Abstract
Status epilepticus (SE) in rats, along with chronic epilepsy, leads to the development of behavioral impairments resembling depressive disorder and/or attention deficit/hyperactivity disorder (ADHD), thus reflecting respective comorbidities in epilepsy patients. Suppressed neurotransmitter tone in the raphe nucleus (RN)-prefrontal cortex (PFC) serotonergic pathway and in the locus coeruleus (LC)-PFC noradrenergic pathway underlies depressive- and impulsive- like behavioral deficits respectively. We examined possible mechanisms leading to the monoamine dysfunction in brainstem efferents, namely modulatory effects of the neuropeptide galanin on serotonin (5-HT) and norepinephrine (NE) signaling. SE was induced in young adult male Wistar rats by LiCl and pilocarpine. Epileptic rats were categorized vis-à-vis behavioral deficits as not impaired, “depressed” and “impulsive”. Depressive- and impulsive- like behaviors were examined in the forced swimming test (FST). The strength of serotonergic transmission in RN-PFC and of noradrenergic transmission in LC-PFC was analyzed using in vivo fast scan cyclic voltammetry. Galanin receptor type 1 (GalR1) / type 2 (GalR2) antagonist M40, and a preferential GalR2 antagonist M871 were administered over 3 days locally into either RN or LC by means of ALZET osmotic minipumps connected to locally implanted infusion cannulas. Intra-RN injection of M40 improved serotonergic tone and depressive-like behavior in epileptic “depressed” rats. Intra-LC injection of M40 improved noradrenergic tone and impulsive-like behavior in epileptic “impulsive” rats. The effects of M40 were only observed in impaired subjects. The treatment did not modify neurotransmission and behavior in naïve and epileptic not impaired rats; in “depressed” rats the effects were limited to serotonergic transmission and immobility, while in “impulsive” rats – to noradrenergic transmission and struggling behavior. Intra-RN administration of M871 exacerbated depressive-like behavior, but had no effects on any other of the examined parameters in any category of animals. These findings suggest that endogenous galanin, acting through GalR1 may be involved in the pathophysiology of epilepsy-associated depression and ADHD via inhibiting RN-PFC serotonergic and LC-PFC noradrenergic transmissions respectively.
Keywords: Epilepsy, comorbidity, depression, attention deficit/hyperactivity disorder, serotonin, norepinephrine, galanin, raphe nucleus, locus coeruleus, prefrontal cortex
1. Introduction
Depressive disorder and attention deficit/hyperactivity disorder (ADHD) are two common comorbidities of epilepsy. Prevalence of depression among epilepsy patients is 30-50% vs. 5-17% in people without epilepsy (Kanner, 2003; Kanner et al., 2012); for ADHD the numbers are 25% and 3-5% respectively (Parisi et al., 2010; Schubert, 2005).
Numbers aside, psychiatric disorders have profound detrimental effects on the quality of life of people with epilepsy, as they exacerbate the severity of the disease and hamper the effectiveness of anticonvulsant interventions (Baca et al., 2011; Kanner, 2016; Luoni et al., 2011).
In a series of studies, we showed that sub-populations of rats with post-status epilepticus (SE) chronic epilepsy consistently presented with either depressive-like, ADHD-like behavioral impairments, or with both (Kumar et al., 2016; Mazarati et al., 2008; Pineda et al., 2014; Pineda et al., 2012). We established that depressive-like behavior stemmed from the suppressed serotonergic transmission in the raphe nucleus (RN) – prefrontal cortex (PFC) pathway. At the same time, ADHD-like impairments developed as a result of deficient noradrenergic transmission in the locus coeruleus (LC)-PFC pathway (Mazarati et al., 2008; Pineda et al., 2014).
Events that lead to the dysfunction of respective monoaminergic systems are of significant interest both from a mechanistic point of view and for their therapeutic implications. For example, the upregulation of presynaptic receptors, specifically 5-HT1A in RN (Pineda et al., 2011; Pineda et al., 2012), and α2A adrenoreceptors in LC (personal unpublished data) may contribute to the observed monoamine deficiencies.
Galanin is a bioactive peptide ubiquitous in the mammalian brain (Gundlach et al., 1990; Merchenthaler et al., 1993; Skofitsch and Jacobowitz, 1986), with a broad spectrum of neurophysiological and neurobehavioral actions (Lang et al., 2015; Mitsukawa et al., 2010). Galanin is a well-established potent modulator of all types of monoaminergic transmission (Hökfelt et al., 1998; Kuteeva et al., 2008; Lundstrom et al., 2005; Picciotto et al., 2010). Three galanin receptor (GalR) subtypes have been cloned, all being G-protein coupled receptors (GPCR). GalR1 is coupled to Gi protein and its activation produces membrane hyperpolarization. GalR2 is coupled to Gq/11 and thus has a depolarizing effect. GalR1 and GalR2 are likely present both in RN and in LC, while Gi/o – coupled GalR3 (Smith et al., 1998) is not (Le Maitre et al., 2013; Lundstrom et al., 2005; Mitsukawa et al., 2010; Webling et al., 2012). Coexistence of galanin with serotonin (5-HT) and norepinephrine (NE) suggests that the peptide may regulate monoamine-dependent behaviors. Indeed, effects of GalR ligands on depression have been well documented, whereby activation of RN GalR1 exerts pro-depressant, and of GalR2-antidepressant effects via negative and positive regulation of serotonergic transmission respectively (Kuteeva et al., 2010; Kuteeva et al., 2008; Lu et al., 2005; Mazarati et al., 2005). Along with regulating behavior, galanin signaling has been implicated in epilepsy and epileptogenesis. In the hippocampus, the activation of both GalR1 and GalR2 had anticonvulsant effects and attenuated neuronal cell death after SE (Mazarati and Lu, 2005; Mazarati et al., 2006; Mazarati et al., 2000; Mazarati et al., 1998). Antiepileptic effects of galanin were observed in the kindling model (Mazarati et al., 2006; Schlifke et al., 2006). In RN, GalR1 facilitated, while GalR2-attenuated seizures via the discussed modulation of the serotonergic RN-hippocampal pathway (Mazarati et al., 2005).
The purpose of the present study was to examine the involvement of endogenous galanin in impairments of monoamine neurotransmission, and in related behavioral deficits associated with chronic epilepsy. We report that in animals with epilepsy, pharmacological blockade of GalR1 in RN improves serotonergic transmission in RN-PFC and exerts antidepressant effect. Blockade of GalR1 in LC improves noradrenergic transmission and attenuates impulsivity.
2. Subjects, materials and methods
2.1. Experimental subjects
The experiments were performed in male Wistar rats (Charles River, Wilmington, MA), fifty days old at the beginning of the study, in accordance with the policies of the National Institutes of Health and of the UCLA Office of Protection of Research Subjects.
2.2. Experiment design
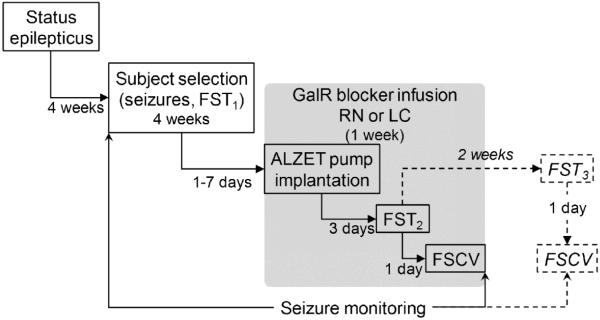
The study consisted of the following steps (Fig. 1). Induction of SE, followed 4 weeks later by animal selection and category assignments based on the spontaneous seizure frequency and the animals’ performance in the forced swimming test (FST). Within each category, the subjects were then randomized for treatments. In the main experiments (solid lines, gray box), GalR blockers were administered over 3 days into either RN or LC, followed by FST and fast scan cyclic voltammetry (FSCV) of 5-HT and NE in the ascending pathways, at the time of drug infusions. In an additional experiment (dashed lines, outside the gray box), FST was repeated two weeks later (i.e. after the one-week washout), and was followed by FSCV.
Fig. 1. Study design.
Experiment design Explanations in Methods.
2.3. Epilepsy model
SE was induced by LiCl (128 mg/kg, i.p., Sigma, St. Louis, MO) followed 24 hours later by pilocarpine (40 mg/kg, s.c., Sigma). In order to alleviate the severity of SE and to decrease the frequency of subsequent spontaneous seizures, rats received i.p. injections of diazepam (10 mg/kg) and phenytoin (50 mg/kg) one and four hours after the SE onset (Kumar et al., 2016; Mazarati et al., 2008; Pineda et al., 2014; Pineda et al., 2012). In control animals, saline was injected instead of pilocarpine. Starting from four weeks after SE and until the end of the study, the animals were continuously video-monitored for documenting spontaneous seizures (Fig. 1).
2.4. Forced swimming test (FST)
FST was used to examine depressive- and impulsive-like behaviors as described by our group (Kumar et al., 2016; Mazarati et al., 2008; Pineda et al., 2014). The first FST (FST1) was performed 4 weeks after SE; the second (FST2) was conducted during the administration of GalR ligands; some animals underwent the third test (FST3) after the drug washout (Fig. 1). Time between the tests was at least 2 weeks, so as to avoid any previous forced swimming experience to affect an animal’s performance (De Pablo et al., 1989; Mezadri et al., 2011). The test consisted of a single 5-minute-long session in a tank filled with water at 22°-25°C. Behavior was video-recorded and analyzed off-line in a blinded fashion. Cumulative durations of three distinct behaviors were calculated: active adaptive behavior (i.e. swimming along the walls, climbing, diving); immobility (i.e. movements were limited to maintaining the head above the surface with no escape attempts); and non-adaptive struggle (i.e. treading water away from the walls with no attempts to escape) (Mazarati et al., 2008; Pineda et al., 2014). The first two behaviors are typical for both normal animals and those with depressive-like impairments. In validated models of depression, the immobility time is increased, and this increment is regarded as both an indicator, and a measure of the inability to cope with the stress (Cryan et al., 2005). Non-adaptive struggle is negligible in normal rats, but occurs in 25-50% of animals with epilepsy (Kumar et al., 2016; Pineda et al., 2014). We established that only those animals which displayed the non-adaptive struggle during FST, presented with impulsivity in an ADHD-specific Lateralized Reaction Time Task (LRTT) (Pineda et al., 2014). The downside of LRTT and similar tasks (such as 5-choice serial reaction time task, 5-CSRT) is that they take weeks to complete (Faure et al., 2014; Jentsch, 2005; Jentsch et al., 2009) and are thus associated with substantial challenges when used in chronic epilepsy (Faure et al., 2014; Pineda et al., 2014). Based on the congruency between ADHD-specific behavior during LRTT and non-adaptive struggle during FST, we proposed that the latter could be used as a simple surrogate marker of impulsivity (Pineda et al., 2014). Such a suggestion was further confirmed by an observation that both impulsivity in LRTT and non-adaptive struggle in the FST bore a specific neurochemical signature in a form of the suppressed noradrenergic tone in the LC-PFC pathway (Kumar et al., 2016; Pineda et al., 2014).
2.5. Subject selection and categorization
In order to standardize experimental conditions, the animals were advanced to, or excluded from further studies and/or data analysis based on the following criteria:
(1) Spontaneous seizures. Only those animals which presented with spontaneous recurrent secondary generalized complex partial seizures (stage 4-5 using the Racine scale) during the first four weeks of the observation were used. Of these animals, rats were excluded at any time during the study, if they presented with more than 5 seizures per week, as such high seizure frequency renders them unamenable to behavioral testing (Mazarati et al., 2010; Pineda et al., 2014).
(2) Behavior. Based on the behavior in the first FST, animals with epilepsy were assigned to one of four categories (Kumar et al., 2016; Pineda et al., 2014). The assignments were based on behavioral patterns consistently observed in naïve (i.e. non-epileptic) animals. (i) In not impaired animals, cumulative immobility time was < 100 s, and struggling behavior- < 10 s. (ii) In “depressed” rats, cumulative immobility time was ≥100, and struggling behavior- < 10 s. (iii) In “impulsive” animals, cumulative immobility time was < 100 s and struggling behavior- ≥ 10 s. (iv) In “depressed/impulsive” rats, cumulative immobility time was ≥100 s and struggle- ≥10 s.
For our studies, we chose only those animals which were either not impaired, or displayed only one behavioral deficit (i.e. either depressive- or impulsive-like behavior). Furthermore, control subjects which had been identified as outliers during FST1., were removed from further experiments (see 2.9, Data analysis).
2.6. GalR blockers
Pharmacological tools for studying GalR subtypes are limited. Subtype-specific ligands do notexist (in that all available ligands show some degree of cross-affinity among receptor subtypes). Based on commercial availability, we used two peptide blockers of galanin receptors: M40 (Galanin [1-13]-Pro-Pro-[Ala-Leu-]2Ala amide), a non-selective GalR1/GalR2 antagonist (Ki for GalR1 1.82 nM, and for GalR2-5.1 nM), and M871 (Galanin-[2-13]-Glu-His-[Pro]3-[Ala-Leu]2-Ala-amide), a preferential GalR2 antagonist (Ki for GalR1 420 nM, for GalR2-13.1 nM; both compounds from Tocris Bioscience, Bristol, UK) (Mitsukawa et al., 2010; Webling et al., 2012). Therefore, the involvement of GalR1 and GalR2 in regulating behavior and monoamine release was deduced from comparing the effects of the two agents.
2.7. Preparation of drug infusion system, surgery and drug delivery
ALZET osmotic mini-pumps (Cupertino, CA) model 1007D (reservoir volume 100 μl, nominal release rate 0.5 μl/hour) were filled with 10 nM solution of M40, 30 nM solution of M871, or saline. The pumps were connected to the infusion cannula (PlasticsOne, Roanoke, VA; 28 GA; length 6.5 mm for RN, 8.0 mm for LC) via a polypropylene catheter placed in sterile saline and primed at 37ºC for 24 hours prior to the implantation.
For drug delivery, animals within each category (i.e. naïve, epileptic not-impaired, epileptic “depressed” and epileptic “impulsive”) were randomized and the implantation of the pumps was performed in a “blinded” fashion. Surgery was performed under isoflurane anesthesia upon placing the animals in the stereotaxic apparatus. A subcutaneous pocket was prepared in the back. For the intra-RN infusion, a single ALZET pump was placed in the pocket and the catheter was guided under the skin to the exposed skull. The cannula was implanted into RN (AP= −7.8; midline; V= 6.0) (Paxions and Watson, 1986). For the intra-LC infusion, two pumps were placed inside the pocket, and guide cannulas were implanted bilaterally into LC (AP= −9.8; L=1.2, V=7.5) (Paxions and Watson, 1986). Cannulas were fixed to the skull using dental cement and the animal was released into the home cage.
Three days after surgery (i.e. after the start of the drug delivery), the animals underwent FST2, which was followed by FSCV in most them. Separate group of rats underwent FST3 two weeks after FST2, (i.e. after 10 days of washout), followed by FSCV (Fig. 1).
2.8. Fast scan cyclic voltammetry (FSCV)
FSCV affords real time measurement of the strength of a monoamine transmission in neuroanatomical pathways of interest. The technique relies on a non-enzymatic oxidation of monoamines to quinones upon passing specific ramp currents through the carbon fiber electrode (CFE) placed in a monoamine-containing milieu, such as brain tissue or a solution. By measuring the oxidation rate, the amount of monoamine released is inferred (Dankoski et al., 2016; Dankoski and Wightman, 2013; Herr et al., 2012; Park et al., 2009; Park et al., 2011; Wood and Hashemi, 2013). The procedure was performed as previously described by our group (Kumar et al., 2016; Mazarati et al., 2008; Pineda et al., 2014; Pineda et al., 2011) with further optimization for directly calculating the amount of the released transmitter.
In Vivo Voltammetry System (Invilog, Kuopio, Finland) include a Potentiostat/Electrical stimulator unit, connected to the two-electrode headstage on the input terminal and to the digital acquisition board on the output terminal. The digital acquisition board was connected to a Windows 10 personal computer equipped with the FSCV analysis software. A CFE (32 μM, sensitivity >20 nA/μM, Nafion-coated) and a dry reference Ag/AgCl electrode (2.5 mm diameter) were connected to the headstage.
Prior to the procedure, CFE was calibrated in incremental concentrations of standards (5-HT and NE, both from Sigma, St. Louis, MO). For 5-HT, the ramp current applied to the CFE consisted of a resting potential 0 V scanned to 1.2 V, then to −0.6 V and then back to 0 V at 300 V/s. For NE, the ramp current consisted of a resting potential −0.4 V, scanned to 1.15 V and back to −400 V at 300 V/s. Ramp currents were applied every 100 ms over 1 s, and average currents for each monoamine concentration were used to generate standard curves. (Kumar et al., 2016; Pineda et al., 2014)
One day after FST2 (or FST3 where applicable), the animal was anesthetized with Urethane (1.5 g/kg s.c.) and placed in the stereotaxic frame. The cannula and the dental cement cap were removed. CFE was lowered into the infralimbic region of PFC (AP=+2.7, L= 0.5, V=5.0 mm) (Paxions and Watson, 1986). The reference electrode was placed on the nasal bone. The concentric stimulating electrode (26 GA) was connected to the stimulator output of the potentiostat/stimulating unit and was placed in RN. For measuring 5-HT release from PFC in response to the RN stimulation, RN was stimulated simultaneously with 5-HT-specific scanning from PFC. The parameters of stimulation were 400 μA 1 ms bipolar square wave pulses, inter-pulse interval 10 ms, number of pulses 100. Stimulation was repeated 5 times at 5 min interval and averages were used for the analysis. Upon completing the acquisition of 5-HT responses, the stimulating electrode was moved to LC and the procedure was repeated, now with applying NE-specific ramp current. Concentrations of 5-HT and NE released from PFC in response to RN and LC stimulations were calculated based on standard curves using interpolating function of Prism 6 software (GraphPad, San Diego, CA).
Upon completing FSCV, the animals were perfused with 4% paraformaldehyde; coronal sections of PFC, RN and LC were stained with cresyl violet in order to verify proper placements of the electrodes and the cannulas.
2.9. Data analysis
Out of a population of approximately 450 chronic epileptic rats, 131 were selected for the study, based on the parameters described in the Methods section (other animals were used for unrelated projects). The study was completed and the results were analyzed in 97 animals with epilepsy. Thirty four animals were excluded at different time points because they presented with more than 5 spontaneous seizures per week. In addition, 41 naïve animals were enrolled as experimental controls. Out of those, 2 rats were removed after FST1 as outliers using Robust regression and outlier removal function of Prism 6 software with the coefficient 5% (i.e. moderately aggressive). Sample sizes are shown in Table 1.
Table 1.
Sample sizes for the experiments.
| Injection site |
Agent | Animal category | |||
|---|---|---|---|---|---|
| Naive | Epilepsy-not impaired |
Epilepsy – “depressed” |
Epilepsy- “impulsive” |
||
| RN | Saline | 7 | 4 | 6 | 4 |
| M40 | 7 | 4 | 6 | 4 | |
| M871 | 7 | 4 | 6 | 4 | |
| M40- washout |
- | 5 | 5 | - | |
| LC | Saline | 6 | 5 | 4 | 6 |
| M40 | 6 | 5 | 4 | 6 | |
| M871 | 6 | 5 | 4 | 6 | |
| Total | 39 | 32 | 35 | 30 | |
Data were analyzed using Prism 6 software. P<0.05 was accepted for statistical significance. When determining sample size, we adhered to the 3Rs reduction principle. First, sample sizes sufficient for yielding statistical significance were estimated prospectively using StatMate 2.0 software (GraphPad). The estimates were based on the historical highest standard deviation for immobility (30 s, being the highest among all outcome measures) and its rounded average value (80 s for naïve and 140 s for “depressed” rats) (Kumar et al., 2016; Mazarati et al., 2008; Pineda et al., 2014) with the goal of achieving a power of 80%. This process yielded 5 animals per group. During the experiment proper, if a sample size reached 4, data were analyzed in order to determine whether further increase of the number of animals to 5-6 would yield statistical differences. If no such prospect was evident, no further studies with the respective treatment were performed.
3. Results
3.1. Subject selection
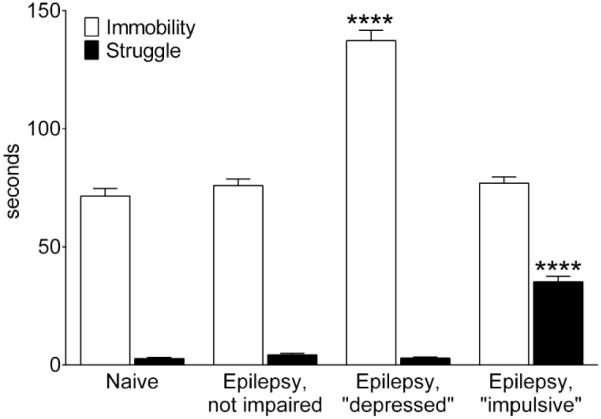
There were no significant differences in the number of secondary generalized complex partial seizures among animals of the three categories during the 4-week selection period (Median/Maximal/Minimal: not impaired- 11/20/5; “depressed”- 11.5/20/4; “impulsive”- 12/19/5, p>0.05, Kruskal-Wallis test). According to the category assignments, epileptic non-impaired animals differed from naïve subjects in neither immobility, nor struggle. Epileptic “depressed” animals showed increased immobility only, while epileptic “impulsive” rats showed increased struggling only (Fig. 2).
Fig. 2. Baseline behavior in the FST.
Immobility and struggle are shown for animals of each category during the FST1, performed at the end of the baseline seizure monitoring and before the randomization for GalR ligand injections. Data are presented as Mean±SEM. Sample sizes: naïve: 39; Epilepsy, not impaired: 32; Epilepsy, “depressed” 30; Epilepsy, “impulsive”: 30. ****- p<0.0001 vs, Naïve (One Way ANOVA plus Dunnet’s multiple comparison test).
3.2. Effects of GalR blockers administered in RN
3.2.1. Behavior
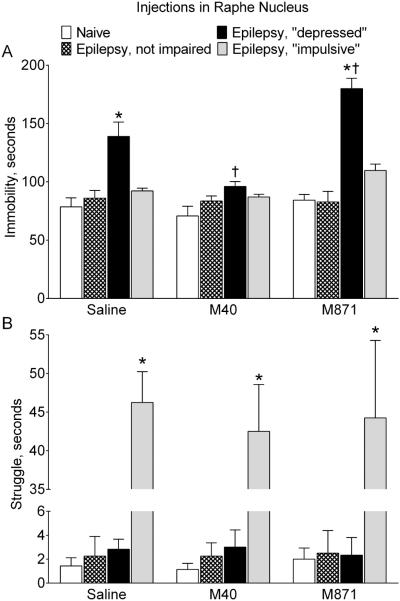
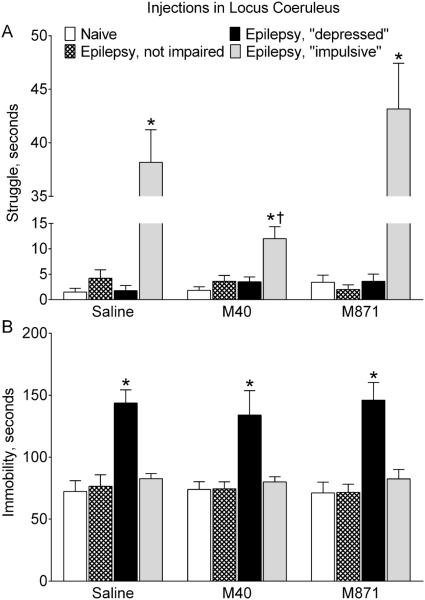
Administration of M40 into RN of epileptic “depressed” rats significantly shortened the immobility time in the FST, as compared with saline-treated subjects of the same category (Fig. 3A). The parameter was now statistically similar to that in naïve animals. Conversely, intra-RN delivery of M871 further increased the immobility time in epileptic “depressed” subjects. Neither M40, nor M871 injection into RN modified immobility in rats of other categories (i.e. naïve, epileptic not impaired and “impulsive”).
Fig. 3. Effects of galanin receptor blockers administered in raphe nucleus on behavior.
Saline, a GalR1/GalR2 blocker M40, or a GalR2 blocker M871 were infused in RN of naïve rats and epileptic animals classified as not impaired, “depressed” and “impulsive” based on their performance in the FST (see Methods). A. Effects on the immobility. Immobility time was significantly increased in epileptic “depressed” rats (Saline). Blockade of RN GalR1/GalR2 (M40) significantly shortened immobility time in these animals only (p>0.05 vs Naïve). Conversely, preferential blockade of GalR2 further increased the immobility time. Neither of the GalR blockers affected immobility in animals of other categories. B. Effects on the struggle. Struggling time was significantly increased in epileptic “impulsive” rats. Neither M40, nor M871 had any effects on struggling behavior in animals of all groups. Data are presented as Mean±SEM. *-p<0.05 vs. Naïve for the same treatment; †- p<0.05 vs. respective category of Saline-treated rats. Two-Way ANOVA plus Tukey’s multiple comparisons test. Immobility: Interaction F (6, 51) = 6.245, p<0.0001; treatment factor F (2, 51) = 14.09, p < 0.0001; behavior factor F (3, 51) = 42.49, p<0.0001. Struggle: Interaction F (6, 51) = 0.1038, p = 0.9956; treatment factor F (2, 51) = 0.1104, p = 0.8957; behavior factor F (3, 51) = 137.0, p < 0.0001.
Intra-RN administration of neither of the GalR blockers affected struggling behavior in animals of all categories (Fig. 3B).
3.2.2. Monoamine transmission
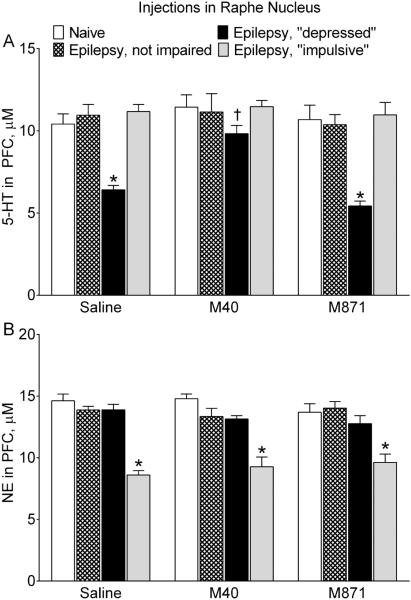
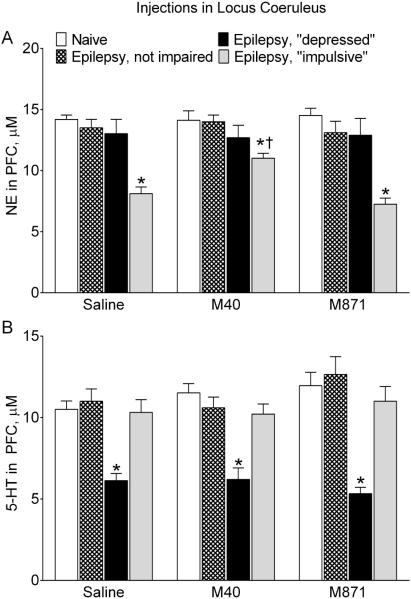
Serotonergic tone in the RN-PFC pathway was selectively suppressed in epileptic “depressed” rats, as compared with naïve and other categories of epileptic animals (Fig. 4A). The administration of M40 in RN of “depressed” animals improved serotonergic tone, which became stronger than in saline-treated rats of the same category and similar to the respective parameter in naïve subjects. In animals of other categories, M40 had no effects on the 5-HT release from PFC. Treatment with M871 did not modify serotonergic transmission in either naïve animals, or any of rats with epilepsy.
Fig. 4. Effects of galanin receptor blockers administered in the raphe nucleus on serotonergic and noradrenergic transmission.
The strength of serotonergic tone in RN-PFC and of noradrenergic tone in LC-PFC are shown for the same animals as in Fig. 1. A. Effects on serotonergic transmission. Epileptic “depressed” rats were characterized by the suppressed serotonergic tone, evident as lower amount of 5-HT released from PFC in response to the RN stimulation (Saline). Blockade of RN GalR1/GalR2 (M40) significantly improved serotonergic tone in “depressed” rats, while blockade of GalR2 (M871) had no effects. Neither of the GalR blockers affected responses in animals of other categories. B. Effects on noradrenergic transmission. Noradrenergic tone in LC-PFC was suppressed in epileptic “impulsive” subjects (Saline). Neither of the treatments had any effects on noradrenergic transmission in animals of all groups. Data are presented as Mean±SEM *-p<0.05 vs. Naïve in the same treatment; †- p<0.05 vs. respective category of Saline-treated rats. Two-Way ANOVA plus Tukey’s multiple comparisons test. Serotonergic transmission: Interaction F (6, 51) = 2.371, p = 0.0426; treatment factor F (2, 51) = 5.952, p = 0.0047; behavior factor F (3, 51) = 25.24, p < 0.0001. Noradrenergic transmission: Interaction F (6, 51) = 1.021, p = 0.4226; treatment factor: F (2, 51) = 0.1458, p = 0.8647; behavior factor F (3, 51) = 44.92, p < 0.0001.
Noradrenergic tone in LC-PFC was suppressed selectively in epileptic “impulsive” rats (Fig. 4B). Neither M40, nor M871 had any effects on noradrenergic transmission in animals of all categories.
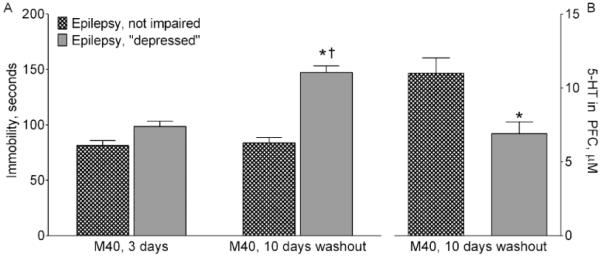
3.2.3. Behavior and serotonergic tone after the M40 washout
In order to establish whether the effects of the intra-RN administration of M40 on behavior and serotonergic transmission were transient, separate group of epileptic not impaired and “depressed” animals were examined in the FST 10 days after the end of the M40 infusion (Fig. 5). In the absence of M40, the immobility time in “depressed” rats was significantly longer than during the drug delivery. FSCV revealed suppressed serotonergic tone in RN-PFC of these animals.
Fig. 5. Behavior and serotonergic transmission after a washout following the administration of M40 in raphe nucleus.
A. Behavior. Immobility in the FST was examined 3 days (i.e. during drug delivery) and 2 weeks (i.e. after a 10 day-washout) after the start of intra-RN infusion of a GalR1/GalR2 blocker M40 in epileptic not-impaired and “depressed” rats. During M40 administration immobility time was similar between the two groups, and in the range observed in naïve animals (compare with Naïve, Fig. 1). After the washout, immobility time in “depressed” animals was significantly longer and in the range observed in “depressed” saline-treated rats (compared with Fig 1, Epilepsy, “depressed”). Data are presented as Mean±SEM *- p<0.05 “M40, 1-week washout” vs. “M40, 3 days”; †- p<0.05 Epilepsy, “depressed” vs. Epilepsy, not impaired. Two Way ANOVA with multiple comparisons plus Sidak’s multiple comparisons test. Interaction: F (1, 8) = 20.20, p = 0.002; time factor F (1, 8) = 24.59, p = 0.0011; Behavior factor F (1, 8) = 70.32, p = 0.0001. B. Serotonergic transmission. After the second FST (shown on A), serotonergic tone was measured in RN-PFC. In epileptic “depressed” subjects, the strength of responses was lowers than in epileptic not impaired rats. In both groups, data were in the same range as in respective saline-treated rats (compare with Fig. 2A). *-p<0.05 (Mann-Whitney test).
3.3. Effects of GalR blockers administered in LC
3.3.1. Behavior
Intra-LC infusion of M40 significantly shortened the duration of struggling behavior in epileptic “impulsive” rats (Fig. 6A) as compared with saline-treated animals of this category. Still, struggle remained more represented in these animals, than in naïve subjects. M40 did not modify struggling behavior in animals of other categories (i.e. naïve, epileptic not impaired and “depressed”), and M871 had no effects on this behavior in any group.
Fig. 6. Effects of galanin receptor blockers administered in the locus coeruleus on behavior.
Saline, a GalR1/GalR2 blocker M40, or a GalR2 blocker M871 were infused in LC of naïve rats (n=6 for each treatment) and epileptic animals classified as not impaired, “depressed” and “impulsive” based on their performance in the FST (see Methods). A. Effects on the struggle. Blockade of LC GalR1/GalR2 (M40) significantly shortened the duration of struggling behavior in epileptic “impulsive” rats, although the behavior was still significantly more represented than in naïve animals. Blockade of GalR2 (M871) did not modify the struggling behavior. None of GalR blockers affected the examined behavior in animals of other groups. B. Effects on the immobility. No effects on either of GalR blockers were observed in animals of all groups. Data are presented as Mean±SEM *-p<0.05 vs. Naïve in the same treatment; †- p<0.05 vs. respective category of Saline-treated rats. Two-Way ANOVA plus Tukey’s multiple comparisons test. Struggle: Interaction F (6, 53) = 17.25, p< 0.0001; treatment factor F (2, 54) = 15.3 , p < 0.0001; behavior factor F (3, 53) = 146.7, p < 0.0001. Immobility: Interaction F (6, 51) = 0.1544, p = 0.9873; treatment factor F (2, 51) = 0.1423, p = 0.8677; behavior factor F (3, 51) = 35.51, p < 0.0001.
None of the GalR blockers, when administered in LC, had any effects on the immobility in naïve and epileptic rats (Fig. 6B).
3.3.2. Monoamine transmission
Intra-LC administration of M40 significantly improved noradrenergic tone in the LC-PFC pathway of epileptic “impulsive” rats, as compared with the saline treatment (Fig. 7A). At the same time, the responses remained compromised in comparison with those in naïve subjects. M871 exerted no effects on noradrenergic transmission in either “impulsive” or other animals. Neither M40, nor M871, affected serotonergic tone in RN-PFC of animals of any group (Fig. 7B).
Fig. 7. Effects of galanin receptor blockers administered in the locus coeruleus on serotonergic and noradrenergic transmission.
The strength of noradrenergic tone in LC-PFC and of serotonergic tone in RN-PFC in LC-PFC are shown in the same animals as in Fig. 6. A. Effects on noradrenergic transmission. In epileptic “impulsive” rats, blockade of LC GalR1/GalR2 (M40) significantly strengthened noradrenergic tone, although it remained weaker than in naïve subjects. Blockade of GalR2 (M871) had no effect on the examined parameter. Neither of the GalR blockers modified noradrenergic transmission in animals of other groups. B. Effects on serotonergic transmission. Neither of the treatments had any effects on serotonergic tone in animals of all groups. Data are presented as Mean±SEM. *-p<0.05 vs. Naïve in the same treatment; †- p<0.05 vs. respective category of Saline-treated rats. Two-Way ANOVA plus Tukey’s multiple comparisons test. Noradrenergic transmission: Interaction F (6, 51) = 2.116, p = 0.0674; treatment factor F (2, 51) = 2.073, p = 0.1363; behavior factor F (3, 51) = 38.83, p < 0.0001. Serotonergic transmission: Interaction F (6, 51) = 0.8145, p = 0.5637; treatment F (2, 51) = 1.107, p = 0.3382; behavior factor F (3, 51) = 31.24, p< 0.0001.
3.4. Spontaneous seizures
Neither M40, nor 871 administered in both RN and LC changed the frequency of secondary generalized complex partial seizures in any animals (data not shown). It should be noted that such specifics of experimental design as short duration of drug delivery and low spontaneous seizure frequency did not allow for objectively assessing effects of the treatments on spontaneous seizures.
4. Discussion
In agreement with earlier reports (Kumar et al., 2016; Pineda et al., 2014), post-SE epilepsy was characterized by either the suppressed serotonergic tone in the RN-PFC pathway, or the suppressed noradrenergic tone in the LC-PFC pathway. Serotonergic deficits translated in depressive-like behavior, and noradrenergic deficits-in impulsive-like behavior. Local pharmacological blockade of GalR1/GalR2 transiently improved monoaminergic perturbations in a site-specific manner, and stemming from these perturbations behavioral impairments. At the same time, preferential blockade of GalR2 had no effects on neurotransmitter and dependent behavioral abnormalities (and in the case of immobility, even further exacerbation was observed).
Galanin has been known for its inhibition of monoamine transmission (Hökfelt et al., 1998; Kuteeva et al., 2008; Lundstrom et al., 2005; Picciotto et al., 2010). Such an umbrella statement, however, does not reflect the complexity of regulatory effects of GalR subtypes. GalR1 is a Gi protein-coupled receptor; its activation inhibits cyclic adenosine monophosphate synthesis, and opens G protein-gated K+ channels (Counts et al., 2002; Wang et al., 1998; Webling et al., 2012). The resulting inhibition of neuronal firing explains inhibitory effects of GalR1 stimulation. GalR2 couples to Gq/11 which leads to the activation of Phospholipase C, increased inositol trisphosphate production and mobilization of intracellular Ca2+, ultimately resulting in increased neuronal activity and transmitter release (Wang et al., 1998).
While the presence of galanin in RN and LC has been well established (Gundlach et al., 1990; Melander et al., 1986; Merchenthaler et al., 1993; Skofitsch and Jacobowitz, 1986), the distribution of different galanin receptor subtypes in RN and LC is still being debated. On the one hand, the presence of both GalR1 and GalR2 binding sites in the rat RN and LC has been corroborated by several reports, including a higher abundance of GalR1 vs. GalR2 in RN (Lu et al., 2005). On the other hand, an in situ hybridization study suggested an exclusive presence of GalR2 in RN (Xu et al., 1998). However, the same study reported that effects of galanin in RN were inhibitory and occurred through increasing K+ conductance, which is a signature of the activation of GalR1, but not of GalR2. Indirect evidence also points towards the presence in RN of both GalR1 and GalR2, with the preponderance of the former. Thus, the injection of galanin (1-29) (an endogenous GalR1=GalR2 agonist) into RN of rats facilitated SE, presumably through suppressing 5-HT release in forebrain efferents. At the same time, the administration of the peptide in RN of GalR1 knockout mice suppressed seizures via increasing 5-HT release in the forebrain, thus implicating GalR2, the effects of which were unmasked though the GalR1 deletion (Mazarati et al., 2005). There appears to be a consensus as to the absence of GalR3 in both RN and LC in rodents (Le Maitre et al., 2013).
Depression-related studies also suggest the presence of both GalR1 and GalR2 in RN. Thus, galanin 1-29 injected in RN decreased, while galanin 2-11 (a GalR2>GalR3 agonist)-increased forebrain level of 5-HT (Mazarati et al., 2005). Intraventricular infusion of a GalR1 agonist M617 and of a GalR2 antagonist M871 exerted pro- and antidepressant effects respectively (Kuteeva et al., 2008). In LC, native galanin induced hyperpolarization of noradrenergic neurons (Pieribone et al., 1995), thus pointing towards a GalR1-mediated effect.
Overall, the existing literature permits to conclude that both GalR1 and GalR2 (but not GalR3) are present in RN and LC, and that GalR1 is more abundant.
Because of the lack of preferential blockers of GalR1, in the present study we inferred the involvement of GalR1 and GalR2 through comparing the effects of a non-selective GalR1/GalR2 antagonist with the effects of a preferential blocker of GalR2. Since the blockade of GalR1/GalR2 produced specific improvements in neurotransmission and behaviors, and at the same time the blockade of GalR2 had no effects, it is reasonable to assume that the regulation of monoamine transmission and respective behaviors by M40 occurs through GalR1. Taken together, the results imply that in epileptic “depressed” rats, galanin acting through GalR1 suppresses the 5-HT release from RN efferents, which leads to depressive-like behavior; in epileptic “impulsive” rats- the NE release from LC efferents leads to impulsive-like behavior.
One important finding was that while blockade of GalR1 in both RN and LC improved neurotransmitter and behavioral deficits in affected animals, it did not modify the examined parameters in unimpaired rats. Thus, no effects were observed in either naïve, or epileptic-not impaired subjects; in “depressed” rats the effects of M40 were limited to serotonergic transmission and immobility, and in “impulsive” rats – to noradrenergic transmission and struggling behavior. The most reasonable explanation is that if a monoaminergic pathway is optimally tuned, its performance cannot be further enhanced. The same stands for monoamine-regulated behaviors: immobility displayed by unimpaired animals reflects not a state of despair/hopelessness, but represents a part of an optimal strategy to engineer the escape, while struggling behavior is all but non-existent.
The situation with GalR2 may be more complex. Based on the earlier discussion (Kuteeva et al., 2008; Mazarati et al., 2005), local blockade of GalR2 should have suppressed monoaminergic tone and consequently should have impaired behavioral performance. However, the only case when the effect of M871 was obvious, was further increase in the immobility time in “depressed” subjects, and even then it occurred in the absence of further suppression of serotonergic transmission. Beyond this one instance, there was high degree of congruency between modulatory effects of galanin on neurotransmitters and behavior, and we cannot offer a satisfactory explanation for the disconnect between behavioral and serotonergic effects of M871. One possible reason is that even a subtle reduction of monoamine transmission by M871, which did not amount to statistical significance, was sufficient to amplify the behavioral deficit.
It is possible that the M871 could have exerted effects on monoamine transmission and behavior if applied at higher concentrations, and judging by its effects on the immobility, the effects would have been detrimental. Nevertheless, even in their present form, the experiments were sufficient to conclude that since M40 was effective, and M871 at the equivalent concentration was not, the activation of a mixed pool of GalR1 and GalR2 in RN and LC by endogenous galanin would lead to monoaminergic deficits via activation of GalR1. This idea is further supported by the earlier discussion that GalR2 activation should improve, rather that disturb, serotonergic and noradrenergic transmission in RN and LC efferents (Mazarati et al., 2005; Wang et al., 1998).
Our results do not align with the previously suggested regulation of depressive behavior by galanin in LC (Epps et al., 2013). In this study, chronic voluntary exercise in rats alleviated depressive-like behavior, and the effect correlated with the increased galanin expression in LC (with the implication that noradrenergic neurotransmission was involved). However, the experimental design was different from ours in that depression was a result of selective breeding rather than a consequence of SE. Furthermore, the authors did not examine the strength of noradrenergic transmission. Finally, neither galanin expression in RN, nor the strength of serotonergic transmission were interrogated. Noradrenergic mechanisms of depression have been established (Chandley and Ordway, 2012), and it is plausible that in certain depression models, norepinephrine plays a major role. It is thus reasonable to assume that in different models, the contribution of different types of monoamine transmission in depression is different, and so is the role of galanin (e.g. GalR2 may be selectively up-regulated as a result of chronic exercise, a possibility which was not explored in the cited work).
One important issue which remains to be resolved is whether transmitter and behavioral impairments in epileptic rats occur at least in part due to the epilepsy-triggered plasticity of galanin signaling, for example local upregulation of galanin, GalR1 and/or the downregulation of GalR2, and if the answer is yes, then which upstream events in turn produce changes in galanin and/or its receptors. Or, do the impairments in question have entirely different underlying mechanisms, and galanin merely by the virtue of its presence and higher abundance of GalR1 exacerbates monoaminergic and behavioral perturbations already in place?
5. Conclusion
Our experiments confirm that galanin is a tangible modulator of both serotonergic and noradrenergic transmission in RN and LC efferents respectively. Furthermore, the studies provide further, albeit indirect, corroboration that both GalR1 and GalR2 are present in both RN and LC; that GalR1 is preponderant over GalR2 and inhibits monoamine release; that GalR2 facilitates monoamine release, although this effect is all but inconsequential due to its lower presence than that of GalR1. The finding, that interfering with the action of endogenous galanin on GalR1 improves epilepsy-associated monoamine perturbations and related comorbidities suggests that endogenous galanin, acting through GalR1, may be involved in the pathophysiology of epilepsy-associated depression and ADHD.
Highlights.
Rats with chronic epilepsy develop depressive- like or impulsive- like behaviors
Suppressed serotonin (5-HT) release from raphe nucleus (RN) underlies depression
Suppressed norepinephrine release from locus coeruleus (LC) underlies impulsivity
Blockade of RN GalR1, but not GalR2 receptor improves 5-HT release and depression
Blockade of LC GalR1, but not GalR2 receptor improves NE release and impulsivity
Acknowledgements
This work was supported by research grants R01NS065783 and R21NS089396 from the National Institutes of Health to AM; and by Sudha Neelakantan and Venky Harinarayan Charitable Fund endowment to Epilepsy Research Laboratories. SM was supported by postdoctoral fellowships No 237168 and 264551 from National Council of Science and Technology (CONACYT, Mexico).
We thank Dr. Udaya Kumar for assistance with behavioral assays.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baca CB, Vickrey BG, Caplan R, Vassar SD, Berg AT. Psychiatric and medical comorbidity and quality of life outcomes in childhood-onset epilepsy. Pediatrics. 2011;128:e1532–1543. doi: 10.1542/peds.2011-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandley MJ, Ordway GA. Noradrenergic Dysfunction in Depression and Suicide. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Counts SE, McGuire SO, Sortwell CE, Crawley JN, Collier TJ, Mufson EJ. Galanin inhibits tyrosine hydroxylase expression in midbrain dopaminergic neurons. J Neurochem. 2002;83:442–451. doi: 10.1046/j.1471-4159.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182:335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Dankoski EC, Carroll S, Wightman RM. Acute selective serotonin reuptake inhibitors regulate the dorsal raphe nucleus causing amplification of terminal serotonin release. J Neurochem. 2016 doi: 10.1111/jnc.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankoski EC, Wightman RM. Monitoring serotonin signaling on a subsecond time scale. Front Integr Neurosci. 2013;7:44. doi: 10.3389/fnint.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Pablo JM, Parra A, Segovia S, Guillamon A. Learned immobility explains the behavior of rats in the forced swimming test. Physiol Behav. 1989;46:229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- Epps SA, Kahn AB, Holmes PV, Boss-Williams KA, Weiss JM, Weinshenker D. Antidepressant and anticonvulsant effects of exercise in a rat model of epilepsy and depression comorbidity. Epilepsy Behav. 2013;29:47–52. doi: 10.1016/j.yebeh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure JB, Marques-Carneiro JE, Akimana G, Cosquer B, Ferrandon A, Herbeaux K, Koning E, Barbelivien A, Nehlig A, Cassel JC. Attention and executive functions in a rat model of chronic epilepsy. Epilepsia. 2014;55:644–653. doi: 10.1111/epi.12549. [DOI] [PubMed] [Google Scholar]
- Gundlach AL, Wisden W, Morris BJ, Hunt SP. Localization of preprogalanin mRNA in rat brain: in situ hybridization study with a synthetic oligonucleotide probe. Neurosci Lett. 1990;114:241–247. doi: 10.1016/0304-3940(90)90570-y. [DOI] [PubMed] [Google Scholar]
- Herr NR, Park J, McElligott ZA, Belle AM, Carelli RM, Wightman RM. In vivo voltammetry monitoring of electrically evoked extracellular norepinephrine in subregions of the bed nucleus of the stria terminalis. Journal of neurophysiology. 2012;107:1731–1737. doi: 10.1152/jn.00620.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hökfelt T, Xu ZQ, Shi TJ, Holmberg K, Zhang X. Galanin in ascending systems. Focus on coexistence with 5-hydroxytryptamine and noradrenaline. Ann N Y Acad Sci. 1998;863:252–263. doi: 10.1111/j.1749-6632.1998.tb10700.x. [DOI] [PubMed] [Google Scholar]
- Jentsch JD. Impaired visuospatial divided attention in the spontaneously hypertensive rat. Behavioural brain research. 2005;157:323–330. doi: 10.1016/j.bbr.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Aarde SM, Seu E. Effects of atomoxetine and methylphenidate on performance of a lateralized reaction time task in rats. Psychopharmacology (Berl) 2009;202:497–504. doi: 10.1007/s00213-008-1181-0. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016;12:106–116. doi: 10.1038/nrneurol.2015.243. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, Caplan R, Ryvlin P, Gilliam F, LaFrance WC., Jr. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24:156–168. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kumar U, Medel-Matus JS, Redwine HM, Shin D, Hensler JG, Sankar R, Mazarati A. Effects of selective serotonin and norepinephrine reuptake inhibitors on depressive- and impulsive-like behaviors and on monoamine transmission in experimental temporal lobe epilepsy. Epilepsia. 2016;57:506–515. doi: 10.1111/epi.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuteeva E, Hökfelt T, Wardi T, Ogren SO. Galanin, galanin receptor subtypes and depression-like behaviour. EXS. 2010;102:163–181. doi: 10.1007/978-3-0346-0228-0_12. [DOI] [PubMed] [Google Scholar]
- Kuteeva E, Wardi T, Lundstrom L, Sollenberg Ü, Langel Ü, Hökfelt T, Ogren SO. Differential role of galanin receptors in the regulation of depression-like behavior and monoamine/stress-related genes at the cell body level. Neuropsychopharmacology. 2008;33:2573–2585. doi: 10.1038/sj.npp.1301660. [DOI] [PubMed] [Google Scholar]
- Lang R, Gundlach AL, Holmes FE, Hobson SA, Wynick D, Hökfelt T, Kofler B. Physiology, signaling, and pharmacology of galanin peptides and receptors: three decades of emerging diversity. Pharmacol Rev. 2015;67:118–175. doi: 10.1124/pr.112.006536. [DOI] [PubMed] [Google Scholar]
- Le Maitre E, Barde SS, Palkovits M, Diaz-Heijtz R, Hökfelt TG. Distinct features of neurotransmitter systems in the human brain with focus on the galanin system in locus coeruleus and dorsal raphe. Proc Natl Acad Sci U S A. 2013;110:E536–545. doi: 10.1073/pnas.1221378110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Barr AM, Kinney JW, Sanna P, Conti B, Behrens MM, Bartfai T. A role for galanin in antidepressant actions with a focus on the dorsal raphe nucleus. Proc Natl Acad Sci U S A. 2005;102:874–879. doi: 10.1073/pnas.0408891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom L, Elmquist A, Bartfai T, Langel Ü. Galanin and its receptors in neurological disorders. Neuromolecular Med. 2005;7:157–180. doi: 10.1385/NMM:7:1-2:157. [DOI] [PubMed] [Google Scholar]
- Luoni C, Bisulli F, Canevini MP, De Sarro G, Fattore C, Galimberti CA, Gatti G, La Neve A, Muscas G, Specchio LM, Striano S, Perucca E, Group SS. Determinants of health-related quality of life in pharmacoresistant epilepsy: results from a large multicenter study of consecutively enrolled patients using validated quantitative assessments. Epilepsia. 2011;52:2181–2191. doi: 10.1111/j.1528-1167.2011.03325.x. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Lu X. Regulation of limbic status epilepticus by hippocampal galanin type 1 and type 2 receptors. Neuropeptides. 2005;39:277–280. doi: 10.1016/j.npep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Lundstrom L, Sollenberg Ü, Shin D, Langel Ü, Sankar R. Regulation of kindling epileptogenesis by hippocampal galanin type 1 and type 2 receptors: The effects of subtype-selective agonists and the role of G-protein-mediated signaling. J Pharmacol Exp Ther. 2006;318:700–708. doi: 10.1124/jpet.106.104703. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Siddarth P, Baldwin RA, Shin D, Caplan R, Sankar R. Depression after status epilepticus: behavioural and biochemical deficits and effects of fluoxetine. Brain. 2008;131:2071–2083. doi: 10.1093/brain/awn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Baldwin RA, Shinmei S, Sankar R. In vivo interaction between serotonin and galanin receptors types 1 and 2 in the dorsal raphe: implication for limbic seizures. J Neurochem. 2005;95:1495–1503. doi: 10.1111/j.1471-4159.2005.03498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Hohmann JG, Bacon A, Liu H, Sankar R, Steiner RA, Wynick D, Wasterlain CG. Modulation of hippocampal excitability and seizures by galanin. J Neurosci. 2000;20:6276–6281. doi: 10.1523/JNEUROSCI.20-16-06276.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Liu H, Soomets U, Sankar R, Shin D, Katsumori H, Langel Ü, Wasterlain CG. Galanin modulation of seizures and seizure modulation of hippocampal galanin in animal models of status epilepticus. J Neurosci. 1998;18:10070–10077. doi: 10.1523/JNEUROSCI.18-23-10070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarati AM, Pineda E, Shin D, Tio D, Taylor AN, Sankar R. Comorbidity between epilepsy and depression: role of hippocampal interleukin-1beta. Neurobiol Dis. 2010;37:461–467. doi: 10.1016/j.nbd.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melander T, Hökfelt T, Rokaeus A, Cuello AC, Oertel WH, Verhofstad A, Goldstein M. Coexistence of galanin-like immunoreactivity with catecholamines, 5-hydroxytryptamine, GABA and neuropeptides in the rat CNS. J Neurosci. 1986;6:3640–3654. doi: 10.1523/JNEUROSCI.06-12-03640.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchenthaler I, Lopez FJ, Negro-Vilar A. Anatomy and physiology of central galanin-containing pathways. Prog Neurobiol. 1993;40:711–769. doi: 10.1016/0301-0082(93)90012-h. [DOI] [PubMed] [Google Scholar]
- Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-de-Oliveira C. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods. 2011;195:200–205. doi: 10.1016/j.jneumeth.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Mitsukawa K, Lu X, Bartfai T. Galanin, galanin receptors, and drug targets. EXS. 2010;102:7–23. doi: 10.1007/978-3-0346-0228-0_2. [DOI] [PubMed] [Google Scholar]
- Parisi P, Moavero R, Verrotti A, Curatolo P. Attention deficit hyperactivity disorder in children with epilepsy. Brain Dev. 2010;32:10–16. doi: 10.1016/j.braindev.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci. 2009;30:2121–2133. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxions G, Watson C. The Rat brain in stereotaxic coordinates. Academic Press; San Diego: 1986. et al. [Google Scholar]
- Picciotto MR, Brabant C, Einstein EB, Kamens HM, Neugebauer NM. Effects of galanin on monoaminergic systems and HPA axis: Potential mechanisms underlying the effects of galanin on addiction- and stress-related behaviors. Brain Res. 2010;1314:206–218. doi: 10.1016/j.brainres.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Xu ZQ, Zhang X, Grillner S, Bartfai T, Hökfelt T. Galanin induces a hyperpolarization of norepinephrine-containing locus coeruleus neurons in the brainstem slice. Neuroscience. 1995;64:861–874. doi: 10.1016/0306-4522(94)00450-j. [DOI] [PubMed] [Google Scholar]
- Pineda E, Jentsch JD, Shin D, Griesbach G, Sankar R, Mazarati A. Behavioral impairments in rats with chronic epilepsy suggest comorbidity between epilepsy and attention deficit/hyperactivity disorder. Epilepsy Behav. 2014;31:267–275. doi: 10.1016/j.yebeh.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda EA, Hensler JG, Sankar R, Shin D, Burke TF, Mazarati AM. Plasticity of presynaptic and postsynaptic serotonin 1A receptors in an animal model of epilepsy-associated depression. Neuropsychopharmacology. 2011;36:1305–1316. doi: 10.1038/npp.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda EA, Hensler JG, Sankar R, Shin D, Burke TF, Mazarati AM. Interleukin-1beta causes fluoxetine resistance in an animal model of epilepsy-associated depression. Neurotherapeutics. 2012;9:477–485. doi: 10.1007/s13311-012-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlifke I, Kuteeva E, Hökfelt T, Kokaia M. Galanin expressed in the excitatory fibers attenuates synaptic strength and generalized seizures in the piriform cortex of mice. Exp Neurol. 2006;200:398–406. doi: 10.1016/j.expneurol.2006.02.124. [DOI] [PubMed] [Google Scholar]
- Schubert R. Attention deficit disorder and epilepsy. Pediatr Neurol. 2005;32:1–10. doi: 10.1016/j.pediatrneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Skofitsch G, Jacobowitz DM. Quantitative distribution of galanin-like immunoreactivity in the rat central nervous system. Peptides. 1986;7:609–613. doi: 10.1016/0196-9781(86)90035-5. [DOI] [PubMed] [Google Scholar]
- Smith KE, Walker MW, Artymyshyn R, Bard J, Borowsky B, Tamm JA, Yao WJ, Vaysse PJ, Branchek TA, Gerald C, Jones KA. Cloned human and rat galanin GALR3 receptors. Pharmacology and activation of G-protein inwardly rectifying K+ channels. J Biol Chem. 1998;273:23321–23326. doi: 10.1074/jbc.273.36.23321. [DOI] [PubMed] [Google Scholar]
- Wang S, Hashemi T, Fried S, Clemmons AL, Hawes BE. Differential intracellular signaling of the GalR1 and GalR2 galanin receptor subtypes. Biochemistry. 1998;37:6711–6717. doi: 10.1021/bi9728405. [DOI] [PubMed] [Google Scholar]
- Webling KE, Runesson J, Bartfai T, Langel Ü. Galanin receptors and ligands. Front Endocrinol (Lausanne) 2012;3:146. doi: 10.3389/fendo.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KM, Hashemi P. Fast-scan cyclic voltammetry analysis of dynamic serotonin reponses to acute escitalopram. ACS Chem Neurosci. 2013;4:715–720. doi: 10.1021/cn4000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, Zhang X, Pieribone VA, Grillner S, Hökfelt T. Galanin-5-hydroxytryptamine interactions: electrophysiological, immunohistochemical and in situ hybridization studies on rat dorsal raphe neurons with a note on galanin R1 and R2 receptors. Neuroscience. 1998;87:79–94. doi: 10.1016/s0306-4522(98)00151-1. [DOI] [PubMed] [Google Scholar]