Abstract
Background
While age-associated changes in LV diastolic function are well-recognized, limited data exist characterizing measures of diastolic function in older adults, including both reference ranges reflecting the older adult population and prognostically relevant values for incident HF, as well as their associations with circulating biomarkers of heart failure (HF) risk.
Methods
Among 5,801 elderly participants in the Atherosclerosis Risk in Communities (ARIC) study (age range 67–90, mean age 76 ± 5, 42% male, 21% black), we determined the continuous association of diastolic measures (TDI e’, E/e’, and left atrial size) with concomitant NT-proBNP and subsequent HF hospitalization or death. We also determined sex-specific 10th and 90th percentile limits for these measures using quantile regression in 401 participants free of prevalent cardiovascular disease and risk factors.
Results
Each measure of diastolic function was robustly associated with NT-proBNP and incident HF or death. ARIC-based reference limits for TDI e’ (4.6 and 5.2 cm/sec for septal and lateral TDI e’, respectively) were substantially lower than guideline cutpoints (7 and 10 cm/sec, respectively), while E/e’ and LA size demonstrated good agreement with guideline cutpoints. TDI e’ was non-linearly associated with incident HF or death, with inflection points for risk supportive of ARIC-based limits. ARIC-based limits for diastolic function improved risk discrimination over guideline-based cutpoints based on the IDI (p<0.001) and continuous NRI (p<0.001), reclassifying 42% of the study population as having normal diastolic function. We replicate these findings in the Copenhagen City Heart Study. Using these limits, 46% had normal diastolic function and were at low risk of HF hospitalization or death (1%/year over a mean 1.7 year follow-up), 49% had 1 or 2 abnormal measures and were at intermediate risk (2.4%/year), and all 3 diastolic measures were abnormal in 5% who were at high risk (7.5%/year).
Conclusions
Our findings suggest that LV longitudinal relaxation velocity declines as a part of healthy aging and is largely prognostically benign. The use of age-based normative values when considering an elderly population improves the risk discrimination of diastolic measures for incident HF or death.
Keywords: echocardiography, cardiac function, epidemiology, aging
Introduction
The prevalence of heart failure (HF) increases exponentially with age, disproportionately burdening the elderly who are projected to account for 20% of the U.S. population by 2030.1,2,3 HF with preserved left ventricular ejection fraction (LVEF; HFpEF), which comprises half of HF cases overall,4,5 accounts for up to 80% of prevalent HF in the elderly.6 Left ventricular (LV) diastolic dysfunction, occurring in parallel with age and accelerated by common CV risk factors, is the primary pathophysiologic abnormality underlying HFpEF although abnormalities of LV systolic deformation have also been implicated.7 Echocardiographic measures of diastolic dysfunction detected in asymptomatic persons are significant, and robust, predictors of incident HF and mortality.8,9,10 Age-related changes in cardiac structure and function are well recognized, and have made the clinical determination of abnormal LV diastolic function particularly challenging in the elderly. These age-associated changes include smaller LV size, greater ejection fraction (LVEF), lower tissue Doppler relaxation velocities (TDI e’), and greater left atrial (LA) size, and – while related to age-associated increases in CV risk factors – are also observed in older adults free of CV risk factors.11,12,13,14,15,16,17,18 Surprisingly few data exist regarding normative values of echocardiographic measures of LV diastolic function in older adults, their association with circulating biomarkers of HF risk, and their prognostic relevance for incident HF in older adults. Indeed, existing estimates are based predominantly on data from younger populations and current guideline recommendations specifically cite the need for more data in the elderly.19,20
We studied community dwelling adults, aged 65 to 90 years old, participating in the Atherosclerosis Risk in Communities (ARIC) study who underwent comprehensive echocardiography at the fifth study visit. We determined normative values for diastolic measures in older adults based on their distribution in a subgroup of participants who were free of prevalent CV disease or major CV risk factors, including hypertension, diabetes, obesity, chronic kidney disease, and active smoking. We then assessed the association of these measures with circulating NT-proBNP levels and with incident HF or mortality in the overall ARIC sample, and validate our findings in independent samples.
Methods
Study Population
ARIC is a prospective epidemiologic cohort study whose design and methods have been previously described.21 Between 1987 and 1989, 15,792 middle-aged subjects were enrolled in 4 communities in the United States: Forsyth County, NC, Jackson, MS, suburban Minneapolis, MN, and Washington County, MD. Between 2011 and 2013, 6,538 participants returned for a fifth study visit that included standardized comprehensive echocardiography in addition to anthropometrics, interviewer-administered questionnaires, and laboratory testing. The study protocol was approved by institutional review boards at each field center, and all participants provided written informed consent. This analysis included 5,801 participants with echocardiographic data at Visit 5 who were free of prevalent HF.
To define a subset of low risk participants free of prevalent cardiovascular disease or risk factors (referred to as the ‘low risk reference subgroup’), we excluded participants with: (1) prevalent CV disease including coronary heart disease (CHD; includes myocardial infarction history or regional wall motion abnormality on echocardiography), prior HF hospitalization or self-report, atrial fibrillation, moderate or greater valvular disease, (2) hypertension, (3) diabetes, (4) Visit 5 body mass index (BMI) of >30 or <18.5 kg/m2, (5) chronic kidney disease defined as an eGFR <60 ml/min/1.73 m2 at Visit 5, (6) QRS duration ≥120 msec at Visit 5, or (7) active smoking.
Prevalent hypertension and diabetes were based on blood pressure and glucose measured from study Visits 1 through 5, self-report, and medication use. ARIC participants undergo surveillance for incident coronary heart disease (CHD) events (including definite or probable MI, or coronary revascularization) as previously described in detail.22 Prevalent HF was based on hospitalization ICD codes prior to 200523 with additional physician adjudication since 2005 as previously described.24 Atrial fibrillation was ascertained based on ECGs at 5 study visits and hospital discharge records as previously described.25
For incident HF and death post-Visit 5, incident HF was based on HF hospitalization or HF death according to ICD codes (code 410 in any position) obtained by ARIC surveillance of hospital discharges.23 Deaths were ascertained by ARIC surveillance or the National Death Index.22
Echocardiography
Design and methods of echocardiography in ARIC Visit 5 have been previously described in detail.26 Briefly, studies were acquired in participants attending Visit 5 at all 4 Field Centers by certified study sonographers using uniform imaging equipment and following a standardized image acquisition protocol. Quantitative measures were performed by blinded analysts at a dedicated core laboratory based on the recommendations of the American Society of Echocardiography.19,20 Reproducibility metrics were excellent, as previously reported.26
Statistical Methods
Echocardiographic measures of LV diastolic function were described in the low risk reference subgroup overall and stratified by sex. As empiric estimates of distribution limits can vary substantially in small to moderate sized samples, we used quantile regression (STATA qreg) to define 10th, 50th, and 90th percentile limits with associated 95% confidence limits in the low risk reference subgroup overall and stratified by sex. Self-reported race was not significantly associated with reference limits for any echocardiographic measure in quantile regression equations adjusting for sex and race. The resulting 10th and 90th percentile limits were considered reference limits for these measures in the overall ARIC sample.
Among ARIC participants attending Visit 5 and free of prevalent HF, atrial fibrillation at the time of echocardiography, or moderate or greater valvular disease (analysis set n=5,356), the cross-sectional continuous association of TDI e’, E/e’, LA width, and LAVi with log-transformed NT-proBNP levels was assessed using restricted cubic splines. The number of knots that minimized model AIC was selected (3 to 6 knots assessed). Four knots were used for TDI e’, and three knots were used for E/e’ ratio, LA width, and LAVi. In the overall ARIC sample free of prevalent HF (n=5,801), the continuous association between incidence rates of HF hospitalization or all-cause mortality and TDI e’, E/e’, LA width, and LAVi were assessed by Poisson regression models. The association of abnormal values, based on either ARIC reference limits or guideline-based cutpoints, with incident HF hospitalization or all-cause mortality was assessed by multivariable Cox proportional hazard models adjusting for age, sex, and race. For cubic splines of incident rates associated with LV functional measures, three knots resulted in the lowest model AIC (3 to 6 knots were assessed).
ARIC-based reference limits were appreciably different from guideline recommendations for TDI e’. We therefore assessed the association of abnormal values of septal and lateral TDI e’ based on either ARIC reference limits or guideline-based cutpoints with incidence of HF hospitalization or death in patients ≥65 years of age in two independent samples with prevalent cardiovascular disease: the echocardiographic sub-studies of the ENGAGE AF-TIMI 48 trial of patients with atrial fibrillation,27 and the TOPCAT trial of patients with HF with preserved ejection fraction.28 Cox proportional hazard models were adjusted for age, sex, and race in analysis of the TOPCAT echo study, and were adjusted for age and sex in the analysis of the ENGAGE AF-TIMI 48 echo study. As an additional confirmatory analysis, we attempted to identify thresholds of TDI e’ to distinguish normal versus abnormal levels in ARIC and in these two independent samples on the basis the ability of a given cut-point to identify patients at increased risk of incident HF hospitalization or death (“optimal cutpoint” analysis). Using a method similar to a one-step CART analysis,29 a sequence of candidate thresholds was considered: TDI e’septal 1 cm/sec increments between 3 and 10 cm/sec; TDI e’lateral 1 cm/sec increments between 3 and 11 cm/sec. The threshold associated with the most significant hazard ratio was selected. All event rates and hazard ratios using ARIC-based limits presented in the Tables and Figures are based on the 10th percentile distribution limits from the low risk subgroup of participants free of cardiovascular disease or risk factors.
To assess the potential clinical impact of employing reference values for diastolic measures derived from ARIC, we compared the prevalence of abnormal diastolic measures using these thresholds compared to guideline-based thresholds,20 and their associated event rates. We then determined the reclassification characteristics of these ARIC-based thresholds and their incremental value relative to guideline-based thresholds using the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) at 2 years using time-to-event data.30 We then sought to replicate these findings in an independent, elderly, population-based study. Reclassification characteristics and incremental value metrics of classification based on ARIC thresholds relative to guideline cutpoints were therefore evaluated in participants ≥65 years of age in the echocardiographic substudy of the 4th examination of Copenhagen City Heart Study (2001–2003; n=563).31,32,33 As TDI e’ was assessed by color TDI in the Copenhagen City Heart Study but by spectral pulsed TDI in ARIC, we converted TDI e’ values to those equivalent to spectral pulsed TDI using a validated equation.34 Continuous NRI and IDI at 8.6 years was calculated using time-to-event data.
A two-sided P value of <0.05 was considered statistically significant. All analyses were performed with STATA 13 (College Station, TX) and R version 3.2.0.
Results
Clinical characteristics of the study population are shown in Table 1. Of the 5,801 participants included in this analysis, 401 (7%) were included in the low risk reference subgroup. The mean age of the low risk reference subgroup was 74.7 ± 4.6, 34% were male, and 7% were black. Compared to the larger population of participants in the risk factor sub-group, the low risk reference subgroup was younger, more likely female, less likely black, and had lower median levels of high sensitivity C-reactive protein, NT-proBNP, and high sensitivity troponin-T. By design, the prevalence of CV risk factors was lower, as were blood pressure and heart rate, while eGFR was higher.
Table 1.
Clinical characteristics of participants in the low risk reference sub-group and the larger ARIC sample with CV risk factors but no prevalent HF.
| Low Risk Reference Subgroup (n=401) |
HF Risk Factors but No Prevalent HF (n=5,400) |
P value | |
|---|---|---|---|
| Age (years) | 74.7 ± 4.6 | 76.0 ± 5.1 | <0.001 |
| Male | 136 (34 %) | 2277 (42 %) | 0.001 |
| Black | 28 (7 %) | 1216 (23 %) | <0.001 |
| Field Center | <0.001 | ||
| Forsyth County, NC | 116 (28.9%) | 1240 (23.0%) | |
| Jackson, MS | 25 (6.2%) | 1104 (20.4%) | |
| Minneapolis, MN | 161 (40.1%) | 1588 (29.4%) | |
| Washington County, MD | 99 (24.7%) | 1468 (27.2%) | |
| Hypertension | 0 | 4795 (89 %) | |
| Diabetes | 0 | 2120 (39 %) | - |
| Obesity | 0 | 1948 (36 %) | - |
| Chronic Kidney Disease | 0 | 1553 (29 %) | - |
| Atrial Fibrillation | 0 | 414 (8 %) | - |
| Current Smoking | 0 | 336 (6 %) | - |
| Any Prior Smoking | 217 (54 %) | 3319 (61 %) | 0.004 |
| BMI (kg/m2) | 24.9 ± 2.7 | 28.8 ± 5.6 | <0.001 |
| Systolic blood pressure (mmHg) | 121 ± 11 | 131 ± 18 | <0.001 |
| Diastolic blood pressure (mmHg) | 64 ± 8 | 67 ± 11 | <0.001 |
| Heart rate (bpm) | 60 ± 8 | 63 ± 11 | <0.001 |
| LV Ejection Fraction (%) | 66.3 ± 4.8 | 65.4 ± 6.4 | 0.007 |
| NT-proBNP (ng/ml) | 90 [54, 153] | 131 [68, 256] | <0.001 |
| hs-Troponin T (ng/ml) | 7 [5, 10] | 11 [7, 16] | <0.001 |
| hs-C reactive protein | 1.3 [0.7, 2.5] | 2.0 [1.0, 4.2] | <0.001 |
| eGFR | 77.5 ± 9.9 | 69.6 ± 17.2 | <0.001 |
The lower reference limits for TDI e’septal and e’lateral, based on our low risk reference sub-group, were 4.6 and 5.2 cm/s respectively (Table 2). Limits for TDI e’septal velocity tended to be similar between the sexes, while limits for TDI e’lateral tended to be lower in women compared to men (5.1 and 5.4 cm/sec, respectively). Reference limits for E/e’septal and E/e’lateral were 14.4 and 12.7 respectively, and tended to be higher in women compared to men. Reference limits for LA width were 3.7 and 4.0 cm in women and men respectively, while the upper reference limit for LAVi was 30 ml/m2 and was similar between sexes. Among the broader ARIC study sample free of prevalent HF, TDI e’ (septal or lateral) was abnormally low in 29%, E/e’ ratio was abnormally high in 31%, and LAVi was abnormally enlarged in 23% using these reference limits. Only modest differences in the prevalence abnormal measures were noted by sex and race (Supplemental Tables 1 and 2).
Table 2.
Percentile limits for the distribution of measures of LV diastolic function among the low risk reference sub-group (n=401).
| 10%-ile (95% CI) | 50%-ile (95% CI) | 90%-ile (95% CI) | |
|---|---|---|---|
| TDI e’septal(cm/sec) | |||
| Overall | 4.6 (4.5 – 4.7) | 6.0 (5.8 – 6.2) | 8.1 (7.8 – 8.4) |
| Female | 4.5 (4.3 – 4.7) | 5.9 (5.7 – 6.1) | 8.4 (8.0 – 8.8) |
| Male | 4.6 (4.4 – 4.8) | 6.1 (5.8 – 6.4) | 7.8 (7.3 – 8.3) |
| TDI e’lateral(cm/sec) | |||
| Overall | 5.2 (5.0 – 5.4) | 7.3 (7.1 – 7.5) | 9.9 (9.5 – 10.3) |
| Female | 5.1 (4.8 – 5.4) | 7.2 (6.9 – 7.5) | 9.9 (9.4 – 10.4) |
| Male | 5.4 (5.0 – 5.8) | 7.7 (7.3 – 8.1) | 9.9 (9.2 – 10.6) |
| E/e’septal | |||
| Overall | 7.2 (6.9 – 7.5) | 10.5 (10.1 – 10.9) | 14.4 (13.6 – 15.2) |
| Female | 7.3 (6.9 – 7.7) | 11.0 (10.6 – 11.4) | 15.1 (14.2 – 16.0) |
| Male | 7.2 (6.6 – 7.8) | 9.7 (9.1 – 10.3) | 13.3 (12.0 – 14.6) |
| E/e’lateral | |||
| Overall | 6.1 (5.8 – 6.4) | 8.7 (8.3 – 9.1) | 12.7 (12.0 – 13.4) |
| Female | 6.3 (5.9 – 6.7) | 9.1 (8.7 – 9.5) | 13.3 (12.5 – 14.1) |
| Male | 5.4 (4.9 – 5.9) | 8.0 (7.4 – 8.6) | 11.5 (10.4 – 12.6) |
| LA A-P dimension (cm) | |||
| Overall | 2.78 (2.70 – 2.86) | 3.25 (3.19 – 3.31) | 3.83 (3.76 – 3.90) |
| Female | 2.72 (2.65 – 2.79) | 3.18 (3.12 – 3.24) | 3.69 (3.61 – 3.77) |
| Male | 2.99 (2.89 – 3.09) | 3.42 (3.33 – 3.51) | 3.96 (3.85 – 4.07) |
| LAVi (ml/m2) | |||
| Overall | 15.2 (14.4 – 16.0) | 22.0 (21.4 – 22.6) | 30.2 (29.2 – 31.3) |
| Female | 15.0 (13.9 – 16.1) | 21.5 (20.7 – 22.3) | 29.9 (28.2 – 31.5) |
| Male | 16.5 (14.9 – 18.1) | 23.7 (22.6 – 24.8) | 31.1 (28.8 – 33.4) |
10th-, 50th-, and 90th-percentile values with associated 95% confidence intervals are derived from quantile regression models in the low risk reference sub-group, overall and separately by sex. Race was not significantly associated with percentile limits independently of sex.
All diastolic measures were robustly associated with NT-proBNP levels in cross-sectional analysis (all p<0.0001; Figure 1) and with incident HF hospitalization or death (all p≤0.003; Figure 2). Although they explained a small proportion of population-level variability of NT-proBNP (Supplemental Figure 1), these measures were highly significantly associated with NT-proBNP levels, with approximately two-fold differences in geometric mean NT-proBNP values for patients with lower versus higher measures of diastolic dysfunction (Figure 1). Both septal and lateral TDI e’ demonstrated non-linear associations with NT-proBNP (Figure 1, panels A and B) and with incident HF hospitalization and death (Figure 2, panels A and B), with a steeper risk in incidence rates noted for e’septal and e’lateral values below approximately 5.4 and 6.4 cm/sec respectively. While E/e’septal and E/e’lateral were statistically non-linearly associated with NT-proBNP (Figure 1, panels C and D), both demonstrated linear association with outcomes, such that higher values were associated with higher incidence rates without evidence of a threshold (Figure 2, panels C and D). Larger LA size was monotonically associated with higher NT-proBNP levels and higher risk of HF hospitalization or death, although this association with outcomes was linear for LA width (Figure 2, panel E) and non-linear for LAVi (Figure 2, panel F). Sex did not significantly modify the association of diastolic measures with incident HF or death (Supplemental Table 1), while the risk associated with abnormal TDI e’ and E/e’ tended to be higher in black compared to white participants (Supplemental Table 2).
Figure 1.
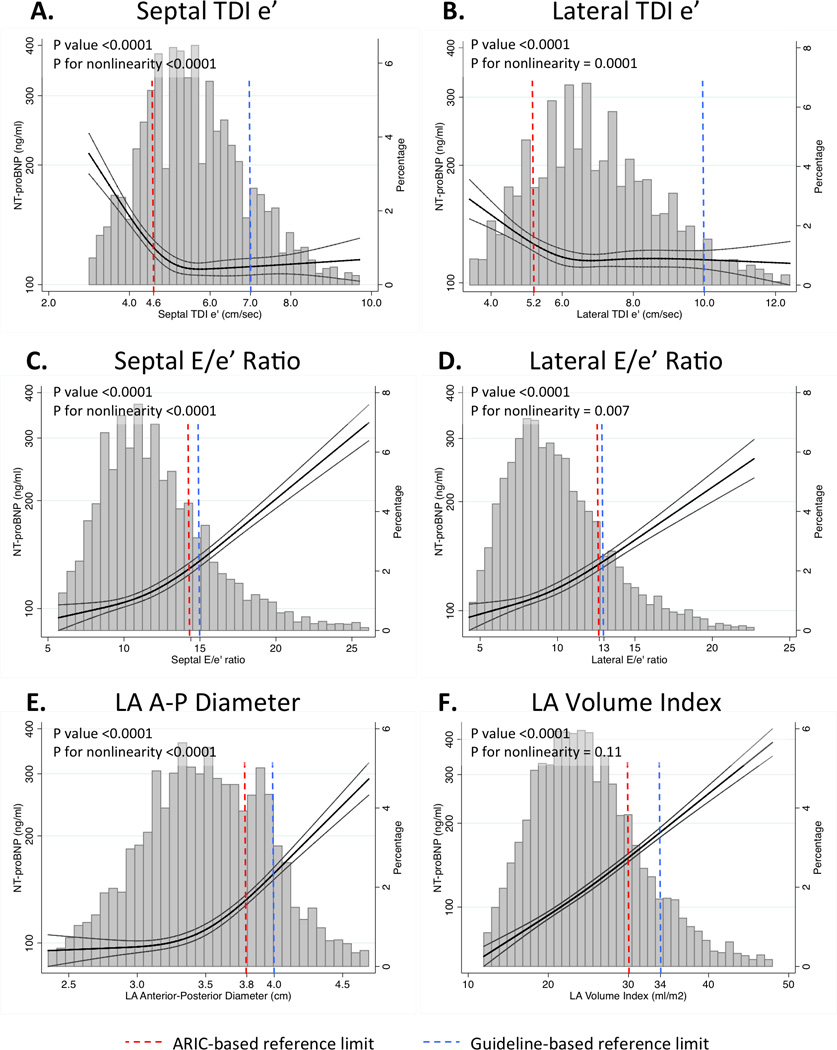
Restricted cubic splines demonstrating the continuous relationship between LV diastolic measures (TDI e’septal [panel A], TDI e’lateral [panel B], E/e’septal ratio [panel C], E/e’lateral ratio [panel D], LA A-P dimension [panel E], and LAVi [panel F]) and NT-proBNP levels assessed concomitantly at Visit 5 among ARIC participants without prevalent heart failure at Visit 5. Y-axis shows geometric mean values of NT-proBNP.
Figure 2.
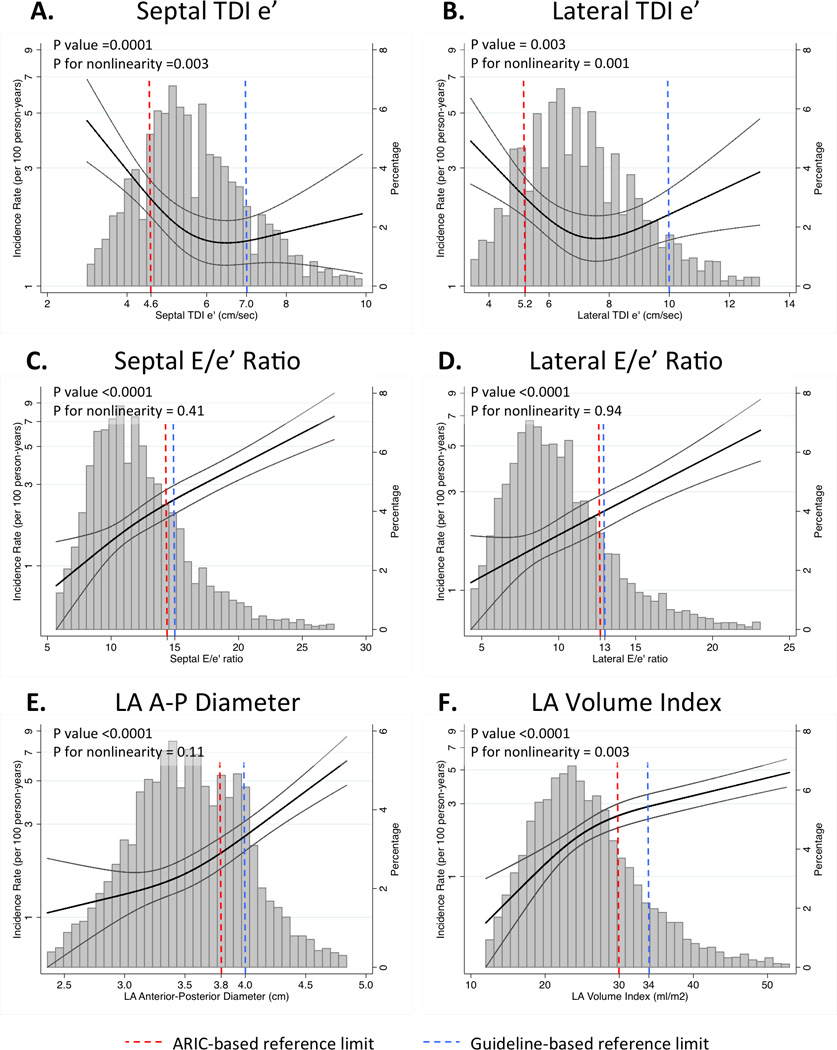
Restricted cubic splines demonstrating the continuous relationship between LV diastolic measures (TDI e’septal [panel A], TDI e’latera [panel B], E/e’septal ratio [panel C], E/e’lateral ratio [panel D], LA A-P dimension [panel E], and LAVi [panel F]) and incident HF hospitalization or death post-Visit 5.
One hundred forty-seven events occurred over a median follow-up of 610 days (25th to 75th percentile range 469–762 days). Abnormal E/e’, LA dimension, and LAVi were significantly associated with incident death or HF hospitalization when classified using either ARIC-based reference limits or guideline cutpoints (Table 3). In contrast, abnormal TDI e’septal and e’lateral were only significantly associated with incident death or HF when defined using ARIC-based reference limits, and were not associated with these outcomes when defined using guideline-based cutpoints. Similarly, in two independent samples, one with HFpEF and the other with atrial fibrillation, abnormal TDI e’septal was only significantly associated with incident HF hospitalization or death when defined using ARIC-based reference limits, and not when defined using guideline-based cutpoints (Table 4). TDI e’lateral was not significantly associated with the composite endpoint in these other studies. Given the large difference in ARIC-based reference limits for TDI e’ compared to guideline recommendations (Figure 3, panel A), we explored the optimal cutpoint values for these measures for predicting death or HF hospitalization (‘optimal cutpoint’ analysis in Table 4). The cutpoints for septal and lateral TDI e’ more closely approximated the ARIC-based limits than the guideline cutpoints.
Table 3.
Association of abnormal measures of LV diastolic function with death or incident HF hospitalization, using ARIC-base reference values versus guideline-based cutpoints.
| ARIC-based | Guideline-based | |||||||
|---|---|---|---|---|---|---|---|---|
| Cutoff | Percent Abnormal |
HR (95% CI) | P value |
Cutoff | Percent Abnormal |
HR (95% CI) | P value |
|
| TDI e’septal (cm/sec) | <4.5 (F), <4.6 (M) | 21% | 1.66 (1.20–2.29) | 0.002 | <7 | 82% | 1.11 (0.74–1.68) | 0.61 |
| TDI e’lateral (cm/sec) | <5.1 (F, <5.4 (M) | 18% | 1.64 (1.18–2.28) | 0.004 | <10 | 91% | 0.80 (0.49–1.30) | 0.37 |
| E/e’septal ratio | >15.1 (F), >13.3 (M) | 25% | 2.09 (1.55–2.83) | <0.001 | >15 | 20% | 1.94 (1.41–2.67) | <0.001 |
| E/e’lateral ratio | >13.3 (F), >11.5 (M) | 20% | 2.09 (1.53–2.85) | <0.001 | >13 | 17% | 2.17 (1.57–3.00) | <0.001 |
| LAD (cm) | >3.7 (F), >4.0 (M) | 28% | 2.37 (1.76–3.19) | <0.001 | >4.0 | 16% | 2.50 (1.80–3.49) | <0.001 |
| LAVi (ml/m2) | > 30 (F), >31 (M) | 23% | 2.31 (1.70–3.13) | <0.001 | >34 | 14% | 2.89 (2.09–3.98) | <0.001 |
All Cox proportional hazard models adjusted for age, sex, and race.
Table 4.
Risk of death or HF hospitalization associated with abnormal septal and lateral TDI e’ based on ARIC-based versus guideline cutpoints, and results of analysis to identify optimal predictive cutpoints, in persons ≥65 years old in the general population (ARIC), with atrial fibrillation and cardiovascular risk factors but no HF (ENGAGE-TIMI 48), and with HFpEF (TOPCAT).
| ARIC | ENGAGE AF-TIMI 48 Echo Sub-study |
TOPCAT Echo Sub-study | |
|---|---|---|---|
| Septal TDI e’ (cm/sec) | <5.0 | <4.0 | <4.0 |
| N | 5,784 | 588 | 348 |
| Events | 177 | 93 | 120 |
| Risk using ARIC limits [<4.5 (F), <4.6 (M)] |
1.66 (1.20–2.29), p=0.002 | 1.74 (1.06–2.86), p=0.028 | 2.03 (1.38–2.99), p<0.001 |
| Risk using Guideline limits [<7] |
1.11 (0.74–1.68), p=0.61 | 1.05 (0.69–1.61), p=0.82 | 1.42 (0.93–2.18), p=0.10 |
| ‘Optimal cutpoint’ analysis | <5.0 | <4.0 | <4.0 |
| Lateral TDI e’ (cm/sec) | |||
| N | 5,774 | 590 | 344 |
| Events | 177 | 94 | 114 |
| Risk using ARIC limits [<5.1 (F, <5.4 (M)] |
1.64 (1.18–2.28), p=0.004 | 1.36 (0.75–2.45), p=0.31 | 1.16 (0.71–1.88), p=0.56 |
| Risk using Guideline limits [<10] |
0.80 (0.49–1.30), p=0.61 | 1.01 (0.65–1.57), p=0.95 | 0.80 (0.52–1.23), p=0.30 |
| ‘Optimal cutpoint’ analysis | <5.0 | <5.0 | <4.0 |
Outcomes: ARIC – incident HF or all cause mortality; ENGAGE-TIMI 48 – incident HF or all cause mortality; TOPCAT – HF hospitalization or all cause mortality. Risk presented as HR (95% CI) adjusted for age, sex, and race in ARIC and TOPCAT, and for age and sex in ENGAGE AF-TIMI 48.
Figure 3.
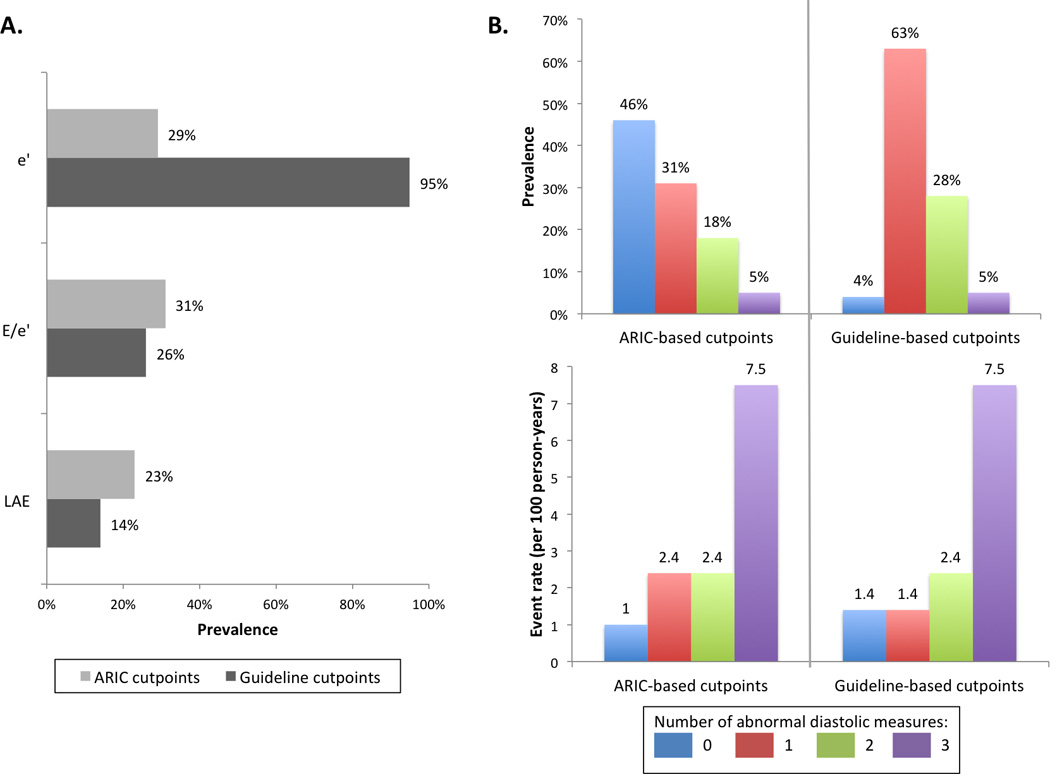
Classification of diastolic measures in the overall ARIC study sample free of prevalent HF using ARIC-based reference limits compared to guideline-based cutpoints. Panel A: Prevalence of abnormal diastolic measures (TDI e’, E/e’, LAVi) by ARIC-based reference limits and guideline-based cutpoints in the overall study sample. Panel B: Prevalence of participant categories based on number of abnormal diastolic measures (TDI e’, E/e’, LAVi) and associated rates of incident HF hospitalization or death using either ARIC-based reference limits or guideline-based cutpoints.
Applying reference limits derived from the 10th percentile limits in our low risk reference sub-group to the larger study sample free of prevalent HF, 46% had normal diastolic measures, 31% had 1 abnormal measure, 18% had 2 abnormal measures, and 5% had 3 abnormal measures (Figure 3, panel B). Participants with normal diastolic measures demonstrated the lowest event rates, those with 3 abnormal measures demonstrated the highest event rates, while those with 1 or 2 abnormal measures demonstrated intermediate event rates (Figure 3, panel B). In contrast, guideline-based cutpoints classified the majority of participants as having 1 abnormality, and only 4% as having normal diastolic function. When compared to classification based on guideline-recommended cutpoints, ARIC-based reference limits reclassified 2,419 participants (42.4% of the total study sample) from having one abnormality to having normal diastolic function (Table 5). These participants reclassified to normal diastolic function experienced incident HF hospitalization or death at a rate similar to those classified as normal by both classifications (1.0 [95% CI 0.7–1.3] versus 0.9 [0.3–2.8] events per 100 person-years respectively, p=0.84), and significantly lower than those who were not reclassified (2.2 [1.6–3.1] events per 100 person-years, p=0.001). Compared to guideline cutpoints, classification employing ARIC reference limits improved the continuous NRI (17.0% [95% CI 9.3–24.3%], p<0.001) and IDI (0.3% [95% CI 0.1 – 0.9%], p<0.001), but did not statistically improve the c-statistic (0.639 versus 0.657 respectively, p=0.22).
Table 5.
Reclassification across diastolic dysfunction score using ARIC-based reference limits from diastolic dysfunction score categories based on guideline recommended cutpoints in the overall ARIC sample.
| Diastolic score based on ARIC-based limits | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | ||
| Diastolic score based on Guideline limits | 0 | 207 (88.9%) 0.9 (0.3–2.8) |
26 (11.1%) 5.7 (1.4–23.0) |
233 1.4 (0.6–3.3) |
||
| 1 | 2419 (67.5%) 1.0 (0.7–1.4) |
992 (27.7%) 2.2 (1.6–3.1) |
164 (4.6%) 2.0 (0.8–4.8) |
8 (0.2%) 7.8 (1.1–55.7) |
3583 1.4 (1.1–1.7) |
|
| 2 | 8 (0.5%) 0 |
727 (45.9%) 2.5 (1.8–3.6) |
735 (46.4%) 2.3 (1.6–3.4) |
114 (7.2%) 2.9 (1.2–7.0) |
1584 2.4 (1.9–3.2) |
|
| 3 | 1 (0.3%) 0 |
138 (45.5%) 3.5 (1.7–7.4) |
164 (54.2%) 11.2 (7.5–16.8) |
303 7.5 (0.5–10.6) |
||
| Total | 2634 1.0 (0.7–1.3) |
1746 2.4 (1.9–3.0) |
1037 2.4 (1.8–3.3) |
286 7.5 (5.3–10.8) |
5,703 | |
Values are presented as absolute numbers and percent reclassified. Event rates per 100 person-years for death or incident HF hospitalization are presented in italics.
Reclassification metrics of ARIC-based versus guideline cutpoints were assessed in an independent population-based study sample of 563 participants ≥65 years of age in the Copenhagen City Heart Study,31,32,33 This population has a mean age of 74.2±6.2 years, 65% were women, all were white, and experienced 274 incident HF hospitalizations or death over a mean follow-up of 8.6±3.3 years. Compared to classification based on guideline cutpoints, ARIC-based reference limits reclassified 289 (51% of the total study population) from having one abnormal diastolic measure to normal diastolic function (Table 6). Participants reclassified to normal diastolic function demonstrated event rates similar to those with normal diastolic function by both classification criteria (5.1 [95% CI 4.3–6.1] versus 5.0 [3.5–7.1] events per 100 person-years respectively, p=0.89), and a significantly lower event rate than those not reclassified (8.5 [6.3–11.5] events per 100 person-years, p=0.002). Furthermore, in the Copenhagen City Heart Study sample, classification employing ARIC reference limits improved the continuous NRI (11.0% [95% CI 2.2–19.8%], p=0.016) and IDI (0.7% [95% CI 0.0 – 2.5%], p=0.032) compared to classification based on guideline cutpoints. Overall annualized event rates in the Copenhagen City Heart Study were higher than in ARIC due to the significantly longer follow-up period (1.7 years in ARIC versus 8.6 years in the Copenhagen City Heart Study) and significant age-related increase in mortality in this age range (mean age 75 years at baseline in both studies).
Table 6.
Reclassification across diastolic dysfunction score using ARIC-based reference limits from diastolic dysfunction score categories based on guideline recommended cutpoints in participants ≥65 years of age in the Copenhagen City Heart Study.
| Diastolic score based on ARIC-based limits | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total | ||
| Diastolic score based on Guideline limits | 0 | 71 (100.0%) 5.0 (3.5–7.1) |
71 5.0 (3.5–7.1) |
|||
| 1 | 289 (81.6%) 5.1 (4.3–6.1) |
60 (17.0%) 8.5 (6.3–11.5) |
5 (1.4%) 6.8 (2.2–21.2) |
354 5.7 (4.9–6.6) |
||
| 2 | 53 (40.8%) 4.2 (2.7–6.4) |
71 (54.6%) 7.1 (5.2–9.6) |
6 (4.6%) 3.4 (0.8–13.7) |
130 5.6 (4.4–7.2) |
||
| 3 | 4 (50.0%) 11.9 (4.5–31.7) |
4 (50.0%) 18.2 (6.8–48.4) |
8 14.4 (7.2–28.7) |
|||
| Total | 360 5.1 (4.4–5.9) |
113 6.3 (4.9–8.1) |
80 7.3 (5.5–9.7) |
10 7.5 (3.4–16.6) |
563 | |
Values are presented as absolute numbers and percent reclassified. Event rates per 100 person-years for death or incident HF hospitalization are presented in italics.
Discussion
This analysis is one of the largest, to our knowledge, to provide normative data on routinely clinically reported measures of LV diastolic function among older adults, to apply these reference limits to a large, bi-racial cohort of elderly community-dwelling persons, and to relate these measures to prognostically relevant biomarkers and clinical outcomes. Each measure of LV diastolic function was robustly associated with circulating NT-proBNP levels and incident HF hospitalization or death. Reference limits for E/e’ and LA size in the elderly are generally in agreement with existing guideline recommendations, while limits for TDI e’ are substantially lower than existing guideline recommendations. TDI e’ demonstrated a non-linear association with NT-proBNP and incident HF hospitalization or death, with inflection points for risk supportive of ARIC-based reference limits. When compared to existing guideline-based cutpoints, ARIC-based reference limits for diastolic function improved risk discrimination and reclassified over one-third of the study population as having normal diastolic function. These participants demonstrated a low risk of HF hospitalization or death.
Age-related decline in LV longitudinal relaxation velocities, reflective of early diastolic relaxation, are well recognized based on data from cross-sectional studies (Supplemental Table 3). Indeed, while ARIC-based reference limits for E/e’ and LAVi were similar to guideline cutpoints, the reference limits for TDI septal and lateral e’ from the ARIC low risk reference group (4.6 and 5.2 cm/sec, respectively) were appreciably lower than guideline recommendations (7 and 10 cm/sec, respectively).20 These ARIC-based reference limits classified TDI e’ as abnormal in 28% of the overall study sample free of prevalent HF, compared to 95% by guideline-based cutpoints. ARIC-based limits for TDI e’ were similar to values from healthy populations of similar age (Supplemental Table 3),35,36,37 including persons >60 years of age in the NORRE study38 and 65–75 years of age in the LOLIPOP study.39 Compared to younger cohorts, these limits derived from our low risk reference subgroup of participants suggest that decline in LV longitudinal relaxation velocities (TDI e’) occurs as a part of healthy aging. Future longitudinal studies with serial echocardiograms over time will be necessary to define the factors influencing decline in longitudinal relaxation velocities with age. Importantly, three of our findings also suggest that much of these age-related changes in TDI e’ are prognostically benign, as opposed to reflecting malignant cardiac senescence. First, our low risk reference subgroup of 401 participants - from whom these limits were derived - demonstrated a very low incidence of HF hospitalization or death during the follow-up period (incidence rate 0.4 [95% CI 0.1–1.3] per 100 person-years). Second, among the full sample of 5,801 ARIC participants free of prevalent HF, the ARIC-based reference limits for TDI e’ identified values below which lower e’ was associated with a higher incidence of death or HF hospitalization. In contrast, abnormal TDI e’ was not robustly associated with incident events at values defined by guideline-based cutpoints. Similar findings were noted when comparing ARIC-based reference limits to guideline cutpoints for abnormal TDI e’ in elderly patients with HFpEF in the TOPCAT echocardiographic substudy and elderly patients with atrial fibrillation in the ENGAGE-TIMI-48 echocardiographic substudy. Finally, these thresholds for risk were not unique to ARIC, with similar thresholds identified in elderly patients in the TOPCAT and ENGAGE-TIMI-48 echocardiographic substudies. Taken together, we believe these findings argue for the use of age-based normative values in interpreting diastolic measures in the elderly. We propose the use of the sex-specific ARIC-based limits specified in Table 3 in persons ≥65 years of age.
Compared to existing guideline-based cutpoints, the application of age-appropriate reference limits allowed for reclassification of a large proportion of the population from having 1 to 0 abnormal diastolic measures. This large group of participants with normal diastolic measures by ARIC-based limits had a low event rate, demonstrating that age-appropriate reference limits allow for the identification of elderly persons with normal diastolic function who are at low risk for incident HF. Furthermore, we replicated these findings in an independent, elderly, community-based cohort with longitudinal follow-up. These are among the first prognostic data to support the recently revised diastolic function guidelines whereby <2 abnormal measures is considered normal, 2 abnormal measures is indeterminate, and >2 abnormal measures is consistent with diastolic dysfunction.20 Furthermore, the application of age-appropriate limits in the elderly helps reframe and refine this classification schema such that those without any abnormality in TDI e’, E/e’, or LA size are at low risk (1% per year over a mean 1.7 year follow-up), those with >2 abnormal diastolic measures are at high risk (7.5% per year), and those with 1–2 abnormal diastolic measures are at intermediate risk (2.4% per year).
Race was not significantly associated with reference limits for any echocardiographic measure in quantile regression analysis, suggesting that these sex-specific ARIC-based reference limits are appropriate for both black and white older adults. The risk of incident HF or death associated with abnormally low e’ and high E/e’ tended to be greater in blacks compared to whites. These findings may help explain the higher incidence of HF in blacks compared to white, Hispanic, and Asian Americans.40,41
Several limitations of this analysis should be recognized. Given the small number of black participants in the low risk reference sub-group, we were unable to determine reference limits separately by race. However, we found no association of self-reported race independent of sex with reference limits for any echocardiographic measure in quantile regression equations. Measurement error may exist in our assessment of risk factors, leading to misclassification. However, the use of data collected serially over five visits to classify risk factors mitigates this limitation. Alterations in LV diastolic function have been observed in overweight persons (BMI 25–30 kg/m2),42 who were included in our low risk reference reference group and may have influenced our ARIC-based reference limits. However, very similar reference limits were observed when restricting the low risk reference sample to the 206 participants with a BMI of 18–25 kg/m2 (Supplemental Table 4) arguing against an appreciable impact of including overweight participants. Follow-up time after echocardiography at ARIC Visit 5 was relatively short, and may have limited our power to assess the prognostic performance of various reference limits for diastolic measures. Data regarding HF diagnosed and managed exclusively in the outpatient setting (i.e. with no hospitalization event) was not uniformly available and, consistent with prior studies of HF in this cohort,23,43 only incident HF hospitalizations were considered.
Conclusions
In a large bi-racial cohort of community dwelling older adults free of HF, LV diastolic measures are robustly associated with NT-proBNP and with incident HF hospitalization or death. Compared to guideline cutpoints, ARIC-based reference limits were appreciably lower for TDI e’, which was non-linearly association with incident outcomes with greater associations at values below the ARIC-based reference limit. Using these limits, 46% had normal diastolic measures and were at low risk of HF hospitalization or death (1%/year at 1.7 year follow-up), 49% had 1 or 2 abnormal measures and were at intermediate risk (2.4%/year), and all 3 diastolic measures were abnormal in 5% who were at high risk (7.5%/year). These findings suggest that a decline in LV longitudinal relaxation velocity occurs as a part of healthy aging and is largely prognostically benign, and support the use of age-based normative values when considering an elderly population.
Supplementary Material
Clinical Perspective.
What is new?
Age-associated changes in LV diastolic function are well-recognized, but limited data exist regarding normative values in the elderly and their association with incident HF.
We relate diastolic measures (TDI e’, E/e’, and left atrial size) to risk of HF hospitalization or death in 5,801 elderly participants in the Atherosclerosis Risk in Communities study, and defined sex-specific 10th-percentile limits in 401 participants free of cardiovascular disease or risk factors.
Each diastolic measure was robustly associated with incident HF hospitalization or death.
Reference limits for E/e’ and LA size were generally in agreement with existing guidelines, while limits for TDI e’ were substantially lower.
What are the clinical implications?
Compared to guideline cutpoints, ARIC-based limits improved risk discrimination, and reclassified over one-third of the study population as having normal diastolic function.
Using these limits, 46% had normal diastolic measures and were at low risk (1%/year), 49% had 1 or 2 abnormal measures and were at intermediate risk (2.4%/year), and 5% had all 3 diastolic measures abnormal and were at high risk (7.5%/year).
These findings suggest that a decline in LV longitudinal relaxation velocity occurs as a part of healthy aging and is largely prognostically benign, and support the use of age-based normative values when considering an elderly population.
Acknowledgments
The authors wish to thank the staff and participants of the ARIC study for their important contributions. The authors also wish to thank the ENGAGE AF-TIMI 48 investigators, the TOPCAT investigators, and the Copenhagen City Heart Study Investigators. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Funding Source: The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The work for this manuscript was also supported by NHLBI grants K08HL116792 (A.M.S.), R00HL107642 (S.C.), R01HL131532 (S.C.), and AHA grant 14CRP20380422 (A.M.S.).
Dr Shah reports receiving research support from Novartis, Gilead, and Actelion, and consulting fees from Myocardia.
Footnotes
Disclosures:
The other authors report no disclosures.
References
- 1.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D’Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics – 2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States: Population estimates and projections. Curr Popul Rep Popul Estim Proj. 2014:P25–P1140. 1–28. [Google Scholar]
- 4.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu P. Outcome of heart failure with preserved ejection fraction in a population-based study. New Engl J Med. 2006;355:260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. New Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL. Importance of heart failure with preserved systolic function in patients ≥65 years of age. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 7.Shah AM, Claggett B, Folsom AR, Lutsey PL, Ballantyne CM, Heiss G, Solomon SD. Ideal cardiovascular health duing adult life and cardiovasculare structure and function among the elderly. Circulation. 2015;132:1979–1989. doi: 10.1161/CIRCULATIONAHA.115.017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redfield MM, Jacobsen SJ, Burnett JC, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 9.Halley CM, Houghtaling PL, Khalil MK, Thomas JD, Jaber WA. Mortality rate in patients with diastolic dysfunction and normal systolic function. Arch Intern Med. 2011;171:1082–1087. doi: 10.1001/archinternmed.2011.244. [DOI] [PubMed] [Google Scholar]
- 10.Kane GC, Karon BL, Mahoney DW, Redfield MM, Roger VL, Burnett JC, Jacobsen SJ, Rodeheffer RJ. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA. 2011;306:856–863. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitzman DW, Scholz DG, Hagen PT, Ilstrup DM, Edwards WD. Age-related changes in normal human hearts during the first 10 decades of life. Part ii (maturity): A quantitative anatomic study of 765 specimens from subjects 20 to 99 years old. Mayo Clin Proc. 1988;63:137–146. doi: 10.1016/s0025-6196(12)64946-5. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Sheikh KH, Beere PA, Philips JL, Higginbotham MB. Age-related alterations of doppler left ventricular filling indexes in normal subjects are independent of left ventricular mass, heart rate, contractility and loading conditions. J Am Coll Cardiol. 1991;18:1243–1250. doi: 10.1016/0735-1097(91)90542-h. [DOI] [PubMed] [Google Scholar]
- 13.Gardin JM, Arnold AM, Bild DE, Smith VE, Lima JA, Klopfenstein HS, Kitzman DW. Left ventricular diastolic filling in the elderly: The cardiovascular health study. Am J Cardiol. 1998;82:345–351. doi: 10.1016/s0002-9149(98)00339-7. [DOI] [PubMed] [Google Scholar]
- 14.Aurigemma GP, Gottdiener JS, Arnold AM, Chinali M, Hill JC, Kitzman D. Left atrial volume and geometry in healthy aging: The cardiovascular health study. Circ Cardiovasc Imaging. 2009;2:282–289. doi: 10.1161/CIRCIMAGING.108.826602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA. Cardiovascular function in multi-ethnic study of atherosclerosis: Normal values by age, sex, and ethnicity. Am J Roentgenol. 2006;186:S357–S365. doi: 10.2214/AJR.04.1868. [DOI] [PubMed] [Google Scholar]
- 16.Lieb W, Xanthakis V, Sullivan LM, Aragam J, Pencina MJ, Larson MG, Benjamin EJ, Vasan RS. Longitudinal tracking of left ventricular mass over the adult life course: Clinical correlates of short- and long-term change in the framingham offspring study. Circulation. 2009;119:3085–3092. doi: 10.1161/CIRCULATIONAHA.108.824243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: Longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: The Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klain AL, Lancellotti P, Marino P, Oh JK, Popescu A, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 22.White A, Folsom A, Chambless L, Sharret R, Yang K, Conwill D, Higgins M, Dale Williams O, Tyroler HA the ARIC Investigators. ScienceDirect - Journal of Clinical Epidemiology : Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: Methods and initial two years' experience. Journal of clinical Epidemiology. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 23.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am. J. Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 24.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: A comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alonso A, Agarwal S, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and Design of a Multicenter Echocardiographic Study to Assess the Relationship between Cardiac Structure and Function and Heart Failure Risk in a Biracial Cohort of Community Dwelling Elderly Persons: The Atherosclerosis Risk in Communities (ARIC) Study. Circ Cardiovasc Img. 2014;7:173–181. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, Deenadayalu N, Hoffman E, Patel I, Shi M, Mercuri M, Mitrovic V, Braunwald E, Solomon SD. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–1465. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function and Prognosis in Heart Failure With Preserved Ejection Fraction: Findings From the Echocardiographic Study of the Treatment Of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. doi: 10.1161/CIRCHEARTFAILURE.114.001583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Breiman L, Friedman J, Stone CJ, Olshen RA. Classification and regression trees. 1st. Boca Raton, FL: Chapman & Hall/CRC; 1984. [Google Scholar]
- 30.Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med. 2013;32:2430–2442. doi: 10.1002/sim.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Biering-Sørensen T, Mogelvang R, Pedersen S, Schnohr P, Sogaard P, Jensen JS. Usefulness of the myocardial performance index determined by tissue Doppler imaging m-mode for predicting mortality in the general population. Am J Cardiol. 2011;107:478–483. doi: 10.1016/j.amjcard.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 32.Biering-Sørensen T, Mogelvang R, Schnohr P, Jensen JS. Cardiac Time Intervals Measured by Tissue Doppler Imaging M-mode: Association With Hypertension, Left Ventricular Geometry, and Future Ischemic Cardiovascular Diseases. J Am Heart Assoc. 2016;5:e002687. doi: 10.1161/JAHA.115.002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mogelvang R, Biering-Sørensen T, Jensen JS. Tissue Doppler echocardiography predicts acute myocardial infarction, heart failure, and cardiovascular death in the general population. Eur Heart J Cardiovasc Imaging. 2015;16:1331–1337. doi: 10.1093/ehjci/jev180. [DOI] [PubMed] [Google Scholar]
- 34.Hummel YM, Klip IJT, Jong RMde, Pieper PG, Veldhuisen DJ van, Voors AA. Diastolic function measurements and diagnostic consequences: a comparison of pulsed wave- and color-coded tissue Doppler imaging. Clin Res Cardiol. 2010;99:453–458. doi: 10.1007/s00392-010-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuznetsova T, Herbots L, Lopez B, Jin Y, Richart T, Thijs L, Gonzalez A, Herregods MC, Fagard RH, Diez J, Staessen JA. Prevalence of left ventricular diastolic dysfunction in a general population. Circ Heart Fail. 2009;2:105–112. doi: 10.1161/CIRCHEARTFAILURE.108.822627. [DOI] [PubMed] [Google Scholar]
- 36.De Sutter J, De Backer J, de Veire NV, Velghe A, De Buyzere M, Gillebert TC. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E’) and the ratio of transmitral early peak velocity to E’ (E/E’) Am J Cardiol. 2005;95:1020–1023. doi: 10.1016/j.amjcard.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 37.Dalen H, Thorstensen A, Vatten LJ, Aase SA, Stoylen A. Reference values and distribution of conventional echocardiographic Doppler measures and longitudinal tissue Doppler velocities in a population free from cardiovascular disease. Circ Cardiovasc Imaging. 2010;3:614–622. doi: 10.1161/CIRCIMAGING.109.926022. [DOI] [PubMed] [Google Scholar]
- 38.Caballero L, Kou S, Dulgheru R, Gonjilashvili N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Gomez de Diego JJ, Oliva MJ, Hagendorff A, Hristova K, Lopez T, Magne J, Martinez C, de la Morena G, Popescu BA, Penicka M, Ozyigit T, Rodrigo Carbonero JD, Salustri A, Van De Veire N, Von Bardeleben RS, Vinereanu D, Voigt JU, Zamorano JL, Bernard A, Donal E, Lang RM, Badano LP, Lancellotti P. Echocardiographic reference ranges for normal cardiac Doppler data: results from the NORRE Study. Eur Heart J Cardiovasc Imaging. 2015;16:1031–1041. doi: 10.1093/ehjci/jev083. [DOI] [PubMed] [Google Scholar]
- 39.Chahal NS, Lim TK, Jain P, Chambers JC, Kooner JS, Senior R. Normative reference values for the tissue Doppler imaging parameters of left ventricular function: a population-based study. Eur J Echocardiogr. 2010;11:51–56. doi: 10.1093/ejechocard/jep164. [DOI] [PubMed] [Google Scholar]
- 40.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lina JAC. Difference in the incidence of congestive heart failure by ethnicity: the Multi-Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. doi: 10.1001/archinte.168.19.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yancy CW. Heart failure in African Americans: pathophysiology and treatment. J Card Fail. 2003;9:s210–s215. doi: 10.1054/s1071-9164(03)00590-6. [DOI] [PubMed] [Google Scholar]
- 42.Russo C, Jin Z, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. J Am Coll Cardiol. 2011;57:1368–1374. doi: 10.1016/j.jacc.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


