 3–4 min), the fraction of 211At activity present as At(2) was considerably lower, being <10% even at doses of about 60,000 Gy.
Fig. 1.
Percentage of 211At activity present as At(2) species as a function of radiation dose absorbed by solvent, determined by reverse-phase HPLC as shown in inset. Studies performed with (grey circles) and without (open circles) addition of NCS (100–200 μg) to 211At in methanol.
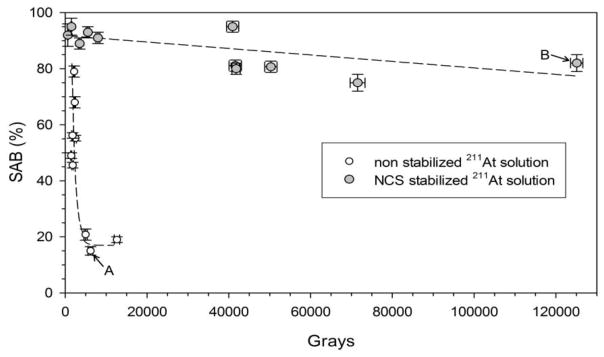
Because the At(2) species is not suitable for electrophilic destannylation reactions [9], we next investigated whether “stabilizing” the 211At/methanol by addition of NCS would lead to improved SAB yields at high radiation doses. As shown in Fig. 2, without prior addition of NCS to the 211At/methanol solution, SAB yields declined precipitously at doses of only a few thousand Gy. In contrast, with NCS stabilization and no subsequent addition of NCS to the labeling reaction, SAB yields remained above 75% even at doses to the reaction mixture greater than 120,000 Gy. Regression analyses of these data by ANOVA [15] yielded a slope of (1.2 ± 0.4) × 10−5 (P <0.05), indicating a decrease in SAB yield of only about 1% per 10,000 Gy accumulated.
Fig. 2.
Effect of the radiation dose delivered to the solvent on SAB production yields for reactions carried out with 211At/methanol solutions with and without addition of NCS to 211At when isolated from PEEK tubing distillation trap. Points A and B designate runs illustrated in Fig. 3
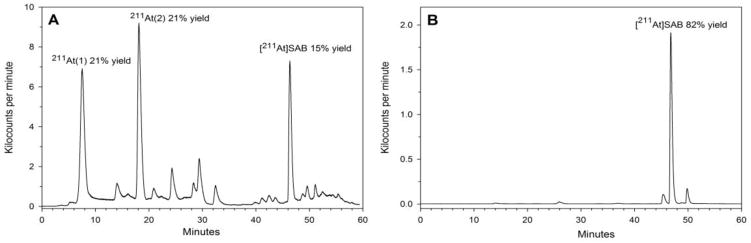
Typical HPLC profiles for SAB reaction mixtures for syntheses performed with and without NCS stabilization are presented in Fig. 3. Fig 3a corresponds to an experiment performed with 383 MBq/135 μL 211At/methanol without prior addition of NCS, with the reaction vial receiving a dose of 6150 Gy before the start of the labeling reaction (This reaction is represented by Point A in Fig. 2). Figure 3b depicts an experiment made with a 211At methanol solution of 398 MBq/155 μL 211At/methanol stabilized by addition of 100 μg of NCS and stored at room temperature for 18.07 h to reach a radiation dose of 125,100 Gy (This reaction is represented by Point B in Fig. 2). Unlike the case with the stabilized preparations, the reaction mixture from SAB reactions performed without NCS stabilized 211At included many peaks on HPLC including peaks corresponding to both At(1) and At(2), which were present in greater abundance than the desired SAB product. The presence of At(2) suggests that the addition of NCS during the destannylation reaction did not efficiently convert this reduced species to At+; however, as we have previously observed [9], comparison of the At(1)/At(2) ratio in Fig. 1 at ~6,000 Gy suggests that some conversion of At(2) to a reactive species had occurred.
Fig. 3.
Typical reverse-phase HPLC chromatograms for SAB synthesis reaction made using non-stabilized (A) and stabilized 211At/methanol solutions (B). HPLC profiles correspond to the experiments plotted in Figure 2 marked as A and B, respectively.
One can envision high radiation doses with 211At being delivered under two different scenarios. First, with high activity level radiopharmaceutical synthesis at/near 211At production sites for clinical studies, radiation dose rate higher than 3–4 Gy/s could be easily reached. And second, when the 211At is shipped to a remote location and used hours after its production, during which dose rate would decline during the course of the exposure due to 211At physical decay during transport. For logistical, cost and radiation safety reasons, most of the experiments we have performed evaluating 211At radiolysis effects were performed mimicking the second case, where the 211At/methanol solution was stored and allowed to decay before being studied.
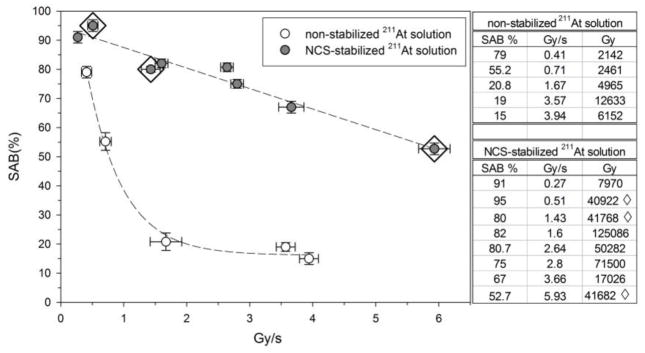
In a preliminary experiment to determine the potential role of dose rate effects, HPLC analysis revealed that the fraction of the 211At activity present as At(2) in the high dose rate solution was 4% vs 28% in the low dose rate solution, motivating further investigation of the importance of dose rate as a variable in the investigation of 211At radiolysis effects. Subsequent studies evaluated the effect of dose rate on SAB synthesis. In Fig. 4, SAB yield as a function of dose rate for reactions run with and without NCS stabilization are compared for dose rates up to ~6 Gy/s. When non-stabilized 211At/methanol solutions were used, a rapid decrease in SAB yield was observed even at dose rates less than 1 Gy/s, although the extent to which this reflects a dose vs. dose rate effect cannot be discerned. In either case, these results suggest that in the absence of 211At stabilization, high yield SAB synthesis would not be feasible at the dose rates that would be anticipated for preparing 211At-labeled radiopharmaceuticals for clinical radiotherapy. Considerably more favorable results were obtained in the experiments with NCS stabilized 211At. A linear decline in SAB yield as a function of dose rate was observed (slope: −7.0 ± 0.6, P <0.0001, ANOVA [15]). Nonetheless, an SAB yield greater than 50% was obtained at the highest dose rate evaluated (~6 Gy/s), which would correspond to an initial 211At activity of about 2200 MBq in the reaction vessel. Comparing the results presented in Fig. 2 and Fig. 4, it appears that SAB yields for NCS stabilized 211At are more dependent on dose rate than dose. In support of this, in three experiments performed at a similar radiation dose (40,900–41,800 Gy; noted by ◇ in Fig. 4), SAB yields declined from 95% at 0.51 Gy/s to 80% at 1.43 Gy/s to 52.7% at 5.93 Gy/s. Further experiments will be required to address the potential role of dose rate in detail; however, it should be noted that separating dose from dose rate effects will be difficult because these variables are strongly related. Although this could be accomplished by varying the exposure time, results would not be directly applicable to the conditions for 211At radiopharmaceutical syntheses, which have optimized reaction periods. Also, experiments performed at varied reaction times might be influenced by the chemical kinetics of the radiolysis induced reactions.
Fig. 4.
Effect of initial radiation dose rate (Gy/s) on SAB production yields for reactions carried out with 211At/methanol solutions with and without stabilization by addition of NCS. The experiments marked with a ◇ on the figure and accompanying data tables correspond to reactions performed at a similar radiation dose (41,000–42,000 Gy) but different initial dose rates (0.51, 1.43 and 5.93 Gy/s).
The optimum conditions for performing halodestannylation reactions generally is includes a pH of about 5–5.5, which is usually accomplished by adding acetic acid to the reaction mixture [3–5]. However, our previous results [8,9] suggest that addition of acetic acid may not be necessary and might even be counterproductive at radiation doses greater than about 3,000 Gy due to radiolysis-induced generation of reducing species in methanol under these conditions [16]. In the current study, with NCS-stabilized 211At and reactions performed at radiation doses of 2,600, 41,200,45,100 and 50,000 Gy in the absence of acetic acid, yields for SAB production were 84, 87, 88 and 85%, respectively. Comparison to the results performed with acetic acid in the reaction mixture (Fig. 2) by t-test indicated no significant difference in SAB yield (null hypothesis: , or ≠ 0; t = 0.537 vs. t0.01, 9 = 3.25). This suggests that addition of acetic acid may not be necessary for SAB synthesis at high radiation doses when stabilized 211At/methanol is used, which may have desirable practical consequences. For example, SAB could be used without purification without the risk that small traces of acetic acid might remain due to incomplete evaporation, thereby reducing the pH of the conjugation reaction, producing lower mAb coupling yields.
Based on the excellent results obtained when NCS was added to the 211At/methanol PEEK tubing wash, we have also evaluated whether other oxidants can be used for the same purpose. Both halogenated as well as other oxidants such as ammonium cerium(IV) nitrate have been studied [17]; for brevity, only the results with the halogenated compounds will be described here. Although these results are anecdotal, they do suggest that oxidants other than NCS might be useful for 211At stabilization. For example, an SAB yield of 91% was obtained with 13.3 mM sodium hypochlorite at 61,300 Gy, 85% with 76.9 mM sodium chlorite at 65,600 Gy, and 76% with 76.9 mM Chloramine-T at 72,800 Gy. Further experiments are planned to determine whether these or other chlorinated oxidants will offer any substantive advantage compared with NCS for 211At stabilization.
In contrast, use of iodine and bromine based oxidants such as N-iodosuccinimide (NIS) resulted in low SAB yields; instead, dependent on the BuSTB:NIS molar ratio, almost complete conversion of the tin precursor to N-succinimidyl 3-iodobenzoate (SIB) was observed. This behavior is consistent with the fact that for N-halogensuccinimide oxidants like Chloramine-T where the halogen is bound to nitrogen, the active oxidant is X+ (X = halogen). The difference in final outcomes reflects the fact that Cl+ is a strong oxidant but a bad electrophile, whereas I+ is a very good oxidant but a very good electrophile as well with the result that iodonium ions (I+) will compete with At+ for reaction with the stannyl groups. The high ratio of I to 211At atoms leads to a high SIB production rate. Even if one used a 100 times smaller amount of NIS than the amount of NCS normally used (for example, 1 μg NIS, equivalent to 2.67 x 1018 I atoms) and a high activity of 211At (for example, 800 MBq, equivalent to 2.99 x 1013 211At atoms), a ratio of I to 211At atoms of about 90,000 would occur. Moreover, because the SIB active ester would be present in much higher concentrations than SAB, it would compete with SAB during the protein conjugation reaction, producing low antibody specific activities and probably compromise immunoreactivity. Similar behavior but to a lesser degree was observed when N-bromosuccinimide was used.
With regard to the potential mechanism of the stabilization effect, it seems likely that the NCS acts by compensating for the radiolytically generated reducing species leading to the formation of At(2), which we have shown previously to likely be astatide [9]. However, that study also indicated that later addition of NCS could partially convert At(2) to the At(1) species required for electrophilic destannylation but that the conversion was not efficient. We also note that even though NCS stabilization permitted SAB synthesis in >50% yields even at dose rates of about 6 Gy/s, an inverse relationship between SAB yield and dose rate was observed. Understanding these observations is complicated by the fact that definitive chemical identification of the astatine species involved in these processes is difficult due to analytical limitations imposed by the lack of a stable astatine isotope that could be used for this purpose. For example, the HPLC chromatogram peak At(1) may or may not corresponds to a single astatine species and if there are multiple species, it is not known whether they would differ in their behavior in electrophilic reaction scenarios.
Studies by other investigators on the redox chemistry of astatine have shown that under oxidizing and acidic pH conditions, AtO+ (At+3 hydroxide species) might be also present [18–20]. Although it possibly could be a component of the At(1) peak, its presence becomes important only at pH much lower than those present in our experiments [21]. Moreover, although AtO+ can produce astatination reactions in trycyanomethanide and azide compounds [22], its suitability for the electrophilic astatination of organostannyl derivatives like BuSTB has not been documented. In contrast, the electrophilic astatination of radiopharmaceuticals via organometallic derivatives using At+ has been widely established, leading us to assume that At+ is the primary and probably the only species present in the At(1) peak.
In previous studies, we demonstrated that methanol was a better solvent than either chloroform or benzene for synthesizing SAB under conditions of high radiation dose [7] but that even in methanol, labeling yields declined due to radiation-induced generation of reducing species [8]. The current study demonstrates that this can be circumvented by addition of NCS to the 211At/methanol solution when the 211At activity is initially exposed to the solvent. The extent to which methanol works in concert with NCS to facilitate subsequent electrophilic astatination reactions is not clear and will be elucidated in subsequent experiments. Based on a theoretical study of complex formation of iodine with methanol [23], we speculate that astatine might interact in a similar fashion. Another possible role of methanol in the stabilization of At+ might be through complex formation with methyl radicals produced by the radiolysis of liquid methanol [22]. Methyl radicals are electron donors and relatively nucleophilic [25]; as nucleophilic species, they would tend to donate electrons and seek electron deficient centers like At+, suggesting another possible role of methanol in the stabilization process.
Finally, we performed a preliminary evaluation of the potential utility of SAB prepared from NCS stabilized 211At/methanol for labeling proteins. Given the relatively low level of precursor compared with previous protocols [5] and the absence of acetic acid, we wished to determine whether SAB could be utilized without purification, simplifying the process and making it more amenable to future automation. However, omitting SAB purification could be problematic because of the possibility that species generated during α-particle mediated radiolysis or other impurities could compromise conjugation efficiency and for mAbs, immunoreactivity. The results of three typical experiments performed with human IgG as the model protein are shown at Table 1. In two runs, the 211At/methanol/NCS solutions were stored overnight and received radiation doses greater than 40,000 Gy and less than 70 MBq was present at the initiation of the coupling reaction. In the third run, SAB production and protein conjugation were performed within 30 min of the elution of the 211At from the PEEK tubing with NCS/methanol. Protein coupling yields for the unpurified SAB preparations receiving a dose of >40,000 Gy were virtually identical to the control run prepared from freshly isolated 211At, suggesting that protein conjugation efficiency was not compromised when SAB prepared from stabilized 211At was used without purification. Moreover, preliminary experiments performed using the procedure outlined at the beginning of this paragraph suggest that this protocol could be used to label chimeric 81C6 mAb with 211At with preservation of immunoreactivity. For example, when mAb labeling was performed with SAB that had been subjected to doses of 1620 and 45,100 Gy, the immunoreactive fractions of 211At-labeled 81C6 mAb were 94 and 79%, respectively, which compare favorably with results previously reported for this labeled mAb [5]. A comprehensive analysis of the application of this labeling strategy for the production of multiple clinical dose levels of 211At-labeled 81C6 mAb is described in a subsequent publication [26].
Table 1.
SAB and protein coupling yields for labeling human IgG (hIgG) by reaction with SAB prepared from NCS stabilized 211At/methanol.
| SAB synthesis reaction | Coupling reaction (hIgG) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| 211At/methanol MBq/μL | Gy/s | Storage time | Gy | SAB (%) | MBq obtained | Coupling (%) | MBq obtained† | |
|
|
||||||||
| 1 | 589/540 | 1.48 | 23 h | 50046 | 85 | 63 | 70 | 41 |
| 2 | 618/680 | 1.23 | 23.1 h | 41768 | 80 | 69 | 72 | 47 |
| 3 | 800/550 | 1.97 | 22 min | 2600 | 84 | 694 | 73 | 477 |
activity obtained after PD10 column purification
4. Conclusions
Radiolysis mediated effects can hinder clinical application of 211At for targeted radiotherapy in two ways. First they can compromise chemical reactivity when a significant radiation dose is received before labeling chemistry can be performed, as would occur when 211At is shipped from its production site to a remote location. And second, electrophilic syntheses of compounds such as SAB at the levels required for clinical trials is difficult. Herein, we describe a simple strategy for circumventing these problems by adding NCS (or possibly other oxidants) to the methanol used to isolate the 211At from the PEEK tubing distillation trap, i.e. before any labeling reaction is attempted. With the standard SAB procedure in which the oxidant is added during the reaction, SAB yields rapidly decline with increasing dose, falling to ~20% at about 5,000 Gy. In contrast, with this 211At stabilization procedure, SAB yields of greater than 80% were obtained with 211At solutions that had been stored for more than three half-lives (23 h) and had receivied radiation doses greater than 100,000 Gy. This suggests that addition of NCS (or similar oxidant) to 211At/methanol before shipment should be considered as a strategy for increasing 211At chemical tractability after arrival. The potential utility of this 211At stabilization strategy for high activity level labeling in the context of 211At-labeled radiopharmaceutical production for clinical application will be addressed in a separate publication [26].
Acknowledgments
The authors thank Dr. Ganesan Vaidyanathan for providing the tin precursor used in this study.
This work was supported in part by National Institute of Health Grants CA42324 and CA184228.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mulford DA, Scheinberg DA, Jurcic JG. The promise of targeted α-particle therapy. J Nucl Med. 2005;46(suppl):199S–204S. [PubMed] [Google Scholar]
- 2.Zalutsky MR. Radionuclide therapy. In: Roesch F, editor. Handbook of Nuclear Chemistry: Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences. Vol. 4. Kluwer Academic; Dordrecht, Netherlands: 2011. pp. 2179–2212. [Google Scholar]
- 3.Vaidyanathan G, Zalutsky MR. Applications of 211At and 223Ra in targeted alpha-particle therapy. Current Radiopharm. 2011;4:283–294. doi: 10.2174/1874471011104040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilbur DS. Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Current Radiopharm. 2011;4:214–247. doi: 10.2174/1874471011104030214. [DOI] [PubMed] [Google Scholar]
- 5.Zalutsky MR, Zhao X-G, Alston KL, Bigner DD. High-level production of α-particle-emitting 211At and preparation of 211At-labeled antibodies for clinical use. J Nucl Med. 2001;42:1508–1515. [PubMed] [Google Scholar]
- 6.Zalutsky MR, Reardon DA, Akabani G, Coleman RE, Friedman AH, Freidman HS, et al. Clinical experience with α-emitting astatine-211: treatment of recurrent brain tumor patients with 211At-labeled chimeric 81C6 anti-tenascin monoclonal antibody. J Nucl Med. 2008;49:30–38. doi: 10.2967/jnumed.107.046938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 1: effects of solvent on the degradation of radiohalogenation precursors by 211At α-particles. J Nucl Med. 2005;46:700–706. [PubMed] [Google Scholar]
- 8.Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 2: radiolytic effects of 211At α-particles influence N-succinimidyl 3-[211At]astatobenzoate synthesis. J Nucl Med. 2005;46:1393–1400. [PubMed] [Google Scholar]
- 9.Pozzi OR, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 3: α-particle induced radiolytic effects on the chemical behavior of 211At. J Nucl Med. 2007;48:1190–1196. doi: 10.2967/jnumed.106.038505. [DOI] [PubMed] [Google Scholar]
- 10.Garg PK, Archer GE, Jr, Bigner DD, Zalutsky MR. Synthesis of radioiodinated N-succinimidyl iodobenzoate: optimization for use in antibody labelling. Int J Rad Appl Instrum [A] 1989;40:485–490. doi: 10.1016/0883-2889(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 11.Larsen RH, Wieland BW, Zalutsky MR. Evaluation of an internal cyclotron target for the production of astatine-211 via the 209Bi(α,2n)211At reaction. Appl Radiat Isot. 1996;47:135–143. doi: 10.1016/0969-8043(95)00285-5. [DOI] [PubMed] [Google Scholar]
- 12.Weber DA, Eckerman KF, Dillman LT, Ryman JC. MIRD: Radionuclide Data and Decay Schemes. New York, NY: Society of Nuclear Medicine; 1989. pp. 406–415. [Google Scholar]
- 13.Friedman AM. Radioastatine: possible uses of a heavy halogen. In: Spencer RP, editor. Therapy in Nuclear Medicine. New York, NY: Grune & Stratton; 1978. pp. 139–144. [Google Scholar]
- 14.Andersson H, Cederkrantz E, Bäck T, Divgi C, Elgqvist J, Himmelman J, et al. Intraperitoneal alpha-particle radioimmunotherapy of ovarian cancer patients: pharmacokinetics and dosimetry of 211At-MX35 F(ab')2--a phase I study. J Nucl Med. 2009;50:1153–60. doi: 10.2967/jnumed.109.062604. [DOI] [PubMed] [Google Scholar]
- 15.De Veaux RD, Velleman PF, Bock DE. Stats Data and Models. 3. Addison-Wesley; 2012. [Google Scholar]
- 16.Baxendale JH, Wardman P. The Radiolysis of methanol: product yields, rate constants, and spectroscopic parameters of intermediates. Washington, DC: National Bureau of Standards; 1975. pp. 1–26. [Google Scholar]
- 17.Zalutsky MR, Pozzi OR. Stabilized composition and methods for radiolabeling pharmaceuticals with alpha-emitters. 8,337,810. United States Patent Number. 2012 Dec 25;
- 18.Champion J, Sabatié-Gogova A, Bassal F, Ayed Tahra, Alliot C, Galland N, et al. Investigation of astatine(III) hydrolyzed species: Experiments and relativistic calculations. J Phys Chem A. 2013;117:1983–1990. doi: 10.1021/jp3099413. [DOI] [PubMed] [Google Scholar]
- 19.Sergentu D-C, David G, Montavon G, Maurice R, Galland N. Scrutinizing “invisible” astatine: a challenge for modern density functionals. J Computational Chem. 2016;37:1345–1354. doi: 10.1002/jcc.24326. [DOI] [PubMed] [Google Scholar]
- 20.Ayed T, Pilmé J, Tézé D, Bassal F, Barbet J, Chérel M, et al. 211At-labeled agents for alpha-immunotherapy: on the in vivo stability of astatine-agent bonds. Eur J Med Chem. 2016;116:156–164. doi: 10.1016/j.ejmech.2016.03.082. [DOI] [PubMed] [Google Scholar]
- 21.Champion J, Alliot C, Renault E, Mokili BM, Chérel M, Galland N, et al. Astatine standard redox potentials and speciation in acidic medium. J Phys Chem A. 2010;114:576–582. doi: 10.1021/jp9077008. [DOI] [PubMed] [Google Scholar]
- 22.Fischer S, Dreyer R, Albrecht S. Pseudohalogen compounds of astatine: Synthesis and characterization of At/I/-tricyanomethanide and At/I/-azide-compounds. J Radioanal Nucl Chem. 1987;117:275–283. [Google Scholar]
- 23.Khan A. Theoretical studies of the complexes of iodine with methanol, ethanol and acetone. J Chem Phys. 1992;96:1194–1198. [Google Scholar]
- 24.Spinks JWT, Woods RJ. Introduction to Radiation Chemistry. 3. John Wiley & Sons Inc; New York: 1990. p. 419. [Google Scholar]
- 25.Ludwig R, Fischer S. Zur Komplexbildung van At(I) mit halogenidionen in alkoholischen Lösungen. Isopenpraxis. 1990;26:27–29. [Google Scholar]
- 26.Vaidyanathan G, Pozzi OR, Choi J, Zhao X-G, Murphy S, Zalutsky MR. Radiopharmaceutical chemistry of targeted radiotherapeutics, part 5: Labeling monoclonal antibody at high activity levels with the α-particle emitter 211At. (to be submitted to Nucl Med Biol) [Google Scholar]