STRUCTURED ABSTRACT
OBJECTIVE
To determine the survival benefit of kidney transplantation in HIV-infected patients with ESRD
SUMMARY BACKGROUND DATA
Although kidney transplantation (KT) has emerged as a viable option for select HIV-infected patients, concerns have been raised that risks of KT in HIV-infected patients are higher than those in their HIV-negative counterparts. Despite these increased risks, KT may provide survival benefit for the HIV-infected patient with ESRD, yet this important clinical question remains unanswered.
METHODS
Data from the Scientific Registry of Transplant Recipients were linked to IMS pharmacy fills (1/1/01–10/1/12) to identify and study 1,431 HIV-infected KT candidates from the first point of active status on the waiting list. Time-dependent Cox regression was used to establish a counterfactual framework for estimating survival benefit of KT.
RESULTS
Adjusted relative risk (aRR) of mortality at five years was 79% lower after KT compared to dialysis (aRR 0.21; 95% CI 0.10–0.42; p <0.001), and statistically significant survival benefit was achieved by 194 days of KT. Among patients coinfected with hepatitis C, aRR of mortality at five years was 91% lower after KT compared to dialysis (aRR 0.09; 95% CI 0.02–0.46; p =0.004); however, statistically significant survival benefit was not achieved until 392 days after KT.
CONCLUSIONS
Evidence suggests that for HIV-infected ESRD patients, KT is associated with a significant survival benefit compared to remaining on dialysis.
MINI ABSTRACT
Although kidney transplantation (KT) has emerged as an option for HIV-infected patients, concerns have been raised that associated risks are higher than those in their HIV-negative counterparts. Despite these increased risks, KT may provide survival benefit for the HIV-infected patient with ESRD, yet this important clinical question remains unanswered.
INTRODUCTION
Human immunodeficiency virus (HIV) infected individuals now comprise 1.5% of the United States end-stage renal disease (ESRD) population,1 and the prevalence of ESRD in this population continues to rise.2–4 In fact, model projections indicate that by 2020 more than 10,000 HIV-infected individuals will be living with ESRD compared to fewer than 200 in 1990.5 Recent efforts have focused on optimizing outcomes in HIV-infected ESRD patients by offering kidney transplantation (KT) to this vulnerable population.6–8
Compared to the uninfected general KT population, KT among HIV-infected recipients has been associated with greater morbidity. Rates of acute rejection have been reported as high as 67% among HIV-infected KT recipients,9 more than double that of their uninfected counterparts, and the National Institutes of Health (NIH) research consortia reported a 2.8-fold increased risk of graft loss among the subset of HIV-infected patients that developed acute rejection (adjusted hazard ratio [aHR] 2.8; 95% confidence interval [CI] 1.2–6.6, p=0.02).8 More recently, a large, retrospective study of national data found that HIV KT recipients co-infected with hepatitis C (HCV) have a 1.57-fold increased risk for death compared to HCV mono-infected KT recipients (aHR 1.57; 95%CI 1.11–2.22, p=0.01).10 Inferior outcomes among HIV-infected KT recipients raise the question of whether HIV-infected ESRD patients would be better served by remaining on dialysis.
However, mortality rates among dialysis dependent HIV-infected individuals are also higher than their uninfected counterparts.11,12 In fact, a recent long-term cohort study with greater than 22-years of follow-up found a 9.9-fold increased risk of mortality among dialysis dependent HIV-infected individuals (aHR 9.9, 95%CI 6.3–14.5, p<0.001).13 These results suggest that KT outcomes of HIV-infected ESRD patients should not be judged based on KT outcomes of the general uninfected KT population, but rather whether KT among HIV-infected individuals is associated with a survival benefit over remaining on dialysis. However, no study has addressed this important question in clinical decision-making for this vulnerable population.
To better understand the impact of KT versus dialysis on survival of HIV-infected ESRD patients, we performed time-to-event survival analyses among a large, national cohort of HIV-infected patients on the KT waitlist as well as HIV-infected KT recipients.
METHODS
Data Source
The study used data from the Scientific Registry of Transplant Recipients, which includes data submitted by members of the Organ Procurement and Transplantation Network (OPTN) on all donors, waitlisted candidates, and transplant recipients in the United States. The Health Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors. Since HIV status is not collected when a patient registers for the waiting list, a novel linkage with pharmacy fill data from IMS Health was used to identify HIV-infected patients. IMS Health collects medication fills through participating pharmacies. Fifty-six percent of incident kidney waitlist candidates from 2001–2012 had pharmacy fills in the linked IMS database that overlapped with the candidate time on the waitlist.
Study Population
Adult KT candidates who filled at least one HIV-specific medication while on the waitlist between January 1, 2001 and October 1, 2012 were identified through IMS pharmacy fills. Patients listed inactive who never changed to active status were excluded, as they were not an appropriate counterfactual. However, patients listed inactive who eventually changed to active were included, but only at the first active date (n=1,431). The candidate’s first listing while HIV+ was kept.
Exploratory Data Analyses
Candidate characteristics were compared by transplant status and donor type (living vs. deceased donor). Donor characteristics were compared by donor type. Continuous variables were analyzed using Wilcoxon rank-sum tests, and categorical variables were examined using chi-square tests of independence.
Outcome Ascertainment
The primary outcome was mortality. Death dates were supplemented by information from the Centers for Medicare and Medicaid Services and the Limited Access Death Master File available from the National Technical Information Service. Multiple simultaneous listings were collapsed. Exposure time began at the later of waitlisting or first HIV medication fill to the earlier of patient death or administrative end of study (October 1, 2012).
Survival Analyses
Survival analyses were performed using the Cox Proportional Hazards model with time-dependent variables for transplantation. Transplant recipients contributed time-at-risk to the waitlist group until receiving their transplant, at which point they began contributing time-at-risk to the transplant group. If the first antiretroviral medication fill was after listing date, the patient was left-truncated until the time of first fill to ensure time on the waitlist was only captured once a candidate was known to be HIV-infected. In order to quantify the mortality risk associated with receiving a transplant versus remaining on the waitlist, we allowed the hazard associated with transplantation to vary as a function of the number of days post-transplant. For every day post-listing, a new record was created for each person to capture whether the person had been transplanted as of that day, as well as the number of days post-transplant (if applicable) at that event time. If the person had not yet been transplanted, both variables were coded as 0. This allowed the reference level for the effect to be a person still on the waitlist; furthermore, a comparison could be made at any time point post-transplant versus the counterfactual of remaining on the waitlist. Because the hazard associated with any factor can only change on days when there is an event (i.e. death), records on a day post-listing when there were no deaths in the cohort were dropped to speed processing time.
To model the hazard, we used the pspline function in R, which fit a series of penalized basis splines to a continuous variable. A major benefit of pspline is the ability to fit an effect without assuming a particular shape to the hazard. While research exists describing the mortality hazard post-transplant for some populations, we did not want to assume that the effect of transplantation on HIV-infected individuals would mirror that previously described for uninfected individuals. Pspline also avoids the pitfalls of user-specified spline knots, where a single misspecified knot can adversely affect the fit of a variable for its entire range. An indicator for transplantation was included; this controlled for the transition between the un-transplanted and transplanted states while allowing the effect of days post-transplant to be as extreme as necessary.
Adjusted analyses included all of the covariates from the most recent SRTR PSR kidney waitlist mortality models, which were run on a national cohort of kidney waitlist candidates from 2012–2013. Covariates were chosen using the LASSO procedure as described by Snyder, et al.14 Covariates significant at P<0.10 are presented. An additional model separated the effect of transplantation in the HIV-infected cohort by donor type. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.1.3 (Vienna, Austria).
RESULTS
Patient and Donor Characteristics
Waitlist candidate and transplant recipient characteristics are shown in Table 1. Between January 1, 2001 and October 1, 2012, 1,431 HIV-infected waitlist candidates were identified who at some point were active status; of these, 113 achieved living donor transplantation, 426 achieved deceased donor transplantation, and 892 remained on the waitlist. HIV-infected waitlist candidates and transplant recipients were commonly male, African American, and between the ages of 35–49 years. Waitlist candidates that were older or blood group B were less likely to achieve transplantation.
Table 1.
Demographic characteristics of HIV+ waitlist candidates and transplant recipients.
| Characteristics | Waiting-list Candidates |
Living Donor Recipients |
Deceased Donor Recipients |
P-value |
|---|---|---|---|---|
| No (%) | No (%) | No (%) | ||
| 892 | 113 | 426 | ||
| Age (years) | < 0.001 | |||
| 18–34 | 83 (9.3) | 10 (8.8) | 46 (10.8) | |
| 35–49 | 440 (49.3) | 70 (61.9) | 255 (59.9) | |
| 50–64 | 337 (37.8) | 30 (26.5) | 118 (27.7) | |
| 65+ | 32 (3.6) | 3 (2.7) | 7 (1.6) | |
| Gender | 0.41 | |||
| Male | 671 (75.2) | 91 (80.5) | 328 (77.0) | |
| Female | 221 (24.8) | 22 (19.5) | 98 (23.0) | |
| Race | < 0.001 | |||
| White | 131 (14.7) | 44 (38.9) | 71 (16.7) | |
| Black | 669 (75.0) | 56 (49.6) | 313 (73.5) | |
| Hispanic | 78 (8.7) | 10 (8.8) | 38 (8.9) | |
| Asian-American | 11 (1.2) | 2 (1.8) | 1 (0.2) | |
| Other/unknown | 3 (0.3) | 1 (0.9) | 3 (0.7) | |
| ESRD cause | 0.39 | |||
| DM | 140 (15.7) | 15 (13.3) | 50 (11.7) | |
| GN | 92 (10.3) | 18 (15.9) | 43 (10.1) | |
| HIV-related | 189 (21.2) | 22 (19.5) | 97 (22.8) | |
| HTN | 283 (31.7) | 32 (28.3) | 134 (31.5) | |
| Other | 188 (21.1) | 26 (23.0) | 102 (23.9) | |
| BMI | 0.016 | |||
| Missing | 19 (2.1) | 5 (4.4) | 18 (4.2) | |
| < 20 | 25 (2.8) | 2 (1.8) | 12 (2.8) | |
| 20–25 | 374 (41.9) | 49 (43.4) | 196 (46.0) | |
| 25–30 | 267 (29.9) | 43 (38.1) | 129 (30.3) | |
| ≥ 30 | 207 (23.2) | 14 (12.4) | 71 (16.7) | |
| PRA ≥ 80 | 78 (8.7) | 9 (8.0) | 34 (8.0) | 0.88 |
| Blood Type | 0.14 | |||
| A | 241 (27.0) | 44 (38.9) | 137 (32.2) | |
| AB | 32 (3.6) | 4 (3.5) | 14 (3.3) | |
| B | 167 (18.7) | 16 (14.2) | 68 (16.0) | |
| O | 452 (50.7) | 49 (43.4) | 207 (48.6) | |
| Retransplant | 30 (3.4) | 3 (2.7) | 18 (4.2) | 0.63 |
| Willing to accept HCV+ kidney | 47 (5.3) | 3 (2.7) | 84 (19.7) | < 0.001 |
DM: Diabetes mellitus; GN: glomerular nephropathy; HTN: hypertension; PRA: panel reactive antibody
Characteristics of living and deceased donors received by HIV-infected candidates are shown in Table 2. Compared to deceased donors, living donors were more often <50years old, less likely to have hypertension, and more likely to be female and African American. Among deceased donor kidney transplants, 12% were extended criteria donors (ECD), 10.6% were donors after cardiac death (DCD), and the median cold ischemia time was 16.4 hours (range 11.9–23.4).
Table 2.
Donor characteristics for HIV+ recipients of living donor and deceased donor transplants
| Characteristics | Living Donor | Deceased Donor | P-value |
|---|---|---|---|
| No (%) | No (%) | ||
| 113 | 426 | ||
| Age (years) | < 0.001 | ||
| 0–17 | 0 | 37 (8.7) | |
| 18–34 | 32 (28.3) | 141 (33.1) | |
| 35–49 | 57 (50.4) | 138 (32.4) | |
| 50+ | 24 (21.2) | 110 (25.8) | |
| Gender | 0.004 | ||
| Male | 51 (45.1) | 257 (60.3) | |
| Female | 62 (54.9) | 169 (39.7) | |
| Race | < 0.001 | ||
| White | 54 (47.8) | 285 (66.9) | |
| Black | 46 (40.7) | 65 (15.3) | |
| Hispanic | 11 (9.7) | 64 (15.0) | |
| Other | 2 (1.8) | 12 (2.8) | |
| ECD (deceased donor only) | - | 51 (12.0) | |
| DCD (deceased donor only) | - | 45 (10.6) | |
| HCV+ (deceased donor only) | - | 59 (13.8) | |
| History of hypertension | 2 (1.8) | 99 (23.2) | < 0.001 |
| History of diabetes | 22 (5.2) | ||
| Cause of death | |||
| Anoxia | - | 119 (27.9) | |
| Cerebrovascular/stroke | - | 143 (33.6) | |
| Head trauma | - | 150 (35.2) | |
| CNS tumor | - | 1 (0.2) | |
| Other | - | 13 (3.1) | |
| Cold ischemia time in hours (median) |
1 (0.5–1.3) | 16.4 (11.9–23.4) | < 0.001 |
ECD: expanded criteria donor; DCD: donor after cardiac death
Unadjusted Death Rates
There were 310 (21.7%) deaths among all HIV-infected patients in the study. Among those who never achieved transplantation, 223 (25.0%) died before study end. There were 9 deaths among 113 living donor transplant recipients (8.0%) and 78 deaths among 426 deceased donor transplant recipients (18.3%). Unadjusted death rates for HIV-infected transplant candidates and recipients were 8.7 per 100 PY (223 per 2555.9 PY) and 3.1 per 100 PY (87 per 2819.6 PY) respectively; furthermore, by donor type, death rates were 1.6 per 100 PY (9 per 578.7 PY) for living donors and 3.5 per 100 PY (78 per 2240.8 PY) for deceased donors.
Adjusted Risk of Death for Transplant Recipients vs. Candidates without Transplant
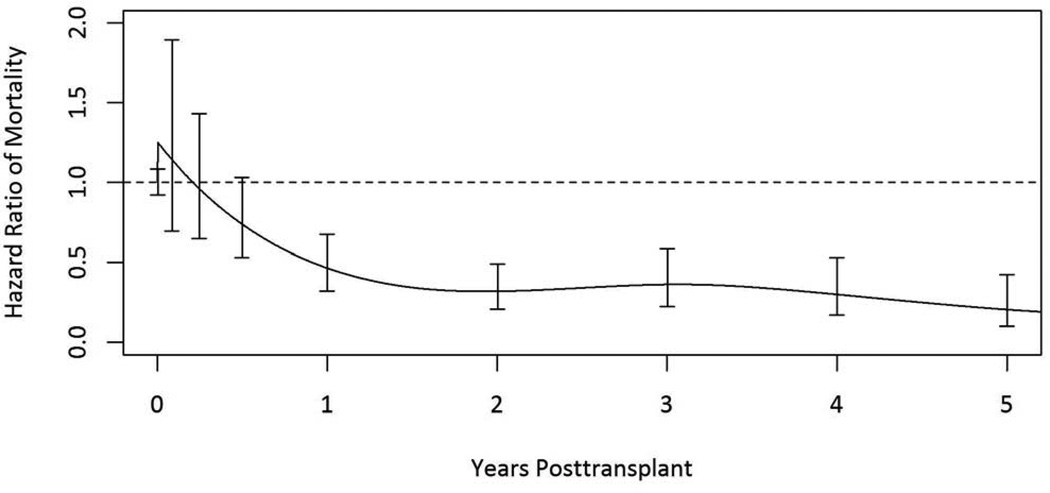
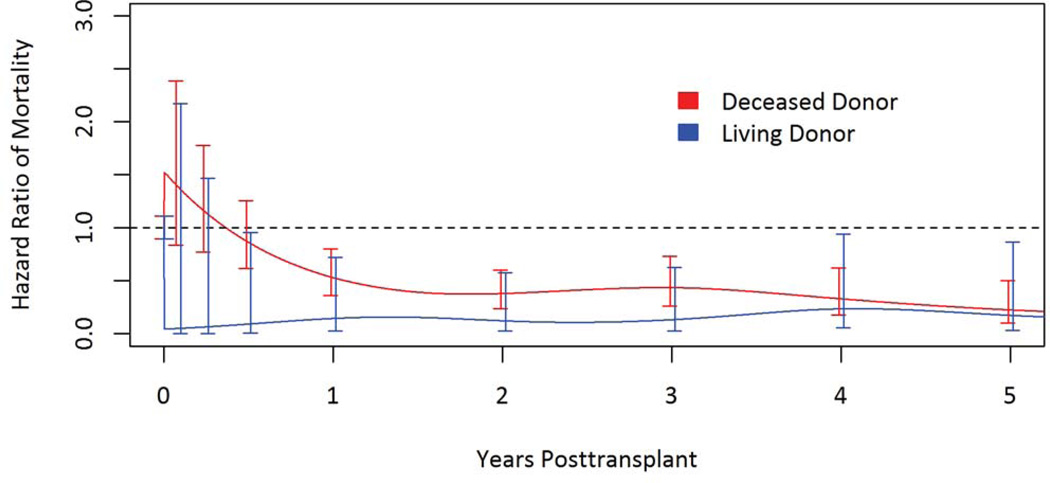
The adjusted relative mortality risk at five years was 79% lower among transplant recipients compared to remaining on dialysis (adjusted relative risk [aRR] 0.21; 95% confidence interval [CI] 0.10–0.42; p <0.001) (Table 3 & Figure 1). More specifically, among living donor recipients the risk was 82% lower (aRR 0.18; 95% CI 0.04–0.87; p =0.03); and among deceased donor kidney transplant recipients the risk was 77% lower (aRR 0.23; CI 0.10–0.50 ; p < 0.001) (Table 4 & Figure 2). Adjusted patient survival at 5 years was 80.1% among waitlist candidates compared with 90.6% among transplant recipients, conditional on survival to the median time to transplant in the cohort (1.7 years post-listing).
Table 3.
Adjusted hazard ratio of post-listing mortality for HIV+ waitlist candidates1
| Characteristic | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Transplant (ref, waitlist) | |||
| 1-year | 0.47 | 0.32–0.67 | < 0.001 |
| 3-year | 0.36 | 0.23–0.59 | < 0.001 |
| 5-year | 0.21 | 0.10–0.42 | < 0.001 |
| Age, per 10 years | 1.30 | 1.10–1.50 | < 0.001 |
| Race (ref, white) | |||
| Black | 0.61 | 0.44–0.86 | 0.005 |
| Hispanic | 0.43 | 0.25–0.75 | 0.003 |
| Asian-American/PI | 0.48 | 0.11–2.00 | 0.32 |
| Other | 0.21 | 0.03–1.60 | 0.13 |
| Diabetes (ref, none) | |||
| Type 1 | 2.30 | 1.10–4.70 | 0.03 |
| Type 2 | 1.70 | 1.10–2.70 | 0.02 |
| Other | 0.85 | 0.45–1.60 | 0.61 |
| Primary payer (ref, Medicaid) | |||
| Medicare | 0.74 | 0.50–1.10 | 0.13 |
| Private/self | 0.58 | 0.37–0.91 | 0.02 |
| Other | 0.85 | 0.31–2.30 | 0.74 |
| Albumin | 0.81 | 0.65–1.00 | 0.06 |
| Dialysis, per 10 years | 2.00 | 1.40–2.70 | < 0.001 |
| Willingness to accept HCV+ donor | 2.30 | 1.60–3.20 | < 0.001 |
Model adjusted for: Transplant (Y/N), days post-transplant, age at listing, race, gender, ABO type, angina or coronary artery disease, BMI, cerebrovascular disease, diabetes type, drug-treated COPD, education level, malignancy, medical condition, peptic ulcer, physical capacity, primary payer type, total albumin, working for income, primary diagnosis, time on dialysis, PRA level, prior solid-organ transplant, prior kidney transplant, willingness to accept an HCV+ kidney
Figure 1.
Adjusted relative mortality risk among HIV+ kidney transplant recipients compared to remaining on dialysis.
Table 4.
Adjusted hazard ratio of post-listing mortality for HIV+ waitlist candidates, by donor type1
| Characteristic | Hazard Ratio | 95% Confidence Interval | p-value |
|---|---|---|---|
| Living donor transplant | |||
| 1-year (vs. waitlist) | 0.15 | 0.03–0.72 | 0.02 |
| 3-year (vs. waitlist) | 0.13 | 0.03–0.62 | 0.01 |
| 5-year (vs. waitlist) | 0.18 | 0.04–0.87 | 0.03 |
| Deceased donor transplant | |||
| 1-year (vs. waitlist) | 0.53 | 0.36–0.80 | 0.002 |
| 3-year (vs. waitlist) | 0.44 | 0.26–0.73 | 0.002 |
| 5-year (vs. waitlist) | 0.23 | 0.10–0.50 | < 0.001 |
| Age, per 10 years | 1.30 | 1.10–1.50 | 0.006 |
| Race (ref, white) | |||
| Black | 0.59 | 0.42–0.83 | 0.002 |
| Hispanic | 0.42 | 0.25–0.73 | 0.002 |
| Asian-American/PI | 0.54 | 0.13–2.30 | 0.40 |
| Other | 0.21 | 0.03–1.60 | 0.13 |
| Diabetes (ref, none) | |||
| Type 1 | 2.30 | 1.10–4.70 | 0.03 |
| Type 2 | 1.70 | 1.10–2.70 | 0.03 |
| Other | 0.84 | 0.44–1.60 | 0.58 |
| Primary payer (ref, Medicaid) | |||
| Medicare | 0.75 | 0.50–1.10 | 0.15 |
| Private/self | 0.60 | 0.38–0.94 | 0.03 |
| Other | 0.90 | 0.33–2.40 | 0.83 |
| Albumin | 0.81 | 0.66–1.00 | 0.07 |
| Dialysis, per 10 years | 1.90 | 1.40–2.70 | < 0.001 |
| Willingness to accept HCV+ donor | 2.20 | 1.50–3.10 | < 0.001 |
Model adjusted for: Transplant (Y/N), days post-transplant, age at listing, race, gender, ABO type, angina or coronary artery disease, BMI, cerebrovascular disease, diabetes type, drug-treated COPD, education level, malignancy, medical condition, peptic ulcer, physical capacity, primary payer type, total albumin, working for income, primary diagnosis, time on dialysis, PRA level, prior solid-organ transplant, prior kidney transplant, willingness to accept an HCV+ kidney
Figure 2.
Adjusted relative mortality risk among HIV-infected living donor and deceased donor kidney transplant recipients compared to remaining on dialysis.
Mortality risk was not statistically different between waitlist candidates and transplant recipients during the first seven months post-transplant. Mortality risk steadily declined among HIV-infected transplant recipients thereafter, with transplantation providing a statistically significant survival benefit by 194 days post-transplant.
HIV-infected Patients Co-infected with HCV
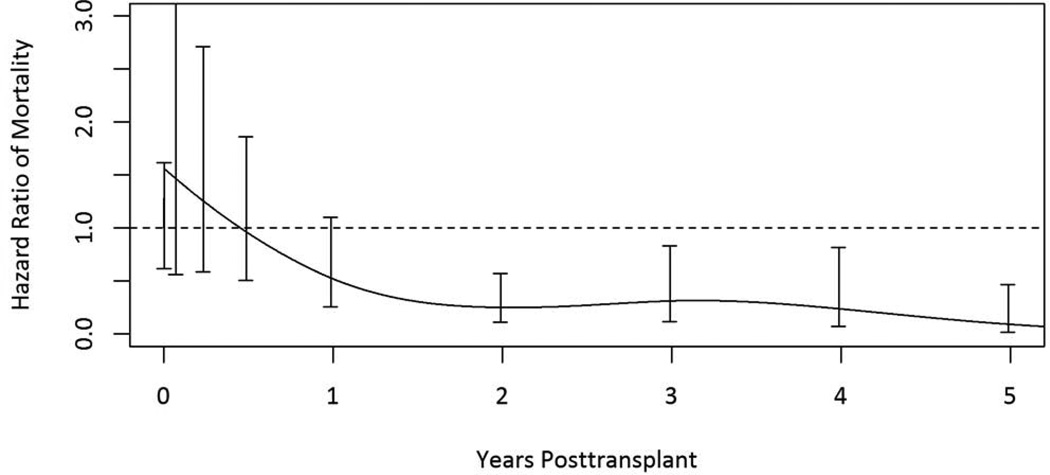
134 HIV-infected patients (9.4%) were willing to accept a kidney from a HCV+ donor and were presumed to be coinfected with HCV. 57 (42.5%) coinfected patients died during the study period, and 35 (61.4%) of those deaths occurred post-transplant. The adjusted relative mortality risk at five years was 91% lower among transplant recipients compared to remaining on dialysis (aRR 0.09; 95% CI 0.02–0.46; p =0.004). Among coinfected patients, mortality risk was not statistically different between waitlist candidates and transplant recipients during the first year post-transplant. Mortality risk, however, steadily declined among co-infected transplant recipients thereafter, with transplantation providing a statistically significant survival benefit 392 days post-transplant (Figure 3).
Figure 3.
Adjusted relative mortality risk among HIV/HCV coinfected kidney transplant recipients compared to remaining on dialysis.
DISCUSSION
In this national study of HIV-infected kidney waitlist candidates, we found that KT was associated with 79% lower risk of mortality compared to dialysis. In fact, 1-year death rates among HIV-infected waitlist candidates were 8.7 per 100 PY (223 per 2,556 person years) compared to just 3.1 per 100 PY (87 per 2,820 person years) with KT. Post-transplant mortality risk steadily declined among HIV-infected transplant recipients compared to HIV-infected waitlist candidates, and KT was associated with a statistically significant survival benefit by 194 days post-transplant. Older age, longer time on dialysis, and coinfection with HCV were each associated with increased risk of death post-listing, regardless of transplant status.
Our finding of an 8.7% yearly death rate among HIV-infected waitlist candidates is significantly higher than the 4.9% annual death rate reported among the uninfected general waitlist population.15 This almost 2-fold increase rate of death among HIV-infected waitlist candidates is consistent with previous published reports indicating significantly higher mortality rates among HIV-infected dialysis patients compared to their uninfected counterparts.11,12 Reasons for higher rates of death among HIV-infected dialysis patients remain elusive, but may in part be explained by the increased cardiovascular risk associated with antiretroviral therapies.16 Importantly, however, annual death rates among HIV-infected KT recipients (3.1%) are similar to their uninfected counterparts (3.3%), and emphasize the benefit of KT in this unique population.
HCV status is not currently captured at the time of waitlisting, and as such, willingness to accept an HCV+ kidney was used as a surrogate marker for HCV status. We identified 134 HIV-infected patients coinfected with HCV. Despite the small sample size, among the identified coinfected subset, we found that KT was associated with a 91% lower risk of mortality compared to dialysis. However, statistically significant benefit was not achieved until 392 days post-transplant, which was 198 days longer than their HIV mono-infected counterparts, and suggests that coinfection with HCV portends more severe morbidity and mortality. Poor outcomes associated with HCV are not limited to HIV-infected KT recipients as infection with HCV independent of HIV-infection has been demonstrated to increase risk of graft loss 1.7-fold compared to HIV-/HCV- KT recipients.17 Given that new direct acting antiviral treatments for HCV lead to sustained virologic response rates in >95% of patients, it may be prudent to continue to offer transplant to coinfected patients with the plan for eradication of HCV in the immediate post-transplant period, and as such, decrease the time from KT to survival benefit.
Importantly, these findings demonstrate that HIV-infected ESRD patients achieve a significant survival benefit with transplantation compared to remaining on dialysis, promoting the continued practice of offering KT to this vulnerable population. Recently, the HIV Organ Policy Equity (HOPE) Act (42 U.S.C. § 274f-5(b)) was signed into law, which has provisions for the recovery of organs from HIV-infected individuals.6,18 Implementation of the HOPE Act will afford HIV-infected ESRD patients the opportunity to achieve transplant using kidneys from HIV-infected donors, and as such, may shorten waiting times for HIV-infected candidates.18,19 Further study will be needed to determine whether HIV-infected ESRD patients achieve the same survival benefit with kidneys from HIV-infected donors.
Inferences based on the results of our study must take into account additional limitations specific to the national transplant registry. The OPTN does not collect data on CD4 count, viral loads, or infections, and collects limited data on acute rejection and malignancies, all of which are factors thought to influence long-term outcomes among HIV-infected dialysis patients and transplant recipients. However, the NIH multi-center protocol, which has been adopted widely across the US, uses relatively restricted criteria for waitlisting and transplantation of HIV-infected ESRD patients, requiring undetectable viral loads and CD4 counts ≥ 200, and it is unlikely that there would be major deviations from this protocol within national data. Moreover, the sample size for subgroup analyses among coinfected patients was small and may limit the accuracy of the time-to-event analyses. Further, IMS Health only captures medication fills for 56% of the incident kidney waitlist, and as such, it is likely that our study underestimated the number of kidney waitlist candidates infected with HIV. Finally, use of IMS data to identify HIV-infected waitlist candidates may have introduced misascertainment bias. However, the data from this unique cohort represent the HIV-infected transplant candidate and recipient population in the real world, and as such, contribute new and important information about the survival benefits of kidney transplantation in this vulnerable population.
To date, this is the first national study examining the survival benefit of KT over dialysis among HIV-infected ESRD patients. Our results suggest that KT is associated with 79% lower risk of death compared to dialysis among HIV-infected ESRD patients. Moreover, HIV-infected ESRD patients achieve this benefit within 7 months post-transplant. These results are encouraging and support the continued use of KT as a lifesaving modality for HIV-infected ESRD patients.
Acknowledgments
Sources of support: NIH grants K24-DK101828 (PI: Segev), K23-DK103918 (PI: Locke), and K23-CA177321-01A1 (PI: Durand); US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau, Division of Transplantation contract HHSH250201000018C (Gustafson, Salkowski, Snyder, and Segev)
Dr. Durand reports grants and personal fees from Gilead Sciences, grants from Bristol Meyers Squibb, personal fees from Roche Diagnostics, and personal fees from Merck outside the submitted work.
The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the SRTR. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or US Government.
Footnotes
DISCLOSURE OF CONFLICTS
The other authors have nothing to disclose.
The research was presented in preliminary forms as abstracts at the 2015 American Transplant Congress.
REFERENCES
- 1.Eggers PW, Kimmel PL. Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol. 2004;15:2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7. [DOI] [PubMed] [Google Scholar]
- 2.United States Renal Data System (USRDS) 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. 2011 doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Mallipattu SK, Wyatt CM, He JC. The New Epidemiology of HIV-Related Kidney Disease. J AIDS Clin Res. 2012;(Suppl 4):001. doi: 10.4172/2155-6113.S4-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abraham AG, Althoff KN, Jing Y, et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz EJ, Szczech LA, Ross MJ, Klotman ME, Winston JA, Klotman PE. Highly active antiretroviral therapy and the epidemic of HIV+ end-stage renal disease. J Am Soc Nephrol. 2005;16:2412–2420. doi: 10.1681/ASN.2005040340. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg EA, Stock PAST. Infectious Diseases Community of Practice. Solid organ transplantation in the HIV-infected patient. Am J Transplant. 2009;9(Suppl 4):S131–S135. doi: 10.1111/j.1600-6143.2009.02903.x. [DOI] [PubMed] [Google Scholar]
- 7.Ando M, Tsuchiya K, Nitta K. How to manage HIV-infected patients with chronic kidney disease in the HAART era. Clin Exp Nephrol. 2012;16:363–372. doi: 10.1007/s10157-012-0585-7. [DOI] [PubMed] [Google Scholar]
- 8.Stock PG, Barin B, Murphy B, et al. Outcomes of kidney transplantation in HIV-infected recipients. N Engl J Med. 2010;363:2004–2014. doi: 10.1056/NEJMoa1001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roland ME, Barin B, Carlson L, et al. HIV-infected liver and kidney transplant recipients:1- and 3-year outcomes. Am J Transplant. 2008;8:355–365. doi: 10.1111/j.1600-6143.2007.02061.x. [DOI] [PubMed] [Google Scholar]
- 10.Locke JE, Mehta S, Reed RD, et al. A National Study of Outcomes among HIV-Infected Kidney Transplant Recipients. J Am Soc Nephrol. 2015;26:2222–2229. doi: 10.1681/ASN.2014070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trullas JC, Cofan F, Barril G, et al. Outcome and prognostic factors in HIV-1-infected patients on dialysis in the cART era: a GESIDA/SEN cohort study. J Acquir Immune Defic Syndr. 2011;57:276–283. doi: 10.1097/QAI.0b013e318221fbda. [DOI] [PubMed] [Google Scholar]
- 12.Khanna R, Tachopoulou OA, Fein PA, Chattopadhyay J, Avram MM. Survival experience of peritoneal dialysis patients with human immunodeficiency virus: a 17-year retrospective study. Adv Perit Dial. 2005;21:159–163. [PubMed] [Google Scholar]
- 13.Bickel M, Marben W, Betz C, et al. End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years. HIV Med. 2013;14:127–135. doi: 10.1111/j.1468-1293.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- 14.Snyder JJ, Salkowski N, Kim SJ, et al. Developing statistical models to assess transplant outcomes using national registries: the process in the United States. Transplantation. 2015 doi: 10.1097/TP.0000000000000891. In press. [DOI] [PubMed] [Google Scholar]
- 15.Ojo AO, Hanson JA, Meier-Kriesche H, et al. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589–597. doi: 10.1681/ASN.V123589. [DOI] [PubMed] [Google Scholar]
- 16.Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8:e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawinski D, Forde KA, Eddinger K, et al. Superior outcomes in HIV-positive kidney transplant patients compared with HCV-infected or HIV/HCV-coinfected recipients. Kidney Int. 2015;88:341–349. doi: 10.1038/ki.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyarsky BJ, Durand CM, Palella FJ, Jr, Segev DL. Challenges and Clinical Decision-Making in HIV-to-HIV Transplantation: Insights From the HIV Literature. Am J Transplant. 2015;15:2023–2030. doi: 10.1111/ajt.13344. [DOI] [PubMed] [Google Scholar]
- 19.Boyarsky BJ, Hall EC, Singer AL, Montgomery RA, Gebo KA, Segev DL. Estimating the potential pool of HIV-infected deceased organ donors in the United States. Am J Transplant. 2011;11:1209–1217. doi: 10.1111/j.1600-6143.2011.03506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]