Abstract
Purpose of review
To provide the reader with an overview of the cognitive-behavioral conceptualization of cancer related cognitive dysfunction (CRCD) and how cognitive behavioral therapy (CBT) can play an important role in treatment.
Recent findings
Recent findings show that Memory and Attention Adaptation training (MAAT), a CBT developed to help cancer survivors develop adaptive skills to improve daily cognitive performance and emotional coping, may be an efficacious treatment of CRCD and can be delivered through videoconference technology to improve survivor access to care.
Summary
The etiology of CRCD remains largely undetermined and likely is produced by multiple mechanisms. This can include neuronal death, microvascular damage, inflammatory processes and psychological factors of perceptions of inadequate cognitive capacity to meet performance demands and related emotional distress. As a result, there are a variety of treatments currently being researched. More research with larger sample sizes, multiple clinicians and multiple sites are needed to confirm efficacy, but CBT approaches such as MAAT that address multiple psychological factors involved may offer a flexible non-pharmacological approach to CRCD that optimizes quality of life outcomes.
Keywords: Cognitive behavioral therapy, cancer, cognitive dysfunction
Introduction
Cancer survival rates over the last 50 years have improved significantly, largely due to early detection of disease and more effective treatment (1). The present 5-year relative survival rate of all cancers is 68%, while worldwide, there are 32.6 million cancer survivors (2). In the midst of this good news is the public health concern of growing numbers of survivors with late effects of disease, treatment or both. Addressing the unique needs of cancer survivors to optimize long-term health function and quality of life well beyond the cessation of active cancer treatment is a priority in cancer care (1, 3).
Late cognitive effects of systemic therapies for non-central nervous system (non-CNS) disease is an ongoing focus of survivorship research and health care (4–6). Reviews of research over the last 2.5 decades estimate rates of long-term cancer-related cognitive dysfunction (CRCD; ≥ 1 year post-treatment) range from about 25% to nearly 50% (4–6).
Neuropsychological domains most commonly affected involve verbal memory, verbal working memory, attention and processing speed (4, 7). In most cases, the level of cognitive impairment when assessed by standardized neuropsychological tests is within a mild or moderate range and often at the lower end of neuropsychological testing norms (6). Higher quality studies of both cross-sectional and longitudinal designs have controlled for confounding factors that contribute to cognitive dysfunction such as depression, fatigue, anxiety and past history of CNS injury, suggesting that systemic therapy (e.g., chemotherapy and/or endocrine therapy) does cause cognitive decline among some individuals (4–6).
While CRCD is objectively assessed as mild to moderate with neuropsychological measurements, the quality of life impact can be substantial (8, 9). When survivors begin to resume pre-cancer vocational or social roles following active treatment, cognitive performance demands increase. It is often at this time point in survivorship when decrements in cognitive function begin to interfere with daily vocational, educational or family function (8–11). Intervening at this stage of survivorship (or ideally, before) is the intention of Memory and Attention Adaptation Training (MAAT) and other CBT approaches.
Cognitive-Behavioral Conceptualization of CRCD
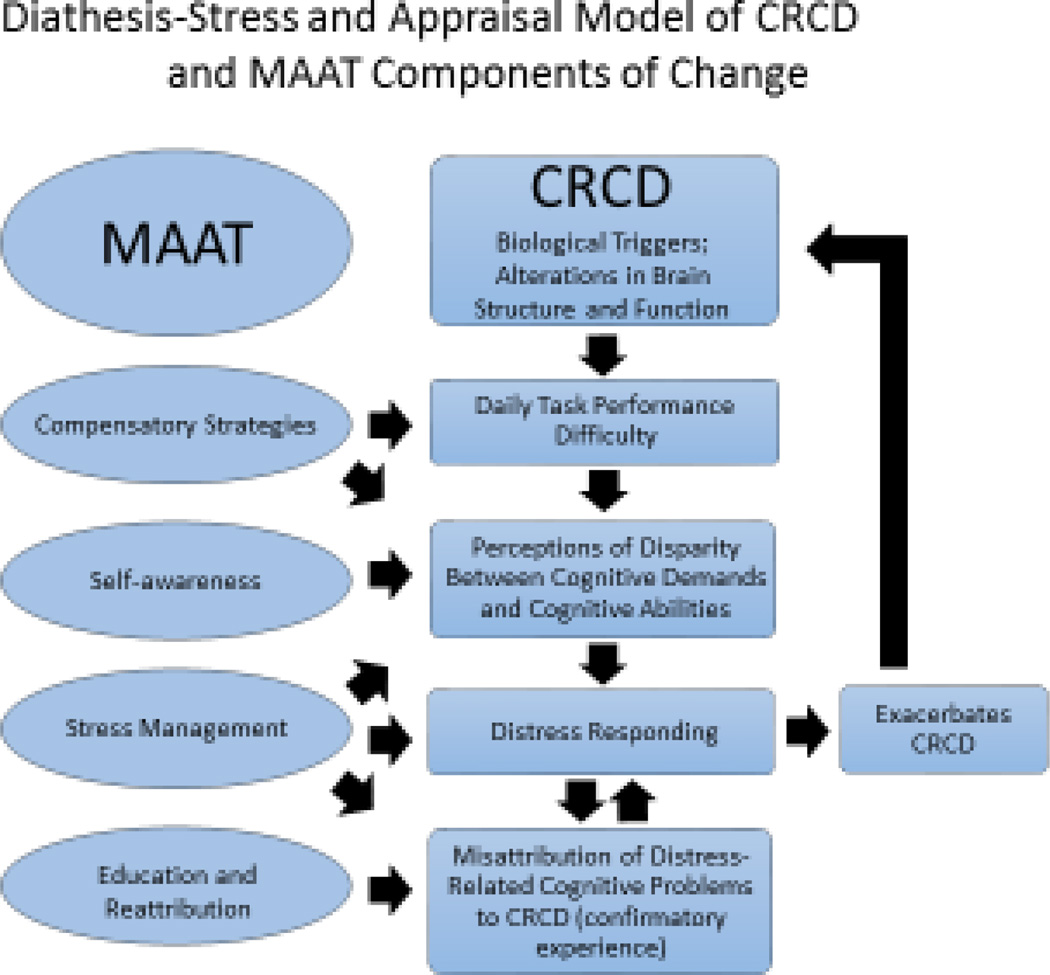
The etiology of CRCD is thought to involve multiple mechanisms, including neuronal death through direct toxicity, microvascular damage, and neural disruption through inflammatory processes (12). A cognitive-behavioral conceptualization is concerned less with the biological event(s) triggering CRCD but more with the individual’s psychological response to injury. MAAT assumes a “diathesis-stress” conceptualization. Under routine and low demand conditions, interacting neurocognitive systems (memory, attention, planned behavior and emotion regulation) function well for survivors. By contrast, under conditions of increased demand, cognitive failures may be more frequent and produce negative consequences. It is at this point where the survivor experiences a perception of disparity between “perceived threat level” (or increased demands on memory) and available resources (lowered perceived cognitive abilities) that leads to increased arousal, distress and in turn reduced cognitive performance. The perception of disparity of memory demand and perceived cognitive abilities is consistent with the Lazarus (13) two-step appraisal model of stress. That is, perceptions of threat or increased demands for memory performance are not enough to trigger stress responses— it also requires the perception that personal or external resources are inadequate to meet the threat. This process is depicted on the right side of Figure 1. Perceptions of inadequate cognitive abilities can increase emotional arousal, leading to cognitive interference with attention, encoding and recall, leading to an attribution of distress-related cognitive interference to CRCD—reinforcing confirmatory beliefs of inadequate cognitive abilities. These psychological responses are hypothesized to not “cause” CRCD but play a maintaining role in the distressing experience. The “diatheses” of CRCD include vulnerabilities in cognitive function produced by the survivor’s exposure to chemotherapy and other factors such as pre-cancer neurocognitive status, education, age, genetic predispositions, cognitive reserve (5) and emotional resilience (3). The more survivors with CRCD can train and make effective use of existing cognitive capacities AND learn and apply new skills, the better the cognitive task performance, perceptions of memory self-efficacy (the belief one has the skills or resources to meet demands) and distress reduction. MAAT’s central aim as a CBT is to build adaptive skills and mastery experience to reduce disparity between perceived coping resources and cognitive demands.
Figure 1.
Diathesis-Stress and Appraisal Model of CRCD and MAAT Components of Change.
There is evidence supporting this model from brain imaging literature. Compared with healthy controls and breast cancer survivors not undergoing chemotherapy, survivors who have had chemotherapy appear to have nearly similar objective memory test performance. Yet, chemotherapy-exposed survivors demonstrate significantly more cortical activation and report more subjective symptoms during task performance, especially with increased task difficulty (14–16). This may explain why survivors report more subjective complaints than healthy counterparts but may score in normal neuropsychological test ranges and encounter greater cognitive failure at the point of resuming pre-cancer work and home roles (6).
CBT and MAAT
In the treatment of CRCD, CBT differs from traditional cognitive retraining. Cognitive retraining involves repetitive tasks to hypothetically enhance neural networking to bypass damaged brain regions. This usually entails computerized exercises with incremental increases in task difficulty based on performance (e.g., once 85% correct response is achieved, the practice task increases in difficulty, and the process is repeated). The “dosing” of daily practice varies but one study suggests daily sessions of 20–30 minutes for 12–15 weeks (17). By contrast, CBT involves the acquisition of skills to make adaptive behavioral and cognitive changes on daily tasks for which memory is used (18, 19). For example, this might include using a verbal rehearsal method to aid working memory for transposing numbers from a computer to a written form at the workplace, or learning how to use and apply a smart phone calendar with alerts to transition to important tasks such as paying a household bill.
In recent reviews, cognitive retraining was found to be helpful but one shortcoming of the approach is the lack of generalizability or “transfer” of cognitive improvement from practice tasks (computer game) to real-world tasks (2, 20–22). CBT enhances self-awareness of where memory problems are likely to occur and directly trains individuals to apply compensatory strategies in those specific cognitive tasks. This might involve self-monitoring of memory failures to better understand the environmental and internal personal factors that contribute to the memory failure risk of a given individual so that compensatory strategies designed to reduce memory failure occurrence can be learned and targeted for specific situations. Two CBT approaches cited in some of these reviews were delivered in 5 or 4 weekly group visits. Both were found to improve neurocognitive outcomes in domains of verbal memory and processing speed and self-reported cognitive symptoms (23, 24). However, the Ercoli study did not involve any control group which limits conclusions due to the effects of practice with repeat neurocognitive testing (23). Similarly, the Schuurs study had limitations. While the investigators used a wait-list control group to account for neurocognitive testing practice effects, participants were not randomized to either the treatment or control condition. This brings into question the possibility of selection bias in the final sample— for instance, participants in the treatment condition (N = 23) could have put forth greater effort in neurocognitive testing and report more favorable self-reports of cognitive function post-treatment. In addition, some of the neurocognitive assessments were conducted by one of the interventionists in the study, which the authors report as a limitation (24).
At present, the MAAT CBT approach has evidence in three small studies (N = 29, 40 and 35, respectively) with improved methodology (e.g., blind assessors, randomization) that demonstrate gains in neurocognitive outcomes, self-reported cognitive symptoms and quality of life (10, 25, 26). This research is highlighted below with a more detailed description of MAAT.
As a CBT, MAAT’s current format involves eight weekly visits of 30–45 minutes duration and can be delivered effectively through videoconference technology (10). Survivors are provided a workbook to reinforce learning and mastery of skills covered in visits. MAAT has four components: 1) Education on chemotherapy-related cognitive problems and other influences on normal attention and memory such as stress “normal forgetting” (27–29). This reduces misattribution that All daily memory failure can be due to chemotherapy—a less controllable causal attribution— and raises the possibility of more controllable causes such as stress response. This causal attributional shift may enhance coping with CRCD through stopping the iterative distress cycle seen in Figure 1. 2) A second key aspect of MAAT is self-awareness training. That is, using self-monitoring to help survivors identify “at risk” environmental, social, physical or emotional conditions under which cognitive failures occur, which enables survivors to tailor compensatory strategies to specific tasks. 3) Compensatory strategies training for application in “at risk” situations that may directly improve cognitive performance. Common compensatory strategies include Self-Instructional Training (SIT), or a method of “self-talk” to enhance on-task attention, using a day planner, verbal rehearsal, active listening and visualization strategies. 4) Stress management methods of applied relaxation training, cognitive restructuring to modify negative appraisals of cognitive failure with activity scheduling for arousal and stress self-regulation (30–32).
MAAT Research: Study 1
MAAT was pilot tested for feasibility and to determine if it could positively influence daily cognitive symptoms, neurocognitive test performance and quality of life. In a single-arm pilot study (25), 29 breast cancer survivors (mean 8.2 years post-chemotherapy) reporting cognitive problems completed MAAT. Principal outcome measures included the Multiple Ability Self-Report Questionnaire (MASQ; self-report of cognitive function in daily life)(33), the Quality of Life-Cancer Survivors scale (QOL-CS)(34), satisfaction ratings and a brief neurocognitive test battery administered at baseline, post-treatment, two-month and six-month follow-up with supplemental funding. Results indicated significant reductions in daily cognitive complaints, improved quality of life and high satisfaction. Neurocognitive improvements in verbal memory and processing speed were also observed. However, with no control group, it was impossible to rule out effects of practice with repeat neurocognitive testing.
MAAT Research: Study 2
The second MAAT study was a small waitlist (no treatment) control RCT (31). Forty breast cancer survivors at least 18 months post-chemotherapy and reporting cognitive problems were randomized to MAAT (n = 19) or waitlist control (n = 21) conditions and assessed at baseline, post-treatment and two-month follow-up. Controlling for education and IQ, MAAT participants made significant improvements over waitlist participants on quality of life and verbal memory as assessed by the California Verbal Learning Test-II (CVLT-II). Subtracting wait-list control effects, the pre-to-post-treatment effect size for the CVLT-2 total score was −.50 and quality of life effect size was −.49 (note that the negative sign does not change the magnitude of effect). CVLT-II scores for MAAT participants also improved at two-month follow-up (d = −.63). While results in this small RCT were encouraging, an active control condition was needed to rule out effects of social attention and demand characteristics of study participation. In addition, improvement in self-report and neurocognitive outcome measures had been improved upon and were incorporated in the next trial. MAAT was also intended to be electronically delivered with videoconference technology to improve access to survivorship care, which has been a challenge for both comprehensive and community cancer centers (1, 35).
MAAT Research: Study 3
In the third MAAT trial, Ferguson, et al. (2016) added an active control condition, supportive therapy (ST) and increased the number of weekly MAAT visits to eight to enhance therapeutic intensity (10). ST is a common active control condition in CBT trials to control for psychological therapy “common factors” such as interpersonal warmth, empathy and treatment expectation. The Functional Assessment of Cancer Therapy-Cognitive scale (FACT-Cog) was added as a primary self-report outcome measure which was available at the time of design of the third MAAT trial (36). Both MAAT and ST were delivered via videoconference network linking rural health centers in the state of Maine. The primary aim of the study was to evaluate electronic delivery feasibility/efficacy with a small final sample size (N=35). Adjusting for baseline differences, MAAT participants improved in FACT-Cog perceived cognitive impairments over ST at two-month follow-up with moderately large effect size (p = .02; d =.52; trend at post-treatment p =.09). In neurocognitive outcomes, MAAT participants made significant improvement in processing speed over controls at post-treatment (p = .03; d = .50) but not on verbal memory. Finally, MAAT participants had a trend (p = .07) of improvement over ST in anxiety about cognitive symptoms (meta-memory in adulthood anxiety scale) but with a large size of effect (d = .90) at two-month follow-up. This suggests MAAT participants continued to make gains in emotion distress reduction through continued mastery and use of coping skills while ST participants (who did not receive skills training) regressed to baseline two-months after cessation of therapeutic attention. MAAT participants reported high satisfaction with treatment and they were highly likely to recommend treatment to a friend. Finally, MAAT participants indicated they likely would have not been able to participate without videoconference delivery due to travel burdens and lack of paid leave from work that was exhausted during active cancer treatment.
Conclusion
At the present time, there are several treatment approaches to CRCD that range from pharmacological approaches, physical activity and computerized cognitive retraining (20–22) but no treatment is empirically established as a standard for clinical use. CBT does offer a practical approach for enhancing cognitive function and quality of life improvement in daily life to address the consequences of CRCD in long-term survivorship. MAAT is one CBT approach that has accumulated preliminary evidence of efficacy and can likely be easily translated to survivorship service given that CBT is reimbursed by third party payers when delivered by trained professionals (e.g., psychologists, psychiatrists, psychiatric nurse practitioners and licensed counselors). Moreover, recent research suggests MAAT can be flexibly delivered using videoconference technology with positive outcomes.
While promising, MAAT research to date has limitations that should be addressed in future investigations. These limitations apply to the other CRCD treatment approaches cited here and include: small sample sizes, only one clinician delivering active treatment or the active control condition (e.g., MAAT and ST/attention control), single research sites and most research has been limited to breast cancer survivors only (8, 20–22). Recent reviews of CRCD treatment research have recommended that future trials utilize multiple sites with multiple clinicians and active treatment control conditions. With these methodological improvements, there will likely be an increase in confidence of results with respect to external validity and generalizability of treatment that can readily translate to clinical practice for survivors. Much of the research funding for CRCD has focused primarily on identifying specific mechanisms of cognitive change in various cancer populations with less funding targeting CRCD treatment research. Larger funding mechanisms, collaborations between institutions with multi-site trials and combining treatment research with imaging research may be one solution to address resource limitations for CRCD treatment and also help identify questions of how treatment can enhance CRCD recovery.
Finally, utilization of various forms of treatment delivery, including videoconference technology, may reduce barriers to survivorship care access and increase dissemination of CBT for CRCD. MAAT does have some evidence of effective videoconference delivery and larger, multisite studies such as those described above are planned. MAAT may also be delivered in group formats to leverage time and space in busy clinical settings. In addition, self-guided mobile device apps of interventions of CRCD may provide even further survivor access to care and may be more convenient and affordable than standard CBT (37). However, some research suggests that web-based or mobile-device self-guided apps are viewed as less than satisfactory for a majority of research participants in studies of such treatments(38). Nonetheless, they do appear to be helpful for some. In future research on CBT for CRCD, it may likely be most fruitful to develop a range of less intensive to more intensive therapeutic approaches in a stepped care fashion that allows more survivor choice and tailoring to individual needs.
Key points.
Cancer related cognitive dysfunction affects many cancer survivors today- because its etiology is not known, there have been a wide array of treatment trials.
Cognitive behavioral therapy is one treatment modality that has been evaluated and can be tailored to the specific areas of concerns that cancer survivors have.
A specific type of CBT, MAAT, has been shown in several studies to help improve patient’s quality of life and improved neuropsychological test outcomes but more research with multiple clinicians and multiple sites is needed to confirm effica
Acknowledgments
Funding for MAAT research has included grants from the National Cancer Institute (R03CA90151; R21CA143619), National Institute’s of Health Office of Research on Women’s Health and the Lance Armstrong Foundation.
Dr. Ferguson extends thanks to Drs. Tim Ahles, Paul Jacobsen and Brenna McDonald for collaboration and consultation on MAAT research and deep gratitude to all participants in MAAT research.
Footnotes
Drs. Kucherer and Ferguson report no conflicts of interest between industry, funding sources and results and conclusions contained within this report.
References
- 1. Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA: a cancer journal for clinicians. 2016 doi: 10.3322/caac.21349. **This article is an important, updated report on vital survivorship stauts and needs going forward that is critical for survivorship research investigators.
- 2.Zeng Y, Cheng AS, Chan CC. Meta-Analysis of the Effects of Neuropsychological Interventions on Cognitive Function in Non–Central Nervous System Cancer Survivors. Integrative cancer therapies. 2016 doi: 10.1177/1534735416638737. 1534735416638737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stanton AL, Rowland JH, Ganz PA. Life after diagnosis and treatment of cancer in adulthood: Contributions from psychosocial oncology research. American Psychologist. 2015;70(2):159. doi: 10.1037/a0037875. **This article is an excellent overview of critical survivorship areas of concern that requrire further clinical investigation to promote well-being of survivors of the future.
- 4.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: a cancer journal for clinicians. 2015;65(2):123–138. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jim HS, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. Journal of Clinical Oncology. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson RJ, Ahles TA. Low neuropsychologic performance among adult cancer survivors treated with chemotherapy. Curr Neurol Neurosci Rep. 2003;3(3):215–222. doi: 10.1007/s11910-003-0081-2. [DOI] [PubMed] [Google Scholar]
- 8.Von Ah D, Habermann B, Carpenter JS, Schneider BL. Impact of perceived cognitive impairment in breast cancer survivors. European Journal of Oncology Nursing. 2013;17(2):236–241. doi: 10.1016/j.ejon.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Boykoff N, Moieni M, Subramanian SK. Confronting chemobrain: an in-depth look at survivors’ reports of impact on work, social networks, and health care response. Journal of Cancer Survivorship. 2009;3(4):223–232. doi: 10.1007/s11764-009-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferguson R, Sigmon ST, Pritchard AJ, LaBrie SL, Goetze RE, Fink CM, Garrett AM. A randomized trial of videoconference-delivered CBT for survivors of breast cancer with self-reported cognitive dysfunction. Cancer. 2016;122:1782–1791. doi: 10.1002/cncr.29891. **An important report on a randomized trial of a cognitive-behavioral treatment that utilizes an active control condition.
- 11.Ottati A, Feuerstein M. Brief self-report measure of work-related cognitive limitations in breast cancer survivors. Journal of Cancer Survivorship. 2013;7(2):262–273. doi: 10.1007/s11764-013-0275-9. [DOI] [PubMed] [Google Scholar]
- 12.Macleod JE, DeLeo JA, Hickey WF, Ahles TA, Saykin AJ, Bucci DJ. Cancer chemotherapy impairs contextual but not cue-specific fear memory. Behav Brain Res. 2007;181(1):168–172. doi: 10.1016/j.bbr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarus RS. Stress and emotion: A new synthesis. Springer Publishing Company; 2006. [Google Scholar]
- 14.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–3870. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. Journal of Clinical Oncology. 2012;30(20):2500–2508. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Archives of neurology. 2011;68(11):1447–1453. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kesler S, Hosseini SH, Heckler C, Janelsins M, Palesh O, Mustian K, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clinical breast cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson B. Compensating for cognitive deficits following brain injury. Neuropsychol Rev. 2000;10(4):233–243. doi: 10.1023/a:1026464827874. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson RJ, Martinson AA. An overview of cognitive-behavioral management of memory dysfunction associated with chemotherapy. Psicooncologia. 2011;8(2/3):385. [Google Scholar]
- 20. Morean DF, O'Dwyer L, Cherney LR. Therapies for cognitive deficits associated with chemotherapy for breast cancer: a systematic review of objective outcomes. Archives of physical medicine and rehabilitation. 2015;96(10):1880–1897. doi: 10.1016/j.apmr.2015.05.012. ** An excellent review of pharmacological and non-pharmacological interventions for cancer-related cognitive dysfunction with detailed summaries of studies.
- 21. Treanor CJ, McMenamin UC, O'Neill RF, Cardwell CR, Clarke MJ, Cantwell M, et al. Non-pharmacological interventions for cognitive impairment due to systemic cancer treatment. Cochrane Database of Systematic Reviews. 2016;(8) doi: 10.1002/14651858.CD011325.pub2. *A comprehensive review of non-pharmacological treatments of cancer-related cognitive dysfunction with descriptions of treatment approaches but perhaps limited by not including recently publications and the need for better quality study in this area.
- 22.Chan R, McCarthy AL, Devenish J, Sullivan KA, Chan A. Systematic review of pharmacologic and non-pharmacologic interventions to manage cognitive alterations after chemotherapy for breast cancer. European Journal of Cancer. 2015;51:437–450. doi: 10.1016/j.ejca.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Ercoli LM, Castellon SA, Hunter AM, Kwan L, Kahn-Mills BA, Cernin PA, et al. Assessment of the feasibility of a rehabilitation intervention program for breast cancer survivors with cognitive complaints. Brain imaging and behavior. 2013;7(4):543–553. doi: 10.1007/s11682-013-9237-0. [DOI] [PubMed] [Google Scholar]
- 24.Schuurs A, Green HJ. A feasibility study of group cognitive rehabilitation for cancer survivors: enhancing cognitive function and quality of life. Psycho-Oncology. 2013;22(5):1043–1049. doi: 10.1002/pon.3102. [DOI] [PubMed] [Google Scholar]
- 25.Ferguson RJ, Ahles TA, Saykin AJ, McDonald BC, Furstenberg CT, Cole BF, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferguson RJ, McDonald BC, Rocque MA, Furstenberg CT, Horrigan S, Ahles TA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psycho-Oncology. 2012;21(2):176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iverson G, Lange R. Examination of "postconcussion-like" symptoms in a healthy sample. Appl Neuropsychol. 2003;10(3):137–144. doi: 10.1207/S15324826AN1003_02. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chan R, Deng Y. Examination of postconcussion-like symptoms in healthy university students: relationships to subjective and objective neuropsychological function performance. Archives of Clinical Neuropsychology. 2006;21(4):339–347. doi: 10.1016/j.acn.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Ferguson RJ, Mittenberg W, Barone DF, Schneider B. Postconcussion syndrome following sports-related head injury: expectation as etiology. Neuropsychology. 1999;13(4):582–589. doi: 10.1037//0894-4105.13.4.582. [DOI] [PubMed] [Google Scholar]
- 30.Thayer J, Hansen AL, Saus-rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: The neurovisceral integration perspective on self-regulation, adaptation and health. Annals of Behavorial Medicine. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 31.Ferguson RJ, McDonald BC, Rocque M, Furstenberg CT, Horrigan S, Ahles T, Saykin AJ. Development of CBT for Chemotherapy-Related Cognitive Change: Results of a Waitlist Control Trial. Psycho-Oncology. 2012;21:176–186. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson R, Cassel A, Dawson R. Cognitive Effects of Cancer Chemotherapy in Adult Cancer Survivors: Cognitive-Behavioral Management. Journal of Rational-Emotive & Cognitive-Behavior Therapy. 2010:1–17. [Google Scholar]
- 33.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 34.Ferrell BR, Dow KH, Grant M. Measurement of the quality of life in cancer survivors. Qual Life Res. 1995;4(6):523–531. doi: 10.1007/BF00634747. [DOI] [PubMed] [Google Scholar]
- 35.Padgett L, McSpadden K, editors. PSYCHO-ONCOLOGY. HOBOKEN 07030-5774, NJ USA: WILEY-BLACKWELL 111 RIVER ST; 2013. Barriers and Facilitators to Implementation of Survivorship Care: Lessons Learned From the National Cancer Institute Community Cancer Centers Program. [Google Scholar]
- 36.Wagner L, Sweet J, Butt Z, Lai J-s, Cella D. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. 2009;7(6):W32–W39. [Google Scholar]
- 37.Mohr DC, Burns MN, Schueller SM, Clarke G, Klinkman M. Behavioral intervention technologies: evidence review and recommendations for future research in mental health. General hospital psychiatry. 2013;35(4):332–338. doi: 10.1016/j.genhosppsych.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Knowles SE, Lovell K, Bower P, Gilbody S, Littlewood E, Lester H. Patient experience of computerised therapy for depression in primary care. BMJ open. 2015;5(11):e008581. doi: 10.1136/bmjopen-2015-008581. [DOI] [PMC free article] [PubMed] [Google Scholar]