Abstract
In the embryo a population of progenitor cells known as the second heart field forms not just parts of the heart but also the jaw muscles of the head. Here we show that it is possible to take skeletal muscle satellite cells from jaw muscles of the adult mouse and to direct their differentiation to become heart muscle cells (cardiomyocytes). This is done by exposing the cells to extracellular factors similar to those which heart progenitors would experience during normal embryonic development. By contrast, cardiac differentiation does not occur at all from satellite cells isolated from trunk and limb muscles, which originate from the somites of the embryo. The cardiomyocytes arising from jaw muscle satellite cells express a range of specific marker proteins, beat spontaneously, display long action potentials with appropriate responses to nifedipine, norepinephrine and carbachol, and show synchronized calcium transients. Our results show the existence of a persistent cardiac developmental competence in satellite cells of the adult jaw muscles, associated with their origin from the second heart field of the embryo, and suggest a possible method of obtaining cardiomyocytes from individual patients without the need for a heart biopsy.
Keywords: Second heart field, muscle satellite cell, cardiomyocytes, myogenesis, BMP, WNT
1. Introduction
In embryonic development the heart forms from three separate regions of the early mesoderm. The primary heart field gives rise to the atria and left ventricle, while the second heart field gives rise to the right ventricle and outflow tract, and, at least in the chick embryo, there is also a third heart field forming the pacemaker region (Bressan et al., 2013; Buckingham, 2005). The second heart field is part of the cranial splanchnic mesoderm and, in addition to forming parts of the heart, also forms the jaw muscles of the head (Buckingham, 2001; Grifone and Kelly, 2007; Lescroart et al., 2010; Nathan et al., 2008). The second heart field is characterized by expression of the transcription factor Islet1 (ISL1) (Black, 2007; Cai et al., 2003; Laugwitz et al., 2008; Laugwitz et al., 2005; Moretti et al., 2006).
In skeletal muscle, stem cells, called satellite cells, are the source of new muscle fibers during normal growth and are also responsible for generating new fibers following muscle damage in adult life (Dhawan and Rando, 2005; Hawke and Garry, 2001; Morgan and Partridge, 2003; Seale and Rudnicki, 2000). As might be expected from the different origins of the parent muscles, the jaw and trunk skeletal muscle satellite cells are also derived from distinct embryologic cell populations and may have different regenerative abilities (Gayraud-Morel et al., 2012). Trunk and limb muscle satellite cells originate from the somites (Daughters et al., 2011; Gros et al., 2005; Relaix et al., 2005), while satellite cells of the jaw muscles originate from the second heart field (Harel et al., 2009; Nathan et al., 2008; Sambasivan et al., 2009). Because of the close developmental relationship between jaw muscles and heart, we postulated that the satellite cells of the jaw muscles in adult animals might retain aspects of secondary heart field developmental competence and still be capable of developing into cardiomyocytes.
Here we show, for the first time, that it is possible to take satellite cells from jaw muscles of the adult head and to direct their differentiation to become embryo-type cardiomyocytes. This is done by exposing the cells to extracellular factors similar to those which heart progenitors would experience during normal embryonic development. By contrast, cardiac differentiation does not occur at all from satellite cells isolated from trunk and limb muscles, which originate from the somites of the embryo. The cardiomyocytes arising from jaw muscle satellite cells express a range of specific marker proteins, beat spontaneously, display long action potentials with appropriate responses to nifedipine, norepinephrine and carbachol, and show synchronized calcium transients. Our results show the existence of a persistent cardiac developmental competence in satellite cells of the adult jaw muscles, associated with their origin from the second heart field of the embryo, and suggest a possible method of obtaining cardiomyocytes from individual patients without the need for a heart biopsy.
2. Materials and Methods
2.1 Cell Cultures
All animal procedures were conducted under IACUC protocol 1002A78295. Satellite cell-derived myoblasts of the jaw (digastric and masseter) or from trunk (limbs and back) muscles were isolated by magnetic cell sorting (MACS) or fluorescence activated cell sorting (FACS) using sequential sorting for CD45, CD31, SCA1 negative, followed by positive selection for integrin β-1 and integrin α-7 cells, as previously described (Hirai et al., 2010). Myoblasts were cultured in myoblast medium containing 20% fetal bovine serum (FBS) (Asakura et al., 2002) supplemented with 100ng/ml bone morphogenetic protein 4 (BMP4) (R&D Systems) plus 10ng/ml basic fibroblast growth factor (bFGF) (R&D Systems) for four days followed by 100ng/ml dickkopf 1 (DKK1), 10ng/ml bFGF and 5ng/ml vascular endothelial growth factor (VEGF) for two days and allowed to differentiate in low serum Dulbecco's minimal essential medium (DMEM) (Gibco) with 5% FBS or serum free (StemPro, Gibco) to a total of twenty-one days. Some initial experiments were conducted using a modified differentiation scheme that consisted of myoblast medium supplemented with 100ng/ml BMP4 for two days followed by 100ng/ml DKK1 for three days.
Movies of beating cardiomyocytes were taken from day 21 cultures isolated from the myotubes and re-plated in differentiation medium for 2 days. Video was recorded at 7 frames/sec for 180 seconds. For single-fiber muscle preparations, masseter or digastric muscles were removed and incubated in 0.2% type I collagenase in DMEM for 45 minutes at 37°C. Isolation of single fibers was achieved through mechanical disruption. They were cultured in myoblast medium for 12 hours with or withour BMP4 (100ng/ml) and then immunostained with antibodies for PAX7 and NKX2.5.
2.2 RNA and gene expression
Two step RT-PCR was performed on an ABI Prism 7500 Real Time PCR System (Applied Biosystems, Foster City, Calif.). Total RNA was isolated from cultured cells during the differentiation protocol using Trizol reagent (Invitrogen). cDNA was generated from 5 μg of total RNA using 1st Strand Synthesis Supermix primed with random hexamers (Invitrogen). Relative qRT-PCR was performed on 1 μl of cDNA using qRT-PCR SYBER Green Master Mix (Invitrogen) with murine specific primers for myogenic (Pax7, M-Cad, Myf5, MyoD), early cardiac progenitor cell (Nkx2.5, Tbx5, Gata4, Isl1,Tbx1), or late cardiomyocyte specific (Myh6, Cav1.2, CX43, MLC2v, cTnT) transcription factor genes. Primer sequences can be furnished upon request. Two stage PCR was performed with each sample cDNA/primer pair done in triplicate. Relative quantification compared to control was estimated using the threshold cycle (CT) of the transcription factor normalized to the CT of the housekeeping gene Gapdh. Dissociation curve and ethidium bromide gel analysis was used to assess PCR product purity at the end of each qPCR run. Graphs are plotted as Relative Quantification Values or ratio to GAPDH.
2.3 Cell lineage analysis
To confirm the second heart field origin of jaw muscles we crossed Isl1-Cre mice (Yang L et al., 2006) to the reporter line mT/mG (Jackson labs) (Muzumdar et al., 2007) to generate Isl1-Cre;mT/mG embryos. E13.5 embryos were collected by perfusion with variable fixation procedure described previously (Daughters et al., 2001). Embryos were embedded in OCT medium and frontal sections through the head region were collected on slides. Slides were visualized for GFP and dTomato staining and further processed for MHC (MF20; DSHB) or Pax7 (DSHB) immunostaining.
For lineage labeling of satellite cells we bred Pax7-CreER mice (Lepper et al., 2009) with mT/mG mice. Pax7-CreER;mT/mG mice were injected with Tamoxifen (3 × 5mg at 3 day intervals) prior to isolation of satellite cells from the masseter and digastric muscles of the head. Satellite cell derived myoblasts were induced to form cardiomyocytes according the above differentiation scheme. Beating aggregates of induced cardiomyocytes were re-plated on glass culture slides and immunostained for cTnT (CT-3; DSHB) using a far-red tagged (Cy5) secondary antibody and visualized for co-localization with GFP.
For studies on the contribution of Isl1 lineage derived satellite cells, jaw derived satellite cells were isolated from Isl1-Cre;mTmG mice generated from crossing Isl1-Cre to the reporter mTmG (Jackson labs). For the Isl1 derived satellite cells experiments, satellite cells were isolated from the masseter muscle of four mice per biological sample by collagenase/dispase digestion. Isl1 lineage derived GFP+ cells were sorted to obtain 100% positive expressing cells using the FACS Aria (BD). Cells were then subjected to the cardiomyocyte differentiation scheme and assayed for colocalization of NKX2.5 expression at day 7, or cTNT expression at day 14, with GFP.
2.4 Immunofluorescence and microscopy
Cell culture slides were fixed with 2% paraformaldehyde (PFA) (pH 8.5) for 15 minutes at room temperature and stored in 1Xphosphate buffered saline A (PBSA) at 4°C until processing. Isl1Cre;mT/mG mouse embryos were fixed using a variable pH PFA fixative procedure modified from (Daughters et al., 2001) overnight at 4°C, washed in 1XPBSA and hardened overnight in 30% sucrose at 4°C. The following day embryos were embedded in OCT (optimal cutting temperature) medium over dry ice and stored at −80°C until processing. Embryos were processed by collecting 10μm thick frontal sections through the head. Both cell culture slides and embryos were processed for expression of markers of myogenesis, cardiogenesis or mature cardiomyocytes. Briefly, slides were washed in 1xPBSA, permeabalized in PBSA containing 0.1% Triton, blocked in 5% normal goat serum (NGS) for 2 hours at RT and incubated overnight with primary antibodies to either PAX7 (1/1000; Developmental Studies Hybridoma Bank, DSHB), MHC (MF20; 1/500; DSHB), NKX2.5 (1/200; Santa Cruz), GATA4 (1/500; ABCAM), Myogenin (F5D, 1:100; DSHB), cTnT (CT-3; 1/500; DSHB) in 5% NGS at 4°C. The next day slides were washed 3 X 1 hour in PBSA, blocked in 5%NGS for 1 hour and incubated overnight in the appropriate secondary antibodies: Alexa-594 anti-rabbit, Alexa-488 anti-mouse or Alexa-634 anti-mouse (1/2000; Invitrogen) at 4°C. Slides were mounted using Vectashield mounting medium containing DAPI (Vector labs) and visualized on a Fluoview 1000 confocal microscope with FV1000 analysis software (Olympus). Photomicrographs are composite images of 1-5μm optical slices through the tissue compressed along the Z-axis. Images for figures were further processed using Photoshop (Adobe Systems) by cropping and by appropriate uniform and linear adjustments of brightness and contrast.
2.5 Electrophysiology
For electrophysiological recordings, induced cardiomyocytes were isolated from cultures on or after day 14 by picking or gentle dissociation with 0.1% collagenase, plated on glass culture slides, and covered in a perfusion chamber. Whole cell current clamp recordings were obtained from cells that were continuously superfused with solution containing 146 mM NaCl, 3 mM KCl, 10 mM HEPES, 2 mM CaCl2, 2 mM MgCl2, 1.25 mM NaH2PO4, 1 mM Na pyruvate, and 10 mM D-glucose (pH 7.4, NaOH). Patch pipettes contained 140 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 11 mM EGTA, 5 mM HEPES, 1 mM glutathione, 3 mM ATP-2K, 2 mM glucose, 0.5 mM GTP-Na (pH 7.2, KOH). Recordings were made at room temperature using pipettes with resistances ranging from 2-5 Mohm, a Multiclamp 700A amplifier, Digidata 1322A, and pClamp 9.2 acquisition software (Molecular Devices, Sunnyvale, CA). Junction potentials and electrode resistance were nulled, data was acquired at 10 kHz, and filtered at 2 kHz for analysis.
For calcium measurements, cells were loaded for 60-90 minutes with 10 μM Fluo-3AM in culture medium or superfusate, at either 37°C or room temperature. Cells were rinsed with superfusate and imaged after 20 or more minutes thereafter. The perfusion chamber was placed on the stage of an Olympus BX-51W microscope outfitted with a Lambda DG-4 fluorescence light source and filter changer (Sutter Instrument, Novato, CA) and a Paultek intensified charge-coupled device. The excitation filter was 470 nm/40 nm and the emission filter was 525 nm/50 nm. Metamorph/Metafluor Imaging Suite (Molecular Devices, Sunnyvale, CA) was used to acquire images at 200 msec intervals. Images were background subtracted and fluorescence intensity in regions corresponding to individual cells was plotted versus time.
3. Results
3.1 Cell lineage of jaw muscles
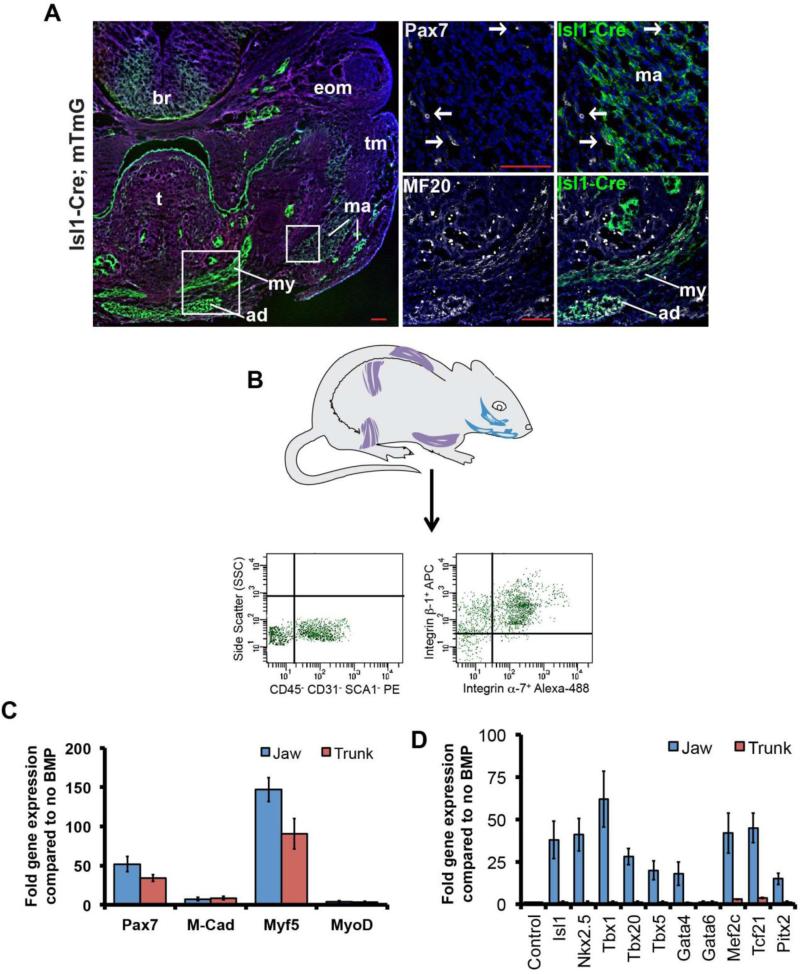
To confirm that the jaw muscles of the head do indeed originate from the secondary heart field, we lineage labeled Isl1-expressing cells using Isl1-Cre mice crossed to a reporter (mTmG), which expresses GFP following Cre-mediated recombination (Muzumdar et al., 2007; Yang L et al., 2006). Consistent with the previous findings (Nathan et al., 2008), we found that the Isl1-positive lineage contributes specifically to the masseter and digastric (jaw) muscles but not to the tongue or the extraocular muscles (Fig.1A). In addition, we confirmed that the satellite cells within the jaw muscles, identified by immunostaining for PAX7, also derived from the Isl1 lineage (Fig.1A).
Fig.1. Satellite cells from jaw muscles are derived from the secondary heart field and express cardiac transcription factors in response to BMP4.
A. Isl1-Cre;mT/mG mouse, frontal section through the head of E13.5 embryo showing that jaw muscles are derived from Isl1-positive precursors. High power views are shown on the right. Myofibers, immunostained for MF20, and satellite cells, immunostained for PAX7, are both derived from Isl1-positive precursors. White boxes on low magnification image indicate the location of the high magnification images on the right. The masseter (ma) consists of both deep and superficial masses; (ad) anterior digastric; (my) mylohyoid; (t) tongue; (tm) temporalis; (eom) extraocular muscles; (br) brain. Scale bars: 100μm. B. Schematic of representative muscles of adult mice used to prepare satellite cells, and typical sequential FACS plot. C. Absence of difference between jaw and trunk-derived satellite cells in myogenic transcription factor gene expression in the presence of BMP4 (day 3). D. Upregulation of cardiogenic transcription factor genes in jaw but not trunk satellite cells in the presence of BMP4 (day 3).
3.2 Myogenic and cardiogenic differentiation in vitro
For the reasons explained above, we tested to find whether the satellite cells of the adult jaw muscles differed in their differentiation potential from those of the trunk. We isolated jaw (masseter and digastric muscles), and trunk (all muscle from fore and hind limbs and back) satellite cells by sequential FACS or MACS using negative selection for CD45, CD31, SCA1, followed by positive selection for β1 and α7-integrin (Hirai et al., 2010) (Fig.1B). In normal low serum myoblast culture conditions, the isolated satellite cells from both jaw and trunk muscle quickly lose expression of PAX7, start to express myogenic transcription factors (MYOD, Myogenin), and then differentiate into multinucleated myotubes. However, we found that culture of isolated satellite cells with BMP4 and 20% serum can maintain cells in a non-differentiating progenitor state in which PAX7 expression is retained by many cells. Under these conditions there was no significant difference in expression of genes encoding myogenic transcription factors (Pax7, Myf5, Myod) between the jaw- and the trunk-derived muscle progenitors (Fig.1C). However, when we looked at expression of a range of markers of early cardiac progenitors we found that satellite cells from jaw, but not the trunk, muscle expressed them in the presence of BMP4 (Fig.1D). Expression of some of these genes, such as Tbx1 and Isl1, is specifically characteristic of the second heart field of the embryo (Laugwitz et al., 2008; Olson, 2006; Plageman and Yutzey, 2005).
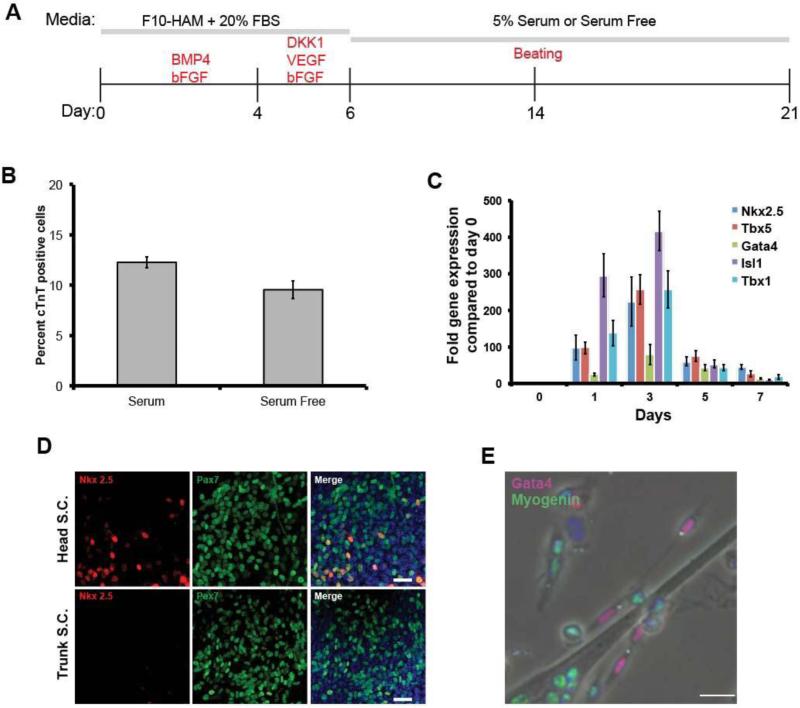
We then cultured isolated jaw muscle satellite cells under conditions that mimic the environment found in the embryo during normal heart field specification (Cohen et al., 2007; Cohen et al., 2008; Kattman et al., 2011; Klaus et al., 2007; Marvin et al., 2001; Prall et al., 2007; van Wijk et al., 2007) (Fig.2A). Cells were first cultured under proliferation conditions with BMP4 and bFGF for four days, followed by DKK1, an inhibitor of Wnt signaling, together with bFGF and VEGF in either 5% serum, or the serum substitute Stempro34. bFGF and VEGF were removed at day 6, followed by culture for a further 8-15 days in the low serum, or serum free, medium.
Fig.2. Formation of cardiomyocyte precursors from jaw muscle satellite cells.
A. Scheme of the growth and differentiation protocol. B. Percentage of cTnT-positive cells generated by the end of the differentiation period, with and without serum. Error bars indicate ± standard error of the mean. C. qRT-PCR of expression of myogenic and cardiogenic transcription factors during the first 7 days. Error bars indicate ± standard error of the mean. D. NKX2.5 (red) is found only in those cells expressing PAX7 (green) at day 3 cultures. Scale bar 25μm. E. At 7 days of culture, myogenin (green) is not co-expressed with GATA4 (red). Scale bar 25μm.
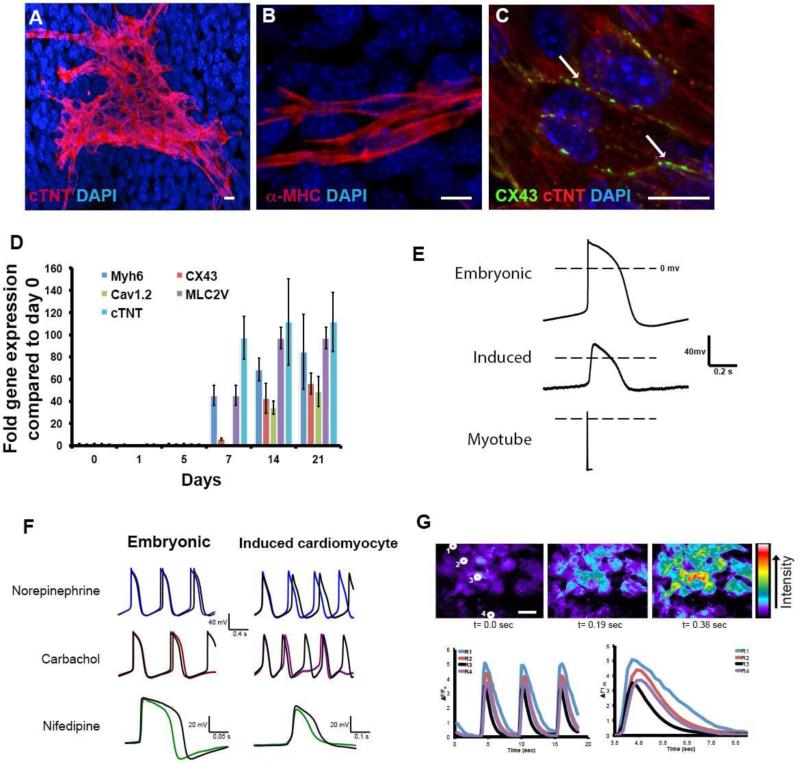
In vivo, jaw muscle satellite cells do not express cardiac transcription factors and this remains the case after isolation (Fig.2C, day 0 timepoint, also Fig.S1). However, by the next day in the proliferation medium early cardiac progenitor cell markers appear (Nkx2.5, Tbx5, Gata4, Isl1, Tbx1) (Fig.2C, later timepoints). In these early stage cultures (up to day 7 of the protocol), NKX2.5 was only found in cells expressing PAX7 (Fig.2D). In the later stages of culture, co-expression of skeletal and cardiac markers was no longer seen. Fig.2E shows expression of a myogenic factor, Myogenin, only in differentiating myotubes and mononuclear cells, while a cardiac transcription factor, GATA4, is seen in separate mononuclear cells. By 7 days, cells were expressing markers of differentiated cardiomyocytes: cardiac troponin T (cTnT), α-myosin heavy chain (=MHC6), myosin light chain 2v, voltage-gated calcium channel 1.2 and connexin 43, all normally found in differentiated cardiomyocytes but not in skeletal myofibers (Fig.3A-D). Starting between 10-15 days, 2.6% cells exhibited spontaneous beating (see supplemental movie 1). Analysis of the final cell population, by RT-PCR and by flow cytometry, indicated that the cells are almost entirely made up of skeletal myoblasts or myotubes together with some cardiomyocytes, and essentially no smooth muscle, endothelial cells or blood cells (Fig.S2) Trunk-derived satellite cells treated in the same way do not show any cardiac differentiation at all, either in terms of early expression of cardiac transcription factors, or in terms of later expression of cardiac differentiation markers. For the jaw-derived satellite cells, the period of WNT inhibition is essential. If the cells are left in BMP4, there is no further differentiation toward either skeletal muscle or cardiomyocytes. If the serum is subsequently lowered to a level (5%) allowing myogenic differentiation, then they undergo the usual differentiation to skeletal myotubes. Only if DKK1 is administered from day 4-6, do some of the cells progress to cardiomyocytes in the low serum or serum-free conditions. By day 7, 23.7% (SEM ±1.09) of cells express cardiac progenitor cell markers. At the end of the culture period, 12.3% (SEM ±0.56) of cells show staining for cardiac troponin T (cTnT, Fig.3A). The cardiomyocyte differentiation proceeds similarly in serum-free medium as it does in 5% serum (Fig.2B).
Fig.3. Characterization of induced cardiomyocytes.
A-C. Induced cardiomyocytes visualized by immunostaining at day 14 of culture. A. Cardiac troponin T (cTnT). B. α-myosin heavy chain. C. Connexin 43 showing gap junctions between adjacent cells D. Expression of five genes for cardiomyocyte terminal differentiation markers (qRT-PCR). Error bars indicate ± standard error of the mean. E. Typical spontaneous action potentials for E13.5 embryonic cardiomyocytes; induced cardiomyocyte; and evoked action potential for skeletal myotube. The duration of the induced cardiomyocyte action potentials is similar to those of the embryonic cardiomyocytes and much longer than those of the myotubes. F. Effects of norepinephrine, carbachol and nifedipine on spontaneous action potentials. Colored overlayed traces indicate spontaneous action potential after addition of each drug. G. Calcium transients in induced cardiomyocytes visualized with Fluo3AM. The traces indicate the signal for the four indicated cells, which are highly synchronized. Scale bar 20μm.All measurements were repeated several times as indicated, with standard errors, in the text.
3.3 Physiological characterization of induced cardiomyocytes
In the induced cardiomyocyte cultures, 2.6% (SEM ±0.92) of the cells exhibit spontaneous beating (supplemental movie 1). Beating appearing from 10-15 days, the same time at which the differentiated cardiomyocyte markers become clearly evident. In order to confirm their identity as bona fide cardiomyocytes we have carried out a range of physiological studies. First we made whole cell current clamp recordings. Spontaneously beating cells show spontaneous action potentials of duration (APD90) 394 msec (SEM ± 36; n=19; Fig.3E). This is a long duration, similar to action potentials observed in embryonic cardiomyocytes in culture (239 ± 87 msec; n=4). By contrast, the skeletal muscle myotubes derived from the trunk satellite cell population had a stable resting membrane potential, and action potentials evoked by current injection were much briefer in duration (APD90 of 10 ±3 msec ; n=3) with a steep depolarization phase, followed by a rapid repolarization and afterhyperpolarization (Fig.3E). Secondly, we have examined the response to various drugs. Nifedipine is a dihydropyridine Ca2+ channel blocker. It reduced the amplitude and duration of spontaneous action potentials and/or reduced the frequency of action potentials in both induced cardiomyocytes (11/15 cells) and cardiomyocytes taken from E13.5 embryos (2/3 cells); (Fig.3F). The reduced amplitude and duration of the action potentials in the presence of nifedipine indicates the involvement of L-type Ca2+ channels in the depolarization and plateau phase of the action potentials. The spontaneous action potentials returned when nifedipine was washed out, demonstrating a clear role of L-type Ca2+ channels in the pacemaker potentials of induced cardiomyocytes. Carbachol, which is a muscarinic cholinergic agonist, reduced the frequency of spontaneous action potentials in induced cardiomyocytes (12/16), while the catecholamine norepinephrine increased the frequency (11/17; Fig.3F). Finally we have examined calcium transients. Cardiomyocytes normally exhibit a rise in internal calcium coincident with the action potential. Cells were loaded with the calcium-sensitive dye fluo-3AM, and fluorescence was measured over time. In 7/7 experiments, cells showed regular calcium oscillations and also showed synchronization of adjacent cell groups, indicating that they are physiologically coupled (Fig.3G and movie S2). In all respects: response to Ca2+ channel blocker, to cholinergic and adrenergic agents, and Ca2+ oscillation, the behavior of the induced cardiomyocytes is very similar to that of embryonic cardiomyocytes (Boheler, 2002; Maltsev et al., 1994; Maltsev et al., 1999).
3.4 Cell lineage of induced cardiomyocytes
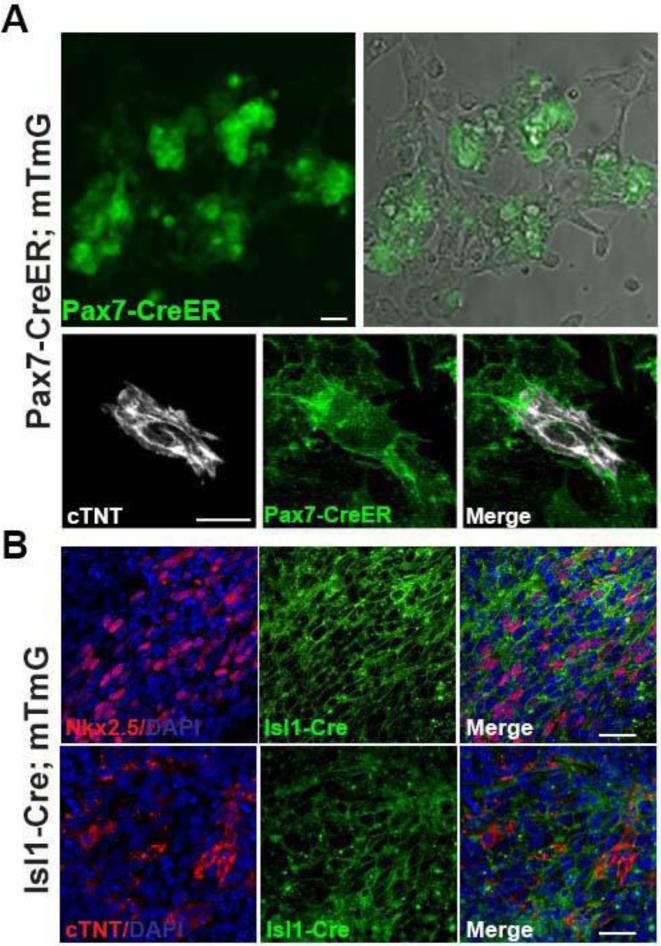
Although we show that there is co-expression of PAX7 and NKX2.5 in the early stages of the cultures, this does not absolutely prove that the cardiomyocytes observed later are actually derived from those cells rather than from some minor contaminant of the preparations. To confirm that the cardiomyocytes from the jaw muscle cultures really do originate from the satellite cells, we permanently labeled Pax7-positive cells using an inducible Pax7-CreER reporter line of mice (Lepper et al., 2009) treated with tamoxifen at least 3 days before sacrifice. In these mice there is no expression of the reporter in the heart (Fig.S3). Jaw muscle satellite cells were isolated using the method described above and 86.4% (SEM ±3.6) of the recovered cells were green, indicating successful recombination. After differentiation following the procedure of Fig.2A, all of the spontaneously beating cells were green, and all of the cTnT-immunopositive cells were green, indicating that they were indeed derived from cells that expressed Pax7 before isolation (Fig.4A).
Fig.4. Cell lineage studies.
A. Evidence that induced cardiomyocytes are derived from satellite cells. Pax7 expressing cells were labeled by treating the Pax7-CreER;mT/mG mice with tamoxifen. The green label indicates that cTnT-positive cardiomyocytes formerly expressed Pax7. Scale bar 25μm. B. Evidence that induced cardiomyocytes are derived from the secondary heart field. Satellite cells were prepared from Isl1-Cre;mTmG mice and then sorted for GFP expression, and subjected to the cardiomyocyte differentiation procedure. The NKX2.5 and cTnT-positive cells must be derived from cells that had expressed Isl1 in vivo. Scale bar: 25μm.
To close the loop on the cell origin, we also examined cells from Isl1-Cre;mTmG mice. Satellite cells were isolated from the jaw muscles and sorted for green fluorescence. Thus all the cells had formerly expressed Isl1. They were subject to the factor treatments of Fig.2A and 33.2% (SEM ± 9.2; n=6) of total cells were obtained positive for NKX2.5, 11.8 % (SEM ± 3.7; n=6) were positive for cTnT, confirming an origin from those cells in the jaw muscles that had once been part of the second heart field of the embryo (Fig.4B).
4. Discussion
This work shows for the first time that cardiomyocytes can be derived in culture from skeletal muscle satellite cells of adult mice. This occurs only if the satellite cells are derived from jaw muscles and not if they are derived from trunk or limb muscles. The mammalian heart itself probably does not possess stem cells and the cell turnover is very limited (Bergmann et al., 2009; Mummery and Passier, 2011; Porrello et al., 2011), so this is a very dramatic phenotypic reprogramming event.
In terms of cell characterization it is important to be sure that the induced cardiomyocytes are really cardiomyocytes. No one marker or behavior is 100% conclusive for cardiomyocyte character, for example skeletal muscle cells can occasionally show spontaneous beating. However the concordance of many markers and characteristics is conclusive. We have demonstrated the presence of several specific molecular markers at the RNA and protein level and several distinct and specific physiological characteristics: long action potentials with appropriate responses to nifedipine, norepinephrine and carbachol, and synchronized calcium transients. All of these properties together indicate that the cells are cardiomyocytes of an embryonic type and not a deviant type of skeletal muscle cell.
The percentage yield of induced cardiomyocytes may seem low. However the figure of 2.6% relates only to beating cells, and many bona fide embryo-derived cardiomyocytes do not beat in culture. The more appropriate figures are 23.7% that express cardiac transcription factors during the proliferation phase and the 12.3% that express cardiac troponin. These are high numbers, for example compared to the yield of cardiomyocytes in some direct reprogramming experiments (Ieda et al., 2010). The remainder of the cells become skeletal myotubes. It is possible that further testing of different types of media might increase the yield of cardiomyocytes closer to 100%, but it is equally possible that a majority of adult jaw muscle satellite cells do have an irreversibly determined commitment to skeletal muscle. In fact the yield is the same when cells are sorted for previous Isl1 expression in vivo, guaranteeing origin from the second heart field, so it cannot be due to the fact that some jaw muscle cells may not come from the second heart field. The yield is certainly high enough to be confident that the cardiomyocytes are really derived from satellite cells and not from some minor contaminant in the preparation. This is further proved by the fact that when the cells were labeled for expression of the satellite cell marker Pax7 in vivo, all of the induced cTnT-positive cells are labeled. This experiment (Fig.4A) clearly excludes a non-satellite cell contaminant as the cell of origin for the induced cardiomyocytes.
We attribute the ability to produce cardiomyocytes from jaw muscle satellite cells to the fact that the jaw muscles, and their satellite cells, arise from the second heart field of the embryo. We presume that the chromatin state of the cells differs from that of the somite-derived satellite cells and enables responsiveness to the BMP, FGF and Wnt signals that control cardiac development. Investigating the difference in chromatin state between the two types of satellite cells will be an important project for the future. The fact that both heart and skeletal muscle emerges from a common region of the pharyngeal mesoderm has in fact been much discussed recently and the molecular basis for the formation of the two cell types is currently being elucidated (Harel et al., 2012; Tzahor and Evans, 2011). There are also some hints from previous work of cardiac features of jaw muscle. In particular, some presence of cardiac myosin heavy chain was noticed in human and rabbit jaw muscles (Bredman et al., 1991), and some transient expression of cardiac transcription factors was seen in embryonic jaw muscles of mice (Grifone and Kelly, 2007; Harel et al., 2009).
Apart from the developmental biology interest of the phenomenon, our result also suggests that it may be possible to generate individual-specific cardiomyocytes from jaw muscle biopsies of human patients, expanded in BMP before differentiation. The ability to expand the cells in BMP is shown here and was also reported by Ono et al. (Ono et al., 2011). Cardiomyocytes derived from such cultures could be used for a variety of diagnostic and drug sensitivity tests. Although it is now possible to make cardiomyocytes from induced pluripotent stem cells prepared from skin biopsies (Freund and Mummery, 2009; Zhang et al., 2009; Zwi et al., 2009), or by direct reprogramming (Ieda et al., 2010) (Islas et al., 2012; Song et al., 2012), our work suggests an alternative potential source for cardiomyocytes without the need for any genetic manipulation.
Supplementary Material
Acknowledgements
Atsushi Asakura for Pax7-CreER mice.
Sylvia Evans and Yasu Kawakami for Isl1-Cre mice, Michael Kyba and Dan Garry for discussions.
Financial support:
NIH (NHLBI) U01HL100407
NIH (NHLBI) U01HL099997 subcontract.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
Randall Daughters: Conception and design, collection of data, data analysis, manuscript writing.
Susan Keirstead: Collection of data, data analysis.
Jonathan Slack: Financial support, intellectual support, manuscript writing.
Competing interests
The authors have no conflicts of interests to disclose.
References
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J. Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL. Transcriptional pathways in second heart field development. Seminars in Cell & Developmental Biology. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boheler KR, Czyz J, Tweedie D, Yang H-T, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ. Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- Bredman JJ, Wessels A, Weijs WA, Korfage JAM, Soffers CAS, Moorman AFM. Demonstration of cardiac-specific myosin heavy-chain in masticatory muscles of human and rabbit. Histochemical Journal. 1991;23:160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Bressan M, Liu G, Mikawa T. Early Mesodermal Cues Assign Avian Cardiac Pacemaker Fate Potential in a Tertiary Heart Field. Science. 2013;340:744–748. doi: 10.1126/science.1232877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. Skeletal muscle formation in vertebrates. Curr. Op. Genetics and Dev. 2001;11:440–448. doi: 10.1016/s0959-437x(00)00215-x. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nature Reviews Genetics. 2005;6:826–837. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang XQ, Shi YQ, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev. Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Wang ZS, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, Morrisey EE. Wnt/beta-catenin signaling promotes expansion of Isl-1 - positive cardiac progenitor cells through regulation of FGF signaling. Journal of Clinical Investigation. 2007;117:1794–1804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Hofbauer RD, Grossman AW, Marshall AM, Brown EM, Hartman BK, Faris PL. Ondansetron attenuates CCK induced satiety and c-fos labeling in the dorsal medulla. Peptides. 2001;22:1331–1338. doi: 10.1016/s0196-9781(01)00460-0. [DOI] [PubMed] [Google Scholar]
- Daughters RS, Chen Y, Slack JMW. Origin of muscle satellite cells in the Xenopus embryo. Development. 2011;138:821–830. doi: 10.1242/dev.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15:666–673. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Freund C, Mummery CL. Prospects for pluripotent stem cell-derived cardiomyocytes in cardiac cell therapy and as disease models. J.Cell Biochem. 2009;107:592–599. doi: 10.1002/jcb.22164. [DOI] [PubMed] [Google Scholar]
- Gayraud-Morel B, Chretien F, Jory A, Sambasivan R, Negroni E, Flamant P, Soubigou G, Coppee JY, Di Santo J, Cumano A, Mouly V, Tajbakhsh S. Myf5 haploinsufficiency reveals distinct cell fate potentials for adult skeletal muscle stem cells (vol 125, pg 1738, 2012). Journal of Cell Science. 2012;125:6198–6198. doi: 10.1242/jcs.097006. [DOI] [PubMed] [Google Scholar]
- Grifone R, Kelly RG. Heartening news for head muscle development. Trends Genet. 2007;23:365–369. doi: 10.1016/j.tig.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimarães-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Dev. Cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel I, Maezawa Y, Avraham R, Rinon A, Ma HY, Cross JW, Leviatan N, Hegesh J, Roy A, Jacob-Hirsch J, Rechavi G, Carvajal J, Tole S, Kioussi C, Quaggin S, Tzahor E. Pharyngeal mesoderm regulatory network controls cardiac and head muscle morphogenesis. Proceedings of the National Academy of Sciences. 2012;109:18839–18844. doi: 10.1073/pnas.1208690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- Hirai H, Verma M, Watanabe S, Tastad C, Asakura Y, Asakura A. MyoD regulates apoptosis of myoblasts through microRNA-mediated down-regulation of Pax3. J. Cell Biol. 2010;191:347–365. doi: 10.1083/jcb.201006025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu J-D, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas JF, Lic Y, Weng K-C, Robertson MJ, Zhang S, Prejusa A, Harger J, Tikhomirova D, Chopra M, Iyer D, Mercola M, Oshima RG, Willerson JT, Potaman VN, Schwartz RJ. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc. Natl. Acad. Sci. USA. 2012;109:13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell. 2011;8:228–240. doi: 10.1016/j.stem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18531–18536. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugwitz K-L, Moretti A, Caron L, Nakano A, Chien KR. Islet1 cardiovascular progenitors: a single source for heart lineages? Development. 2008;135:193–205. doi: 10.1242/dev.001883. [DOI] [PubMed] [Google Scholar]
- Laugwitz KL, Moretti A, Lam J, Gruber P, Chen YH, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JXJ, Evans S, Chien KR. Postnatal isl1+cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepper C, Conway SJ, Fan CM. Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. 2009;460:627–631. doi: 10.1038/nature08209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Kelly RG, Le Garrec J-F, Nicolas J-F, Meilhac SM, Buckingham M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137:3269–3279. doi: 10.1242/dev.050674. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Wobus AM, Rohwedel J, Bader M, Hescheler J. Cardiomyocytes differentiated in vitro from embryonic stem cells developmentally express cardiac-specific genes and ionic currents. Circ. 1994;75:233–44. doi: 10.1161/01.res.75.2.233. [DOI] [PubMed] [Google Scholar]
- Maltsev VA, Ji GJ, Wobus AM, Fleischmann BK, Hescheler J. Establishment of β-Adrenergic Modulation of L-Type Ca2+ Current in the Early Stages of Cardiomyocyte Development. Circ.Res. 1999;84:136–145. doi: 10.1161/01.res.84.2.136. [DOI] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes & Development. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen YH, Qyang YB, Bu L, Sasaki M, Martin-Puig S, Sun YF, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic Isl1(+) progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–1165. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Morgan JE, Partridge TA. Muscle satellite cells. Int. J. Biochem. Cell Biol. 2003;35:1151–1156. doi: 10.1016/s1357-2725(03)00042-6. [DOI] [PubMed] [Google Scholar]
- Mummery CL, Passier R. New perspectives on regeneration of the heart. Circulation Research. 2011;109:828–829. doi: 10.1161/RES.0b013e3182349a8a. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo LQ. A global double-fluorescent cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nathan E, Monovich A, Tirosh-Finkel L, Harrelson Z, Rousso T, Rinon A, Harel I, Evans SM, Tzahor E. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Calhabeu F, Morgan JE, Katagiri T, Amthor H, Zammit PS. BMP signalling permits population expansion by preventing premature myogenic differentiation in muscle satellite cells. Cell Death Diffn. 2011;18:222–234. doi: 10.1038/cdd.2010.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plageman TF, Yutzey KE. T-box genes and heart development: Putting the “T” in heart. Developmental Dynamics. 2005;232:11–20. doi: 10.1002/dvdy.20201. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient Regenerative Potential of the Neonatal Mouse Heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prall OWJ, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, Stennard FA, Wise N, Schaft D, Wolstein O, Furtado MB, Shiratori H, Chien KR, Hamada H, Black BL, Saga Y, Robertson EJ, Buckingham ME, Harvey RP. An Nkx2-5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Rocancourt D, Mansouri A, Buckingham M. A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. 2005;435:948–53. doi: 10.1038/nature03594. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Dev. Cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Seale P, Rudnicki MA. A new look at the origin, function, and “stem-cell” status of muscle satellite cells. Dev. Biol. 2000;218:115–124. doi: 10.1006/dbio.1999.9565. [DOI] [PubMed] [Google Scholar]
- Song K, Nam Y-J, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahor E, Evans SM. Pharyngeal mesoderm development during embryogenesis: implications for both heart and head myogenesis. Cardiovascular Research. 2011;91:196–202. doi: 10.1093/cvr/cvr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk B, Moorman AFM, van den Hoff MJB. Role of bone morphogenetic proteins in cardiac differentiation. Cardiovascular Research. 2007;74:244–255. doi: 10.1016/j.cardiores.2006.11.022. [DOI] [PubMed] [Google Scholar]
- Yang L, Cai CL, Lin L, Qyang Y, Chung C, Monteiro RM, Mummery CL, Fishman GI, Cogen A, S. E. Isl1Cre reveals a common Bmp pathway in heart and limb development. Development. 2006;133:1575–1585. doi: 10.1242/dev.02322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JH, Wilson GF, Soerens AG, Koonce CH, Yu JY, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ. Res. 2009;104:E30–E41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, Gepstein L. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–1523. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.