Abstract
The Hippo signaling pathway, also known as the Salvador–Warts–Hippo pathway, is a regulator of organ size. The pathway takes its name from the Drosophila protein kinase, Hippo (STK4/MST1 and STK3/MST2 in mammals), which, when inactivated, leads to considerable tissue overgrowth. In mammals, MST1 and MST2 negatively regulate the transcriptional co-activators yes-associated protein 1 (YAP) and WW domain containing transcription regulator 1 (WWTR1/TAZ), which together regulate the expression of genes that control proliferation, survival, and differentiation. YAP and TAZ activation have been associated with liver development, regeneration, and tumorigenesis. How their activity is dynamically regulated in these contexts, however, is just beginning to be elucidated. We review the mechanisms of Hippo signaling in the liver and explore outstanding questions for future research.
The ability of the liver to regenerate after injury is widely known and was even noted by the ancient Greeks. As punishment for helping mankind, Prometheus from Greek mythology was chained to a rock and had his liver eaten by an eagle, only for it to regenerate throughout the night and the cycle to be repeated the next day. Indeed, within hours of a partial hepatectomy, the remaining lobes rapidly grow larger through a combination of hepatocyte hypertrophy and replication1, 2. Ninety-five percent of the mass lost from a hepatectomy can be recovered within a month, thus enabling procedures such as split-liver transplantation3, 4. Conversely, transplantation of an oversized liver graft into a recipient leads to a gradual reduction of the graft through apoptosis5–7. How organs such as the liver sense and regulate their size is central to our understanding of development, regeneration, and disease.
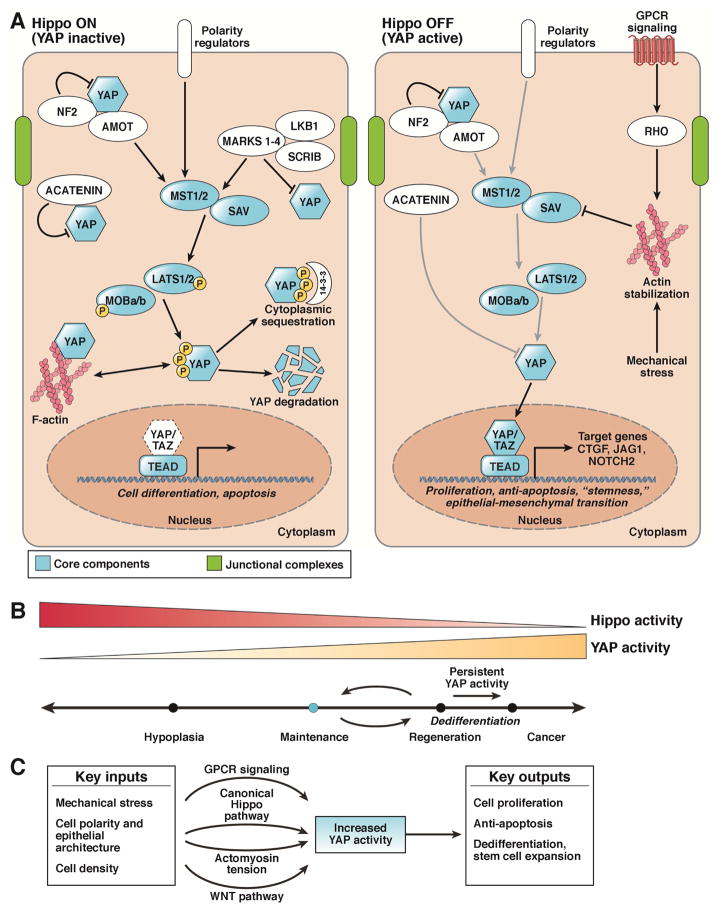
The Hippo signaling pathway has emerged as an important biochemical pathway in this context. Originally described in the fruit fly, Drosophila melanogaster, this pathway is named for the serine/threonine kinase Hippo (STK3/MST2 and STK4/MST1, in mammals), which when lost, results in enlarged organs from excessive proliferation and decreased apoptosis8. MST1 and MST2, in partnership with its scaffolding molecule salvador family WW domain containing protein 1 (SAV1), regulate the activity of the large tumor suppressor kinases 1 and 2 (LATS1 and 2). In turn, LATS1 and 2 and their partners, the MOB kinase activators 1A and B (MOB1A and MOB1B), phosphorylate the co-transcriptional activators, Yes-associated protein 1 (YAP1) or its paralog, WW domain containing transcription regulator 1 (WWTR1/TAZ), at several serine residues (Figure 1A, Hippo ON and YAP inactive).
Figure 1. Regulation of the Mammalian Hippo Signaling Pathway.
A. The Hippo pathway (mammalian) consists of the core components STK3 and STK4, SAV, LATS1 and 2, MOB1A and B, YAP, TAZ, and TEAD. Upon activation of the canonical Hippo pathway, STK3/4 phosphorylates and activates Lats1/2, which subsequently phosphorylates cytoplasmic Yap. During homeostasis, Hippo signaling is ON resulting in Yap phosphorylation (S112 in mice, S127 in humans) causing 14-3-3 binding and cytoplasmic sequestration. Phosphorylation of Yap can also lead to proteasomal degradation. When Hippo is off, YAP (in the unphosphorylated for) translocates to the nucleus and binds to the TEAD family of transcription factors, leading to the transcription of genes involved in cell survival, growth, and proliferation. Proposed cell activities for each state are found beneath the gene in italics. Arrows indicate positive relationships, while bars indicate negative activity.
B. YAP activity at various levels and for various time periods differentially modulates cell state and phenotype.
C. Multiple physiologic and pathologic inputs modulate YAP activity. Mechanical stress, cell polarity, and cell density are all factors that have been shown to modulate Yap activity. Additionally, knowledge of these different states is communicated via various signaling modalities, including the aforementioned canonical Hippo pathway, the Wnt pathway, GPCRs, and changes in cytoskeletal tension.
These phosphorylation sites regulate the position and activity of YAP1 in the cell. The most well-studied serine phosphorylation site is S127 in the human protein (S112 in the mouse protein). When phosphorylated at this amino acid, YAP binds the scaffolding molecule 14-3-3 and is eventually shuttled to the proteasome for degradation9–13. When not phosphorylated at this amino acid, YAP translocates into the nucleus to activate various gene expression programs (Figure 1A, Hippo OFF).
Although slightly smaller, TAZ has similar sites of regulation and activity. YAP and TAZ seem to have redundant roles in the liver, supported by the fact that they bind the same genomic targets14, 15, but whether there are nuances in how they regulate their target genes remains to be explored.
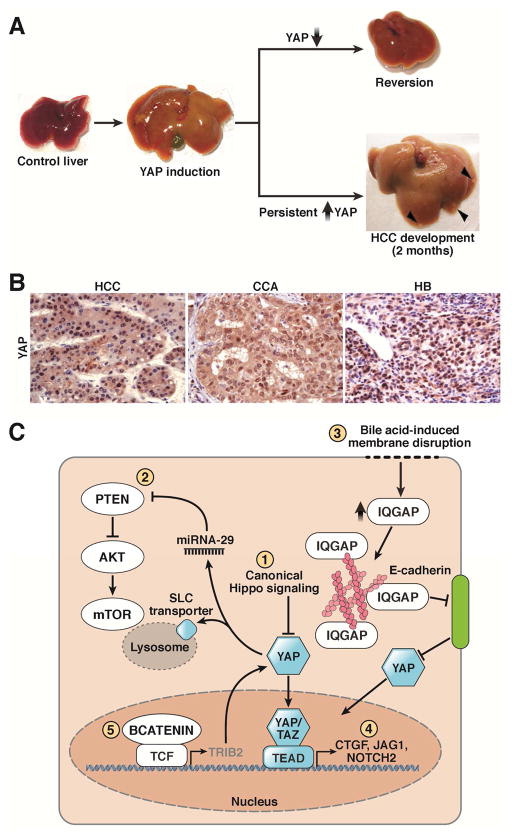
Reports that yorkie, the Drosophila homolog of YAP, promotes proliferation and prevents apoptosis16 spurred researchers to investigate whether manipulation of YAP and other homologues in the mammalian Hippo pathway produced similar results in vertebrates. Simultaneously, the genomic region containing YAP was found to be amplified in breast and liver cancer17, 18, supporting the idea that increased levels of this protein could contribute to excessive growth and oncogenesis. Shortly thereafter, transgenic overexpression of YAP in mice confirmed this hypothesis: these mice developed rapid hepatomegaly and, over time, hepatocellular carcinoma (HCC)19, 20. Notably, YAP-induced hepatomegaly is reversible. Restoring endogenous YAP levels after a period of overexpression leads to a rapid decrease in liver size (Figure 3A) and normalization of the parenchymal architecture, suggesting that the Hippo pathway appears to have an important role in maintaining liver size.
Figure 3. Effects of Yap Overexpression in the Liver.
A. Liver-specific overexpression of YAP leads to massive hepatomegaly with livers approaching 4–5x their original size. Upon restoration of endogenous levels of YAP, the liver returns to its usual size19. Persistent YAP activation for 2 months frequently results in HCC development (Arrowheads).
B. Increased overall YAP and nuclear YAP is a feature of several liver cancers, including HCC, CCA, and HB115.
C. YAP can mediate its tumorigenic effects either autonomously or through synergy with other pathways. YAP can be activated through canonical Hippo inactivation (1) or non-canonical membrane-associated signaling (2). YAP can also interact with the PI3K–Akt–mTOR pathway through a microRNA-mediated mechanism or via upregulation of lysosomal SLC transporters (3). Finally, YAP can interact with the NOTCH and Wnt pathways, as evidenced through upregulation of NOTCH ligands and receptors (4) and YAP’s stabilization by the Wnt target gene TRIB2 (5).
Since then, multiple groups have reported that genetic liver deletion of the upstream Hippo kinases such as MST1 and MST221–23 and LATS1 and LATS224, or their scaffold molecules SAV23, 25, MOB1A, and MOB1B,26, 27 decreases phosphorylation of YAP, causing its translocation to the nucleus, hepatomegaly, and liver cancer (Figure 1A, Hippo OFF). Because overall YAP levels and localization within the cell correlate with the transcriptional output of the Hippo Pathway, it can be thought of as the gatekeeper of the pathway. Often, YAP cellular localization is interpreted to be a proxy for Hippo activity (Hippo ON and YAP inactive or Hippo OFF and YAP active), which may be an oversimplification of YAP’s more complex regulation.
Nuclear localization of YAP or TAZ is associated with the activation of target genes. Genes important for liver growth and regeneration, such as CTGF28, JAG1,29, 30 and NOTCH2,30 are direct targets of Hippo signaling. As transcriptional co-activators, YAP and TAZ do not bind to DNA directly but instead act to recruit machinery to their transcription factor partners. In most cases, the TEAD family of transcription factors that bind YAP and TAZ are required for the phenotypes controlled by Hippo signaling28, 31–34.
The kinases, scaffolding proteins, and transcriptional partners described above form the core of the canonical Hippo pathway with its construction and downstream activity highly conserved throughout evolution (Figure 1A, blue). During homeostasis, Hippo signaling is thought to be active, resulting in high phosphorylation of YAP and low levels of nuclear YAP. The Hippo pathway is therefore considered tumor suppressive, as loss of Hippo signaling results in YAP accumulation, translocation into the nucleus, and activation of genes that promote proliferation and prevent apoptosis (Figure 1B).
Pathway Regulation
The Hippo pathway responds not only to the biochemical but also the physical milieu of the cell35, 36. Plating cells at low-density results in YAP activation and proliferation35, 36. Once these cells reach confluence, YAP is shuttled out of the nucleus and cell proliferation ceases. Furthermore, increasing the tension experienced by confluent epithelial cells can reactivate YAP/TAZ and restart cell proliferation37, 38.
Junctional complexes between cells are important sensors that coordinate tissue integrity and growth. These complexes are ideally positioned to detect changes in tissue tension or density and transduce these signals into various signaling pathways, so it would be logical to identify a role for YAP/TAZ at these sites. Whether similar themes are also observed in the liver following injury remains to be determined: indeed, partial hepatectomy results in a dramatic change in the shear stress experienced by hepatocytes due to increased portal venous pressure39. The ability of YAP and TAZ to sense changes in tissue architecture and rapidly promote expression of genes that promote organ growth requires further exploration into the cytoskeletal elements that may transduce these external cues.
Cytoskeletal regulators
One of the first upstream regulators identified was NF2 (merlin), mutations in which are associated with the spontaneous development of schwannomas and meningiomas in humans40. A member of the FERM family of plasma membrane-associated proteins41, NF2, when deleted, leads to potent YAP activation and liver overgrowth. This overgrowth phenotype is reduced by hemizygous deletion of Yap, indicating that much of the resulting proliferation is due to YAP activity42, 43. Recent work has elucidated how NF2 interacts with downstream Hippo components: in Drosophila and mammals, NF2 complexes with LATS1 and 2 at the plasma membrane—an event that facilitates phosphorylation of LATS1 and 2 by MST1 and MST2 in complex with SAV44. Intriguingly, disruption of the actin cytoskeleton promotes interactions among NF2 and LATS1 and 2,44 indicating the importance of the plasma membrane as a site of Hippo signal transduction.
A small interfering RNA (siRNA) screen has identified human kinases associated with junctional complexes that affect YAP phosphorylation and activity45. Several microtubule affinity-regulating kinases (MARKs) were found to tightly associated with YAP as well as scribble, LGL, DLG and LKB1, all members of the adherens junction (AJ). In prior reports, scribble and LKB1 have been reported to regulate cell growth and proliferation46, 47. Accordingly, disruption of Lkb1 in mice activates YAP, resulting in liver overgrowth48.
α-catenin, another intracellular member of the AJ complex, results in excessive growth and cancer when deleted in the skin49, 50. α-catenin directly binds to YAP, with its loss resulting in YAP nuclear localization and cellular proliferation35. In the liver, α-catenin helps organize the liver parenchyma. When α-catenin is reduced through siRNA knockdown, livers are significantly larger in size after injury than controls; moreover, liver sinusoids and bile canaliculi are disorganized, resulting in increased serum bile51. In keratinocytes, α-catenin physically interacts with NF252, but studies are needed to determine whether a similar regulatory complex exists in the liver.
Angiomotin (AMOT) is yet another protein found at the AJ that strongly binds YAP and TAZ53–55. AMOT also binds NF2, regulating its tumor suppressive functions56. Because NF2 loss leads to liver overgrowth, it was proposed that AMOT loss would lead to a similar phenotype. In contrast, livers of AMOT-knockout mice are indistinguishable from controls and have reduced regenerative responses after injury, indicating that AMOT instead facilitates YAP mediated gene activation57.
It is not clear how these membrane complexes interact and integrate various cues into functional transcriptional changes. Liver epithelial cells have apical, basolateral, and tight junction complexes, all of which contain YAP and TAZ, and to varying degrees, associated regulatory proteins. At the subcellular level, how do these various plasma membrane compartments transmit extracellular signals through YAP and TAZ—particularly since they each serve slightly different roles? The example of AMOT facilitating downstream YAP activation in certain contexts despite its ability to also bind NF2 illustrates the complexity of YAP and TAZ regulation at the plasma membrane.
Studies have provided evidence for the differential regulation of Yki by AJ and basolateral complexes. AJ loss is predominantly associated with non-cell autonomous accumulation but a cell-autonomous decrease of Yki, whereas basolateral loss results in cell autonomous accumulation of Yki58. That cytoskeletal components can dynamically alter the cell state of a neighboring cell could help explain, in part, how tissues coordinate maintenance or regenerative programs.
Notably, these interactions are not unidirectional: YAP, through its close association with actin filaments, can also regulate cytoskeletal proteins. Recent work has shown that YAP overexpression in hepatocytes promotes the formation of a contractile actin structure that destabilizes E-cadherin-mediated cell-cell junctions59. In a similar vein, TAZ knockdown in vitro reduces cellular invasion and results in upreglation of E-cadherin60. This evolving crosstalk between YAP/TAZ and the cellular cytoskeleton has important implications for cellular organization and tissue homeostasis61.
Receptor-mediated signaling
Bile duct ligation often leads to rapid biliary proliferation, suggesting that one of bile’s components directly stimulates cell proliferation62, 63. Mouse knockout models of the nuclear receptor farnesoid X receptor (FXR) and the small hetero-dimer partner (SHP) result in a marked accumulation of hepatic bile acids without biliary obstruction. FXR- or SHP-knockout mice have enlarged livers and develop liver tumors through a YAP-dependent process that requires the scaffolding molecule IQ motif containing GTPase activating protein 1 (IQGAP1)64. Notably, IQGAP1 is greatly upregulated in these mice following the disruption of the plasma membrane through chronic bile acid exposure.
While their role in activating YAP within the liver is not well understood, G protein-coupled receptors (GPCRs) have been recently identified as important modulators of the Hippo pathway in other tissues65, 66. Small molecule screening has identified several serum-bound ligands that can modulate Hippo signaling, including epinephrine, estrogen, lyosphosphatidic acid, sphingosine 1-phosphate65, 66, thrombin,67 Wnt3a, Wnt5a, and Wnt5b68. Moreover, mutant GPCR signaling in uveal melanoma is one example in which normal Hippo signaling has been coopted63,64, but studies are needed to determine whether HCC can develop via similar mechanisms.
Development, Homeostasis and Regeneration
YAP and TAZ have critical roles during development. YAP-knockout mice die at embryonic day 8.5 and present with defects in yolk sac vasculogenesis, chorioallantoic attachment, and body axis elongation69; TAZ-knockout mice develop renal cysts and emphysema70, 71.
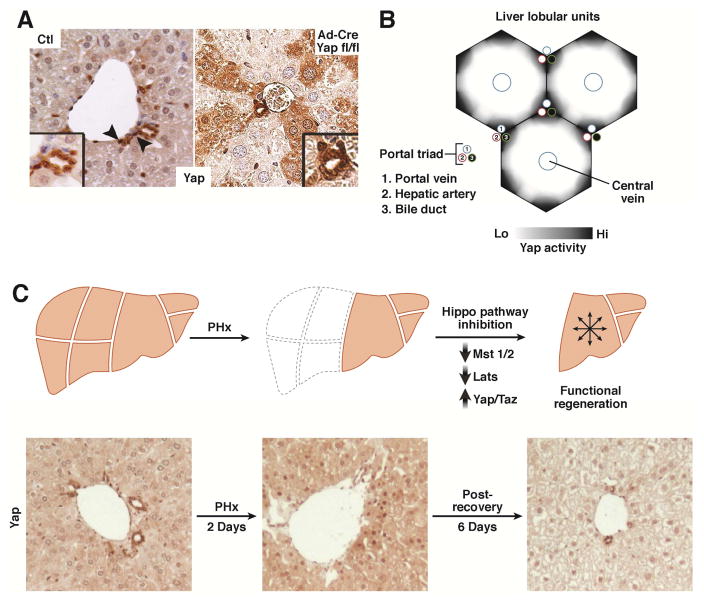
YAP is found throughout the adult liver, although biliary cells demonstrate the highest levels of YAP protein and activity (Figure 2A)30, 72, 73. The remainder of the liver parenchyma has a graded distribution of YAP, highest in the portal area and lowest in the central venous region (Figure 2B)74. The biliary ducts are most profoundly affected by liver-specific YAP deletion: these mice are born with hypoplastic biliary ducts that are progressively lost over time42. Gradually, hepatitis and fibrosis develop, likely due to cholestatic liver injury from the immature biliary system and hepatocyte hypersensitivity to injury42.
Figure 2. Yap Expression During Homeostasis and Regeneration.
A. YAP is present in the epithelial cells of mouse liver (hepatocytes and biliary cells). YAP expression and nuclear-localization is more prominent in biliary cells (arrowhead) as compared to hepatocytes. Ad-Cre Yap fl/fl illustrates that YAP is present in hepatocytes as documented by mosaic Yap staining after deletion30.
B. Schematic of YAP activity in the liver. YAP activity is highest in the biliary cells/portal hepatocytes, diminishing in the hepatocytes toward the central vein.
C. Hippo/Yap activity dynamically changes after partial hepatectomy. Yap levels increase with an associated decrease in MST1, MST2, LATS1 and LATS2 activity. These return to their normal levels as the liver reaches its appropriate size. Partial hepatectomy in mice results in YAP enrichment and an increase in nuclear localization (Day 2). After 8 days of recovery, YAP expression is reduced to below baseline levels.
Studies of the role of the Hippo pathway components during early liver development are limited. The Albumin-Cre mouse line that is commonly used to inactivate components of the Hippo pathway has moderate but limited activity in embryonic hepatoblasts/hepatocytes, which makes interpretation for a role of this pathway during development difficult. Using this model, inactivation of LATS1 and 2 during development leads to a prominent expansion of the ductal plate (the precursor of mature bile ducts) at E17.5 and an increase in immature biliary epithelial cells at the expense of mature hepatocytes at P1. Additionally, LATS1 and 2-knockout hepatoblasts are more likely to differentiate into biliary-like cells in vitro75, a result consistent with our observation that YAP overexpression in adult hepatocytes causes transdifferentiation into biliary epithelium30.
In the adult liver, YAP deletion in hepatocytes is inconsequential during homeostasis (Figure 2A, Ad-cre Yap fl/fl) as there appears to be compensation through increased expression of TAZ76. Adult YAP liver knockouts do not acutely develop biliary duct loss or hepatocyte necrosis. However, mice with hepatocyte-specific deletion of YAP have extensive hepatic necrosis and high mortality after bile duct ligation, indicating that the compensatory increase in TAZ is insufficient to promote normal regeneration30, 77.
After partial hepatectomy, an injury associated with a rapid and widespread proliferative response, overall YAP protein levels increase, YAP phosphorylation decreases, and Hippo target genes are upregulated78–80. YAP becomes localized to the hepatocyte nucleus immediately after injury, with overall YAP levels eventually decreasing several days later (Figure 2C). The coordination of hepatocyte proliferation after partial hepatectomy may be partially due to hedgehog pathway ligands derived from hepatic stellate cells (HepSC). YAP was recently reported to be a downstream target of the hedgehog pathway. By blocking hedgehog signaling in HepSCs, hepatocyte proliferation is blunted and nuclear YAP accumulation in neighboring hepatocytes does not occur81, evidence of the critical contribution of stromal cells in promoting regeneration.
HepSCs often have high levels of nuclear YAP, indicating that their activity is particularly sensitive to the Hippo pathway. Moreover, YAP is important in promoting their activation into myofibroblasts, which secrete factors that help coordinate the regenerative response following liver injury82. Protracted YAP signaling may lock these HepSCs into a myofibroblastic fate, resulting in defective repair mechanisms that contribute to liver cirrhosis. A small molecule inhibitor of YAP82, as well as omega-3 polyunsaturated acids (which accelerate YAP and TAZ degradation83), reduce development of fibrosis in mice, so agents that target this pathway may slow the development of cirrhosis.
Emerging Roles in Liver Zonation
Homeostasis and regeneration
Nuclear localization of YAP is mostly restricted to the periportal zone of the liver, with biliary cells demonstrating the highest levels23. Conversely, the pericentral zone has little to no nuclear YAP. Interestingly, this Hippo signaling gradient appears in opposition to that of WNT signaling, which is highest in the central venous area and diminishes towards the portal area84. The presence of these opposing gradients, which help define specific liver zones, raises the question as to how these 2 pathways interact in regulating liver development, homeostasis, and regeneration.
Liver growth can be driven through activation of both pathways independently. Injection of R-spondin 1 (RSPO1), which potentiates WNT signaling, causes increased proliferation and increased overall liver size80; YAP overexpression in hepatocytes likewise leads to hepatomegaly. However, whether hepatocytes from different zones effect these changes remains to be determined. With regards to YAP, we observed that YAP overexpression specifically activates periportal hepatocytes30. During homeostasis, all 3 zones (periportal, pericentral, and midlobular) have equivalent proliferation rates, measured by EDU incorporation85, indicating that zonal dominance is not a particular feature of liver homeostasis.
In the context of regeneration, the type and duration of injury seem to dictate which cells are recruited for proliferation85–87. Partial hepatectomy, an acute injury that affects the liver indiscriminately, leads to the approximately equal expansion of all 3 zones. Regeneration post-PH seems to progress in multiple waves, with periportal hepatocytes proliferating first (possibly due to a shorter G1 phase)88, followed by midlobular and pericentral hepatocytes. Chronic CCl4 administration, conversely, which predominantly damages the central venous zone and results in fibrosis, leads to the activation and expansion of periportal hybrid hepatocytes (hybrid because they also express biliary markers in addition to hepatocyte markers). These hepatocytes reconstituted approximately 67% of the new hepatocytes generated in response to chronic CCl4 injury73. Fundamentally, hepatocytes in all 3 zones have the capacity to replicate in response to injury; when this capacity in blocked in one zone, hepatocytes from other zones can seemingly compensate.
Within these contexts, it is not known how Hippo and WNT interact. The glutamine synthase zone (a marker of WNT activity that serves to define the pericentral zone) increases following YAP knockout and decreases following knockout of MST1 and MST274. Additionally, the hepatocytes with increased YAP activity (in MST1 and MST2-knockout mice) have a decreased nuclear localization of β-catenin74, so the 2 pathways may inhibit each other. Further studies are required to resolve how these pathways interact during homeostasis and regeneration.
Metabolism
In addition to variable proliferative capacity, hepatocytes have different metabolic functions depending on their position on the pericentral–periportal axis: pericentral hepatocytes are largely involved in glycolysis, bile synthesis, and glutaminogenesis, whereas periportal hepatocytes function mainly in gluconeogenesis and ammonia clearance. It is not clear whether the opposing Hippo and WNT signaling gradients also help define a particular metabolic state.
A study of a YAP-overexpressing zebrafish model found, surprisingly, that YAP can directly increase expression of glutamine synthase, independent of the WNT pathway. The resultant increase in glutamine increases nucleotide biosynthesis, expanding the available nucleotide pool that fuels YAP-mediated liver growth89. In mice, however, liver-specific deletion of MST1 or MST2 reduces the glutamine synthase zone74, suggesting that upstream Hippo kinases might regulate other substrates that help to determine regulation of gene expression by YAP. Nevertheless, it is clear that YAP can modify the expression of genes that regulate metabolism within a cell. Determining the mechanisms of this process will require a more clear definition of the specific contexts in which gene expression is controlled.
Modifications of other components of the Hippo pathway can also affect liver metabolism. Mice that have a liver-specific disruption of Lats2 have increased expression of SREBP target genes, resulting in increased cholesterol synthesis and the development of steatosis90. This phenotype occurs independently of YAP, indicating that the Hippo pathway can regulate metabolic homeostasis through multiple effectors.
Mechanisms that Moderate YAP/TAZ Activity
Persistent high-level expression of YAP leads to the development of liver cancer within 1–2 months (Fig 3A), whereas mutations in factors upstream of Hippo often require a much longer timescale (9–12 months) to develop. Additional YAP inhibitory and cell protective mechanisms are likely responsible for this disparity, with loss of such feedback contributing to cancer development.
One potential mechanism is inhibiting the binding of YAP and TAZ to their transcriptional partner TEAD, either through peptidomimetics or small molecular inhibitors91, 92. Vestigial-like4 (VGLL4) is an important competitive inhibitor of TEAD activation, acting to partially mask the YAP–TEAD binding site and interfering with its ability to activate downstream target genes93, 94. Peptides that mimic VGLL4 and interfere with YAP–TEAD interaction slow the growth of YAP-dependent gastric tumors93.
MicroRNAs offer a mechanism to downregulate RNA expression. Depending on the Hippo pathway component that is targeted, this can up- or down-regulate YAP/TAZ activity. miR-9-3p has been reported to specifically target TAZ in hepatoma cell lines95, an activity which will need to be validated in vivo. Several other microRNAs to be considered with respect to the liver include LATS2 targeting miR-135b96 and miR-3197, and miR-375 which regulates YAP198. These microRNAs are reported to have activity in other tissues.
Gene expression signatures associated with high YAP activity often include altered expression of its upstream regulators37, 45: as YAP and TAZ activity increases, expression of proteins that reduce their activity also increase, generating a negative-feedback loop. One such target is the protein LATS2, a kinase that inactivates YAP and TAZ76. Knockout of YAP from livers of mice reduces expression of LATS2, resulting in reduced inactivation of TAZ. This would help explain the compensatory increase in levels of TAZ in YAP-knockout mice.
YAP has multiple potential regulatory sites, although serine 127 has garnered the most focus, because of its role in facilitating nuclear YAP localization. Mice that express transgenic YapS112A (mouse homolog of S127) do not have liver overgrowth and have livers with a normal appearance. Although Yap is predominantly localized to the nucleus, the total amount is decreased. These mice are particularly prone to injury—exposure to diethylnitrosoamine results in hepatomegaly and increased development of liver tumors. This demonstrates that additional mechanisms exist that maintain the total transcriptional output of the Hippo pathway. Further work has identified serine 366 as a phosphodegron site that can be exploited by the cell to downregulate total levels of YAP24.
Additionally, SIRTUIN1 (SIRT1), a protein associated with longevity and tumor suppressive activity is associated with YAP levels in the liver. Using knockout and overexpressing mice as well as complementary cell lines, the expression of YAP directly correlates well with SIRT1 expression. Whether YAP is a direct enzymatic target of SIRT1 or a secondary downstream target has yet to be resolved99.
These mechanisms highlight the extent to which the Hippo pathway has developed multiple means to temporally limit YAP activity. Understanding and measuring their overall contribution to Hippo signaling will be useful for designing targeted cancer therapies.
Liver Cancer Development
YAP is overexpressed in a number of solid tumors, including those of the colon100, breast17, lung101, 102, and ovary103, 104, as well as in medulloblastoma105, cholangiocarcinoma, hepatoblastoma, and HCC (Figure 3B)21, 72 indicating that it promotes cancer progression. In HCC, the core Hippo constituents are typically not mutated; instead, the increase in YAP levels is largely due to gene amplification and post-transcriptional regulation18.
Increased YAP activity is an early event in liver cancer development92. In mice, overexpression of YAP alone results in HCCs20. Hepatocytes that express high levels of YAP dedifferentiate and acquire features of hepatic progenitor cells30, which could allow them to rapidly accumulate oncogenic mutations through increased proliferation. Knockouts of Hippo pathway components such as MST1, MST221, SAV23, 25, LATS24, or NF242, 43 lead to similar phenotypes in mice (See Table 1), but there is little evidence that they contribute to HCC in humans21. Consequently, analyses of downstream factors in the Hippo pathway could provide more information about the role of YAP in HCC development and progression.
In this vein, a gene expression signature associated with loss of Hippo signaling has been identified that predicts reduced survival time of patients with HCC. This silence of Hippo signaling (SOH) signature was developed and validated in 4 independent international cohorts of patients with HCC. The SOH gene expression signature can be used to aid pathology staging criteria106. Notably, the SOH tumor signature overlaps partially with a previously reported hepatic stem cell gene signature107 associated with an aggressive clinical course. There is evidence that increased YAP can lead to dedifferentiation and progenitor cell expansion.
Testing for components of the Hippo pathway by immunohistochemistry or mRNA expression is likely to be the most accessible means of delivering prognostic information to patients with HCC. The presence of nuclear YAP staining was strongly associated with a shorter disease-free survival time in a European cohort108. Studies from smaller Asian cohorts demonstrated that high levels of YAP, TAZ and TAZ mRNA were each independently associated with a lower overall rate of survival60, 109.
YAP seems to be involved in several pathways that control liver development, regeneration, and disease. One of its signaling partners is NOTCH, which is required for biliary expansion and specification during liver development110–112. During tumorigenesis, YAP increases transcription of jagged1 and notch2 which promote hepatocyte dedifferentiation and cancer growth29, 30.
HCC and HB also commonly have active WNT signaling113, 114. Mice with hepatocytes that express activated forms of YAP and β-catenin (but not either protein alone) rapidly develop hepatoblastoma115. Moreover, WNT signaling can stabilize YAP to potentiate its downstream effects, through upregulation of TRIB2, which downregulates a ubiquitin ligase that normally targets YAP for degradation116. Additionally, in certain subtypes of HCC, loss of Hippo signaling correlates with mTOR pathway activation117, 118, which is thought to proceed through activation of the PI3K pathway via a YAP-induced microRNA that reduces expression of PTEN119. YAP has also been shown to activate the mTOR pathway through the upregulation of amino acid transporters, SLC38A1 and SLC7A5118. This mechanism, in part, sustains YAP1-mediated proliferation of HCC cells. Interestingly, reduction of SLC38A1 (which encodes a glutamine transporter) reduces proliferation of HCC cells. Studies of this dependency on glutamine and the potential role of YAP in effecting this metabolic change (also supported by89) could help identify new targets for HCC therapy.
YAP activity is also associated with human cholangiocarcinoma and reduction in its activity in vitro limits cholangiocarcinoma growth120. About 12% of cholangiocarcinomas are associated with focal deletions at the SAV1 locus, providing a mechanism by which YAP can become activated121. Mice that express transgenic YAP and activated PI3K, or its downstream effector AKT, in liver develop cholangiocarcinomas. Transcriptional profiles of these cholangiocarcinomas are similar to those of patient tumor tissues122. Patients with cholangiocarcinoma have high mortality and few therapeutic options, so studies of this mouse model are important.
Targeting the Hippo pathway may be an effective strategy for treating HCC. Liposome-mediated administration of a YAP siRNA to HCC causes cells to redifferentiate into hepatocytes, supporting the observation that high expression of YAP increases the stemness of cells74. As these experiments were performed in cells with mutations in the Hippo pathway, further work is needed to determine whether hepatocyte redifferentiation is effective in HCC cells with other driver mutations (in WNT, PI3K, p53, etc.), although there is evidence that the Hippo pathway interacts with these other pathways during liver carcinogenesis.
It is unclear if alterations in the activity of TAZ, the other co-activator of the Hippo pathway, contribute to the development or progression of liver cancer and should be explored as therapeutic targets. TAZ is an important transactivator of the Hippo pathway in liver cancer cells95, 109, although there has been no in-depth study to determine the specific roles for YAP and TAZ in the liver. Several genome-wide, chromatin immunoprecipitation studies of YAP and TAZ binding found these molecules to be interchangeable, binding to the same sites across the genome and presumably regulating the expression of similar genes14, 15.
An exome sequencing study of 243 HCCs identified 161 genes that promote tumorigenesis, via 11 different pathways123, but none were in the Hippo pathway. Small studies have shown that YAP amplification18, deletion of STK3 or STK421, or deletions in SAV1121 contribute to development of HCCs and cholangiocarcinomas. High tumor levels of YAP and TAZ have been associated with shorter disease-free survival times21, 72, 101, 106, 108, 109, 115, 124–126. Studies are needed to determine the mechanisms that activate YAP and TAZ, and other features of their regulation.
Altogether, these studies suggest that inactivation of Hippo signaling forms a baseline upon which other signaling systems affect cancer phenotype. Undoubtedly, understanding and targeting each of these pathways during therapy could significantly advance our ability to develop effective and durable liver cancer therapy.
Future Directions
The Hippo signaling pathway has been progressively recognized as a potent growth regulator. Over the last several years, we have increased our understanding of the function of this pathway during development and regeneration, and now have rationale for design of therapeutic agents. A large number of signaling inputs into the Hippo pathway have been identified, but it is not clear how these affect YAP activity to regulate disparate phenotypes (Figure 3C).
Many questions remain and continue to emerge from the study of this pathway. These include: What determines the zonation of YAP and TAZ expression in the liver? What is the consequence of hippo pathway zonation? Does this pattern of signaling affect zonation defined by Wnt signaling? How do junction complexes differ in their regulation of YAP and TAZ? How is YAP activity maintained during homeostasis? What factors increase YAP activity after liver injury and then subsequently down regulate it? Although YAP is often overexpressed in cancers, why are so few mutations found in the hippo pathway? How does hippo signaling interact with other biochemical pathways, such as NOTCH and WNT signaling? And finally, what are the downstream targets of YAP/TAZ that mediate their biological functions?
Answers to these and other questions will facilitate a better understanding of liver homeostasis and pathogenesis.
Table I.
Phenotypes of Hippo Pathway Mutants in the Liver
| Mutation | Genotype | Phenotype | References |
|---|---|---|---|
| YAP overexpression | ApoE-rtTA; TRE Yap | hepatomegaly, HCC | 20 |
| LAP1-tTA;TetO-Yap(S127A) | hepatomegaly, HCC | 19 | |
| Ck19-CreERT2;TetO-Yap(S127A) | ductal hyperplasia | 30 | |
| AAV-Cre; TetO-Yap(S127A) | dedifferentiation, hepatomegaly, HCC | 30 | |
| Yap(S112A)/Yap(S112A) | no change at baseline, hypertrophy after injury | 24 | |
| YAP knockout | AAV-TBG-Cre; Yap fl/fl | hepatocyte sensitivity to stress | 30 |
| Alb-Cre; Yap fl/fl | biliary and hepatocyte hypoplasia | 42 | |
| MOB1A and MOB1B | Mob1a Δ/Δ 1b tr/+ | HCC development | 26, 27 |
| Knockout of MST1 and MST2 | Ad-Cre; Mst1−/−; Mst fl/fl | HCC development | 21 |
| Alb-Cre; Mst1−/− Mst fl/- | 22 | ||
| Alb-Cre; Mst1−/− Mst fl/fl | 23 | ||
| Knockout of NF2 | Alb-Cre; Nf2 fl/fl | biliary ductular reaction, HCC | 42 |
| Ad-Cre; Nf2 fl/fl | 43 | ||
| Knockout of SAV | Alb-Cre; Sav1 fl/fl | Hepatic progenitor expansion, HCC, CC | 25 |
| Knockout of NF2 and SAV | Alb-Cre; NF2 fl/fl Sav1 fl/fl | Biliary cell hyperplasia | 44 |
| Knockout of LATS1 and LATS2 | Ad-Cre; Lats1−/−; Lats 2 fl/fl | Hepatomegaly, biliary cell expansion | 24 |
| Knockout of α-catenin | ΛNΠ α-catenin siRNA | Hepatomegaly, disorganized sinusoids, cholestasis, | 51 |
Acknowledgments
We appreciate the members of the Camargo laboratory for stimulating and insightful discussions, Mark Puder (Boston Children’s Hospital) for sharing samples of mouse hepatectomies, and Satdarshan Monga (University of Pittsburgh) for allowing presentation of their work115. DY is a Gilead Sciences Scholar in Liver Disease and is supported by the Harvard Digestive Disease Center (P30 DK034854). This work was supported by awards from the NIH K08 DK105351 (DY), R01 AR064036 (FDC) and R01 DK099559-01 (FDC).
Footnotes
Author Contribution: SP, FDC and DY conceived of the work. SP generated the initial draft. DY reviewed and gave final approval.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Author names in bold designate shared co-first authorship.
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 3.Dutkowski P, Linecker M, DeOliveira ML, et al. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307–23. doi: 10.1053/j.gastro.2014.08.045. [DOI] [PubMed] [Google Scholar]
- 4.Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011;43:884–7. doi: 10.1016/j.transproceed.2011.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka A, Tanaka K, Tokuka A, et al. Graft size-matching in living related partial liver transplantation in relation to tissue oxygenation and metabolic capacity. Transpl Int. 1996;9:15–22. doi: 10.1007/BF00336807. [DOI] [PubMed] [Google Scholar]
- 6.Fukazawa K, Nishida S. Size mismatch in liver transplantation. J Hepatobiliary Pancreat Sci. 2016;23:457–66. doi: 10.1002/jhbp.371. [DOI] [PubMed] [Google Scholar]
- 7.Francavilla A, Ove P, Polimeno L, et al. Regulation of liver size and regeneration: importance in liver transplantation. Transplant Proc. 1988;20:494–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Wu S, Huang J, Dong J, et al. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 9.Kanai F, Marignani PA, Sarbassova D, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–91. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yagi R, Chen LF, Shigesada K, et al. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999;18:2551–62. doi: 10.1093/emboj/18.9.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui CB, Cooper LF, Yang X, et al. Transcriptional coactivation of bone-specific transcription factor Cbfa1 by TAZ. Mol Cell Biol. 2003;23:1004–13. doi: 10.1128/MCB.23.3.1004-1013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu S, Totty N, Irwin M, et al. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14-3-3 and attenuation of p73-mediated apoptosis. Molecular Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhao B, Li L, Tumaneng K, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF -TRCP. Genes & Development. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli GG, Carrara M, Yuan WC, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 2015;60:328–37. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zanconato F, Forcato M, Battilana G, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–27. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, Wu S, Barrera J, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Overholtzer M, Zhang J, Smolen GA, et al. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zender L, Spector MS, Xue W, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Current Biology. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 Maintain Hepatocyte Quiescence and Suppress Hepatocellular Carcinoma Development through Inactivation of the Yap1 Oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song H, Mak KK, Topol L, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proceedings of the National Academy of Sciences. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Li Y, Kim SM, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proceedings of the National Academy of Sciences. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Zhang N, Xie R, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015;29:1285–97. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee K-P, Lee J-H, Kim T-S, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proceedings of the National Academy of Sciences. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishio M, Hamada K, Kawahara K, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–18. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishio M, Sugimachi K, Goto H, et al. Dysregulated YAP1/TAZ and TGF-beta signaling mediate hepatocarcinogenesis in Mob1a/1b-deficient mice. Proc Natl Acad Sci U S A. 2016;113:71–80. doi: 10.1073/pnas.1517188113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & Development. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tschaharganeh DF, Chen X, Latzko P, et al. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530–1542. doi: 10.1053/j.gastro.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo Pathway Activity Influences Liver Cell Fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao B, Kim J, Ye X, et al. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer research. 2009;69:1089–1098. doi: 10.1158/0008-5472.CAN-08-2997. [DOI] [PubMed] [Google Scholar]
- 32.Goulev Y, Fauny J, Gonzalez-Marti B, et al. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Current Biology. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 33.Wu S, Liu Y, Zheng Y, et al. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental Cell. 2008;14:388–98. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Ren F, Zhang Q, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Developmental Cell. 2008;14:377–87. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schlegelmilch K, Mohseni M, Kirak O, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 38.Aragona M, Panciera T, Manfrin A, et al. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–59. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 39.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–21. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 41.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;75:826. doi: 10.1016/0092-8674(93)90501-g. [DOI] [PubMed] [Google Scholar]
- 42.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Developmental Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benhamouche S, Curto M, Saotome I, et al. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes & Development. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin F, Yu J, Zheng Y, et al. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–55. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohseni M, Sun J, Lau A, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–17. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baas AF, Smit L, Clevers H. LKB1 tumor suppressor protein: PARtaker in cell polarity. Trends Cell Biol. 2004;14:312–9. doi: 10.1016/j.tcb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- 48.Nguyen HB, Babcock JT, Wells CD, et al. LKB1 tumor suppressor regulates AMP kinase/mTOR-independent cell growth and proliferation via the phosphorylation of Yap. Oncogene. 2013;32:4100–9. doi: 10.1038/onc.2012.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasioukhin V, Bauer C, Degenstein L, et al. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell. 2001;104:605–17. doi: 10.1016/s0092-8674(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 50.Kobielak A, Fuchs E. Links between alpha-catenin, NF-kappaB, and squamous cell carcinoma in skin. Proc Natl Acad Sci U S A. 2006;103:2322–7. doi: 10.1073/pnas.0510422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herr KJ, Tsang YH, Ong JW, et al. Loss of alpha-catenin elicits a cholestatic response and impairs liver regeneration. Sci Rep. 2014;4:1–11. doi: 10.1038/srep06835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gladden AB, Hebert AM, Schneeberger EE, et al. The NF2 tumor suppressor, Merlin, regulates epidermal development through the establishment of a junctional polarity complex. Dev Cell. 2010;19:727–39. doi: 10.1016/j.devcel.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan SW, Lim CJ, Chong YF, et al. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. The Journal of biological chemistry. 2011;286:7018–26. doi: 10.1074/jbc.C110.212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao B, Li L, Lu Q, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes & development. 2011;25:51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364–70. doi: 10.1074/jbc.C110.205401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi C, Troutman S, Fera D, et al. A tight junction-associated Merlin-angiomotin complex mediates Merlin’s regulation of mitogenic signaling and tumor suppressive functions. Cancer Cell. 2011;19:527–40. doi: 10.1016/j.ccr.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi C, Shen Z, Stemmer-Rachamimov A, et al. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal. 2013;6:1–12. doi: 10.1126/scisignal.2004060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang CC, Graves HK, Moya IM, et al. Differential regulation of the Hippo pathway by adherens junctions and apical-basal cell polarity modules. Proc Natl Acad Sci U S A. 2015;112:1785–90. doi: 10.1073/pnas.1420850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bai H, Zhu Q, Surcel A, et al. Yes-associated protein impacts adherens junction assembly through regulating actin cytoskeleton organization. Am J Physiol Gastrointest Liver Physiol. 2016;311:G396–411. doi: 10.1152/ajpgi.00027.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao H, Jiang N, Zhou B, et al. TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci. 2015;106:151–9. doi: 10.1111/cas.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Apte U, Gkretsi V, Bowen WC, et al. Enhanced liver regeneration following changes induced by hepatocyte-specific genetic ablation of integrin-linked kinase. Hepatology. 2009;50:844–51. doi: 10.1002/hep.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–77. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Glaser SS, Gaudio E, Miller T, et al. Cholangiocyte proliferation and liver fibrosis. Expert Rev Mol Med. 2009;11:e7. doi: 10.1017/S1462399409000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anakk S, Bhosale M, Schmidt VA, et al. Bile acids activate YAP to promote liver carcinogenesis. Cell Rep. 2013;5:1060–9. doi: 10.1016/j.celrep.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu FX, Zhao B, Panupinthu N, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–91. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miller E, Yang J, DeRan M, et al. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem Biol. 2012;19:955–62. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Mo JS, Yu FX, Gong R, et al. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs) Genes Dev. 2012;26:2138–43. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Park HW, Kim YC, Yu B, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–94. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morin-Kensicki E, Boone B, Howell M. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Molecular and cellular biology. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hossain Z, Ali SM, Ko HL, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A. 2007;104:1631–6. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Makita R, Uchijima Y, Nishiyama K, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–53. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 72.Li H, Wolfe A, Septer S, et al. Deregulation of Hippo kinase signalling in Human hepatic malignancies. Liver Int. 2012;32:38–47. doi: 10.1111/j.1478-3231.2011.02646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bai H, Gayyed MF, Lam-Himlin DM, et al. Expression of Yes-associated protein modulates Survivin expression in primary liver malignancies. Hum Pathol. 2012;43:1376–85. doi: 10.1016/j.humpath.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fitamant J, Kottakis F, Benhamouche S, et al. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep. 2015;10:1692–1707. doi: 10.1016/j.celrep.2015.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee DH, Park JO, Kim TS, et al. LATS-YAP/TAZ controls lineage specification by regulating TGFbeta signaling and Hnf4alpha expression during liver development. Nat Commun. 2016;7:11961. doi: 10.1038/ncomms11961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moroishi T, Park HW, Qin B, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015;29:1271–84. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bai H, Zhang N, Xu Y, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang C, Zhang L, He Q, et al. Differences in Yes-associated protein and mRNA levels in regenerating liver and hepatocellular carcinoma. Mol Med Rep. 2012;5:410–4. doi: 10.3892/mmr.2011.640. [DOI] [PubMed] [Google Scholar]
- 79.Wu H, Xiao Y, Zhang S, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell Rep. 2013;3:1663–77. doi: 10.1016/j.celrep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grijalva JL, Huizenga M, Mueller K, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 81.Swiderska-Syn M, Xie G, Michelotti GA, et al. Hedgehog regulates yes-associated protein 1 in regenerating mouse liver. Hepatology. 2016;64:232–44. doi: 10.1002/hep.28542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–88. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Zhang K, Chang Y, Shi Z, et al. omega-3 PUFAs ameliorate liver fibrosis and inhibit hepatic stellate cells proliferation and activation by promoting YAP/TAZ degradation. Sci Rep. 2016;6:30029. doi: 10.1038/srep30029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benhamouche S, Decaens T, Godard C, et al. Apc tumor suppressor gene is the “zonation-keeper” of mouse liver. Dev Cell. 2006;10:759–70. doi: 10.1016/j.devcel.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 85.Planas-Paz L, Orsini V, Boulter L, et al. The RSPO-LGR4/5-ZNRF3/RNF43 module controls liver zonation and size. Nat Cell Biol. 2016;18:467–79. doi: 10.1038/ncb3337. [DOI] [PubMed] [Google Scholar]
- 86.Wang B, Zhao L, Fish M, et al. Self-renewing diploid Axin2(+) cells fuel homeostatic renewal of the liver. Nature. 2015;524:180–5. doi: 10.1038/nature14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Font-Burgada J, Shalapour S, Ramaswamy S, et al. Hybrid Periportal Hepatocytes Regenerate the Injured Liver without Giving Rise to Cancer. Cell. 2015;162:766–79. doi: 10.1016/j.cell.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–30. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 89.Cox AG, Hwang KL, Brown KK, et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat Cell Biol. 2016;18:886–896. doi: 10.1038/ncb3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aylon Y, Gershoni A, Rotkopf R, et al. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 2016;30:786–97. doi: 10.1101/gad.274167.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu-Chittenden Y, Huang B, Shim JS, et al. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes & development. 2012;26:1300–5. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perra A, Kowalik MA, Ghiso E, et al. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088–96. doi: 10.1016/j.jhep.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 93.Jiao S, Wang H, Shi Z, et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166–80. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 94.Zhang W, Gao Y, Li P, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014;24:331–43. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Higashi T, Hayashi H, Ishimoto T, et al. miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br J Cancer. 2015;113:252–8. doi: 10.1038/bjc.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin CW, Chang YL, Chang YC, et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat Commun. 2013;4:1877. doi: 10.1038/ncomms2876. [DOI] [PubMed] [Google Scholar]
- 97.Mitamura T, Watari H, Wang L, et al. microRNA 31 functions as an endometrial cancer oncogene by suppressing Hippo tumor suppressor pathway. Mol Cancer. 2014;13:97. doi: 10.1186/1476-4598-13-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang ZW, Men T, Feng RC, et al. miR-375 inhibits proliferation of mouse pancreatic progenitor cells by targeting YAP1. Cell Physiol Biochem. 2013;32:1808–17. doi: 10.1159/000356614. [DOI] [PubMed] [Google Scholar]
- 99.Wang Y, Cui R, Zhang X, et al. SIRT1 increases YAP- and MKK3-dependent p38 phosphorylation in mouse liver and human hepatocellular carcinoma. Oncotarget. 2016;7:11284–98. doi: 10.18632/oncotarget.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barry ER, Morikawa T, Butler BL, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–10. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steinhardt AA, Gayyed MF, Klein AP, et al. Expression of Yes-associated protein in common solid tumors. Human Pathology. 2008:1–8. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kim JM, Kang DW, Long LZ, et al. Differential expression of Yes-associated protein is correlated with expression of cell cycle markers and pathologic TNM staging in non-small-cell lung carcinoma. Hum Pathol. 2011;42:315–23. doi: 10.1016/j.humpath.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 103.Hall CA, Wang R, Miao J, et al. Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res. 2010;70:8517–25. doi: 10.1158/0008-5472.CAN-10-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang X, George J, Deb S, et al. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810–22. doi: 10.1038/onc.2011.8. [DOI] [PubMed] [Google Scholar]
- 105.Fernandez-L A, Northcott PA, Dalton J, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–41. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sohn BH, Shim JJ, Kim SB, et al. Inactivation of Hippo Pathway Is Significantly Associated with Poor Prognosis in Hepatocellular Carcinoma. Clin Cancer Res. 2016;22:1256–64. doi: 10.1158/1078-0432.CCR-15-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–6. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 108.Reis H, Bertram S, Pott L, et al. Markers of Hippo-Pathway Activity in Tumor Forming Liver Lesions. Pathol Oncol Res. 2016 doi: 10.1007/s12253-016-0079-0. [DOI] [PubMed] [Google Scholar]
- 109.Hayashi H, Higashi T, Yokoyama N, et al. An Imbalance in TAZ and YAP Expression in Hepatocellular Carcinoma Confers Cancer Stem Cell-like Behaviors Contributing to Disease Progression. Cancer Res. 2015;75:4985–97. doi: 10.1158/0008-5472.CAN-15-0291. [DOI] [PubMed] [Google Scholar]
- 110.Hofmann JJ, Zovein AC, Koh H, et al. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–72. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zong Y, Panikkar A, Xu J, et al. Notch signaling controls liver development by regulating biliary differentiation. Development. 2009;136:1727–39. doi: 10.1242/dev.029140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–92. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Armengol C, Cairo S, Fabre M, et al. Wnt signaling and hepatocarcinogenesis: the hepatoblastoma model. Int J Biochem Cell Biol. 2011;43:265–70. doi: 10.1016/j.biocel.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Semin Cancer Biol. 2011;21:44–58. doi: 10.1016/j.semcancer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tao J, Calvisi DF, Ranganathan S, et al. Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang J, Park JS, Wei Y, et al. TRIB2 acts downstream of Wnt/TCF in liver cancer cells to regulate YAP and C/EBPalpha function. Mol Cell. 2013;51:211–25. doi: 10.1016/j.molcel.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hansen CG, Ng YL, Lam WL, et al. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park YY, Sohn BH, Johnson RL, et al. Yes-associated protein 1 and transcriptional coactivator with PDZ-binding motif activate the mammalian target of rapamycin complex 1 pathway by regulating amino acid transporters in hepatocellular carcinoma. Hepatology. 2016;63:159–72. doi: 10.1002/hep.28223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tumaneng K, Schlegelmilch K, Russell RC, et al. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–9. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Marti P, Stein C, Blumer T, et al. YAP promotes proliferation, chemoresistance, and angiogenesis in human cholangiocarcinoma through TEAD transcription factors. Hepatology. 2015;62:1497–510. doi: 10.1002/hep.27992. [DOI] [PubMed] [Google Scholar]
- 121.Sia D, Hoshida Y, Villanueva A, et al. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yamada D, Rizvi S, Razumilava N, et al. IL-33 facilitates oncogene-induced cholangiocarcinoma in mice by an interleukin-6-sensitive mechanism. Hepatology. 2015;61:1627–42. doi: 10.1002/hep.27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schulze K, Imbeaud S, Letouze E, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–11. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang T, Zhang J, You X, et al. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051–9. doi: 10.1002/hep.25899. [DOI] [PubMed] [Google Scholar]
- 125.Xu MZ, Yao T-J, Lee NPY, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–4585. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pei T, Li Y, Wang J, et al. YAP is a critical oncogene in human cholangiocarcinoma. Oncotarget. 2015:1–15. doi: 10.18632/oncotarget.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]