ABSTRACT
Staphylococcus aureus TF2758 is a clinical isolate from an atheroma and a super-biofilm-elaborating/polysaccharide intercellular adhesin (PIA)/poly-N-acetylglucosamine (PNAG)-overproducing strain (L. Shrestha et al., Microbiol Immunol 60:148–159, 2016, https://doi.org/10.1111/1348-0421.12359). A microarray analysis and DNA genome sequencing were performed to identify the mechanism underlying biofilm overproduction by TF2758. We found high transcriptional expression levels of a 7-gene cluster (satf2580 to satf2586) and the ica operon in TF2758. Within the 7-gene cluster, a putative transcriptional regulator gene designated rob had a nonsense mutation that caused the truncation of the protein. The complementation of TF2758 with rob from FK300, an rsbU-repaired derivative of S. aureus strain NCTC8325-4, significantly decreased biofilm elaboration, suggesting a role for rob in this process. The deletion of rob in non-biofilm-producing FK300 significantly increased biofilm elaboration and PIA/PNAG production. In the search for a gene(s) in the 7-gene cluster for biofilm elaboration controlled by rob, we identified open reading frame (ORF) SAOUHSC_2898 (satf2584). Our results suggest that ORF SAOUHSC_2898 (satf2584) and icaADBC are required for enhanced biofilm elaboration and PIA/PNAG production in the rob deletion mutant. Rob bound to a palindromic sequence within its own promoter region. Furthermore, Rob recognized the TATTT motif within the icaR-icaA intergenic region and bound to a 25-bp DNA stretch containing this motif, which is a critically important short sequence regulating biofilm elaboration in S. aureus. Our results strongly suggest that Rob is a long-sought repressor that recognizes and binds to the TATTT motif and is an important regulator of biofilm elaboration through its control of SAOUHSC_2898 (SATF2584) and Ica protein expression in S. aureus.
IMPORTANCE
During the search for molecular mechanisms underlying biofilm overproduction of Staphylococcus aureus TF2758, we found a putative transcriptional regulator gene designated rob within a 7-gene cluster showing a high transcriptional expression level by microarray analysis. The deletion of rob in non-biofilm-producing FK300, an rsbU-repaired derivative of NCTC8325-4, significantly increased biofilm elaboration and PIA/PNAG production. The search for a gene(s) in the 7-gene cluster for biofilm elaboration controlled by rob identified ORF SAOUHSC_2898. Besides binding to its own promoter region to control ORF SAOUHSC_2898 expression, Rob recognized the TATTT motif within the icaR-icaA intergenic region and bound to a 25-bp DNA stretch containing this motif, which is a critically important short sequence regulating biofilm elaboration in S. aureus. Our results strongly suggest that Rob is a long-sought repressor that recognizes and binds to the TATTT motif and is a new important regulator of biofilm elaboration through its control of SAOUHSC_2898 and Ica protein expression in S. aureus.
INTRODUCTION
Staphylococcus aureus is among the most common human pathogens, causing a wide range of infections, from superficial skin and mucosal infections to bone or lung infections, as well as serious systemic diseases. S. aureus colonization has been regarded as a risk factor for developing subsequent infections. Some chronic infections, such as endocarditis, osteomyelitis, and those on implanted medical devices, are characteristically associated with biofilm elaboration (1–3). Development of biofilms has been divided into at least three physiologically different stages: initial attachment, biofilm maturation, and detachment (or dispersal), which involves specific factors (4). The matrix of a staphylococcal biofilm is mainly composed of polysaccharides, cell surface and secreted bacterial proteins, and extracellular DNA (5). Cells encased in the matrix are protected from antibiotic therapy and host immune responses (3, 4, 6). Dispersal of cells from a biofilm may be important for the dissemination of the bacteria (7).
The main exopolysaccharide of the S. aureus biofilm matrix is poly-N-acetylglucosamine (PNAG), which is also known as polysaccharide intercellular adhesin (PIA) (8). The synthesis and accumulation of PIA/PNAG on the cell surface are carried out by the products of four genes: icaA, icaD, icaB, and icaC (9). These genes are located in one operon and were first identified by Heilmann et al. (10). Recent studies have indicated that the expression of icaADBC is affected by a number of regulatory and environmental factors (11–14). The icaR gene is located adjacent to icaADBC but is divergently transcribed from this operon (15). The protein encoded by icaR belongs to the TetR family of transcriptional regulators and represses icaADBC transcription by binding to a region immediately upstream of the icaA start codon (16). Additionally, environmental factors, including glucose, ethanol, high temperatures, and high osmolarity, have been reported to affect biofilm elaboration (11–14). Ethanol increases the expression of icaA by repressing icaR transcription (15). In contrast, enhancement of icaA expression by high glucose or NaCl levels was found to occur independently of icaR.
A 5-nucleotide motif (TATTT) within the icaR-icaA intergenic region was previously shown to play a key role in the transcription of the ica locus (16). This study also demonstrated that IcaR binds to a 42-bp sequence within the ica promoter region but not the TATTT sequence. Hence, the effects of the TATTT motif on icaADBC expression have been suggested to be controlled by another, as-yet-unidentified repressor(s).
We evaluated the biofilm-elaborating ability of clinical isolates in Japan and found that TF2758, which was isolated from an atheroma, is an extremely high biofilm producer (17). Whole-genome sequencing and a microarray analysis of TF2758 discovered a spontaneous mutation in a putative transcriptional regulator gene, within a 7-gene cluster, which was expressed at markedly higher levels than in a non-biofilm-elaborating control strain. We designated this gene rob, regulator of biofilm. In the present study, we demonstrate that Rob is a long-sought repressor that recognizes and binds to the TATTT motif and suggest that Rob is an important regulator of biofilm elaboration through its control of the expression of as-yet-uncharacterized hypothetical protein SAOUHSC_2898 (SATF2584) and IcaADBC.
RESULTS
Identification of rob from a super-biofilm-elaborating strain.
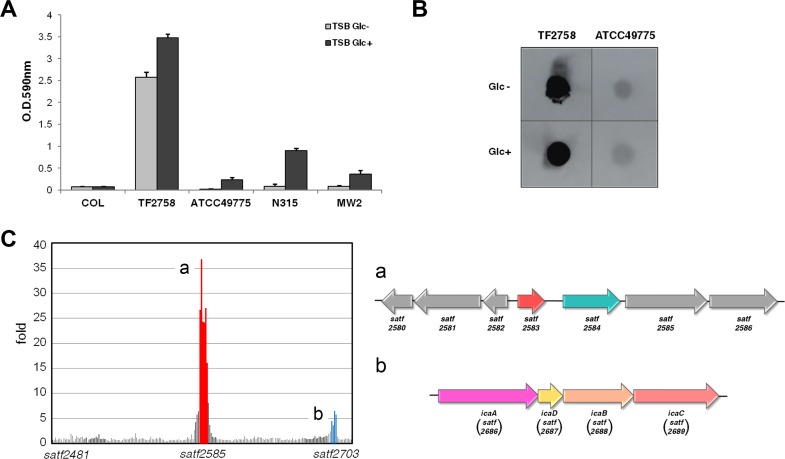
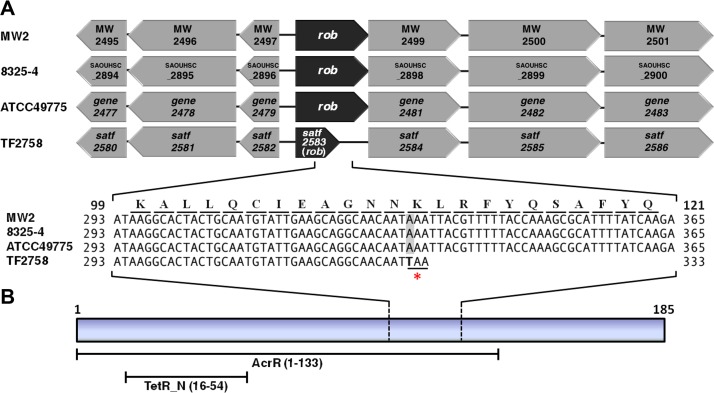
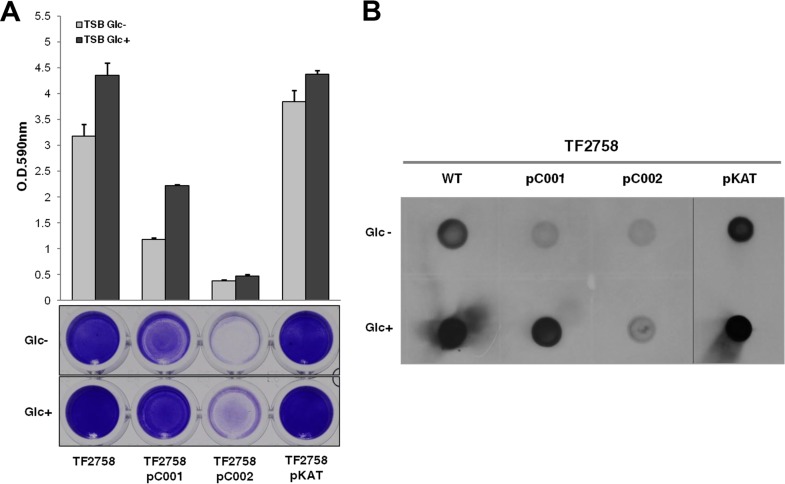
As shown in Fig. 1, one of the clinically isolated strains, TF2758, showed a strong biofilm-elaborating ability and hyperproduction of PIA/PNAG (17). In order to elucidate the mechanism underlying the overproduction of biofilms in TF2758, a gene expression analysis using a custom microarray was performed. We used ATCC 49775 as a control because it was the strain most closely related to TF2758 by comparative genomic hybridization and a very-low-biofilm-elaborating strain (Fig. 1A and B). The results obtained showed that there were two strongly upregulated gene clusters: satf2580 to satf2586 (15- to 40-fold) and the ica operon (satf2686 to satf2689; 2- to 10-fold) (Fig. 1C; see also Table S1 in the supplemental material). Sequencing of the TF2758 genome and comparisons with complete genomes of other S. aureus strains indicated that TF2758 possessed a nonsense mutation in the gene satf2583 (Fig. 2A) and a missense mutation in the gene icaR, which resulted in the creation of a stop codon and an alteration in a nucleotide (A to T), respectively (Fig. S1). SATF2583 possessed regions homologous to the TetR family and AcrR family of transcriptional regulators, suggesting that it acts as a DNA-binding protein (Fig. 2B). In order to clarify the impact of SATF2583 on biofilm elaboration in S. aureus, we transformed TF2758 with the plasmid pC001, which is pKAT carrying open reading frame (ORF) SAOUHSC_2897 with a 5′-flanking region cloned from FK300, the rsbU-repaired derivative of NCTC8325-4. As shown in Fig. 3A, TF2758 carrying pC001 significantly lost its biofilm-elaborating ability, particularly in the absence of 1% glucose. PIA/PNAG detection by anti-PNAG revealed more clear-cut data (Fig. 3B). In the presence of 1% glucose, TF2758 carrying pC001 still retained its PIA/PNAG-producing ability; however, the amount produced was markedly smaller than that by the wild type. However, the production of PIA/PNAG was almost completely inhibited in the absence of 1% glucose. These results suggest that SATF2583 is a negative regulator of biofilm elaboration and also that the satf2583 gene from FK300 is functional.
FIG 1 .
Biofilm elaboration and PIA/PNAG production by Staphylococcus aureus TF2758. (A) Biofilm elaboration. Bacteria were grown in trypticase soy broth (TSB) in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors for each sample are shown. (B) PIA/PNAG production. Extracts from overnight cultures were spotted on a membrane, and PIA/PNAG was detected by rabbit anti-PNAG, as described in Materials and Methods. Non-biofilm-elaborating strain ATCC 49775 was used as a control in the comparative microarray analysis. Figures were compiled from separate images on the same film. (C) Comparative gene expression analysis of TF2758 and ATCC 49775. TF2758 gene expression was represented as a fold change from that of ATCC 49775. Two gene clusters exhibiting marked increases in gene expression are colored (red and blue), and these gene clusters are depicted at right (a and b).
FIG 2 .
Identification of a nonsense mutation in the satf2583 (rob) gene of TF2758 and the predicted domain structure of its transcript. (A) Comparison of the satf2580-to-satf2586 region with those of MW2, 8325-4, ATCC 49775, and TF2758. A part of the nucleotide sequence of each strain and the amino acid sequence are shown. The numbers shown on both sides indicate the nucleotide sequence and amino acid sequence positions in the ORF of rob. The nonsense codon created by the mutation (A to T) is indicated by an asterisk. (B) Structural characteristics of Rob. It contains a TetR_N superfamily domain within an AcrR domain.
FIG 3 .
Rob and IcaR from FK300 reduce biofilm elaboration and PIA/PNAG synthesis in strain TF2758. Biofilm elaboration (A) and PIA/PNAG production (B) of TF2758 and TF2758 carrying pC001 (pKAT-rob [FK300]), pC002 (pKAT-icaR [FK300]), or pKAT. Bacteria were grown in TSB in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors from each sample are shown. Extracts from overnight cultures were spotted on a membrane, and PIA/PNAG was detected by rabbit anti-PIA, as described in Materials and Methods. WT, wild type. Figures (WT, pC001, pC002, and pKAT) were compiled from two separate images on the same film.
List of genes upregulated in microarray experiments. Download TABLE S1, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Identification of a missense mutation in the icaR gene of TF2758 and the domain structure of its transcript. (A) Comparison of the nucleotide sequence and amino acid sequence of the icaR gene among MW2, 8325-4, ATCC 49775, and TF2758. The numbers shown on both sides are the nucleotide sequence and amino acid sequence positions in the ORF of icaR. Amino acids (A to T) altered by the mutation at nucleotide position 103 (G to A) are indicated in red. (B) Structural characteristics of IcaR. It contains a TetR_N superfamily domain within an AcrR domain. Download FIG S1, TIF file, 0.2 MB (199.5KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The icaR gene, located adjacent to the ica operon, is a member of the TetR family of transcriptional regulators (15, 18). IcaR was previously reported to repress icaADBC transcription by binding to a 42-bp region within the ica promoter (16). The missense mutation that we identified in icaR occurs in the helix-turn-helix (HTH) domain (Fig. S1 in the supplemental material) and may affect protein function. Therefore, we complemented TF2758 with pC002, which is pKAT carrying icaR from FK300. The resulting strain significantly decreased biofilm elaboration, and PIA/PNAG production was inhibited regardless of the presence or absence of glucose (Fig. 3A and B). These results suggest that icaR from FK300 is functional and that the satf2583-involved biofilm elaboration pathway occurs through and upstream of the ica operon. We tentatively named this ORF rob (regulator of biofilm).
Effects of Rob on biofilm elaboration, PIA/PNAG production, and ica operon expression in S. aureus FK300.
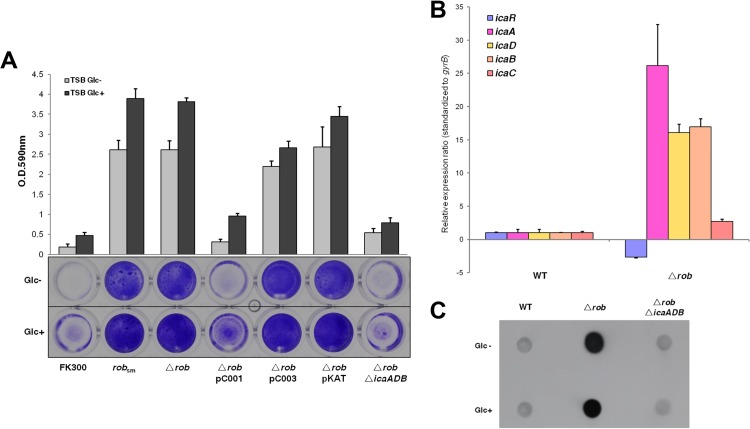
TF2758 was resistant to transformation by the plasmids pKFT and pKOR1, which are used for allelic exchange in S. aureus. Since the rob gene in FK300 is functional, we selected strain FK300 for further studies on rob function. We introduced the same mutation found in TF2758 into rob in FK300 by allelic replacement. As shown in Fig. 4A, this mutation in rob resulted in a marked increase in biofilm elaboration in the presence or absence of 1% glucose. The deletion of the rob gene also increased biofilm elaboration in FK300. We complemented the rob deletion mutant with plasmids carrying the rob gene (SAOUHSC_2897) from FK300 (pC001) or the truncated rob gene (satf2583) from TF2758 (pC003). We found that the transformant of the rob deletion mutant with pC001 exhibited repressed biofilm elaboration, similarly to the control, FK300. In contrast, pC003 was unable to complement the rob deletion phenotype, which was similar to that of the rob deletion mutant carrying the mock vector pKAT (Fig. 4A).
FIG 4 .
Effects of the rob deletion on biofilm elaboration and ica operon expression in FK300. (A) Biofilm elaboration in wild-type FK300 and its derivatives was assessed using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors from each sample are shown. sm, FK300 carrying a stop mutation at adenine nucleotide position 331 (A331 to T331); pC001, pKAT with rob (FK300); pC003, pKAT with rob (TF2758). (B) Quantitative measurements of icaR and icaADBC transcription by quantitative PCR. Total RNA preparation, cDNA synthesis, and then quantitative PCR were performed as described in Materials and Methods. Transcript levels in the rob deletion mutant compared to those in wild-type (WT) strain FK300 were assigned. The expression of the gyrB gene was used for sample normalization. Error bars indicate standard errors. (C) PIA/PNAG production was measured as described in the legend to Fig. 1.
In order to examine whether rob regulates biofilm elaboration through the ica operon, we measured icaR and icaADBC expression by reverse transcription-quantitative PCR (qRT-PCR) and PIA/PNAG production in wild-type and rob mutant strains of S. aureus FK300 (Fig. 4B). The results obtained indicated that the rob deletion mutant decreased icaR expression and increased icaADBC expression with a concomitant increase in PIA/PNAG production (Fig. 4C). The deletion of the ica operon in the FK300 rob deletion mutant abolished biofilm elaboration and PIA/PNAG production (Fig. 4A and C). Taken together, these results suggest that biofilm elaboration in the rob deletion mutant is ica dependent and that Rob, at least in part, represses icaADBC transcription.
SAOUHSC_2898 (SATF2584) is involved in biofilm elaboration, which is under the control of Rob.
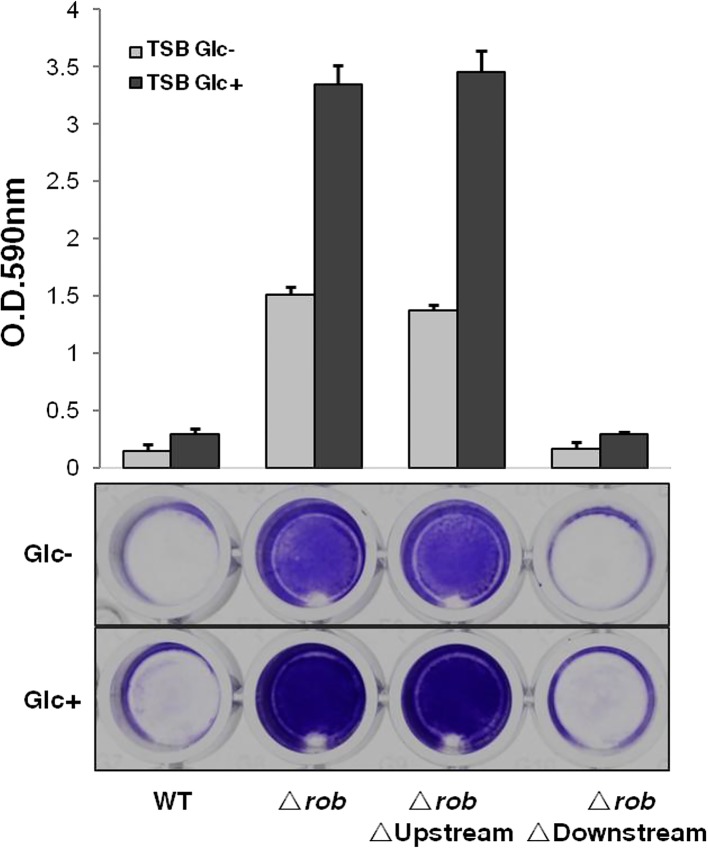
The results of a preliminary microarray analysis suggested that Rob suppresses the expression of the surrounding 7-gene cluster (satf2580 to satf2586) and the ica operon in TF2758 (Fig. 1C; Table S1). Our RNA-sequencing (RNA-seq) data showed that these genes form operons (Fig. S2 in the supplemental material). Therefore, we hypothesized that Rob affects biofilm elaboration by repressing one or more genes in the satf2580-to-satf2586 gene cluster. In order to test this possibility, we deleted upstream genes (SAOUHSC_2894 and SAOUHSC_2895) and downstream genes (SAOUHSC_2898, SAOUHSC_2899, and SAOUHSC_2900) in the FK300 rob deletion mutant. The results, shown in Fig. 5, revealed that the deletion of the upstream genes had no significant effect whereas the deletion of the downstream genes significantly reduced biofilm elaboration in the rob deletion mutant to a level similar to that of wild-type FK300.
FIG 5 .
Biofilm elaboration in the rob deletion mutant requires a downstream gene(s) but not upstream genes. Bacteria were grown in TSB in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors from each sample are shown. Δrob, FK300 rob deletion mutant; ΔrobΔUpstream, FK300 with deletions of rob and its upstream genes SAOUHC_ 2894, SAOUHC_ 2895, and SAOUHSC_2896; ΔrobΔDownstream, FK300 with the deletion of rob and its downstream genes SAOUHSC_2898, SAOUHSC_2899, and SAOUHSC_2900. WT, wild type.
Identification of rob operon and transcription start site of rob using RNA-seq analysis. (A) Visualization of RNA transcript identified by RNA-seq. Total RNA of FK300, FK300 Δrob, and TF2758 was prepared from cultures grown for 6 h at 37°C. After removal of DNA contaminants and rRNA, libraries were generated and purified as described in Materials and Methods. RNA-seq reads were mapped to S. aureus NCTC8325. Genes with continuous coverage were considered to belong to the same operon. The ORFs of NCTC8325 are shown at the top of the figure. Transcripts identified by RNA-seq are represented as dashed arrows. The sequence from the predicted transcription start site (TSS) to the start codon of rob was shown at the bottom of the figure. (B) Diagrammatic representation of the rob promoter region. GENETYXMAC v.15 (Software Development Co., Ltd., Tokyo, Japan) was used for prediction of the −35, −10 sequence. The start codons of genes are indicated by arrows. The Rob-binding site is indicated by the open rectangle. The transcription start site of rob is highlighted by a bent arrow. Download FIG S2, TIF file, 1.5 MB (1.6MB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
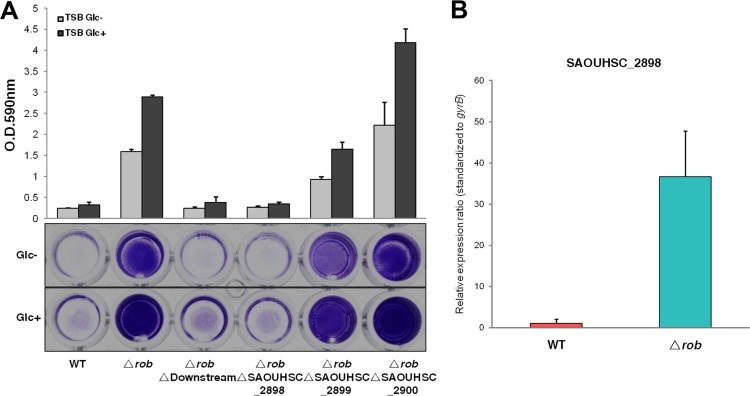
As described above, there are three adjacent genes located immediately downstream of rob that are under the control of one promoter, forming an operon. We deleted each gene individually in the FK300 rob deletion mutant (Fig. 6A). We found that only the SAOUHSC_2898 deletion caused a marked reduction in biofilm elaboration. The SAOUHSC_2899 deletion had a slight effect, whereas the SAOUHSC_2900 deletion had no effect on biofilm elaboration in the rob deletion mutant. Additionally, biofilm elaboration could be restored through complementation of the rob SAOUHSC_2898 double mutant with pC004, which carries the SAOUHSC_2898 gene from FK300 (Fig. S3 in the supplemental material). These results suggest that SAOUHSC_2898 is a critical factor mediating biofilm elaboration regulated by Rob.
FIG 6 .
Contribution of SAOUHSC_2898 to biofilm elaboration by the rob deletion mutant and regulation of SAOUHSC_2898 expression by rob in FK300. Bacteria were grown in TSB in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. (A) Effects of SAOUHSC_2898, SAOUHSC_2899, and SAOUHSC_2900 deletions on biofilm elaboration in the FK300 rob deletion mutant. The averages and standard errors from each sample are shown. (B) Transcription of SAOUHSC_2898 in the FK300 wild-type strain and its rob deletion mutant. Transcript levels in the rob deletion mutant compared to those in the wild-type strain were assigned. The expression of the gyrB gene was used for sample normalization. Error bars indicate standard errors. WT, wild type.
Reduced biofilm elaboration in the rob SAOUHSC_2898 double mutant was restored through complementation with the SAOUHSC_2898 gene. Bacteria were grown in TSB in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors from each sample are shown. pC004, pKAT with SAOUHSC_2898 (FK300). Download FIG S3, TIF file, 0.5 MB (565.3KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
In order to further confirm the regulation of SAOUHSC_2898 by rob, qRT-PCR was performed with RNA isolated from wild-type FK300 and the rob deletion mutant. The deletion of rob resulted in the increased expression of SAOUHSC_2898 (Fig. 6B). SAOUHSC_2898 is predicted to encode a 2-deoxy-d-gluconate 3-dehydrogenase that belongs to the oxidoreductase family (http://aureowiki.med.uni-greifswald.de/SAOUHSC_02898). Our results suggest that this enzyme is involved in some unknown biosynthetic pathway impacting biofilm elaboration. Rob may repress biofilm elaboration in FK300 by downregulating the transcription of the SAOUHSC_2898 gene.
Rob recognizes a palindromic motif in its own promoter.
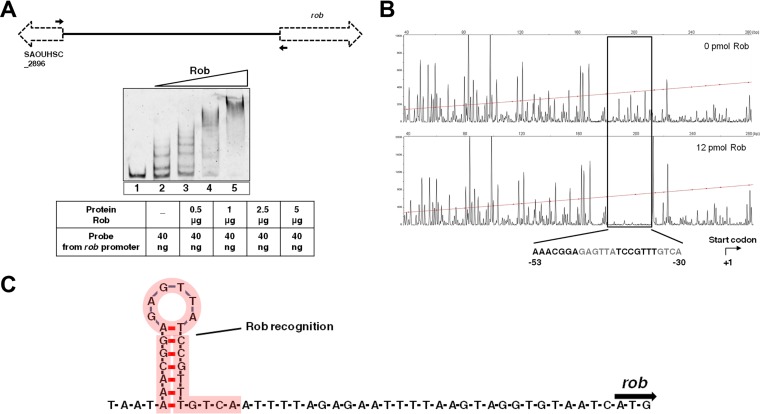
A microarray analysis showed that the inactivation of rob in TF2758 resulted in the increased expression of surrounding genes. Therefore, using electrophoretic mobility shift assay (EMSA) and DNase I footprint analyses, we investigated whether Rob directly binds to its own promoter and regulates this gene cluster’s transcription. We purified His-tagged Rob from Escherichia coli and analyzed its binding to an intergenic fragment between SAOUHSC_2896 and rob (Fig. 7A). Rob induced significant dose-dependent shifts in the probe’s mobility. A DNase I footprint analysis clearly demonstrated that Rob bound to a 24-nucleotide DNA (Fig. 7B). We then analyzed the secondary structure of the binding sequence. We found that it contained an almost perfect palindromic sequence (Fig. 7C). Interestingly, the transcription start site (TSS) of rob was predicted to be within the binding region of Rob by RNA-sequencing (RNA-seq) analysis (Fig. S2 in the supplemental material). These results suggest that Rob represses SAOUHSC_2898 transcription by recognizing the possible palindromic sequence present in the intergenic region of SAOUHSC_2896 and rob.
FIG 7 .
Rob binds to a palindromic motif in its own promoter. (A) EMSA for the DNA-binding activity of Rob to the intergenic region between SAOUHSC_2896 and rob. EMSA was performed in the absence (lane 1) or presence (lanes 2 to 5) of the Rob protein. The primers used to amplify the intergenic region for Rob binding are indicated by black arrows. (B) DNase I footprinting assay. The 6-FAM-labeled DNA probe was incubated with or without recombinant Rob (12 pmol) and then subjected to DNase I digestion. The rectangle indicates the region protected by Rob. The palindromic motif is shown in bold. (C) Schematic representation of the secondary structure of the binding region by Rob. The sequence bound by Rob is highlighted by red shading.
Recombinant Rob binds to the ica promoter region.
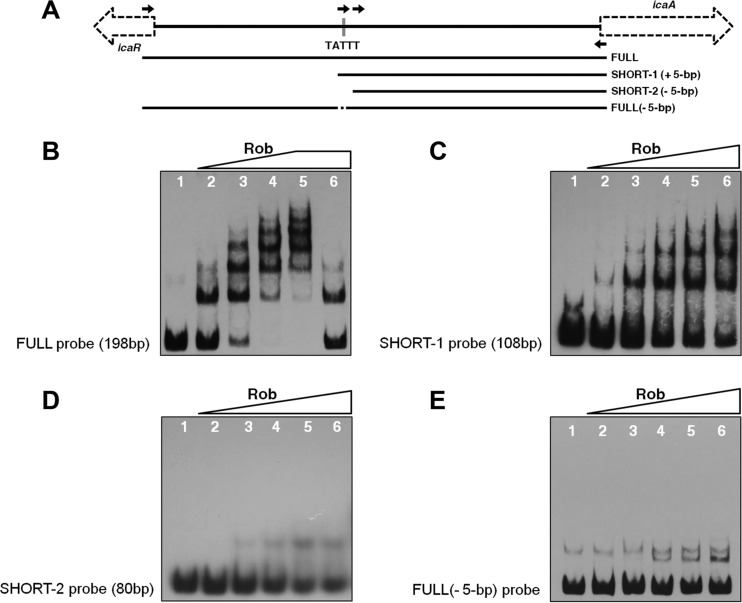
As shown in Fig. 4, the transcription levels of icaADBC were also significantly increased in the rob mutant. In order to investigate whether Rob directly modulates icaADBC expression by binding to the ica promoter, we used EMSA to analyze the Rob protein binding to a 198-bp probe (FULL) that contained the entire icaR-icaA intergenic region (Fig. 8A). As shown in Fig. 8B, the recombinant Rob protein induced several shifts, even with only 0.1 μg of FULL. Rob-DNA complex bands migrated in a ladder-like pattern with increases in Rob protein concentrations. Rob-DNA complexes were outcompeted with a 100-fold excess of unlabeled specific competitor DNA. These results suggest that Rob binds to the icaR-icaA intergenic region in a dose-dependent manner.
FIG 8 .
Rob binds to the ica promoter region and its binding is TATTT motif dependent. (A) Schematic representation of the design of DNA probes used in EMSAs. (B to E) EMSAs of Rob. Recombinant Rob was incubated with FULL (B), SHORT-1 (C), SHORT-2 (D), or FULL(−5-bp) (E) (2 ng/reaction mixture). The amounts (micrograms per reaction mixture) of Rob were as follows: lanes 1, 0; lanes 2 to 5, 0.1, 0.5, 1.0, and 1.5, respectively; lanes 6, 1.5 (with 100-fold excess of unlabeled specific competitor) (B) and 2.0 (C to E).
Jefferson et al. previously identified a 5-bp (TATTT) motif within the icaR-icaA intergenic region that controls the transcriptional regulation of the ica locus (16) (Fig. 8A). They suggested that an unknown repressor(s) utilize(s) the TATTT sequence in order to regulate icaADBC expression. Since Rob represses the ica transcription of the ica locus, we investigated whether Rob recognizes this 5-bp motif. We designed several additional probes for DNA-binding assays (Fig. 8A). A 108-bp probe (SHORT-1), the shortest oligonucleotide containing the 5-bp sequence lacking the 5′ 90-bp sequence of FULL, was dose dependently shifted by Rob (Fig. 8C). We then generated an oligonucleotide (SHORT-2) with a 28-bp deletion from the 5′ end of the SHORT-1 probe. As shown in Fig. 8D, SHORT-2 had no significant shift in the presence of Rob. In order to further investigate whether Rob recognizes the 5-bp motif, we made a 193-bp FULL(−5-bp) probe lacking the 5-bp TATTT sequence of FULL. As shown in Fig. 8E, FULL(−5-bp) was not shifted, as observed in FULL migration in Fig. 8B, suggesting that Rob was unable to bind to the 193-bp FULL(−5-bp) probe. Taken together, these results suggest that Rob recognizes and binds to the 5-bp motif within the ica promoter region.
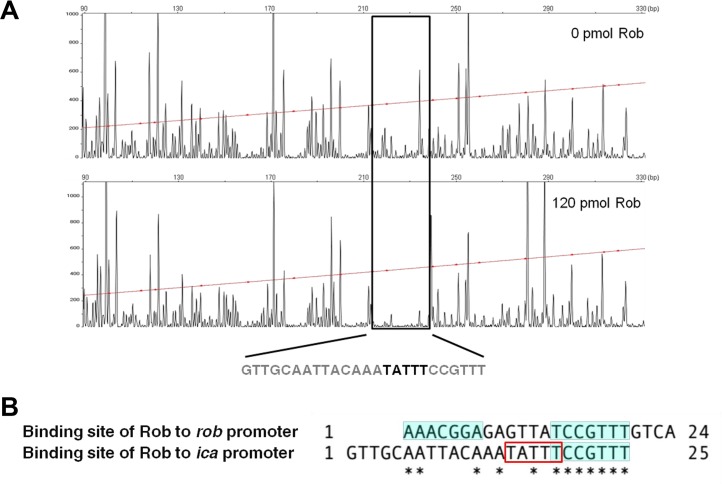
We performed a DNase I footprint analysis to identify a Rob-binding site(s). As shown in Fig. 9, Rob protected a region of approximately 25 bp that included the 5-bp motif. In order to further confirm that the 5-bp motif is necessary for the binding of Rob to the icaR-icaA intergenic region, we attempted to screen for proteins bound to the icaR-icaA intergenic DNA fragment with or without the 5-bp motif using cytosolic proteins of FK300. The cell extract of the wild-type strain FK300 was mixed with magnetic beads conjugated with either the 198-bp FULL probe or 193-bp FULL(−5-bp) probe, and the bound proteins were then analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). We found that Rob was present in proteins bound to the 198-bp probe but was absent in proteins bound to 193-bp FULL(−5-bp) (data not shown). Overall, these results strongly suggest that Rob recognizes and binds to the 5-bp TATTT motif within the promoter region of the ica locus.
FIG 9 .
DNase I footprinting assay of Rob binding to the ica promoter region. (A) Footprint analysis of Rob binding to the ica promoter region. The sequence of the protected region is shown. (B) Comparison of the sites of Rob binding to the rob promoter region and ica promoter region. The palindromic sequence within the Rob-binding region is shaded. The bold letters (A) and rectangle (B) indicate the 5-bp TATTT motif.
DISCUSSION
Biofilm elaboration is an important virulence determinant in certain types of S. aureus infections, particularly those involving implanted medical devices. Biofilm growth is influenced by a number of regulatory mechanisms. However, it is becoming increasingly apparent that the transcriptional regulation of biofilm-associated genes, such as icaADBC, is complex. Staphylococcal regulatory factors, including SarA, SigB, IcaR, TcaR, SrrAB, and Rbf, were previously shown to regulate icaADBC expression (11, 15, 19–22). In the present study, we identified a novel TetR/AcrR family regulator, Rob, which is a repressor of biofilm elaboration, by controlling SAOUHSC_2898, within a 7-gene cluster under the control of Rob. Furthermore, we demonstrated that Rob directly binds to the icaR-icaA intergenic region and represses icaADBC. The binding site in the icaR-icaA intergenic region contained the 5-bp motif, which has been suggested to control the transcriptional regulation of icaADBC (see Fig. S4 in the supplemental material) (16).
Diagrammatic representation of the icaR-icaA intergenic region. The start sites of icaR and icaA are indicated by arrows. The Rob-binding site is indicated by the open rectangle. The gray-shaded rectangle indicates the IcaR-binding site (16). The 5-bp TATTT motif, which has a functional role in the transcriptional regulation of the ica locus, is highlighted by a red frame (16). The Shine-Dalgarno sequence of icaR is underlined. The 5′ UTR of icaR is boxed (dashed line) in the sequence (31). The bent arrow indicates the transcriptional start site of icaA (45). Download FIG S4, TIF file, 0.2 MB (225.6KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
BLAST analysis showed that the satf2580-to-satf2586 gene cluster, which was upregulated in the super-biofilm-elaborating strain TF2758, is also present in several other staphylococcal strains, but not in Staphylococcus epidermidis, which is among the best-studied and most clinically relevant biofilm-elaborating organisms (see Fig. S5 in the supplemental material). Therefore, a novel regulatory pathway appears to be involved in biofilm elaboration in S. aureus.
Comparison of the satf2580-to-satf2586 region among different Staphylococcus species. The red frame represents the 7-gene cluster highlighted in this study. Download FIG S5, TIF file, 0.3 MB (320.1KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
SAOUHSC_2898 is predicted to encode a 2-deoxy-d-gluconate 3-dehydrogenase, which belongs to the oxidoreductase family. Oxidoreductases specifically act on the CH-OH group of donors with NAD+ or NADP+ as an acceptor. This enzyme participates in pentose and glucuronate interconversions, a metabolic pathway that has recently been shown to be significantly enriched in biofilm elaboration (23). An increase in the expression of oxidoreductase was previously reported to induce staphylococcal biofilm elaboration (24). The detailed characterization of SAOUHC_2898 will provide an insight into ica-dependent biofilm elaboration.
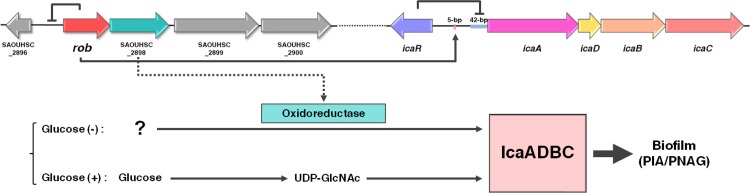
SAOUHSC_2897 and SAOUHSC_2898 were previously reported to be glucose induced biofilm accessory genes of an operon designated gbaAB (25). However, in our assay, the complementation of TF2758 with SAOUHSC_2897 (rob) completely canceled PIA/PNAG production in the absence of 1% glucose. Furthermore, the addition of glucose did not alter the amount of PIA/PNAG produced by the FK300 rob deletion mutant (Fig. 4C). Thus, it is reasonable to assume that an SAOUHSC_2898-catalyzed pathway controlled by SAOUHSC_2897 (rob) affects biofilm elaboration in a glucose-independent manner (Fig. 10).
FIG 10 .
Proposed model for regulation of PIA/PNAG synthesis by Rob in S. aureus FK300. The rob gene product represses the expression of the surrounding 7-gene cluster, including rob and SAOUHSC_2898. The gene product of SAOUHSC_2898 may function as an oxidoreductase in a hypothetical pathway through which glucose-independent, icaADBC-dependent polysaccharide accumulation occurs. rob also recognizes the TATTT motif in the ica promoter region and binds to this region. The binding of Rob to the ica promoter region may suppress the expression of the icaADBC locus. 5-bp, the TATTT motif important for the expression of ica locus; 42-bp, the IcaR-binding region. Arrows correspond to activation, and bars correspond to repression.
A number of regulators, including SigB, SarA, and SarX, and two-component signal transduction systems (TCSs) have been shown to affect staphylococcal biofilm elaboration (26–29). Our genetic analyses showed that the deletion of ica genes or SAOUHSC_2898 resulted in a loss in the ability to elaborate biofilms in the FK300 rob mutant. The rob mutant showed decreased icaR expression and increased icaADBC transcription, suggesting that rob regulates an ica-dependent pathway for biofilm elaboration, at least in part by activating icaR expression.
Some factors regulate icaADBC expression by binding to the icaR-icaA intergenic region (15, 16, 22, 28). Although Rob is one of the TetR/AcrR family regulators in S. aureus, its role in the regulation of biofilms is not completely clear. Jefferson et al. previously reported that the TATTT sequence has a functional role in the transcriptional regulation of the ica locus (16). The simple deletion of the TATTT motif in S. aureus MN8m markedly increased biofilm elaboration and the transcription of icaADBC. Jefferson et al. (16) hypothesized the presence of an uncharacterized repressor(s) recognizing and binding to the motif. Most recently, Schwartbeck et al. also showed that the S. aureus isolates carrying the 5-bp deletion exhibited a mucoid phenotype and strong biofilm formation (30). These mucoid isolates were protected against phagocytosis and survived better under starvation conditions. The results of the present study demonstrated that Rob binds to an icaR-icaA intergenic region of approximately 25 bp, including the 5-bp TATTT motif, strongly suggesting that Rob is the postulated repressor reported by Jefferson et al., and further support the idea of rob regulating biofilm elaboration in an ica-dependent manner. A comparison of the Rob-binding site in the icaR-icaA intergenic region with that in the rob promoter revealed that the right half of the palindrome sequence was also present in the icaR-icaA intergenic region (Fig. 9). This palindrome-like sequence may be recognized by Rob. A previous study showed that the TATTT motif has a functional role in the transcriptional regulation of the ica locus but not icaR transcription (16). Ruiz de los Mozos et al. (31) recently demonstrated that the 5′ and 3′ untranslated region (UTR) base pairings of icaR mRNA control its transcription in S. aureus. The 5-bp motif is located within the 5′ UTR of icaR (Fig. S4 in the supplemental material). The possibility of an interaction between Rob and 5′ UTR to control base pairing remains elusive.
Taken together, the results of the present study suggest that Rob controls the two different pathways of biofilm elaboration in S. aureus. The TetR-family transcriptional regulator Rob affects biofilm elaboration through SAOUHSC_2898 and by recognizing/binding the TATTT motif in an ica-dependent manner. These results provide additional insights into the transcriptional regulation of the ica locus. Both Rob-mediated pathways will be investigated in more detail in future studies.
MATERIALS AND METHODS
Bacterial strains and growth media.
The bacterial strains and plasmids used in the present study are listed in Table 1. The S. aureus strain designated TF2758 is a clinical isolate from an atheroma in Japan. S. aureus ATCC 49775 served as a negative-control, non-biofilm-producing strain. S. aureus FK300, an rsbU-repaired derivative of strain NCTC8325-4, was used in a functional study of the role of rob. S. aureus RN4220 (32) was used as the initial recipient for the manipulation of recombinant plasmids. S. aureus was routinely grown in tryptic soy broth (TSB; Becton, Dickinson Microbiology Systems, Cockeysville, MD) or on tryptic soy agar (TSA) plates. Tetracycline (Tc; 5 μg/ml) or chloramphenicol (Cp; 10 μg/ml) was added as necessary. Escherichia coli strain DH5α was used for the construction and maintenance of plasmids. E. coli was grown in lysogeny broth (LB) (5 g yeast extract, 10 g polypeptone, and 10 g NaCl per liter; pH 7.2) or on LB agar. When required, ampicillin (Ap; 100 μg/ml), kanamycin (Kn; 30 μg/ml), Tc (10 μg/ml), or Cp (10 μg/ml) was added to the culture medium.
TABLE 1 .
Strains and plasmids used in the present study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| S. aureus | ||
| TF2758 | Wild-type clinical isolate, biofilm positive | This study |
| ATCC 49775 | Wild-type clinical isolate, biofilm negative | ATCC |
| FK300 | Derivative of NCTC8325-4 (rsbU repaired) | Laboratory stock |
| RN4220 | Restriction-negative strain, NCTC8325-4 derivative | 32 |
| TF2758 pC001 | TF2758 complemented with pC001 | This study |
| TF2758 pC002 | TF2758 complemented with pC002 | This study |
| robsm strain | FK300 with a stop mutation in rob | This study |
| Δrob strain | FK300 Δrob | This study |
| Δrob pC001 strain | FK300 Δrob complemented with pC001 | This study |
| Δrob pC003 strain | FK300 Δrob complemented with pC003 | This study |
| Δrob pKAT strain | FK300 Δrob complemented with pKAT | This study |
| Δrob ΔicaADB strain | FK300 Δrob ΔicaADB | This study |
| Δrob ΔUpstream strain | FK300 Δrob ΔSAOUHSC_2894 ΔSAOUHSC_2895 ΔSAOUHSC_2896 | This study |
| Δrob ΔDownstream strain | FK300 Δrob ΔSAOUHSC_2898 ΔSAOUHSC_2899 ΔSAOUHSC_2900 | This study |
| Δrob ΔSAOUHSC_2898 strain | FK300 Δrob ΔSAOUHSC_2898 | This study |
| Δrob ΔSAOUHSC_2899 strain | FK300 Δrob ΔSAOUHSC_2899 | This study |
| Δrob ΔSAOUHSC_2900 strain | FK300 Δrob ΔSAOUHSC_2900 | This study |
| E. coli | ||
| DH5α | Cloning strain | TaKaRa |
| BL21(DE3) | Host for recombinant protein production | Novagen |
| Plasmids | ||
| pGEM-T Easy | Cloning vector | Promega |
| pKAT | E. coli-S. aureus shuttle vector | 36 |
| pC001 | Vector for complementation experiments; containing rob from FK300 cloned in pKAT | This study |
| pC002 | Vector for complementation experiments; containing icaR from FK300 cloned in pKAT | This study |
| pC003 | Vector for complementation experiments; containing rob from TF2758 cloned in pKAT | This study |
| pC004 | Vector for complementation experiments; containing SAOUHSC_2898 from FK300 cloned in pKAT | This study |
| pKFT | Vector for allele replacement | 34 |
| pET-28a(+) | E. coli expression plasmid | Novagen |
| pET-22b(+) | E. coli expression plasmid | Novagen |
| pET28a-rob | His-Rob expression plasmid | This study |
| pET22b-icaR | His-IcaR expression plasmid | This study |
Plasmid and strain construction.
Routine DNA manipulations were performed as previously described (33). FK300 mutants were constructed by allele replacement using pKFT (34). PCR was performed using KOD-Plus-Neo (Toyobo, Japan) under appropriate cycling conditions. The oligonucleotides used in this study are listed in Table 2. Fragments were cloned into the plasmid pKFT using restriction enzymes and transformed into E. coli DH5α. Recombinant plasmids were then introduced into DNA restriction system-deficient S. aureus RN4220 by electroporation (35). Modified plasmids were electroporated into S. aureus FK300 for allele replacement. Markerless deletion mutants were screened by PCR from tetracycline-sensitive colonies. Fragments were confirmed by DNA sequencing using the BigDye Terminator v 3.1 cycle sequencing kit (Applied Biosystems, USA).
TABLE 2 .
Primers used in the present study
| Purpose and primer | Sequence (5′ to 3′) |
|---|---|
| Plasmid and strain construction | |
| robsm-1 | ACAACGCCCTTAATTGTTGCC |
| robsm-2 | GCAACAATTAAGGGCGTTGTTACCAAAG |
| rob-1 | TACCAAGCTTCCTCTAACAACTGTTTTAC |
| rob-2 | CATCAACTAGTTTGTGCGCTATTTCTTC |
| rob-3 | GCTGTTGCAATCATTATCAACTAGTG |
| rob-4 | AGGTAAAGCTTTAGCGTATTGTAGCG |
| robUp-1 | AACTAAGCTTTGCCATCGTACTACTAG |
| robUp-2 | GAGCAAAGACGCATCACAGCGGTCTGCTAAAATGAAATTC |
| robUp-3 | GAATTTCATTTTAGCAGACCGCTGTGATGCGTCTTTGCTC |
| robUp-4 | CGGCAAGCTTAATGAGGATATCAAGACG |
| robDown-1 | AACTAAGCTTATCACTCAGATCACCTTC |
| robDown-2 | GCGGAATCAGGGAGTGGTTCGTGCGCTATTTCTTCAATTC |
| robDown-3 | GAATTGAAGAAATAGCGCACGAACCACTCCCTGATTCCGC |
| robDown-4 | GTAAACAAAAATAAGCTTGGTCAGCC |
| SAOUHSC_2898-1 | AACTAAGCTTATCACTCAGATCACCTTC |
| SAOUHSC_2898-2 | GGCTTGATTCCTTCAGAAACGTGCGCTATTTCTTCAATTC |
| SAOUHSC_2898-3 | GAATTGAAGAAATAGCGCACGTTTCTGAAGGAATCAAGCC |
| SAOUHSC_2898-4 | GCGAATAAAGCTTCATCCATACG |
| SAOUHSC_2899-1 | GCCGTCTTGGGATCCTCATTAAC |
| SAOUHSC_2899-2 | GGATAATCAGCAGCATAAAGCGGTACACCTTTAGGATCTG |
| SAOUHSC_2899-3 | CAGATCCTAAAGGTGTACCGCTTTATGCTGCTGATTATCC |
| SAOUHSC_2899-4 | CTATGGATCCTTCTTCAGTATC |
| SAOUHSC_2900-1 | TTAGGATCCAAAGGTGCGCTCATTATG |
| SAOUHSC_2900-2 | GAATATAACCTAAGTGACCGCCAGGAATAAAGATGAGCAC |
| SAOUHSC_2900-3 | GTGCTCATCTTTATTCCTGGCGGTCACTTAGGTTATATTC |
| SAOUHSC_2900-4 | CTATTTTGGATCCGTTTACAAC |
| icaR-1 | TGGTGAAGCTTGATCAACGATAGTATC |
| icaR-4 | TAATAAAGCTTGATACCATCGTACTC |
| ica-1 | AATTGGATCCTCATTGAACAAGAAGCC |
| ica-2 | TAATACTAGTTGTCCCCCTTGAGCCCATC |
| ica-3 | GATGAAACTAGTTATGAAAATGCTTATCC |
| ica-4 | AATTGTAACACTAAGGATCCACCCTCC |
| SAOUHSC_2898-comF | AGGTGGATCCTTCGAAATGTGCTTGC |
| SAOUHSC_2898-comR | ACATAAGCTTGATCTACCAAGGC |
| Quantitative PCR | |
| gyrB for | AGGTCTTGGAGAAATGAATG |
| gyrB rev | CAAATGTTTGGTCCGCTT |
| icaR for | CGCCTGAGGAATTTTCTG |
| icaR rev | GGATGCTTTCAAATACCAAC |
| icaA for | AGTTGTCGACGTTGGCTAC |
| icaA rev | CCAAAGACCTCCCAATGT |
| icaD for | ACCCAACGCTAAAATCATCG |
| icaD rev | GCGAAAATGCCCATAGTTTC |
| icaB for | ATACCGGCAACTGGGTTTAT |
| icaB rev | TGCAAATCGTGGGTATGTGT |
| icaC for | CTTGGGTATTTGCACGCATT |
| icaC rev | GCAATATCATGCCGACACCT |
| SAOUHSC_2898 for | ATTGACACCTCGTGACGTTG |
| SAOUHSC_2898 rev | CCACTTGATACGTTGACGAC |
| EMSA and DNase I footprint analysis | |
| ica-p-F | ATTGCGTTATCAATAATCTTATCCTTC |
| ica-p-R (5-biotin) | TTGCAATTCCTTTACCTACCTTTC |
| ica-p-R′ | TTGCAATTCCTTTACCTACCTTTC |
| ica-p-F-s1 | ACAAATATTTCCGTTTAATTATAACAAC |
| ica-p-F-s2 | AATCTATTGCAAATTAAAATACTATC |
| 5bp-deletion-2 | TTGTTGTTATAATTAAACGGTTTGTAATTGCAACTTAATT |
| 5bp-deletion-3 | AATTAAGTTGCAATTACAAACCGTTTAATTATAACAACAA |
| rob-p-F | CGTCTTTGCTCTCTAGTTAAAGAC |
| rob-p-R | CTATTCTCTTTTGCATCTTTTCGC |
| T7 promoter-1 Cy3a | TAATACGACTCACTATAGGG |
| Fp-M13-F | GTTTTCCCAGTCACGAC |
| Fp-M13-R 6-FAMb | CAGGAAACAGCTATGAC |
| pET-28a-Rob-F | AGGTGGATCCATGCGAAAAGATGC |
| pET-28a-Rob-R | TAACAAGCTTTTAGTCATTACGTCCCACC |
| pET-22b-IcaR-F | GGAATTCCATATGCACCACCACCACCACCACTTGAAGGATAAGATTATTGATAACGC |
| pET-22b-IcaR-R | CCCAAGCTTTTATTTCTTCAAAAATATATTTAGTAGCG |
Cy3 labeled at the 5′ end.
6-FAM labeled at the 5′ end.
In complementation experiments, genes were amplified by PCR using the corresponding primer pairs and then cloned into the HindIII site of pKAT (36). The plasmids pC001, pC002, and pC003 carrying rob-FK300, icaR-FK300, and rob-TF2758 genes, respectively, were constructed and transformed into the S. aureus strains listed in Table 1 by electroporation. The inserts in all plasmid constructs were verified by PCR and DNA sequencing.
Biofilm assay.
A biofilm assay using polystyrene plates was performed as described previously (37) with a few modifications. In brief, overnight cultures were diluted 1:100 with TSB. Ten microliters of this dilution was then transferred, in triplicate, into flat-bottom 96-well polystyrene plates (TrueLine; Nippon Genetics Co., Ltd., Japan) containing TSB or TSB plus 1% glucose. After incubation at 37°C for 24 h, the wells were gently washed three times with 300 μl of sterile phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4⋅12H2O, and 1.8 mM KH2PO4, pH 7.4), and the biofilm was stained with 1% crystal violet for 15 min. Unbound crystal violet was then removed by washing the plate in a container by immersing and agitating it gently 10 times in tap water. Biofilm-bound crystal violet was solubilized in 200 μl of 33% glacial acetic acid at room temperature for 15 min. The extracts were diluted 10-fold, and absorbance at 590 nm was measured with an Immuno-Mini NJ-2300 spectrophotometer (Nalge Nunc International K.K., Tokyo, Japan).
PIA/PNAG detection.
The ability of S. aureus strains to produce PIA/PNAG was tested according to a previously described protocol (9). Briefly, S. aureus strains were grown at 37°C overnight with shaking in 3 ml of TSB. Cultures were then diluted 1:1,000 in the appropriate medium, and 4 ml of this cell suspension was used to inoculate sterile 12-well polystyrene plates (TrueLine; Nippon Genetics Co., Ltd., Japan). After a 24-h static incubation at 37°C, the cells were resuspended in 50 μl of 0.5 M EDTA (pH 8.0) and incubated for 5 min at 100°C. Cells were removed by centrifugation, and 40 μl of the supernatant was incubated with 10 μl of proteinase K (20 mg/ml; Nacalai Tesque, Kyoto, Japan) at 37°C for 30 min. After the addition of 10 μl of Tris-buffered saline (20 mM Tris-HCl, 150 mM NaCl [pH 7.4]) containing 0.01% bromophenol blue, 5 μl was immobilized on a nitrocellulose membrane (Amersham Protran NC 0.45; GE Healthcare, Buckinghamshire, United Kingdom) and dried at room temperature. The membrane was blocked with 5% skimmed milk in PBS with 0.1% Tween 20, and this was followed by a 2-h incubation with rabbit anti-PNAG antiserum (38) diluted at 1:10,000. Bound antibodies were detected with peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) antibodies (MP Biomedicals, LLC-Cappel Products, Ohio, USA) diluted 1:10,000 and developed with Pierce enhanced chemiluminescence (ECL) Western blotting substrate (Thermo Scientific, Rockford, IL).
RNA isolation, RT, and real-time PCR.
Overnight S. aureus cultures were diluted in fresh TSB containing 1% glucose to an initial optical density (OD) of 0.02 at 660 nm and harvested after a 6-h incubation with shaking at 37°C. Total RNA was isolated using the FastRNA Pro Blue kit (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. DNA was removed by a treatment with RQ1 RNase-free DNase (Promega, Madison, WI) at 37°C for 30 min. After inactivation of DNase, PCR was performed to confirm the absence of contaminating DNA. RNA was then reverse transcribed with a Transcriptor First-Strand cDNA synthesis kit (Roche, Mannheim, Germany). The resulting cDNA was diluted 10-fold with Tris-EDTA (TE) buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) and then used as a template in the real-time PCR reaction. Quantitative real-time reverse transcription-PCR (qRT-PCR) was performed with the SsoAdvanced Universal SYBR green SuperMix (Bio-Rad, Hercules, CA) using a CFX96 real-time PCR detection system (Bio-Rad). The thermal cycling conditions used were as follows: 95°C for 1 min, followed by 40 cycles of 95°C for 15 s, 60°C (icaR and icaA) or 62°C (gyrB and SAOUHSC_2898) for 15 s, and 72°C for 30 s. All PCR runs were performed in triplicate, and data were analyzed using the CFX Manager software (version 3.0; Bio-Rad) according to the manufacturer’s instructions. The housekeeping gene gyrase subunit B (gyrB) was used as a reference gene to normalize the expression level of the target gene in each reaction. Real-time PCR primers are listed in Table 2.
Microarray analysis.
The design and preparation of probes, which cover more than 98% of the open reading frames (ORFs) of S. aureus MW2, and their immobilization on the glass slide were described elsewhere (39). RNA extraction (after a 2-h incubation) and cDNA synthesis were performed as described above. cDNA was fluorescently labeled with Alexa Fluor 555 (Cy3) and Alexa Fluor 647 (Cy5) (Thermo Fisher Scientific, Oregon, USA). Labeled cDNA samples were mixed and hybridized to the slides. After washing, fluorescent signals were detected using a GenePix 4000B microarray scanner (Axon Instruments). Data were then normalized and analyzed using Array Vision 8.0 software (Imaging Research, CT, USA). The non-biofilm-elaborating strain ATCC 49775, the genotype of which is the most closely related to TF2758 in our Japanese clinical isolate collection, was used as the reference strain.
Transcriptomic analysis of rob operon via RNA-seq.
Overnight S. aureus FK300 (wild-type, Δrob) and TF2758 cultures were diluted in fresh TSB to an initial density of 0.02 at 660 nm and harvested after a 6-h incubation with shaking at 37°C. Total RNA was isolated using the FastRNA Pro Blue kit (MP Biomedicals, Santa Ana, CA, USA) according to the manufacturer’s instructions. To eliminate DNA contamination, 1 µg total RNA in each sample was treated with 3 µl (1 U/µl) of RQ1 RNase-free DNase (Promega, Madison, WI) at 37°C for 30 min. After digestion by DNase, PCR of the gyrB gene was performed to confirm the absence of contaminating DNA. The concentration and quality of total RNA were determined using a Qubit 2.0 fluorometer (Thermo Fisher Scientific) and Agilent 2200 TapeStation (Agilent Techonogies), respectively. rRNA was removed using the Ribo-Zero bacterial kit (Epicentre). Removal of rRNA was confirmed by the Agilent 2200 TapeStation.
Libraries were generated using the ScriptSeq v2 RNA-Seq (Epicentre) and purified using the Minelute PCR purification kit (Qiagen) according to the manufacturer’s instructions. Libraries were sequenced using the index sequences of TruSeq v2 LT sample preparation kit on the Illumina MiSeq platform. Sequence reads were preprocessed for quality, trimmed, and mapped to S. aureus strain NCTC8325 (GenBank accession number NC_007795) as the reference genome using CLC Genomics Workbench software platform ver. 9 (Qiagen) and Integrative Genomics Viewer (IGV) ver. 2.
Sequencing of the TF2758 genome.
Genomic DNA was extracted using the lysostaphin and QIAamp DNA minikit (Qiagen, Germany) according to the manufacturer’s instructions. Libraries were prepared for sequencing with Nextera DNA kits (Illumina, USA) and were sequenced with the Illumina GAIIx system according to Illumina protocols. The raw reads were trimmed and assembled using a SOAPdenovo assembler. The draft genome sequence was automatically annotated using the Microbial Genome Annotation Pipeline (MiGAP) (40) and was manually curated using IMC-GE software (In Silico Biology, Inc., Kanagawa, Japan).
Protein purification.
To elucidate the DNA-binding properties of Rob, the full-length open reading frame (ORF) of rob was amplified from FK300 genomic DNA using primers pET-28a-Rob-F/pET-28a-Rob-R (Table 2) and cloned into the expression vector pET-28a(+) (Novagen) to obtain pET28a-rob. The plasmid was then transformed into E. coli BL21(DE3), and bacteria were grown at 37°C in 300 ml LB containing 30 μg/ml kanamycin to an optical density (OD) of 0.5 at 600 nm. Expression of Rob was induced with 0.5 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Nacalai Tesque, Kyoto, Japan) and with incubation at 37°C for another 6 h. Cells were harvested by centrifugation and frozen at −80°C. Cell pellets were thawed in lysis buffer (50 mM NaH2PO4 and 300 mM NaCl, pH 8.0) and lysed by sonication on ice. Cell debris was removed by centrifugation (10,000 × g at 4°C for 20 min), and the supernatant was used for isolation of His6-tagged Rob fusion protein by using Talon metal affinity resins (Clontech Laboratories, Inc.) according to the company’s protocol. The expression and purity of the protein were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using a 12% polyacrylamide gel. Protein concentrations were measured using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as the standard protein. The recombinant His-tagged IcaR protein was purified as described elsewhere (18).
Electrophoretic mobility shift assays (EMSAs).
Gel shift assays were performed as described previously (16) with the following modifications. DNA fragments corresponding to the icaR-icaA intergenic region and promoter region of rob were amplified by PCR with the primers listed in Table 2. PCR products were purified using the QIAquick gel extraction kit (Qiagen). A 20-μl binding reaction mixture containing 0.1 to 2 μg of purified recombinant protein and 1 μg of sonicated salmon sperm DNA as well as 1 μg of poly(dI-dC) in binding buffer (10 mM HEPES [pH 8.0], 60 mM KCl, 4 mM MgCl2, 0.1 mM EDTA [pH 8.0], 0.1 mg/ml BSA, 0.25 mM dithiothreitol [DTT], and 5% glycerol) was incubated at room temperature for 15 min before the addition of 2 μg of the biotin-labeled probe. The reaction mixtures were incubated for an additional 20 min and then electrophoresed in a 5% polyacrylamide gel in prechilled 1× Tris-borate-EDTA (TBE) buffer. DNA was then transferred onto a nylon membrane (Biodyne B; Pall, USA), and band shifts were detected by exposing dried membranes to X-ray films. In order to measure the binding of Rob to its promoter region, a gel shift assay was performed using an alternative method as described previously (41).
DNase I footprint analysis.
Footprinting was performed according to a previously described method (42). DNA fragments were generated by PCR with TaKaRa LA Taq (TaKaRa Bio Inc., Shiga, Japan). PCR products were purified and ligated with pGEM-T Easy (Promega) using Ligation High ver. 2 (Toyobo, Osaka, Japan). The resulting plasmids were then used as a template for the amplification of DNA probes using the primer pair Fp-M13-F and Fp-M13-R (5′ 6-carboxyfluorescein [FAM] labeled). DNA fragments (0.45 pmol) were mixed with purified proteins in 50 μl of a reaction mixture containing the same buffer used for gel shift assays. After a 20-min incubation at room temperature, the reaction mixtures were treated with 0.3 U of DNase I (Promega, Madison, WI) for 1 min and then purified by phenol-chloroform-isoamyl alcohol (CIAA) extraction and ethanol precipitation. After purification, the samples were analyzed using an ABI 3130xl Genetic Analyzer equipped with the Peak Scanner software (Applied Biosystems).
Isolation and identification of proteins binding to the ica promoter.
A cell extract was isolated from strain FK300 as previously described with some modifications (43). Briefly, cultured S. aureus cells were pelleted and then washed with buffer A (20 mM Tri-HCl, 5 mM MgCl2, 0.1 M EDTA, and 5% glycerol, pH 7.8). Cell pellets were resuspended in 10 ml of buffer A and treated with lysostaphin (0.1 mg/ml) at 4°C for 1 h. After freezing at −80°C and thawing at 4°C twice, 6 ml of buffer A (containing KCl at a final concentration of 1.3 M) was added and incubated on ice for 40 min. The cell lysate was treated with DNase I (10 μg/ml) and RNase A (10 μg/ml) at room temperature for 30 min. After centrifugation for 30 min at 40,000 × g, the supernatant was dialyzed against distilled water overnight and stored at −80°C.
Biotinylated DNA was prepared as described above. DNA was immobilized on 2 mg of streptavidin-coated magnetic beads (Dynabeads M-280 streptavidin; Life Technologies, Inc.) according to the manufacturer’s protocol. After washing, 100 μl of the cell extract was added and incubated at room temperature for 30 min in gel shift binding buffer. The beads were washed twice with buffer B (10 mM HEPES [pH 8.0], 60 mM KCl, 4 mM MgCl2, 1 mM EDTA [pH 8.0], 1 mM DTT, and 5% glycerol) containing 0.5 μg/ml of salmon sperm DNA and then washed twice with buffer B. The bound proteins were eluted from immobilized DNA with buffer B containing 0.5 M NaCl. The eluates from two binding reactions were pooled and concentrated by methanol-chloroform precipitation. Proteins were separated by SDS-PAGE, followed by Coomassie blue or silver staining. Prior to in-gel trypsin digestion, excised gel pieces were destained and submitted to reduction with DTT and alkylation with iodoacetamide as described previously (44). After being dried, the gel pieces were subjected to trypsin digestion at 35°C overnight with XL-TrypKit (APRO Sci, Japan). Digested peptides were transferred to new tubes and evaporated to <10 μl in a vacuum centrifuge evaporator, and this was followed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analyses for protein identification. LC-MS/MS analyses were performed on a nanoflow liquid chromatograph coupled with nano-electrospray MS, a Triple TOF 5600 system (AB Sciex, Concord, Ontario, Canada) equipped with an Eksigent cHiPLC-nanoflex system (AB Sciex). The nano-high-performance liquid chromatography (HPLC) columns used were the cHiPLC trap column (200 μm by 0.5 mm; ChromXP C18-CL; 3 μm) and nano-cHiPLC analytical capillary column (75 μm by 15 cm; ChromXP C18-CL; 3 μm, 120 Å). Tryptic peptides (2 μl) were loaded, and trapping and desalting were performed at 2 μl/min for 10 min with 0.1% formic acid. The trapped peptides were separated by a linear gradient at a flow rate of 0.3 μl/min, followed by their introduction into the source of the mass spectrometer online. Mobile phase A (0.1% formic acid in H2O) and mobile phase B (0.1% formic acid in acetonitrile) were used to establish a 45-min gradient comprising 25 min of 2 to 32% B, 1 min of 32 to 90% B, 4 min of 90% B, and a final decrease to 2% B, which was followed by reequilibration at 2% B for 15 min. Eluted peptides from the column were analyzed with a Triple TOF 5600 using an ion spray voltage of 2.2 kV. Product ions were scanned in a mass range from 230 m/z up to 1,500 m/z. MS/MS data acquisition was performed using Analyst 1.5.2 (AB Sciex), and proteins were identified by means of an automated database search using ProteinPilot software (AB Sciex).
ACKNOWLEDGMENTS
We thank Hidetada Hirakawa, Gunma University, for his skillful suggestion on DNase I footprint analysis.
This work is supported in part by a grant from the Health and Labor Sciences Research Grants for Research on Allergic Disease and Immunology from the Ministry of Health, Labor and Welfare of Japan (201322025A) and a grant from the Japan Agency for Medical Research and Development (AMED) (924711).
Footnotes
Citation Yu L, Hisatsune J, Hayashi I, Tatsukawa N, Sato’o Y, Mizumachi E, Kato F, Hirakawa H, Pier GB, Sugai M. 2017. A novel repressor of the ica locus discovered in clinically isolated super-biofilm-elaborating Staphylococcus aureus. mBio 8:e02282-16. https://doi.org/10.1128/mBio.02282-16.
REFERENCES
- 1.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayberry-Carson KJ, Tober-Meyer B, Smith JK, Lambe DW Jr, Costerton JW. 1984. Bacterial adherence and glycocalyx formation in osteomyelitis experimentally induced with Staphylococcus aureus. Infect Immun 43:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Götz F. 2002. Staphylococcus and biofilms. Mol Microbiol 43:1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 4.Otto M. 2008. Staphylococcal biofilms. Curr Top Microbiol Immunol 322:207–228. doi: 10.1007/978-3-540-75418-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lister JL, Horswill AR. 2014. Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front Cell Infect Microbiol 4:178. doi: 10.3389/fcimb.2014.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones SM, Morgan M, Humphrey TJ, Lappin-Scott H. 2001. Effect of vancomycin and rifampicin on meticillin-resistant Staphylococcus aureus biofilms. Lancet 357:40–41. doi: 10.1016/S0140-6736(00)03572-8. [DOI] [PubMed] [Google Scholar]
- 7.Otto M. 2013. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med 64:175–188. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- 8.Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear beta-1,6-linked glucosaminoglycan: purification and structural analysis. J Bacteriol 178:175–183. doi: 10.1128/jb.178.1.175-183.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cramton SE, Gerke C, Schnell NF, Nichols WW, Götz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun 67:5427–5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Götz F. 1996. Molecular basis of intercellular adhesion in the biofilm-formation Staphylococcus epidermidis. Mol Microbiol 20:1083–1091. doi: 10.1111/j.1365-2958.1996.tb02548.x. [DOI] [PubMed] [Google Scholar]
- 11.Lim Y, Jana M, Luong TT, Lee CY. 2004. Control of glucose- and NaCl-induced biofilm formation by rbf in Staphylococcus aureus. J Bacteriol 186:722–729. doi: 10.1128/JB.186.3.722-729.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dobinsky S, Kiel K, Rohde H, Bartscht K, Knobloch JK, Horstkotte MA, Mack D. 2003. Glucose-related dissociation between icaADBC transcription and biofilm expression by Staphylococcus epidermidis: evidence for an additional factor required for polysaccharide intercellular adhesin synthesis. J Bacteriol 185:2879–2886. doi: 10.1128/JB.185.9.2879-2886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knobloch JK, Bartscht K, Sabottke A, Rohde H, Feucht HH, Mack D. 2001. Biofilm formation by Staphylococcus epidermidis depends on functional RsbU, an activator of the sigB operon: differential activation mechanisms due to ethanol and salt stress. J Bacteriol 183:2624–2633. doi: 10.1128/JB.183.8.2624-2633.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramton SE, Ulrich M, Götz F, Döring G. 2001. Anaerobic conditions induce expression of polysaccharide intercellular adhesin in Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun 69:4079–4085. doi: 10.1128/IAI.69.6.4079-4085.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conlon KM, Humphreys H, O’Gara JP. 2002. icaR encodes a transcriptional repressor involved in environmental regulation of ica operon expression and biofilm formation in Staphylococcus epidermidis. J Bacteriol 184:4400–4408. doi: 10.1128/JB.184.16.4400-4408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jefferson KK, Cramton SE, Götz F, Pier GB. 2003. Identification of a 5-nucleotide sequence that controls expression of the ica locus in Staphylococcus aureus and characterization of the DNA-binding properties of IcaR. Mol Microbiol 48:889–899. doi: 10.1046/j.1365-2958.2003.03482.x. [DOI] [PubMed] [Google Scholar]
- 17.Shrestha L, Kayama S, Sasaki M, Kato F, Hisatsune J, Tsuruda K, Koizumi K, Tatsukawa N, Yu L, Takeda K, Sugai M. 2016. Inhibitory effects of antibiofilm compound 1 against Staphylococcus aureus biofilms. Microbiol Immunol 60:148–159. doi: 10.1111/1348-0421.12359. [DOI] [PubMed] [Google Scholar]
- 18.Jeng WY, Ko TP, Liu CI, Guo RT, Liu CL, Shr HL, Wang AH. 2008. Crystal structure of IcaR, a repressor of the TetR family implicated in biofilm formation in Staphylococcus epidermidis. Nucleic Acids Res 36:1567–1577. doi: 10.1093/nar/gkm1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ulrich M, Bastian M, Cramton SE, Ziegler K, Pragman AA, Bragonzi A, Memmi G, Wolz C, Schlievert PM, Cheung A, Döring G. 2007. The staphylococcal respiratory response regulator SrrAB induces ica gene transcription and polysaccharide intercellular adhesin expression, protecting Staphylococcus aureus from neutrophil killing under anaerobic growth conditions. Mol Microbiol 65:1276–1287. doi: 10.1111/j.1365-2958.2007.05863.x. [DOI] [PubMed] [Google Scholar]
- 20.Valle J, Toledo-Arana A, Berasain C, Ghigo JM, Amorena B, Penadés JR, Lasa I. 2003. SarA and not sigmaB is essential for biofilm development by Staphylococcus aureus. Mol Microbiol 48:1075–1087. [DOI] [PubMed] [Google Scholar]
- 21.Cue D, Lei MG, Luong TT, Kuechenmeister L, Dunman PM, O’Donnell S, Rowe S, O’Gara JP, Lee CY. 2009. Rbf promotes biofilm formation by Staphylococcus aureus via repression of icaR, a negative regulator of icaADBC. J Bacteriol 191:6363–6373. doi: 10.1128/JB.00913-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson KK, Pier DB, Goldmann DA, Pier GB. 2004. The teicoplanin-associated locus regulator (TcaR) and the intercellular adhesin locus regulator (IcaR) are transcriptional inhibitors of the ica locus in Staphylococcus aureus. J Bacteriol 186:2449–2456. doi: 10.1128/JB.186.8.2449-2456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Chen Y, Liu D, Zhao N, Cheng H, Ren H, Guo T, Niu H, Zhuang W, Wu J, Ying H. 2015. Involvement of glycolysis/gluconeogenesis and signaling regulatory pathways in Saccharomyces cerevisiae biofilms during fermentation. Front Microbiol 6:139. doi: 10.3389/fmicb.2015.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kulkarni R, Antala S, Wang A, Amaral FE, Rampersaud R, Larussa SJ, Planet PJ, Ratner AJ. 2012. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun 80:3804–3811. doi: 10.1128/IAI.00689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You Y, Xue T, Cao L, Zhao L, Sun H, Sun B. 2014. Staphylococcus aureus glucose-induced biofilm accessory proteins, GbaAB, influence biofilm formation in a PIA-dependent manner. Int J Med Microbiol 304:603–612. doi: 10.1016/j.ijmm.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Lauderdale KJ, Boles BR, Cheung AL, Horswill AR. 2009. Interconnections between sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect Immun 77:1623–1635. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tormo MA, Martí M, Valle J, Manna AC, Cheung AL, Lasa I, Penadés JR. 2005. SarA is an essential positive regulator of Staphylococcus epidermidis biofilm development. J Bacteriol 187:2348–2356. doi: 10.1128/JB.187.7.2348-2356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cue D, Lei MG, Lee CY. 2013. Activation of sarX by Rbf is required for biofilm formation and icaADBC expression in Staphylococcus aureus. J Bacteriol 195:1515–1524. doi: 10.1128/JB.00012-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma-Kuinkel BK, Mann EE, Ahn JS, Kuechenmeister LJ, Dunman PM, Bayles KW. 2009. The Staphylococcus aureus LytSR two-component regulatory system affects biofilm formation. J Bacteriol 191:4767–4775. doi: 10.1128/JB.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwartbeck B, Birtel J, Treffon J, Langhanki L, Mellmann A, Kale D, Kahl J, Hirschhausen N, Neumann C, Lee JC, Götz F, Rohde H, Henke H, Küster P, Peters G, Kahl BC. 2016. Dynamic in vivo mutations within the ica operon during persistence of Staphylococcus aureus in the airways of cystic fibrosis patients. PLoS Pathog 12:e1006024. doi: 10.1371/journal.ppat.1006024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruiz de los Mozos I, Vergara-Irigaray M, Segura V, Villanueva M, Bitarte N, Saramago M, Domingues S, Arraiano CM, Fechter P, Romby P, Valle J, Solano C, Lasa I, Toledo-Arana A. 2013. Base pairing interaction between 5′- and 3′-UTRs controls icaR mRNA translation in Staphylococcus aureus. PLoS Genet 9:e1004001. doi: 10.1371/journal.pgen.1004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreiswirth BN, Löfdahl S, Betley MJ, O’Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch EF, Maniatis T. 1989, Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 34.Kato F, Sugai M. 2011. A simple method of markerless gene deletion in Staphylococcus aureus. J Microbiol Methods 87:76–81. doi: 10.1016/j.mimet.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer GR, Iandolo JJ. 1990. High-frequency transformation of Staphylococcus aureus by electroporation. Curr Microbiol 21:373–376. doi: 10.1007/BF02199440. [DOI] [Google Scholar]
- 36.Kato F. 2004. PhD thesis University of Tohoku, Sendai, Japan. [Google Scholar]
- 37.Heilmann C, Gerke C, Perdreau-Remington F, Götz F. 1996. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun 64:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skurnik D, Merighi M, Grout M, Gadjeva M, Maira-Litran T, Ericsson M, Goldmann DA, Huang SS, Datta R, Lee JC, Pier GB. 2010. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest 120:3220–3233. doi: 10.1172/JCI42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aburatani S, Tashiro K, Savoie CJ, Nishizawa M, Hayashi K, Ito Y, Muta S, Yamamoto K, Ogawa M, Enomoto A, Masaki M, Watanabe S, Maki Y, Takahashi Y, Eguchi Y, Sakaki Y, Kuhara S. 2003. Discovery of novel transcription control relationships with gene regulatory networks generated from multiple-disruption full genome expression libraries. DNA Res 10:1–8. doi: 10.1093/dnares/10.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Sugawara H, Ohyama A, Mori H, Kurokawa K. 2009. Microbial Genome Annotation Pipeline (MiGAP) for diverse users, poster S001-1-2 20th Int Conf Genome Informatics (GIW2009). [Google Scholar]
- 41.Sato’o Y, Hisatsune J, Nagasako Y, Ono HK, Omoe K, Sugai M. 2015. Positive regulation of staphylococcal enterotoxin H by Rot (repressor of toxin) protein and its importance in clonal complex 81 subtype 1 lineage-related food poisoning. Appl Environ Microbiol 81:7782–7790. doi: 10.1128/AEM.01936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hirakawa H, Oda Y, Phattarasukol S, Armour CD, Castle JC, Raymond CK, Lappala CR, Schaefer AL, Harwood CS, Greenberg EP. 2011. Activity of the Rhodopseudomonas palustris p-coumaroyl-homoserine lactone responsive transcription factor RpaR. J Bacteriol 193:2598–2607. doi: 10.1128/JB.01479-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oscarsson J, Harlos C, Arvidson S. 2005. Regulatory role of proteins binding to the spa (protein A) and sarS (staphylococcal accessory regulator) promoter regions in Staphylococcus aureus NTCC 8325-4. Int J Med Microbiol 295:253–266. doi: 10.1016/j.ijmm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 44.Hara K, Ohara M, Hayashi I, Hino T, Nishimura R, Iwasaki Y, Ogawa T, Ohyama Y, Sugiyama M, Amano H. 2012. The green tea polyphenol (−)-epigallocatechin gallate precipitates salivary proteins including alpha-amylase: biochemical implications for oral health. Eur J Oral Sci 120:132–139. doi: 10.1111/j.1600-0722.2012.00947.x. [DOI] [PubMed] [Google Scholar]
- 45.Cue D, Lei MG, Lee CY. 2012. Genetic regulation of the intercellular adhesion locus in staphylococci. Front Cell Infect Microbiol 2:38. doi: 10.3389/fcimb.2012.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of genes upregulated in microarray experiments. Download TABLE S1, DOCX file, 0.02 MB (16.8KB, docx) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Identification of a missense mutation in the icaR gene of TF2758 and the domain structure of its transcript. (A) Comparison of the nucleotide sequence and amino acid sequence of the icaR gene among MW2, 8325-4, ATCC 49775, and TF2758. The numbers shown on both sides are the nucleotide sequence and amino acid sequence positions in the ORF of icaR. Amino acids (A to T) altered by the mutation at nucleotide position 103 (G to A) are indicated in red. (B) Structural characteristics of IcaR. It contains a TetR_N superfamily domain within an AcrR domain. Download FIG S1, TIF file, 0.2 MB (199.5KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Identification of rob operon and transcription start site of rob using RNA-seq analysis. (A) Visualization of RNA transcript identified by RNA-seq. Total RNA of FK300, FK300 Δrob, and TF2758 was prepared from cultures grown for 6 h at 37°C. After removal of DNA contaminants and rRNA, libraries were generated and purified as described in Materials and Methods. RNA-seq reads were mapped to S. aureus NCTC8325. Genes with continuous coverage were considered to belong to the same operon. The ORFs of NCTC8325 are shown at the top of the figure. Transcripts identified by RNA-seq are represented as dashed arrows. The sequence from the predicted transcription start site (TSS) to the start codon of rob was shown at the bottom of the figure. (B) Diagrammatic representation of the rob promoter region. GENETYXMAC v.15 (Software Development Co., Ltd., Tokyo, Japan) was used for prediction of the −35, −10 sequence. The start codons of genes are indicated by arrows. The Rob-binding site is indicated by the open rectangle. The transcription start site of rob is highlighted by a bent arrow. Download FIG S2, TIF file, 1.5 MB (1.6MB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Reduced biofilm elaboration in the rob SAOUHSC_2898 double mutant was restored through complementation with the SAOUHSC_2898 gene. Bacteria were grown in TSB in the presence (Glc+) or absence (Glc-) of 1% glucose. Biofilm elaboration was measured using the polystyrene microtiter plate assay described in Materials and Methods. The averages and standard errors from each sample are shown. pC004, pKAT with SAOUHSC_2898 (FK300). Download FIG S3, TIF file, 0.5 MB (565.3KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Diagrammatic representation of the icaR-icaA intergenic region. The start sites of icaR and icaA are indicated by arrows. The Rob-binding site is indicated by the open rectangle. The gray-shaded rectangle indicates the IcaR-binding site (16). The 5-bp TATTT motif, which has a functional role in the transcriptional regulation of the ica locus, is highlighted by a red frame (16). The Shine-Dalgarno sequence of icaR is underlined. The 5′ UTR of icaR is boxed (dashed line) in the sequence (31). The bent arrow indicates the transcriptional start site of icaA (45). Download FIG S4, TIF file, 0.2 MB (225.6KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Comparison of the satf2580-to-satf2586 region among different Staphylococcus species. The red frame represents the 7-gene cluster highlighted in this study. Download FIG S5, TIF file, 0.3 MB (320.1KB, tif) .
Copyright © 2017 Yu et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.