Abstract
Objective
Ictal SPECT is promising for accurate non-invasive localization of the epileptogenic brain tissue in focal epilepsies. However, high quality ictal scans require meticulous attention to the seizure onset. In a relatively large cohort of pediatric patients, this study investigated the impact of the timing of radiotracer injection, MRI findings and seizure characteristics on ictal SPECT localizations, and the relationship between concordance of ictal SPECT, scalp EEG and resected area with seizure freedom following epilepsy surgery.
Methods
Scalp EEG and ictal SPECT studies from 95 patients (48 males and 47 females, median age = 11 years, (25th, 75th) quartiles = (6.0, 14.75) years) with pharmacoresistant focal epilepsy and no prior epilepsy surgery were reviewed. The ictal SPECT result was examined as a function of the radiotracer injection delay, seizure duration, epilepsy etiology, cerebral lobe of seizure onset identified by EEG and MRI findings. Thirty two patients who later underwent epilepsy surgery had postoperative seizure freedom data at <1, 6 and 12 months.
Results
Sixty patients (63.2%) had positive SPECT localizations - 51 with a hyperperfused region that was concordant with the cerebral lobe of seizure origin identified by EEG and 9 with discordant localizations. Of these, 35 patients (58.3%) had temporal and 25 (41.7%) had extratemporal seizures. The ictal SPECT result was significantly correlated with the injection delay (p<0.01) and cerebral lobe of seizure onset (specifically frontal versus temporal; p = 0.02) but not MRI findings (p = 0.33), epilepsy etiology (p ≥ 0.27) or seizure duration (p = 0.20). Concordance of SPECT, scalp EEG and resected area was significantly correlated with seizure freedom at 6 months after surgery (p=0.04).
Significance
Ictal SPECT holds promise as a powerful source imaging tool for presurgical planning in pediatric epilepsies. To optimize the SPECT result the radiotracer injection delay should be minimized to ≤ 25 s, although the origin of seizure onset (specifically temporal versus frontal) also significantly impacts the localization.
Keywords: Ictal SPECT, pediatric epilepsies, radiotracer injection, postsurgical outcome
1. Introduction
More than 750,000 children in the US suffer from epileptic seizures, with approximately 50,000 new cases being diagnosed every year1. Over 30% of these patients do not respond to antiepileptic medications2 and suffer from medically intractable seizures, which are associated with 4-5 times higher morbidity and mortality than that of the generation population3. There are very few curative treatments for medically refractory epilepsy. Surgical resection of the brain area that is responsible for seizure generation is a treatment option available only to select patients with focal epilepsy. Its success largely depends on accurate localization of the epileptogenic tissue. Eligible patients undergo a complex and extensive preoperative evaluation that includes implantation of invasive electrodes to accurately localize the seizure focus, minimize long-term neurological/cognitive impairment and maximize seizure freedom. Invasive monitoring carries significant risk. To date, there are no sufficiently accurate non-invasive monitoring tools that could optimize, complement and even minimize invasive monitoring, thus reducing the morbidity risk, cost and overall burden of the preoperative evaluation4,5.
There is an unmet clinical need for improved noninvasive tools for presurgical evaluation in pediatric epilepsy. Scalp electroencephalography (EEG) remains the primary diagnostic tool in the field and is typically used to guide additional studies. However, its spatial specificity is poor (≥ 2 cm) and cannot be used by itself for surgical planning. Imaging modalities hold promise for this purpose6 but have various limitations, particularly in pediatric patients. Magnetic Resonance Imaging (MRI) can only image structural abnormalities potentially associated with seizures or structural changes, such as loss of hippocampal volume that occur as the result of seizures7,8,9. A substantial number of epilepsy patients have normal MRI scans10, in which case MRI is not useful for localizing the epileptogenic brain tissue.
Positron Emission Tomography (PET) is a promising modality for imaging changes in cerebral metabolism (hypometabolism) rather than blood flow in the epileptogenic tissue.
The more commonly performed interical PET (in contrast to rare ictal PET) has been shown to have good sensitivity (≥ 70%) to identify the epileptogenic brain tissue as a broad area of hypometabolism14,15, but may have limited spatial specificity. Overall, interictal PET has higher specificity in TLE14,20 and is thus of lesser utility in children given that over 50% of pediatric patients have extratemporal seizures, for whom interictal PET may have lower sensitivity and higher inter-observer variability21.
Ictal perfusion Single-Photon Emission Computed Tomography (SPECT) is another promising non-invasive imaging modality that can detect the seizure focus with spatial accuracy that is comparable to that of invasive EEG, but at significantly lower risk to patients5,22,23,24. Its spatial resolution is 5-10 mm, and thus superior to that of clinical scalp EEG (∼2.5 cm)25,26. The minimum amount of brain tissue resected during surgery is typically ∼3-4 cm3, when the seizure focus is precisely localized. Thus, ictal SPECT has sufficiently high spatial resolution to accurately localize the epileptogenic region based on changes in cerebral blood flow (focal cerebral hyperperfusion - see Figure 1) induced by ictal discharges. Overall, it has higher sensitivity than interictal PET independently of the location of the seizure focus20,25,27,28. Previous studies have shown that ictal SPECT registered to MRI may be valuable for localizing the epileptogenic brain tissue in patients with extratemporal seizures and nonlesional MRI28,29. Given the high incidence of extratemporal seizures in children, ictal SPECT could become particularly useful for surgical planning. However, perfusion may change as seizures propagate and spread to large areas of the brain and thus the sensitivity and spatial specificity of ictal SPECT largely depend on the timing of radiotracer injection. If a seizure is not accurately detected, the radiotracer may not be delivered sufficiently early during seizure evolution in which case SPECT images show diffuse seizure propagation and postictal effects (see Figure 1) and are of limited clinical utility30. Currently, the success rate of ictal SPECT is ∼50% but could substantially increase if reliable seizure detection and consequently the radiotracer injection are automated31.
Figure 1.
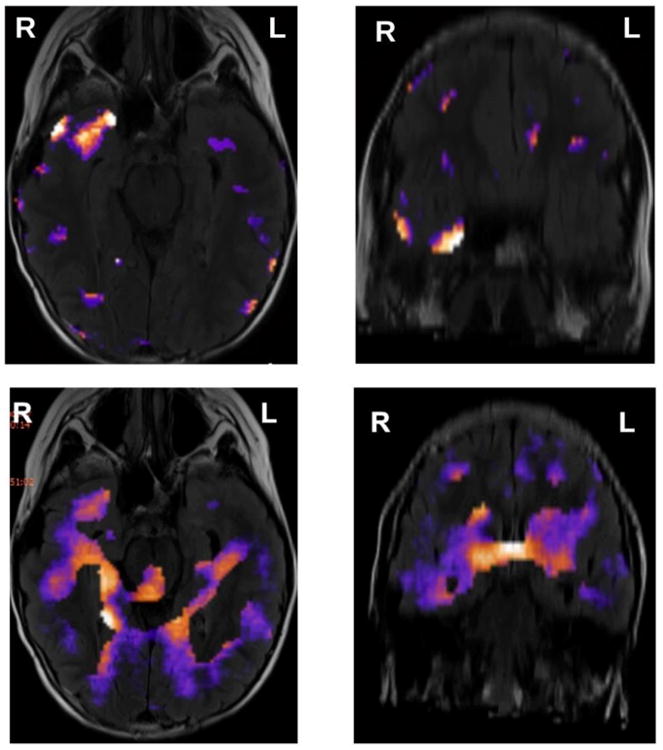
Examples (axial and coronal views) of (a) successful (focal) SPECT localization and (b) unsuccessful (diffuse) SPECT, from the same pediatric patient with medication intractable focal epilepsy and nonlesional MRI. In the images shown in top panels, the radiotracer was injected 2 s after ictal onset, whereas in the images shown in bottom panels it was injected 30 s after ictal onset, resulting in a focal area of hyperperfusion in the first case and a diffuse area associated with seizure propagation in the second case. The same empirical threshold was used in both cases.
The clinical utility of ictal SPECT in children with epilepsy has not been extensively investigated32,33 and to date it is unclear whether the timing of the radiotracer injection is the primary or sole predictor of its outcome34. Also, only few studies have compared SPECT- and intracranial EEG-based estimates of the epileptogenic region33,35,36 and/or have assessed the relationship between the epilepsy surgery outcome and the ictal SPECT result. It has been previously shown that concordance between the hyperperfused region in SPECT images and electrocorticography (ECoG) was useful in predicting the long-term postsurgical outcome37.
In a relatively large cohort of 95 pediatric patients with pharmacoresistant focal epilepsy, this study systematically investigated the ictal SPECT result as a function of multiple potentially affecting factors, including the injection delay, MRI findings and seizure characteristics. The overarching goal of the study was to determine whether the injection delay individually or in combination with other factors predict the SPECT result in pediatric patients who have significantly more heterogeneous seizures than adults. The cohort included 32 patients who underwent epilepsy surgery and had additional information on the postsurgical outcome. The study also investigated the relationship between concordance of the ictal SPECT result, scalp EEG and ECoG with seizure freedom following epilepsy surgery.
2. Materials and Methods
2a. Patient cohort
This retrospective study was approved by the Institutional Review Board. Scalp electroencephalograms (EEG), MRI and ictal SPECT images for 95 consecutive pediatric patients with pharmacoresistant localization-related epilepsy and focal onset seizures irrespective of etiology were reviewed (patient records from June 1, 2009 to May 31, 2012 were examined). The only exclusion criterion was epilepsy surgery prior to the ictal SPECT study. Forty eight males and 47 females were included. No patient had repeated studies. Age at imaging was 1-20 years, median = 11 years, (25th, 75th) quartiles = (6.0, 14.75) years. Based on visual examination of scalp EEG at the time of imaging, 38 patients (40%) had frontal lobe seizures, 47 patients (49.5%) had temporal lobe seizures, 8 patients (8.4%) had parietal lobe seizures, and 2 patients (2.1%) had occipital lobe seizures. Magnetic Resonance Imaging (MRI) was available for all patients. Thirty two patients (33.7%) had undergone epilepsy surgery at a later date following the ictal SPECT study.
2b. Ictal SPECT
All patients were first evaluated for the need of sedation, to minimize motion artifacts, and over 50% were sedated prior to ictal SPECT. Nuclear Medicine physicians at our institution are blinded to the EEG findings when reading ictal SPECT studies.
Radiotracer
Tc99m-ECD is the radiotracer almost exclusively used at our institution. In the very few cases when it was not available Tc-99m HMPAO was used, which typically gives the same results as with Tc-99m ECD except that it has higher soft tissue uptake in the calvarium. However, this does not affect the diagnosis of focal ictal activity in then cortex on SPECT. Tc99m-ECD is a lipophilic radiopharmaceutical that is rapidly taken up into neurons. Its physical and shelf lives are ∼6 hrs, respectively. Following injection, it takes 15-30 sec for the tracer to reach the brain. Its initial distribution is according to the first pass and reflects regional cerebral blood flow. Once taken up by the brain, the tracer is trapped and does not re-distribute to extracerebral regions for hours24. Thus, the duration of cerebral uptake may be significantly longer than a typical seizure duration (∼20 sec - 3 min). If the tracer is injected soon after ictal onset, it is anticipated that the area of hyperperfusion at ictal onset will correspond to the epileptogenic region38.
Image Processing
Ictal SPECT images were subtracted from interictal SPECT (available for all patients and acquired during the admission) and were registered to MRI. A home-developed software package is used, which enables multimodality image fusion, substaction, etc and is similar to SISCOM. The operator (a physician) empirically controls the image thresholding. Previous studies have shown that SISCOM images have superior spatial specificity than ictal or interictal images individually32. Ictal and interictal images were normalized prior to the subtraction to correct for respective differences in radiotracer dose during these studies. A SISCOM SPECT was considered positive when a focal area of hyperperfusion was identifiable, irrespective of its agreement with scalp EEG or other modalities.
Injection delay
This was defined as the difference between the electrographic seizure onset (identified by the clinical electroencephalographer by visual examination of the EEG) and the time of radiotracer injection.
2c. Data
In addition to demographic data, MRI outcome (lesional/nonlesional), SPECT results (positive/negative), cerebral lobe of seizure onset identified by scalp EEG, seizure duration and injection delay were collected. For patients who later underwent epilepsy surgery, additional information on the resected area, pre- and post-resection seizure frequency and seizure freedom (at <1 month, 6, 12 months and for a small patient subset also at 18 and 24 months following surgery) was available. Follow up data at <1 month were collected for the purpose of examining potential correlations between ictal SPECT findings and acute post-operative seizures.
2c. Statistical analysis
Three sub-cohorts were identified: a) patients with a positive SPECT localization of the epileptogenic tissue in agreement with the EEG (n = 51); b) patients with negative SPECT (n = 35); c) patients with positive SPECT localization in disagreement with the EEG (n = 9).
Logistic regression models were developed to assess the relationship between the SPECT result and age, sex, injection delay, seizure duration, MRI findings (nonlesional = 0, lesional = 1) and brain region of seizure onset (frontal = 1, temporal = 2, parietal = 3, occipital = 4). In the statistical analysis and models, the SPECT result was categorized in two different ways: a) negative or discordant = 0, positive and concordant = 1; b) negative = 0; positive (concordant or discordant) = 1), i.e., in one set of models the discordant positive SPECT was treated as unsuccessful and in the other as successful given specificity of the hyperperfusion to a lobe or region. Lobe of seizure onset (a categorical variable) was modeled assuming the temporal lobe onset as the reference, i.e., temporal-versus-frontal, temporal-versus-parietal and temporal-versus-occipital. Given substantial heterogeneity in the cohort, epilepsy etiology was first modeled either as idiopathic or cryptogenic (= 0) or symptomatic (= 1). In separate models it was also represented in more detailed categories: idiopathic/cryptogenic = 0, focal cortical dysplasia = 1, mesial temporal sclerosis = 2, cortical dysplasia = 3, tumor = 4, other = 5. For patients who underwent epilepsy surgery, additional models were developed to assess the relationship between seizure freedom with EEG and ictal SPECT concordance, resected area, seizure origin, etiology, pre-resection seizure frequency and MRI.
The resected area was categorized as: frontal = 1, temporal =2, parietal = 3, occipital = 4, multiple regions or hemispherectomy = 5. For the purposes of statistical modeling, the temporal area was assumed as the reference. Concordance of the resected area was categorized as: agreement with scalp EEG and positive concordant SPECT = 1, agreement with EEG but negative SPECT = 2, disagreement with EEG and negative SPECT = 3, disagreement with EEG but agreement with positive SPECT = 4.
The probability density functions in Figure 2 were estimated using a non-parametric kernel approach (a Gaussian kernel, with bandwidth = 1.8). Confidence intervals were estimated using bootstrapping with replacement (2000 draws).
Figure 2.
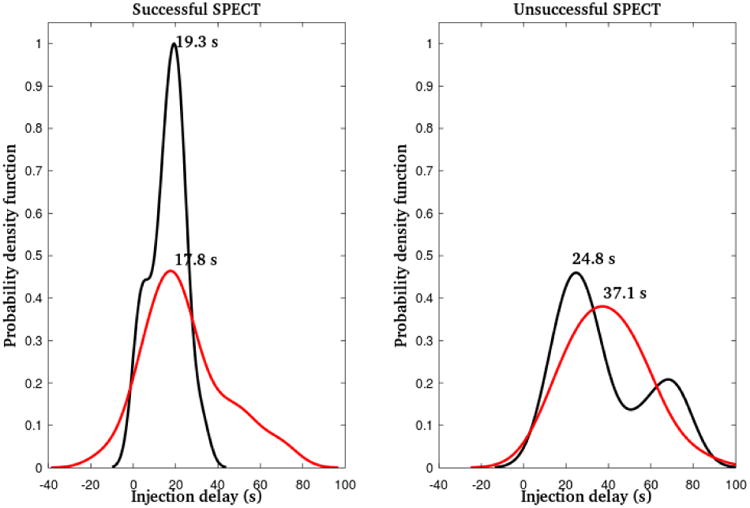
Estimated injection delay distributions (probability density functions) for successful (left panel) and unsuccessful (right panel) ictal SPECT, separately for patients with nonlesional (black) and lesional (red) MRI. Injection delays at the peaks (global maxima) of these distributions are superimposed.
3. Results
Fifty one patients (53.7%), 22 males (43.1%) and 29 females (56.9%), had a positive ictal SPECT concordant with the EEG (group A). These included 33 patients (64.7%) with lesional MRI and 18 (35.3%) with nonlesional MRI. Thirty five patients (36.8%), 22 males (62.9%) and 13 females (37.1%), had a negative ictal SPECT (group B). Nineteen patients (54.3%) had lesional MRI and 16 (45.7%) had nonlesional MRI. Nine patients (9.5%), including 1 male and 1 female (data were missing for 7 patients) had a positive ictal SPECT discordant with the EEG (group C). Three patients (33.3%) had lesional MRI and 6 (66.7%) had nonlesional MRI. For 41 patients (43.2% of the cohort and 74.5% of those with lesional MRI) the identified structural abnormality was unrelated to their epilepsy. As expected in a large pediatric cohort, epilepsy etiologies varied significantly across patients. Thirty one patients (32.6%) had either idiopathic (n = 18; 18.9%) or cryptogenic (n = 13; 13.7%) epilepsy. Ten patients (10.5%) had focal cortical dysplasia, 8 (8.4%) had mesial temporal sclerosis (MTS) and 7 (7.4%) had various tumors. The remaining etiologies included Tuberous Sclerosis Complex (TSC), traumatic brain injury (TBI), and brain malformations and were distributed across small (n<5) patient subgroups. No statistically significant correlation was found between seizure etiology and the SPECT result either when dichotomized as symptomatic versus idiopathic/cryptogenic or classified in more detailed categories (p = 0.68 and p ≥ 0.27, respectively). Summary statistics for age, sex and MRI outcome are presented in Table 1. The SPECT result was not significantly correlated with any of these parameters (p = 0.82 for age, p = 0.07 for sex, p = 0.33 for MRI, based on univariate models and p ≥ 0.09 for all parameter combinations in multivariate models).
Table 1.
Summary statistics for patients grouped according to their ictal SPECT result. Median age and corresponding inter-quartile range (IQR), and percentages for sex (male (M), female (F)) and MRI outcome (positive (+), negative (-)) are provided for the 3 groups.
| Group | Age (median; IQR) (years) | sex (M; F) | MRI Outcome nonlesional(NL); Lesional (L) |
|---|---|---|---|
| Positive SPECT Concordant with EEG (n = 51) | 11 | M: 22 (43.1%) F: 29 (56.9%) |
NL: 18 (35.3%) L: 33 (64.7%) |
| (6.25, 15) | |||
| Positive SPECT discordant with EEG (n = 9) | 9 | M: 4 (44.4%) F: 5 (55.6%) |
NL: 6 (66.7%) L: 3 (33.3%) |
| (6.0, 14.0) | |||
| Negative SPECT (n = 35) | 11 | M: 22 (62.9%) F: 13 (37.1%) |
NL: 16 (45.7%) L: 19 (54.3%) |
| (5.25, 14.0) |
Assuming scalp EEG as the gold standard, the origin of the seizure being imaged was localized to a cerebral lobe for all patients. The sensitivity (and in this case also accuracy) of ictal SPECT to image this region was 53.7% (51 of 95). Specificity could not be estimated in the traditional sense given that all patients had a localized seizure focus by the EEG and thus there were no true negatives. There were 44 false negative SPECT results, i.e., no or discordant localizations and thus the false negative rate was 46.3%.
The injection delay was in the range -13 to 71 s for patients in group A (median = 18 s), 13-79 s for patients in group B (median = 35 s), and 18-74 s for patients in group C (median = 27 s). Additional statistics for this parameter are summarized in Table 2. There was a statistically significant difference between group A and B delays even when the single outlying pre-seizure injection (and thus negative injection delay) was excluded from group A (p<0.001). The radiotracer was injected prior to seizure onset (based on visual EEG examination) only in 1 patient (13 s prior to ictal onset). The injection delay was significantly correlated with the SPECT result (p < 0.01, Wald statistic = 14.46), irrespective of whether positive discordant SPECT localizations were treated as successful (==1) or unsuccessful (==0) outcomes in the regression models. The distributions of injection delays as a function of the SPECT result and MRI are shown in Figure 2. Groups B and C are shown together as a patient group with unsuccessful (non-localizable or discordant) SPECT. There was no statistically significant correlation between MRI outcome and injection delay (p = 0.19 and p = 0.52 for patients with successful and unsuccessful SPECT, respectively). The distribution of injection delays in patients with successful SPECT localization and nonlesional MRI had a substantially narrower peak compared to all other groups, indicating less variability of delays in this group.
Table 2.
Summary statistics for seizure characteristics (duration and cerebral lobe of origin) and the injection delay for patients grouped according to their ictal SPECT result. Data on seizure duration was missing for 11 patients with positive SPECT concordant with the EEG, 8 patients with negative SPECT and 1 patient with a positive but discordant SPECT.
| Group | Injection delay (s) (Median; 95% Confidence interval (CI) for Median; IQR range) | Seizure duration (s) (Median; 95% CI for Median; IQR range) | Origin (cerebral lobe) of seizure onset (F, T, P, O) |
|---|---|---|---|
| Positive SPECT Concordant with EEG (n = 51) | 18.0, CI: [14.0, 21.0] | 84.0, CI: [75.6, 115.0] | F: 23.5% T: 66.7% P: 7.8% O: 2.0% |
| IQR: (10.5, 25.0) | (68.5, 128.5) Missing: n=11 |
||
| Positive SPECT discordant with EEG (n = 9) | 27.0, CI: [31.0, 51.0] | 245.5, CI: [58.0, 724.00] | F: 66.7% T: 11.1% P: 22.2% O: None |
| IQR: (19.8, 56.8) | (58.5, 500.5) Missing: n = 8 |
||
| Negative SPECT (n = 35) | 35.0, CI: [19.0, 71.0] | 80.0, CI: [67.0, 107.0] | F: 57.1% T: 34.3% P: 5.7% O: 2.9% |
| IQR: (23.8, 54.5) | (60.25, 134.25) Missing: n = 1 |
The distributions of the cerebral lobes of seizures onset identified by EEG in the 3 groups are summarized in Table 2. The majority (n=34; 66.7%) of patients in group A had temporal lobe seizures, followed by frontal (n=12; 23.5%), parietal (n=4; 7.8%) and occipital (n=1; 2%). The majority of patients (n=20; 57.1%) in group B had frontal lobe seizures, followed by temporal (n=12; 34.3%), parietal (n=2; 5.7%) and occipital (n=1; 2.9%). Similarly, the majority of patients in group C had frontal lobe seizures (n=6; 66.7%), followed by parietal (n=2; 22.2%) and temporal (n=1; 11.1%). No patient in this group had occipital lobe seizures. The cerebral lobe of seizure onset (specifically temporal versus frontal) was significantly correlated with the SPECT result (p = 0.024, Wald statistic = 5.11). Furthermore, there was a statistically significant combinatorial relationship between the SPECT result and both the injection delay and the origin of seizure onset (p <0.01 for in injection delay, Wald statistic = 15.19; p = 0.02 for temporal versus frontal lobe, Wald statistic = 5.86), irrespective of the categorization (successful or unsuccessful) of positive discordant SPECT localizations in the statistical models. The two predictors were statistically independent of each other (p = 0.9 for the correlation between the two). There were no significant correlations between the SPECT result and other comparisons between lobes of seizure onset (parietal versus temporal (p = 0.63) and occipital versus temporal (p = 0.80)), possibly due to the small number of patients with seizures originating in these brain regions and thus lack of statistical power to detect such changes.
Seizure duration was in the range 12 - 500 s (median = 84 s) for group A, 34 – 299 s (median = 80 s) for group B and 30 – 1312 s (median = 245.5 s) for group C. Additional summary statistics for seizure characteristics are presented in Table 2. The radiotracer was injected at or prior to seizure offset in all patients. In 12 patients (23.5%) in group A, 10 patients (28.6%) in group B and 1 patient (11.1%) in group C, the radiotracer was injected at seizure offset. There was no significant correlation between the SPECT result and seizure duration (p = 0.20) or the ratio of injection delay to seizure duration (p = 0.18). Furthermore, there was no significant correlation between seizure duration and the origin of seizure onset (p = 0.59). However, injection delay was significantly longer for longer seizures (p = 0.01, Wald statistic = 6.98). A scatter plot of the two parameters is shown in Figure 3. Finally, the interval from the time of radiotracer injection to seizure offset was not significantly correlated with the SPECT result (p = 0.28).
Figure 3.
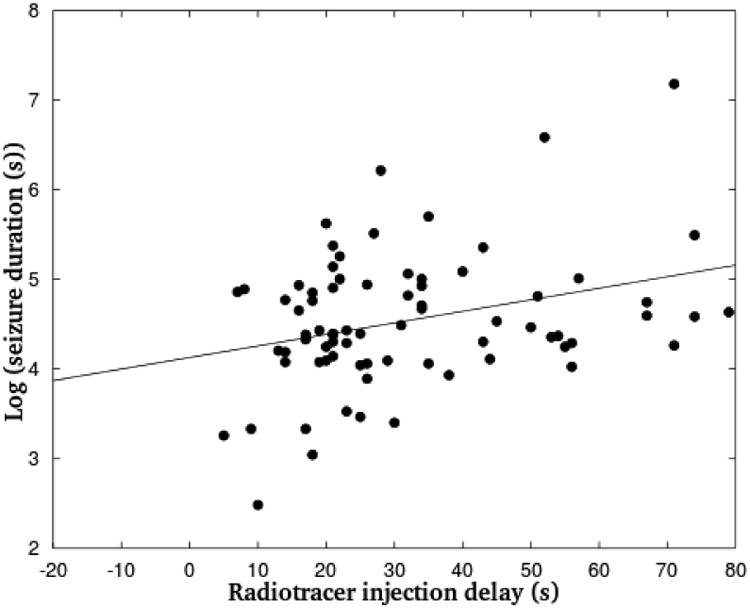
Scatter plot of injection delay as a function of the natural logarithm of seizure duration. Best linear model fitted to the data is superimposed. Due to the spread of seizure duration estimates and several extreme outliers in the linear scale, the logarithm of this parameter is more
Seventeen males (53.1%) and 15 females (46.9%) underwent epilepsy surgery following the ictal SPECT study. Twenty (62.5%) had positive SPECT concordant with the EEG, 10 (31.2%) had negative SPECT and 2 (6.3%) had positive SPECT discordant with the EEG. Median injection delay for patients with positive SPECT was 18 s (CI: [15.0, 32.0] s) and 38.5 s (CI: [26.0, 56.2] s) for patients with negative or discordant SPECT. Twenty seven patients (84.4%) had lesional MRI and 5 (15.6%) had nonlesional MRI. Nine patients (28.1%) had seizures originating in frontal regions, 20 patients (62.5%) had temporal lobe seizures, 2 (6.3%) had parietal lobe seizures and 1 patient (3.1%) had occipital lobe seizures. Summary statistics for preoperative monthly seizure frequencies and seizure freedom immediately (< 1 month), 6 months and 12 months following surgery are summarized in Table 3. The number of patient seizures per month (actual or equivalent, i.e., estimated from a shorter time interval) prior to surgery were in the range 1-750 (median = 27, (25th, 75th) quartiles = (5.0, 330.0)). There was no statistically significant correlation between seizure frequency and cerebral lobe of seizure origin (p = 0.46) or MRI outcome (p = 0.5) or the duration of the seizure imaged by SPECT (p = 0.13).
Table 3.
Preoperative seizure frequency, postoperative seizure freedom (as a binary variable) at <1 month, 6 months and 12 months following epilepsy surgery and distribution of cerebral origin of seizure onset for patients grouped according to their ictal SPECT result.
| Group | Origin (cerebral lobe) of seizure onset (F, T, P, O) | Preoperative seizure frequency (monthly) (Median, IQR range) | Postoperative Seizure Freedom (% patients) <1 mo | Postoperative Seizure Freedom (% patients) 6 mo | Postoperative Seizure Freedom (% patients) 12 mo |
|---|---|---|---|---|---|
| Positive SPECT Concordant with EEG (n = 20) | F: 20.0% T: 75.0% P: 5.0% O: None |
27.0 | 90% | 78.6% (n = 14) |
72.7% (n = 11) |
| (8.0, 405.0) | |||||
| Positive SPECT discordant with EEG (n = 2) | F: 50.0% T: None P: 50.0% O: None |
4.0 | 100% | 100% (n = 1) |
100% (n = 1) |
| (1.0, 6.0) | |||||
| Negative SPECT (n = 10) | F: 40.0% T: 50.0% P: None O: 10.0% |
60.0 | 100% | 90% (n = 10) |
83.3% (n = 6) |
| (3.0, 300.0) |
For 8 patients (25.0%) the resected brain tissue was in the cerebral lobe of seizure origin identified by scalp EEG but not SPECT, for 19 patients (59.4%) it was in lobe identified by both EEG and SPECT, for 2 patients (6.25%) it was in the lobe identified by SPECT but not EEG. Thus, the SPECT localization was concordant with the resected area in 21 patients (65.6%). Two patients (1 with occipital seizures and negative SPECT, 1 with frontal seizures and positive SPECT) underwent a hemispherectomy and for 1 patient brain tissue was resected from both the left temporal lobe (localized by SPECT but not scalp EEG) and in the left frontal lobe (localized neither by SPECT nor EEG).
Immediately following surgery (< 1 month), all but 2 patients (6.3%) were seizure free. These 2 patients had 2 and 60 seizures per month, respectively. Both patients had temporal lobe seizures and ictal SPECT that was concordant with scalp EEG. At 6 months following surgery, information on seizure freedom was available for 25 patients, and 21 (84.0%) were seizure free whereas 4 (16.0%) had recurrent seizures. At 12 months following surgery, data were available for 18 patients and 14 patients (77.8%) were seizure free. There was no significant correlation between postoperative seizure freedom at any time point and preoperative seizure frequency (p ≥ 0.44), cerebral lobe (p ≥ 0.13), MRI outcome (p ≥ 0.22) or SPECT result (p ≥ 0.37). Although there was no significant correlation between the concordance of the resected area with the EEG and SPECT localization and seizure freedom immediately following surgery or at 12 months (p = 0.84 and p = 0.71, respectively) there was a significant correlation between concordance of scalp EEG, ictal SPECT and the resected area with seizure freedom at 6 months (p = 0.04, Wald statistic = 4.35). Data on seizure freedom at 18 and 24 months were missing for >50% of patients, possibly due to loss to follow-up. However, the same 4 patients with recurrent seizures at 12 months also had seizures at 18 and 24 months.
4. Discussion
Ictal SPECT is a very promising non-invasive, high-resolution imaging modality that can image blood flow changes associated with ictal discharges and consequently identify the epileptogenic brain tissue with superior spatial resolution to that of scalp EEG. Consequently, it can be a powerful tool in the presurgical evaluation of pediatric epilepsies and poses significantly lower morbidity risk that invasive monitoring. The present study has systematically evaluated ictal SPECT in a large cohort of pediatric patients as a function of multiple potentially affecting factors, including the radiotracer injection delay, seizure characteristics and MRI findings.
A hyperperfused SPECT focus was identified in 60 patients (63.2%), including 51 concordant with the scalp EEG findings and 9 discordant. Twenty four patients (40%) had a negative MRI. The ictal SPECT was not significantly correlated with patient age, sex, MRI findings, epilepsy etiology (either dichotomized as symptomatic versus idiopathic/cryptogenic or classified in more detailed categories) or seizure duration, but was significantly correlated with the injection delay and cerebral lobe of seizure onset (identified by scalp EEG). Overall, 39 of 60 patients (65.0%) with a positive SPECT localization (concordant or discordant with the EEG) had injection delays <25 s, whereas only 9 of 35 patients (25.7%) with negative SPECT had injection delays <25 s. Similar results have been reported by previous studies34. These results were consistent irrespective of how positive discordant SPECT localizations were treated in the statistical analysis (as successful or unsuccessful). The majority of patients with positive concordant SPECT had temporal lobe seizures (66.7%), whereas the majority of patients with negative or discordant SPECT had extratemporal seizures (predominantly frontal seizures in 57.1% of patients with negative SPECT and 66.7% in patients with positive discordant SPECT). When positive concordant or discordant SPECT were grouped together, 35 of 60 patients (58.3%) had temporal lobe seizures and 25 (41.7%) had extratemporal seizures. Depending on their origin of onset among other factors, seizures may have distinct propagation dynamics that could impact the SPECT result. In fact, there was a statistically significant correlation between temporal versus frontal seizures and the SPECT localization. Frontal seizures may propagate faster and quickly spread to large areas of the brain resulting in poor SPECT localizations. Although seizure propagation dynamics were not measured, seizure duration was available for all patients and was not correlated with the SPECT result. However, longer injection delays were significantly correlated with longer seizures. It is possible that rapidly evolving seizures may be more easily visualized in scalp EEG, prompting the clinical encephalographer examining the EEG to quickly call for radiotracer injection.
The ictal SPECT result was significantly correlated with injection delay and the cerebral lobe of seizure origin (specifically frontal in comparison to temporal) also in the subset of 32 patients who underwent epilepsy surgery. Of these patients, 22 (68.75%) had a positive SPECT (20 patients had a SPECT result that was concordant with the EEG and 2 patients had discordant SPECT localizations), and 10 (31.25%) had negative SPECT. All but 2 patients achieved seizure freedom immediately following surgery and over 70% were seizure-free at 12 months. No statistically significant correlation was found between seizure freedom at < 1 month, 6 months or 12 months and preoperative seizure frequency, resected area, origin of seizure onset, MRI findings or the SPECT result. However, seizure freedom at 6 months was significantly correlated with concordance between the resected area, the SPECT localization and the scalp EEG localization. Similar results have been previously reported even at longer follow-up intervals29,37. Given substantial missing data at 12 months, it is possible that the sample did not have sufficient statistical power at this time point to detect correlations between multi-modal data concordance and seizure freedom.
There are several limitations to this study. First, MRI was categorized as nonlesional or lesional. It is possible that more detailed categories of structural abnormalities could be correlated with the SPECT result, although previous studies have highlighted the value of this modality specifically for cases of nonlesional MRI rather than for distinct structural abnormalities20,29. Also, in 41 of 55 patients with lesional MRI, the identified abnormality was not associated with their epilepsy. Second, a substantial amount of data on seizure freedom were missing at 12, 18 and 24 months following surgery, making it difficult to assess the impact of concordance of the SPECT localization with EEG and ECoG and the resected area on the long-term surgical outcome. These data were assumed to be missing at random, possibly due to loss to follow-up. Finally, the SPECT localizations were compared to scalp EEG localizations, which although sometimes incorrect, were available for all 95 patients. In contrast, information on the resected area (potentially a better gold standard than EEG-based localization) was only available for the 32 patients who underwent surgery following the SPECT study. Given that a relatively small number of epilepsy patients typically undergo surgery, lack of subsequent surgical information was not an exclusion criterion, to ensure inclusion of as many patients as possible in the study.
Despite these limitations, this relatively large study confirms previous findings on the impact of the injection delay on the localization value of ictal SPECT in children and highlights the importance of automating the radiotracer injection using robust EEG-based seizure detectors and predictors39. Finally, this study also highlights that imaging a dynamically evolving seizure is a complex process that may depend on multiple factors beyond the radiotracer injection, including seizure dynamics and the origin of seizure onset. Improved knowledge of these dynamics could be incorporated into the seizure detector to further improve the SPECT result.
Highlights.
Ictal SPECT is a powerful non-invasive imaging modality for seizure localization in pediatric epilepsies
Successful ictal SPECT localization depends both on the timing of radiotracer injection and the cerebral lobe (particularly temporal versus frontal) of seizure onset
MRI abnormalities, seizure characteristics and epilepsy etiology do not significantly affect the ictal SPECT localization
Early radiotracer injection (within ≤ 25 s from seizure onset) is critical for optimizing the ictal SPECT result
Acknowledgments
This work was conducted with support from the Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1 TR001102) and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, or the National Institutes of Health.
Footnotes
Disclosure: None of the authors has any conflict of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Russ SA, Larson K, Halfon N. A National Profile of Childhood Epilepsy and Seizure Disorder. Pediatrics. 2012;129(2):256–264. doi: 10.1542/peds.2010-1371. [DOI] [PubMed] [Google Scholar]
- 2.Berg AT, Rychlik K, Levy SR, Testa FM. Complete remission of childhood-onset epilepsy: stability and prediction over two decades. Brain. 2014;137:3213–3222. doi: 10.1093/brain/awu294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perucca P, Hesdorffer DC, Gilliam FG. Response to first antiepileptic drug trial predicts health outcome in epilepsy. Epilepsia. 2011;52(12):2209–15. doi: 10.1111/j.1528-1167.2011.03283.x. [DOI] [PubMed] [Google Scholar]
- 4.Duncan R, Patterson J, Roberts R, Hadley DM, Bone I. Ictal/Postictal SPECT and the pre-surgical localization of complex partial seizures. J Neurol Neurosurg Psychiatry. 1993;56(2):141–148. doi: 10.1136/jnnp.56.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Brien TJ, So EL, Mullan BP, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50(2):445–454. doi: 10.1212/wnl.50.2.445. 1998. [DOI] [PubMed] [Google Scholar]
- 6.Duncan JS, Winston GP, Koepp MJ, Ourselin S. Brain imaging in the assessment for epilepsy surgery. Lancet. 2016;15(4):420–433. doi: 10.1016/S1474-4422(15)00383-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barr W, Ashtari M, Schaul N. Bilateral reductions in hippocampal volume in adults with epilepsy and a history of febrile seizures. J Neurol Neurosurg Psychiatry. 1997;63(4):461–467. doi: 10.1136/jnnp.63.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woermann FG, Sisodiya SM, Free SL, Duncan JS. Quantitative MRI in patients with idiopathic generalized epilepsy. Evidence of widespread cerebral structural changes. Brain. 1998;121(Pt 9):1661–1667. doi: 10.1093/brain/121.9.1661. [DOI] [PubMed] [Google Scholar]
- 9.Briellmann RS, Berkovic SF, Syngeniotis A, King MA, Jackson GM. Seizure-associated hippocampal volume loss: A longitudinal magnetic resonance study of temporal lobe epilepsy. Ann Neurol. 2002;51(5):641–644. doi: 10.1002/ana.10171. [DOI] [PubMed] [Google Scholar]
- 10.Cascino GD, Jack CR, Parisi JE, Sharbrough FW, Hirschorn KA, Meyer FB. Magnetic resonance imaging-based volume studies in temporal lobe epilepsy: pathological correlation. Ann Neurol. 1991;30:31–36. doi: 10.1002/ana.410300107. [DOI] [PubMed] [Google Scholar]
- 11.Meltzer CC, Adelson PD, Brenner RP, et al. Planned ictal FDG PET imaging for localization of extratemporal epileptic foci. Epilepsia. 2000;41(2):193–200. doi: 10.1111/j.1528-1157.2000.tb00139.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Sriram S, Delbeke D, et al. Aphasic or amnesic status epilepticus detected on PET but not EEG. Epilepsia. 2009;50(2):251–255. doi: 10.1111/j.1528-1167.2008.01782.x. [DOI] [PubMed] [Google Scholar]
- 13.Barrington SF, Koutroumanidis M, Agathonikou A, et al. Clinical Value of ‘Ictal’ and the Routine Use of FDG-Positron Emission Tomography Simultaneous Scalp EEG Studies in Patients with Intractable Partial Epilepsies. Epilepsia. 1998;39(7):753–766. doi: 10.1111/j.1528-1157.1998.tb01162.x. [DOI] [PubMed] [Google Scholar]
- 14.Won HJ, Chang KH, Cheon JE, et al. Comparison of MR Imaging with PET and Ictal SPECT in 118 Patients with Intractable Epilepsy. AJNR Am J Neuroradiol. 1999;20:593–599. [PMC free article] [PubMed] [Google Scholar]
- 15.Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy: A meta-analysis. Seizure. 2007;16:509–520. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Carne RP, O'Brien TJ, Kilpatrick CJ, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127(Pt 10):2276–2285. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- 17.Henry TR, Mazziotta JC, Engel J. Interictal Metabolic Anatomy of Mesial Temporal Lobe Epilepsy. Arch Neurol. 1993;50(6):582–589. doi: 10.1001/archneur.1993.00540060022011. [DOI] [PubMed] [Google Scholar]
- 18.Chugani DC, Chugani HT, Muzik O, et al. Imaging epileptogenic tubers in children with tuberous sclerosis complex using alpha-[11C]methyl-L-tryptophan positron emission tomography. Ann Neurol. 1998;44(6):858–866. doi: 10.1002/ana.410440603. [DOI] [PubMed] [Google Scholar]
- 19.Choi JY, Kim SJ, Hong SB, Seo DW, Hong SC, Kim BT, Kim SE. Extratemporal hypometabolism on FDG PET in temporal lobe epilepsy as a predictor of seizure outcome after temporal lobectomy. Eur J Nucl Med Mol Imaging. 2003;30(4):581–587. doi: 10.1007/s00259-002-1079-8. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Mountz JM. SPECT Imaging of Epilepsy: An Overview and Comparison with F-18 FDG PET. Int J Mol Imaging. 2011;2011:1–9. doi: 10.1155/2011/813028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drzezga A, Arnold S, Minoshima S, Noachtar S, Szecsi J, Winkler P, Romer W, Tatsch K, Weber W, Bartenstein P. 18F-FDG PET studies in patients with extratemporal and temporal epilepsy: evaluation of an observer-independent analysis. J Nucl Med. 1999;40(5):737–746. [PubMed] [Google Scholar]
- 22.Lee HW, Hong SB, Tae WS. Opposite ictal perfusion patterns of subtracted SPECT. Hyperperfusion and hypoperfusion. Brain. 2000;123(Pt 10):2150–2159. doi: 10.1093/brain/123.10.2150. [DOI] [PubMed] [Google Scholar]
- 23.Van Paesschen W. Ictal SPECT. Epilepsia. 2004;45(Suppl 4):35–40. doi: 10.1111/j.0013-9580.2004.04008.x. [DOI] [PubMed] [Google Scholar]
- 24.Treves ST, Chugani HT, Bourgeois BFD, Kuruc A. Central Nervous System: The Brain and the Cerebrospinal Fluid, Chapter 4. In: Treves ST, editor. Pediatric Nuclear Medicine and Molecular Imaging. Springer; New York, NY: 2014. [Google Scholar]
- 25.Ho S, Berkovic SF, Berlangieri SU, Newton MR, Egan GF, Tochon Danguy HJ. Comparison of ictal SPECT and interictal PET in the presurgical evaluation of temporal lobe epilepsy. Ann Neurol. 1995;37:738–745. doi: 10.1002/ana.410370607. [DOI] [PubMed] [Google Scholar]
- 26.Hwang S, Kim JH, Park SW, et al. Comparative analysis of MR imaging. Positron Emission Tomography, and Ictal Single-Photon Emission CT in patients with neocortical epilepsy. AJNR Am J Neuroradiol. 2001;22:937–946. [PMC free article] [PubMed] [Google Scholar]
- 27.Desai A, Bekelis K, Thadani VM, Roberts DW, Jobst BC, Duhaime AC, Gilbert K, Darcey TM, Studholme C, Siegel A. Interictal PET and ictal subtraction SPECT: sensitivity in the detection of seizure foci in patients with medically intractable epilepsy. Epilepsia. 2013;54(2):341–350. doi: 10.1111/j.1528-1167.2012.03686.x. [DOI] [PubMed] [Google Scholar]
- 28.Perissinotti Am, Setoain X, Aparicio J, et al. Clinical Role of Subtraction Ictal SPECT registered to MR Imaging and 18F-FDG PET in Pediatric Epilepsy. J Nucl Med. 2014;55(7):1099–1105. doi: 10.2967/jnumed.113.136432. [DOI] [PubMed] [Google Scholar]
- 29.Von Oertzen TJ, Mormann F, Urbach H, Reichmann K, Koening R, Clusmann H, Biersack HJ, Elger CE. Prospective use of subtraction ictal SPECT registered to MRI (SISCOM) in presurgical evaluation of epilepsy. Epilepsia. 2011;52(12):2239–2248. doi: 10.1111/j.1528-1167.2011.03219.x. [DOI] [PubMed] [Google Scholar]
- 30.La Fougere C, Rominger A, Forster S, Geisler J, Bartenstein P. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. 2009;15:50–55. doi: 10.1016/j.yebeh.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 31.Shoeb A, Edwards H, Connolly J, Bourgeois B, Treves ST, Guttag J. Patient-specific seizure onset detection. Epilepsy Behav. 2004;5:483–498. doi: 10.1016/j.yebeh.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Vera P, Kaminska A, Cieuta C, Hollo A, Stievenant JL, Gardin I, Ville D, Mangin JF, Plouin P, Dulac O, Chiron C. Use of subtraction ictal SPECT co-registered to MRI for optimizing the localization of seizure foci in children. J Nucl Med. 1999;40(5):786–792. [PubMed] [Google Scholar]
- 33.Kaminska A, Chiron C, Ville D, Dellatolas G, Hollo A, Cieuta C, Jalin C, Delalande O, Fohlen M, Vera P, Soufflet C, Dulac O. Ictal SPECT in children with epilepsy: comparison with intracranial EEG and relation to postsurgical outcome. Brain. 2003;126(1):248–260. doi: 10.1093/brain/awg013. [DOI] [PubMed] [Google Scholar]
- 34.Lee SK, Lee SY, Yun CH, et al. Ictal SPECT in neocortical epilepsies: clinical usefulness and factors affecting the pattern of hyperperfusion. Neuradiology. 2006;48(9):678–684. doi: 10.1007/s00234-006-0106-z. [DOI] [PubMed] [Google Scholar]
- 35.Thadani VM, Siegel A, Lewis P, Siegel AM, Jobst BC, Gilbert KL, Darcey TM, Roberts DW, Williamson PD. Validation of ictal single photon emission computed tomography with depth encephalography and epilepsy surgery. Neurosurg Rev. 2004;27(1):27–33. doi: 10.1007/s10143-003-0289-2. 2004. [DOI] [PubMed] [Google Scholar]
- 36.Barba C, Di Giuda D, Policicchio D, Bruno I, Papacci F, Colicchio G. Correlation between provoked ictal SPECT and depth recordings in adult drug-resistant epilepsy patients. Epilepsia. 2007;48(2):278–85. doi: 10.1111/j.1528-1167.2007.00935.x. 2007. [DOI] [PubMed] [Google Scholar]
- 37.Jalota A, Rossi MA, Pylypyuk V, et al. Resecting critical nodes from an epileptogenic circuit in refractory focal-onset epilepsy patients using subtraction ictal SPECT registered to MRI. J Neurosurg. 2016;18:1–12. doi: 10.3171/2015.6.JNS141719. [DOI] [PubMed] [Google Scholar]
- 38.Packard A, Roach PJ, Davis RT, Carmant L, Davis R, Riviello J, Holmes G, Barnes PD, O'Tuama LA, Bjornson B, Treves ST. Ictal and interictal technetium-99m-bicisate brain SPECT in children with refractory epilepsy. J Nucl Med. 1996;37(7):1101–1106. [PubMed] [Google Scholar]
- 39.Kim S, Holder DL, Laymon CM, Tudorascu DL, Deeb EL, Panigrahy A, Mountz JM. Clinical value of the first dedicated, commercially available automatic injector for ictal brain SPECT in presurgical evaluation of pediatric epilepsy: comparison with manual injection. J Nucl Med. 2013;54(5):732–738. doi: 10.2967/jnumed.112.105189. [DOI] [PubMed] [Google Scholar]


