Abstract
Objectives
Test whether hyperglycemic critically ill children with cardiovascular and/or respiratory failure experience more ICU-free days when assigned to tight glycemic control with a normoglycemic versus hyperglycemic blood glucose target range.
Design
Multi-center randomized clinical trial.
Setting
Pediatric ICUs at 35 academic hospitals.
Patients
Children aged 2 weeks to 17 years receiving inotropic support and/or acute mechanical ventilation, excluding cardiac surgical patients.
Interventions
Patients receive intravenous insulin titrated to either 80–110 mg/dL (4.4–6.1 mmol/L) or 150–180 mg/dL (8.3–10.0 mmol/L). The intervention begins upon confirmed hyperglycemia and ends when the patient meets study-defined ICU discharge criteria or after 28 days. Continuous glucose monitoring, a minimum glucose infusion, and an explicit insulin infusion algorithm are deployed to achieve the BG targets while minimizing hypoglycemia risk.
Measurements and main results
The primary outcome is ICU-free days (equivalent to 28-day hospital mortality-adjusted ICU length of stay). Secondary outcomes include 90-day hospital mortality, organ dysfunction scores, ventilator-free days, nosocomial infection rate, neurodevelopmental outcomes, and nursing workload. To detect an increase of 1.25 ICU-free days (corresponding to a 20% relative reduction in 28-day hospital mortality and a one-day reduction in ICU length of stay), 1414 patients are needed for 80% power using a two-sided 0.05 level test.
Conclusions
This trial tests whether hyperglycemic critically ill children randomized to 80–110 mg/dL benefit more than those randomized to 150–180 mg/dL. This study implements validated bedside support tools including continuous glucose monitoring and a computerized algorithm to enhance patient safety and ensure reproducible bedside decision-making in achieving glycemic control.
Keywords: Pediatric critical care, Stress hyperglycemia, Insulin therapy, Randomized clinical trial
1. Introduction
Stress hyperglycemia, a state of abnormal metabolism with elevated blood glucose (BG) concentrations, is common in critically ill patients with cardiovascular and/or respiratory failure [1]. Tight glycemic control (TGC) to 80–110 mg/dL (4.4–6.1 mmol/L) was originally shown to reduce morbidity and mortality in a large randomized clinical trial (RCT) of critically ill adult surgical patients [2] but results were not reliably replicated with subsequent adult studies, including one large multi-center study which demonstrated increased mortality [3] as well as independent risk of hypoglycemia [4] from TGC. Similarly, RCTs in critically ill children to date, largely or exclusively enrolling cardiac surgical populations, have demonstrated inconsistent results from TGC. While the first pediatric RCT [5] showed substantial benefit, including significant reductions in mortality, length of stay (LOS), and infection rate despite relatively high rates of severe hypoglycemia (<40 mg/dL; 2.2 mmol/L), subsequent pediatric trials demonstrated no reductions in mortality, LOS, or infections [6,7]. In the small subgroup of non-cardiac-surgical patients, there was narrow benefit detected in reduction of healthcare costs over a period of one year [6]. A smaller study of critically ill children with severe burns showed a significant reduction in burn-related morbidities with TGC [8]. Thus, no study of TGC has yet been conducted exclusively in critically ill children who are not cardiac surgical or burn patients. In addition, a survey of practicing pediatric intensivists identified wide variability in glycemic control practices, with stated equipoise for study of TGC for this population [9].
The unique goal of the Heart and Lung Failure – Pediatric INsulin Titration trial (HALF-PINT; NTC01565941) is to target the pediatric intensive care unit (ICU) population with cardiovascular and/or respiratory failure who are not post-operative cardiac surgical patients. HALF-PINT is also distinctively designed to minimize hypoglycemia and maximize safety by taking advantage of the latest glucose monitoring technologies, as well as an explicit computer-guided insulin dosing spreadsheet. These safeguards are designed to maintain severe hypoglycemia rates at rates lower than the usual care rates for hypoglycemia in ICUs [7]. Eligibility criteria are designed to include pediatric ICU patients with cardiovascular and/or respiratory failure who have new-onset hyperglycemia. We thereby enroll the target cohort of pediatric ICU patients with the greatest risk of mortality and longest lengths of stay who could benefit from this intervention [10,11].
This manuscript describes the design and rationale for this National Institutes of Health-funded (U01 HL107681) multi-center, international randomized clinical trial. The study compares outcomes of hyperglycemic critically ill children aged 2 weeks to 17 years with cardiovascular and/or respiratory failure treated with an explicit insulin dosing algorithm to titrate intravenous insulin, together with continuous glucose monitoring and a minimum recommended glucose infusion rate, who are randomized to achieve BG target ranges of 80–110 mg/dL (4.4–6.1 mmol/L; TGC-1) vs. 150–180 mg/dL (8.3–10.0 mmol/L; TGC-2).
2. Material and methods
2.1. Study overview
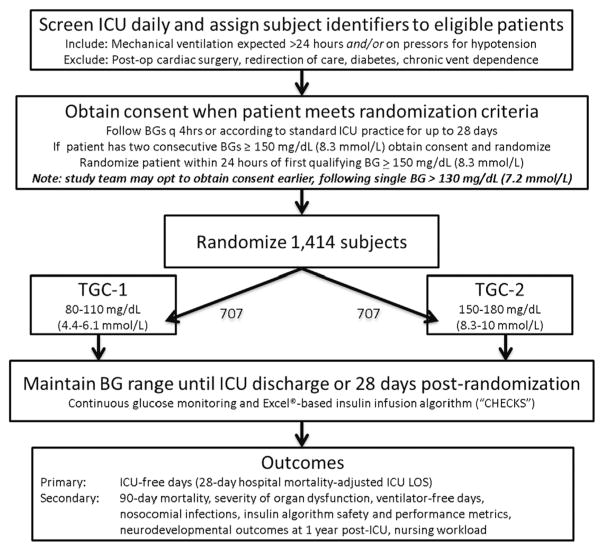
The study is coordinated by a Clinical Coordinating Center (CCC) with staff from both CCC Principal Investigators’ sites (PIs MA, VN) and a Data Coordinating Center (DCC; PI DW, U01 HL108028). Patients are screened and recruited at 35 sites in the United States, Canada, and Australia. Central ethics review was arranged at the Boston Children’s Hospital Institutional Review Board (IRB) to be available for sites willing to cede ethics review authority with appropriate signed institutional agreements. Ten sites have established this reliance relationship, while the remaining sites are conducting local IRB review and oversight. Study protocol modifications are developed collaboratively between the study’s ten-member leadership committee and a steering committee comprised of co-investigators from all participating sites. We plan to enroll 1414 patients randomly assigned to one of the two TGC target groups. Patient screening began in March 2012. A study flow chart is presented in Fig. 1.
Fig. 1.
Flow diagram for trial.
2.2. Patient selection
Study inclusion criteria were chosen to ensure a critically ill non-cardiac-surgery pediatric population with confirmed hyperglycemia and at high risk for hospital mortality and prolonged ICU LOS. Exclusion criteria were chosen to exclude patients who had the greatest risk of harm from hypoglycemia, the least potential for benefit due to low acuity, as well as patients who would not be able to meet the study criteria for ICU discharge within 28 days. Inclusion and exclusion criteria are enumerated in Table 1.
Table 1.
Heart and Lung Failure – Pediatric INsulin Titration trial inclusion and exclusion criteria.
| Inclusion criteria |
|
|
|
| Exclusion criteria |
|
|
|
Families of potential study patients are approached by the local study team upon confirmation of hyperglycemia [two consecutive BGs of ≥150 mg/dL (8.3 mmol/L)] after obtaining permission from the attending physician to approach the family. However, from the onset of the study in March 2012 to November 2014, families were approached when patients had one BG > 130 mg/dL (7.2 mmol/L) so that the patients could be more quickly randomized if and when they develop two consecutive BGs of ≥150 mg/dL. This process was abandoned when it became clear that families, across multiple sites, were more reluctant to grant consent when their child did not yet qualify for the intervention.
Attending physicians are asked to confirm that eligible female patients are not pregnant prior to their families being approached to minimize any risk to the fetus from study-induced hypoglycemia. An informed consent and/or assent discussion with the family and/or patient takes place in the ICU or in a nearby private room. Patients whose families decline participation in the study are managed according to usual practice by the local clinical team, which may or may not include therapy for glycemic control. Consented subjects with confirmed hyperglycemia are randomized to one of the two treatment arms.
Patients are not approached for enrollment if they are already enrolled in a study that has been determined to be a competing clinical trial. This assessment is made formally by the HALF-PINT leadership committee based upon a structured analysis of the potential interactions between the two trials. If both allocation arms of an RCT are well within the usual standard of care, co-enrollment is allowed. For oncologic non-randomized protocols and observational trials without a therapeutic intervention, co-enrollment is also allowed.
2.3. Study protocol
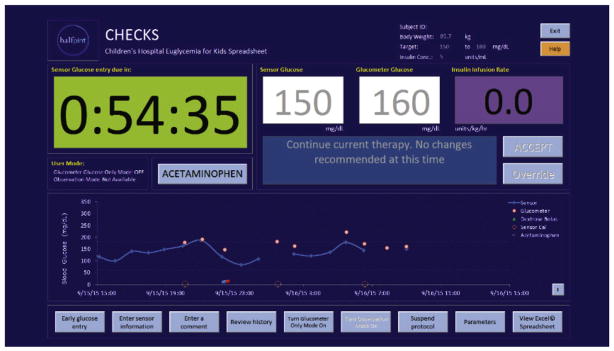
After a patient with confirmed hyperglycemia is enrolled, the study protocol begins with randomization of the patient into one of the two TGC groups. Randomization is accomplished using a web-based, DCC-guided process. Using permuted blocks of random sizes of 4, 6, or 8 and an allocation ratio of 1:1, treatment assignment lists for each site were generated by the DCC and stored in a secure database that was queried only at the time of randomization. To obtain the treatment group assignment, site staff log into the study website and complete a series of questions to confirm the patient’s eligibility for the trial. At the culmination of this process, the next treatment group assignment in the site-specific list is issued. Timing of BG measurements, dosing of insulin, and dextrose rescue boluses, are guided by the Children’s Hospital EuglyCemia for Kids Spreadsheet (CHECKS) [7,12] with a user interface specifically designed for the HALF-PINT study. To facilitate ease of use, reproducibility, and scalability into the large number of participating ICUs, the user interface, shown in Fig. 2, places instructions at the same location on the screen when the program is running and only accepts values entered in specific locations on the screen. CHECKS calculates insulin therapy dose recommendations from discrete BG values, which are measured at the bedside with a glucose meter and entered into the spreadsheet by trained bedside staff at time intervals recommended by the CHECKS algorithm. Timing is guided by sensor glucose (SG) measurements obtained from a continuous glucose monitor (CGM) that reports interstitial glucose concentrations every 5 min [13,14]. SG values are first used to estimate whether the insulin infusion rate should be changed or whether a dextrose rescue bolus is needed, and if so to prompt the study staff to obtain a bedside BG measurement. For safety reasons, only the BG measurement from the glucometer is used to effect changes in insulin infusion dosing rates or the amount of glucose needed in the rescue bolus.
Fig. 2.
Children’s Hospital EuglyCemia for Kids Spreadsheet (CHECKS) interface.
The CHECKS algorithm recommends insulin infusion rates and/or the need for a dextrose rescue bolus based on rules derived from proportional-integral-derivative control theory [15]. In this approach, the recommended insulin infusion rate is calculated as the sum of three components. The first component, proportional, is based on the difference between the measured glucose value and the upper target range (110 or 180 mg/dL depending on treatment arm). The second component, integral, serves as a background infusion rate and provides insulin to maintain the glucose level within target range. The third component, derivative, adjusts the rate up or down depending on whether the glucose values are increasing or decreasing. The infusion is suspended whenever the sum of the three components is negative, and a dextrose rescue bolus is given whenever the glucose concentration is anticipated to fall below 60 mg/dL within 30 min (anticipated glucose value based on the current glucose value and its rate-of-change; amount of dextrose proportional to the negative insulin infusion rate calculated from the three components).
Additional rules are used to instruct the user when to check the SG and when to obtain a blood sample for measurement of BG. Recommended time intervals for obtaining blood samples range between 30 and 360 min, with the frequency depending on the estimated risk of hypoglycemia. For example, following a dextrose rescue bolus, the algorithm recommends a blood sample be taken within 30 min of the previous sample to confirm the rescue was successful. During insulin infusion, BG measurements are requested a minimum of once every 120 min. Instances in which a patient is not receiving insulin and SG measurements are within the assigned TGC range, the algorithm allows up to 360 min between BG entries. The bedside clinician is requested to enter SG and/or BG data at these time points and thereafter follow instructions provided on the user interface screen which was programmed as a Microsoft Excel® VBA (Visual Basic for Applications) form.
In order to enhance safety, an initial insulin dosing limit is imposed by default at 0.3 units/kg/h; however, this may be altered once the physiologic needs of the particular patient are confirmed by local study personnel. All manipulations to the safety features of an individual’s dosing algorithm are made with the knowledge of HALF-PINT clinical leadership as well as the bedside clinical team. Such changes are necessary in order to provide safety limits for the population at large, but allow for occasional extreme physiologic variability when specific clinical situations are encountered.
Data validation is performed to confirm that entries are within the analytical range for the glucose meter (for BG measurement) and glucose sensor (for SG measurement). These safety measures are intended to minimize user errors that commonly occur with spreadsheet applications. Values entered by the user are copied to their correct cell locations located on a Microsoft Excel® spreadsheet which performs all of the algorithm calculations. All calculation steps are transparent to the user and can be manually verified for accuracy. A prominently displayed timer on the user interface shows the time remaining (in hours/minutes) until the next required SG and/or BG entry. All bedside computers are configured to enable live monitoring as well as troubleshooting by the CCC staff at any time (LogMeIn Inc., Boston MA). Defined events, such as hypoglycemia, or overrides by bedside staff of a CHECKS recommendation, trigger an immediate and automatic email to site study staff and CCC staff members to maintain continuous situational awareness.
Insulin therapy begins once the CGM sensor has been placed and insulin has arrived at the patient’s bedside from pharmacy. The nurse, with an institution-specific standard prescriber order set, initiates the intravenous infusion according to the study algorithm spreadsheet if the glucose concentration is ≥110 mg/dL (6.1 mmol/L) in TGC-1, or ≥180 mg/dL (10.0 mmol/L) in TGC-2. Thereafter, insulin is recommended by CHECKS anytime the BG is anticipated to be above the upper limit of the assigned TGC range, based on the rate of change of BG and the patient’s calculated basal insulin requirement. In TGC-2, insulin is discontinued when BG drops below 150 mg/dL, but no attempt is made to raise BG above 150 mg/dL using dextrose or other carbohydrates. The alarm on the receiver of the CGM is set to notify bedside clinicians of a glucose level < 70 mg/dL (3.9 mmol/L) in both study groups. The alarm level is set conservatively to warn of impending hypoglycemia (<60 mg/dL) and enable earlier assessment and intervention to prevent the occurrence of hypoglycemia. CHECKS prompts for calibration of the CGM every 6 to 12 h; if 6 h have passed since the most recent calibration, CHECKS will require calibration on the next glucose meter BG entered into the spreadsheet. The insulin infusion continues until the patient meets study-defined ICU discharge criteria or after 28 days of study intervention or loses sufficient venous/arterial access for insulin infusion and blood samples, at which point the insulin infusion is discontinued. Insulin infusion is also discontinued if CGM is terminated for any reason, for example in the rare event of multiple sensor failures.
In order to ensure a continuous supply of glucose, avoid metabolic starvation, and minimize the risk of hypoglycemia, the protocol recommends the following minimum glucose infusion rate: patients < 6 years of age should receive a continuous dextrose infusion of at least 5 mg/kg/min and patients ≥6 years of age should receive a continuous dextrose infusion of at least 2.5 mg/kg/min [16]. The glucose infusion rate may be delivered via dextrose intravenous infusion, dextrose content in other medications, dextrose-containing parenteral nutrition infusions, or as equivalent carbohydrate content in enteral feeds.
We have incorporated several devices into the study design to mitigate the risk of hypoglycemia, to ensure consistency across all participating sites, and to address limitations of prior studies. First, a CGM is placed in the subcutaneous tissue, most commonly in the lateral thigh, occasionally in the abdomen. The Medtronic Enlite® Continuous Glucose Monitoring System (Medtronic Diabetes, Northridge CA), approved by the Food and Drug Administration (FDA) for use in patients aged 18 years and older with diabetes mellitus, was used in the first 20 patients. Due to unforeseen technical failures, the Dexcom G4 Platinum CGM system (Dexcom, San Diego CA), approved by the FDA for use in patients 18 years and older with diabetes mellitus, is in use for the remainder of patients. The Dexcom system uses a platinum electrode coated with glucose oxidase and generates a current which is proportional to the glucose concentration in the interstitial fluid and displays the glucose concentration every 5 min.
The CGM provides an advantage over conventional TGC protocols that use periodic BG measurements in that it provides continuous glucose trending information and alarms that can be acted on rapidly, prompting a BG check potentially minutes to hours before a scheduled BG check would have otherwise been due. Thus, CGM offers protection against sudden unexpected decreases in BG, which are common in the ICU environment [17]. The Dexcom system is not labeled for use with acetaminophen as acetaminophen is known to falsely elevate the glucose measurement. To ensure these readings do not affect the CHECKS algorithm measurement error, a software “button” has been installed in the CHECKS Excel® spreadsheet (see Fig. 2 – acetaminophen button) to block the provider from entering SG readings for three hours after the administration of acetaminophen, whether oral, rectal, or intravenous. The bedside nurse is responsible for activating this mode with each acetaminophen administration, which alerts the CHECKS algorithm to suspend reliance on the CGM values for 3 h.
The second device in HALF-PINT to standardize practice and minimize site differences is the Nova StatStrip (Nova Biomedical, Waltham MA) bedside glucose meter for BG determination. This device, which meets standards for precision and accuracy in a wide range of conditions, requires a small sample size of 1.2 μL, provides a result in 6 s, and is the only point-of-care hospital BG measurement device approved by the FDA for use in the critical care setting. The manufacturer sequestered a supply of a single test strip manufacturing lot to be shared across all sites in order to minimize variability over the course of the trial. Using manufacturer-supplied hardware and software, data from each meter is periodically uploaded directly into the CCC central database, thereby protecting against human error with data entry. Blood is drawn preferentially via a pre-existing arterial line, or alternatively from a freely-flowing venous line, if local site practice allows. Capillary blood samples are only allowed on a temporary basis while improved vascular access is being actively sought. Nurses or study personnel were required to perform quality control procedures on the glucose meters at least once every 24 h while in use.
Third, to control for dilution or contamination of blood samples and to mitigate infection risk due to frequent blood sampling, we employ the VAMP Jr.® venous/arterial blood management protection system (Edwards Lifesciences, Irvine CA) to obtain blood samples [7]. This device allows for reinfusion of “waste” volumes, thereby minimizing the volume of blood wasted in comparison to standard practice. In addition, while standard nursing practice involves accessing a line three times for each blood draw (i.e., once for waste withdrawal, once for sample withdrawal, and once for flush administration), the VAMP Jr.® requires only a single line access for each blood draw. As there are multiple equivalent FDA-approved products and local practices, sites are required either to use the VAMP Jr.® or a locally preferred system that is judged by CCC leadership to be functionally equivalent.
Protocols and study documents for the trial are maintained at a secure study website, designed with multiple levels of access, on which regulatory, training resource, and informed consent documents are stored and available to study staff. Study staff supports a 24-h international toll-free hotline which may be accessed by local site study staff if their local leadership is unable to successfully troubleshoot any issue, ranging from a protocol question to a technical issue with the computer or CGM. CCC staff maintains the ability to securely and remotely access all laptops in the study in order to troubleshoot and remedy any computer issue that may arise.
2.4. Nurse training
As facile manipulation of study devices by bedside staff is a critical part of the conduct of the HALF-PINT protocol, nurse training is a requirement for certification to care for a HALF-PINT patient. This is accomplished through a multimodal infrastructure including on-site training by manufacturer representatives where appropriate (VAMP Jr.®, Nova StatStrip®), and using live local or remote training sessions or recorded videos for the remainder of devices (CGM, CHECKS). All nurses complete an initial competency test, as well as annual tests, in order to establish and maintain certification with all devices and with the HALF-PINT insulin infusion algorithm. Records for each nurse at every site are maintained centrally by the CCC on the password-protected study website. One device, the Nova StatStrip®, requires a unique login for each nurse, only allowing use if active certification status is verified. Refresher videos for all devices are offered during the online web-based randomization process.
2.5. Study measurements and outcomes
The primary outcome of this trial is ICU-free days to Day 28, which is equivalent to 28-day hospital mortality-adjusted ICU LOS. Although many trials conducted within the pediatric critical care setting have measured surrogate markers, we believe that the potential improvements in clinical outcomes provide the best comparison by which to evaluate the benefits, costs, and risks of the intervention. The 28-day mortality outcome has been recognized as the standard in several adult TGC and other ICU trials [3,18–21]. ICU LOS has also been a primary target in several additional important studies [22,23]. Secondary outcomes include 90-day hospital mortality, severity of organ dysfunction, ventilator-free days to Day 28, incidence of nosocomial infections, insulin algorithm safety, insulin algorithm performance, neurodevelopmental health, and nursing workload.
Baseline, clinical, and laboratory data are collected daily by study staff from Day 0 (day of randomization) until the patient meets study-defined ICU discharge criteria or Day 28 is reached, whichever occurs first. Data collected beyond Day 28 includes ICU and hospital discharge dates as well as 90-day hospital mortality. Secondary outcomes are detailed in Table 2. ICU LOS is calculated based upon the following site-independent discharge criteria that must be true for at least 24 consecutive hours: patient is extubated, off of any non-invasive ventilation that provides ≥5 cm H2O pressure or has reached pre-morbid tracheostomy, ventilator, or non-invasive ventilator settings, and is able to maintain age-appropriate mean arterial pressures without the use of vasopressors or inotropes. High flow nasal cannula ≥ 5 L/min is considered a form of non-invasive ventilation, and needs to be discontinued in order for the patient to be considered discharged from the study.
Table 2.
Trial secondary outcomes.
|
Severity of organ dysfunction during the study period is measured daily. The PEdiatric Logistic Organ Dysfunction (PELOD) scores are used to describe the severity of organ dysfunction [24,25]. A documented relationship exists between severity of organ dysfunction as measured by PELOD score and the risk of death in critically ill children [26,27]. Ventilator-free days during the 28 days following randomization is reported as a useful outcome in clinical trials because the measure encompasses both reduction in the duration of mechanical ventilation and improvement in mortality [28,29]. The end of the patient’s duration of mechanical ventilation is defined as the date/time of extubation for patients who are intubated or, for patients with a tracheostomy, the date/time of the discontinuation of mechanical ventilation or date/time of resumption of pre-morbid ventilator settings where appropriate. Mechanical ventilation is only considered to be discontinued when it is not reinstituted within 24 h.
We use the 2008 Centers for Disease Control’s published definitions [30] for the following nosocomial infections attributable to the ICU stay: total bloodstream infections including central venous line-associated bloodstream infections, respiratory tract infections including ventilator-associated pneumonias, urinary tract infections, and wound infections that occur >24 h post-randomization in the ICU or within 48 h of discharge to the non-ICU inpatient unit. Device-related infections will be counted per 1000 device days, and non-device-related infections will be counted per 1000 ICU days.
Neurodevelopmental health is assessed at baseline and at approximately one-year post-ICU discharge. The Vineland Adaptive Behavioral Scales-II (Vineland-II) [31] is assessed at follow-up to evaluate the child’s overall adaptive functioning. The Vineland-II focuses on the child’s ability to appropriately adapt to conceptual, social, and physical circumstances. It includes domain scores in communication, daily living skills, and socialization as well as a maladaptive behavior index, and as such it provides a comprehensive understanding of the child’s development by assessing typical performance in day-to-day activities required for personal and social sufficiency. The Child Behavior Checklist (CBCL) [32] and Pediatric Quality of Life Inventory (PedsQL) [33] are completed by the parent/guardian after HALF-PINT enrollment to evaluate pre-ICU behavioral problems and health-related quality of life. They are also completed one-year post-ICU discharge to determine if any changes in neurodevelopmental status are modulated by the level of TGC or exposure to hypoglycemia in the ICU. The CBCL consists of several age-dependent behavioral syndrome scales (e.g., aggressive behavior, anxious/depressed) as well as internalizing and externalizing problems scales to describe their child’s ability to appropriately internally process and externalize emotions and social circumstances. The PedsQL consists of physical and psychosocial health summary scores and an overall health-related quality of life measure to describe their child’s general functional health. All instruments are validated in English and Spanish for children aged 2–18 years.
TGC protocols are typically managed by bedside nurses. In practice, there has been substantial resistance to adherence with TGC by nursing staff due both to the increased workload stemming from frequent BG monitoring and changes in infusion rate, and to concerns about the risk of hypoglycemia [34,35]. While critical care nurses continuously multi-task and prioritize care based on patient safety and treatment efficacy, little is known about how best to implement and sustain TGC practices. To evaluate protocol implementation and the potential burden placed on bedside nurses when managing a patient on TGC, we use the Subjective Workload Assessment Technique (SWAT) [36] and National Aeronautics and Space Administration-Task Load Index (NASA-TLX) [37] to characterize the nursing staff experience while using TGC protocols. The SWAT and NASA-TLX are two widely-adaptable assessment tools that have been used to describe perceived workload. Both tools place a task in context of a respondent’s baseline rating of time, cognitive, and stress burdens. An anonymous baseline survey that contains demographic variables and ratings scales for the SWAT and NASA-TLX are given to all nurses trained on the HALF-PINT protocol. Once a patient has been randomized into a HALF-PINT TGC protocol, bedside nurses are randomly selected to complete an anonymous follow-up survey describing their perceived workload associated with managing a patient on TGC [38]. The follow-up survey will provide modified SWAT and NASA-TLX scores to create a perceived workload profile for TGC-related nursing activities over several intervals of the study period. Additional survey questions that describe the nurse’s ability to complete non-TGC-related nursing activities, compare TGC-related to general ICU nursing workload, as well as describe a nurse’s overall perception of TGC will also be reported. One item comparing TGC-related nursing activities to ICU-specific tasks has been adapted from the Nine Equivalents of Nursing Manpower Use Score (NEMS) [39], a validated assessment tool representative of usual critical care bedside nurse workload. De-identified data (by nurse and site) will be reported using both qualitative and summary statistics.
2.6. Statistical analysis
All primary analyses will be performed on an intention-to-treat basis. We hypothesize that patients randomized to TGC-1 and insulin titration to 80–110 mg/dL will experience lower mortality and shorter ICU LOS (and so higher ICU-free days) than those managed with TGC-2 (150–180 mg/dL). In our power calculations, for the TGC-2 group we conservatively hypothesized an 8% 28-day hospital mortality rate and, among survivors, used the same ICU LOS distribution as the Paediatric Intensive Care Audit Network (PICANet) survivors (mean 8.5 days) [40,41]. A survey of the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI), a US collaborative of 79 pediatric ICUs, indicated a willingness to change practice based upon results of a clinical trial which demonstrated a 20% mortality reduction combined with a one-day reduction in ICU LOS [5,9]. Thus, for the TGC-1 group we assumed a 6.4% mortality rate (20% decrease from 8%) and, among survivors, an ICU LOS distribution with mean 7.5 days (a one-day, or 11.8% decrease, from 8.5 days). The TGC-1 group ICU LOS distribution among survivors was derived from a proportional odds model, effectively shifting the distribution of ICU LOS among survivors in the TGC-2 group to yield a new distribution with mean ICU LOS of one fewer day. This equates to an expected increase of 1.25 ICU-free days, from 17.94 to 19.19, if TGC-1 is superior to TGC-2, and requires a sample size of 1274 randomized patients (637 per group) for 80% power with a two-sided alpha level of 0.05. These sample size calculations needed adjustment to allow for interim analyses and potential early stopping for efficacy or futility. Based on East Version 5.4 (Cytel Statistical Software, Cambridge MA), allowing looks after 50%, 67%, 83%, and 100% of the data have accrued and assuming general endpoints based on treatment effects estimated through generalized linear regression models requires a sample size of 1414 patients (707 per group).
This clinically important reduction in mortality and ICU LOS appears to be plausible based on the results achieved in the Leuven pediatric ICU trial [5]. The Leuven trial, conducted in a predominantly pediatric cardiac surgical population (N = 700), used comparably lower BG target ranges and was complicated by 44% incidence of severe hypoglycemia in children < 1 year of age. Nonetheless, the Leuven trial achieved a 55% relative decrease in 30-day ICU mortality (2.3% vs. 5.1%, p = 0.047) and a 10% reduction in ICU LOS (5.51 vs. 6.15 days, p = 0.017).
ICU-free days will be considered to be zero for patients who did not meet study-defined ICU discharge criteria or were transferred or died (in the ICU or hospital) by Day 28. Due to the equivalent but inverse relationship between ICU-free days to Day 28 and hospital mortality-adjusted ICU LOS, we will analyze the latter using proportional hazards regression in order to adjust for age group (<2, 2–6, and ≥7 years of age) and severity of illness. In particular, an ICU-free day value of zero will be analyzed as having a hospital mortality-adjusted ICU LOS of 28 days. Whether patients are censored at 28 days or have this outcome of 28 days will lead to the same statistical inferences, since no observations of the primary outcome will be longer than 28 days.
Analysis of the primary outcome will be performed on an intention-to-treat basis. The primary outcome will be collected for all patients who were randomized including those who never received the intervention, except if the guardian withdrew full consent. Patients whose guardian withdrew full consent will be assigned the worst outcome (i.e., zero ICU-free days) in the intention-to-treat analysis. The primary outcome and all secondary outcomes will be analyzed on a per-protocol basis, excluding randomized patients who never received the intervention and those whose guardian withdrew full consent. These analyses will use proportional hazards regression for time to event outcomes (using the exact method to handle tied observations), linear regression for continuous outcomes, and logistic regression for binary outcomes adjusting for age group and severity of illness.
2.7. Study oversight
An independent Data and Safety Monitoring Board (DSMB), recruited and coordinated by the National Heart, Lung, and Blood Institute, monitors the trial for adverse events, adherence to the study protocol, and potential for early stopping. The DSMB met after the first 50 randomized patients were studied, and will continue to meet approximately every six months until completion of the trial. Following each meeting the DSMB makes a recommendation regarding continuation, modification, or discontinuation of the trial. The first interim look for potential early stoppage for efficacy or futility is scheduled after accrual of outcome variables for 50% of patients. Changes to the protocol are recommended by the DSMB based upon its independent review of study data as well as newly available TGC data from other populations.
The study core leadership committee collaborated with the DSMB to create rules to classify important adverse events in terms of severity and their relatedness to the study protocol. Particular attention is given to hypoglycemia events, which were most concerning to the DSMB as a potential risk of the study insulin infusion protocol. All hypoglycemic glucose meter BG measurements < 60 mg/dL are reviewed by the CCC, classified, and reported to the DSMB. Severe hypoglycemia events (BG < 40 mg/dL) are reported to the DSMB chair on an expedited timeline. Hypoglycemia events occurring three or more hours after discontinuation of the study-guided insulin infusion are classified as unrelated to the study. Hypoglycemia events occurring within three hours of the patient’s most recent dose of insulin are classified as possibly, probably, or definitely related to the study as determined by the proximity of the event to the last insulin dose.
3. Discussion
HALF-PINT addresses an important gap in the field of glycemic control in the pediatric ICU. Prior trials of TGC in this population have raised important questions about the use of this therapeutic modality in our critically ill patients, specifically those with cardiovascular and/or respiratory failure unrelated to cardiac surgical disease. Many studies have demonstrated the association between hyperglycemia and poor outcomes in this population [22,42–44], but only three prior publications described prospective, randomized interventions which have the ability to assess causality, not including an RCT in burned children [8]. In a single center trial enrolling 75% cardiac surgical patients, Vlasselaers found a marked 55% relative reduction in 30-day ICU mortality, attributed to TGC implementation, a magnitude of improvement no other RCT in modern pediatric critical care has been able to achieve with any intervention [5]. The subsequent Control of Hyperglycaemia in Paediatric intensive care (CHiP) trial, published after the initiation of the HALF-PINT trial, did not demonstrate any survival benefit in the entire cohort, notably with a similar percentage of cardiac surgical patients (61%) [6]. Both trials were complicated by significant severe hypoglycemia (Vlasselaers, 25%; CHiP, 7.3%), and neither utilized CGM technology or explicit electronic bedside decision support. Of note, roughly one-third of the randomized CHiP patients never became hyperglycemic. The Safe Pediatric Euglycemia after Cardiac Surgery (SPECS) trial examined TGC in an exclusive cardiac surgical population and did not demonstrate any outcome benefit, even with a substantially lower rate of severe hypoglycemia (3.3%) using the explicit methodology CHECKS algorithm and CGM [7]. Taken together, there is substantial evidence both for [5] and against [6,7] the benefits of TGC.
Although a meta-analysis of the above studies demonstrated no net benefit in critically ill children [45], a total of only 706 non-cardiac-surgical pediatric ICU patients had been studied as of the start of the HALF-PINT trial (550, if non-hyperglycemic CHiP patients are excluded), as compared with 2343 cardiac surgical patients. HALF-PINT was designed to address the gaps in the spectrum of high risk critically ill patients who may potentially benefit from TGC. These design features include recruitment of patients who are critically ill and hyperglycemic, but not cardiac surgery patients, and use of a TGC protocol with bedside decision support which incorporates the latest technology in order to maximize achievement of target TGC ranges while minimizing the risk of severe hypoglycemia.
HALF-PINT was designed to test the hypothesis that 80–110 mg/dL is the superior target glucose range compared with 150–180 mg/dL. The higher target range was chosen based upon a survey of pediatric intensivists [9] and consensus recommendations reported in statements from multiple critical care and endocrine societies (150–180 [46]; 140–180 [47]; 140–200 [48]). A summary of surveyed opinions of pediatric intensivists indicated that physicians had equipoise between higher and lower target ranges, that they did not believe “no glycemic control” represented standard of care, and that they were unwilling to infuse dextrose to purposefully elevate glucose concentration above 150 mg/dL.
Taking all these factors into account, we designed HALF-PINT as a practical, replicable test of insulin therapy to two target ranges in an enriched population of hyperglycemic critically ill children. We did not intend to conduct a glycemic clamp trial comparing glycemia at two ranges (80–110 vs. 150–180 mg/dL).
3.1. Rationale for outcome measures
The primary outcome variable in the HALF-PINT trial is ICU-free days to Day 28, which is equivalent to 28-day hospital mortality-adjusted ICU LOS. Mortality, the common primary outcome in adult TGC studies, is an impractical endpoint in the pediatric cohort, with an expected mortality in this population of 10% or less. The primary benefit of choosing ICU-free days is that it incorporates mortality, but also takes into account global resolution of all ICU interventions, primarily respiratory and cardiac supportive interventions. Our definition of ICU LOS is operationalized by physiologic features, and does not require the patient to be physically out of the ICU, merely off ICU therapies. This choice is in contrast to the primary outcome in the Vlasselaers trial (change in C-reactive protein from baseline) [5], but very similar to the primary outcome in the CHiP trial (ventilator-free days) [6].
A key emerging secondary outcome measure for all pediatric critical care trials is long-term neurodevelopmental outcomes following an ICU intervention. The most significant concern for implementing TGC stems from insulin-induced hypoglycemia, which has been associated with severe and long-term effects on the developing brain [49] as well as mortality [4,50]. This risk of TGC, above and beyond the high baseline rate of hypoglycemia seen in critically ill children even without insulin infusion, has served as a deterrent to physicians treating hyperglycemia in the pediatric ICU. Previous trials exploring TGC in both adults and children have achieved mixed results in reducing mortality at the cost of frequent and excessive hypoglycemia in the treatment arm targeting lower BG. The Leuven group reported that children experiencing severe hypoglycemia fared no worse in neurodevelopmental status than patients who did not have hypoglycemia, but the Normoglycemia in Intensive Care Evaluation-Survival Using Glucose Algorithm Regulation (NICE-SUGAR) study in adults reported the opposite to be true [3]. More recent data from SPECS in the pediatric cardiac surgical population demonstrated worse neurodevelopmental outcomes at follow-up in patients one year of age with moderate or severe hypoglycemia (<50 mg/dL [2.8 mmol/L]) compared with mild or no hypoglycemia [51]. These inconsistent findings underscore the importance of measuring long-term neurodevelopmental status to gain a better understanding of the long-term effects of TGC as well as hypoglycemia in the pediatric ICU population. Without data supporting the long-term safety of TGC protocols, it is unlikely that TGC will be adopted purely on the basis of short-term efficacy.
3.2. Protocol considerations
In designing the HALF-PINT trial, we sought to expand on the successful and safe implementation of TGC from the SPECS study by adopting the most current glucose sensor technology and making improvements to the computerized insulin titration algorithm previously used in our SPECS trial [7]. In our previous iterations of this spreadsheet, clinical staff had to enter blood glucose values directly into an Excel® spreadsheet, limiting options for error checking and data verification. Modifications for CHECKS in the HALF-PINT study, therefore, included user interface features and automatic range checking which allow for the user to double-check for possible keystroke errors prior to entering values into the algorithm. The interface also features a graph showing the entered BG values and insulin infusion rates over the span of 24 h, with the intent of enabling clinical staff to more readily identify trends in the patient’s BG. Finally, the ability of the CCC staff to remotely monitor each patient at the bedside via LogMeIn also enables a high level of technical support as well as quality assurance that the trial is being actively and appropriately managed by bedside clinicians.
A critical tool used in this study to maintain patient safety is the CGM. It is implemented in HALF-PINT as an off-label use of an FDA-approved device, since it is not labeled for use in the ICU, nor in non-diabetics, nor in children below 18 years of age. Local or central IRBs approve use of the CGM as a Non-Significant Risk device that serves as an ‘early warning system’ for hypoglycemia by audibly alarming when the glucose drops below 70 mg/dL (3.9 mmol/L), allowing the bedside clinician to intervene sooner than is possible with conventional TGC protocols that employ periodic blood sampling for BG measurement. The combination of CGM with open loop electronic decision support tool provides the safest study protocol delivery with optimal data collection and ability to easily track protocol deviations for any reason.
The HALF-PINT study protocol was specifically designed to avoid inconsistencies with glucose measurement methods that have been subject to criticism in other TGC trials. This is achieved by standardizing devices across all sites; i.e., same glucose meter at all sites, same test strip manufacturing lot across sites, same CGM system, and same insulin titration algorithm. Uniform training is also implemented across all sites, including international sites. Central administration of all glucose meters from the CCC headquarters allows for active monitoring of device upkeep and seamless, reliable data collection. Use of the VAMP Jr.® (or an equivalent device) to ensure undiluted sampling mitigates the risks of anemia and infection and also leads to greater consistency and reliability of the blood glucose measurement.
4. Conclusions
Since the value of TGC in the ICU was first demonstrated fifteen years ago, there have been many studies and much debate over the extent of its efficacy and which populations may derive benefit or harm. HALF-PINT is another important trial in this process of thoroughly examining our care and testing key practices using large multi-center RCTs. HALF-PINT is expected to provide an important contribution to this body of work, with its focus on hyperglycemic critically ill, non-cardiac-surgical children.
Acknowledgments
Funding
This work is supported by the National Institutes of Health [U01 HL107681 and U01 HL108028].
The HALF-PINT CCC and DCC acknowledge the extraordinary commitment of all the following sites who participated in HALF-PINT by obtaining IRB approval, training their staff, and screening and/or enrolling patients: Boston Children’s Hospital, The Children’s Hospital of Philadelphia, New York-Presbyterian Morgan Stanley Children’s Hospital, Children’s Hospital Colorado, Cincinnati Children’s Hospital Medical Center, Penn State Children’s Hospital at The Milton S. Hershey Medical Center, Westchester Medical Center, Children’s Hospital Los Angeles, Women & Children’s Hospital of Buffalo, Medical City Dallas Children’s Hospital, Duke Children’s Hospital and Health Center, Yale-New Haven Children’s Hospital, Primary Children’s Hospital, Kosair Children’s Hospital, Children’s Hospital of Orange County, University of Maryland Hospital for Children, Ann & Robert Lurie Hospital Chicago, Johns Hopkins Medical Center, UCSF Benioff Children’s Hospital-Oakland, Children’s Healthcare of Atlanta, University of Michigan C.S. Mott Children’s Hospital, Mattel Children’s Hospital UCLA, Children’s Hospital at Dartmouth-Hitchcock, Children’s Medical Center Dallas, Comer Children’s Hospital-University of Chicago, Nemours Alfred I. duPont Hospital for Children, Steven and Alexandra Cohen Children’s Medical Center of New York, The Children’s Hospital at Montefiore, UCSF Benioff Children’s Hospital- San Francisco, Miller Children’s Hospital Long Beach, Children’s Hospital- St Louis, Seattle Children’s Hospital, The Children’s Hospital at Oklahoma University Medical Center, Centre Hospitalier Universitaire Sainte-Justine, and The Royal Children’s Hospital Melbourne.
The HALF-PINT trial was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (U01 HL107681-MA/VN, U01 HL108028-DW), endowed chairs (VN, MA), and departmental investigatorship (GS). Nova Biomedical Co. provided glucose meters (Nova StatStrip®), test strips, management software, and training support at no cost; Edwards Lifesciences Co. provided closed blood-sparing sampling systems (VAMP Jr.®) at no cost; Dexcom, Inc. provided continuous glucose monitoring systems and sensors (G4® Platinum) at a reduced rate; and Medtronic MiniMed, Inc. provided continuous glucose monitoring systems and sensors (Guardian REAL-Time®, Enlite®) at a reduced rate.
Abbreviations
- BG
blood glucose
- CBCL
Child Behavior Checklist
- CCC
Clinical Coordinating Center
- CGM
continuous glucose monitor
- CHECKS
Children’s Hospital EuglyCemia for Kids Spreadsheet
- CHiP
Control of Hyperglycaemia in Paediatric intensive care
- DCC
Data Coordinating Center
- DSMB
Data and Safety Monitoring Board
- FDA
Food and Drug Administration
- ICU
Intensive Care Unit
- IRB
Institutional Review Board
- LOS
length of stay
- NASA-TLX
National Aeronautics and Space Administration-Task Load Index
- NEMS
Nine Equivalents of Nursing Manpower Use Score
- PALISI
Pediatric Acute Lung Injury and Sepsis Investigators
- PedsQL
Pediatric Quality of Life Inventory
- PELOD
PEdiatric Logistic Organ Dysfunction
- PICANet
Paediatric Intensive Care Audit Network
- RCT
randomized clinical trial
- SG
sensor glucose
- SPECS
Safe Pediatric Euglycemia after Cardiac Surgery
- SWAT
Subjective Workload Assessment Technique
- TGC
tight glycemic control
- VBA
Visual Basic for Applications
Footnotes
ClinicalTrials.gov Identifier: NCT01565941.
References
- 1.Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. 2009;373:1798–1807. doi: 10.1016/S0140-6736(09)60553-5. http://dx.doi.org/10.1016/S0140-6736(09)60553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. http://dx.doi.org/10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Chittock DR, Su SYS, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. http://dx.doi.org/10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 4.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, Mitchell I, Foster D, Dhingra V, Henderson WR, Ronco JJ, Bellomo R, Cook D, McDonald E, Dodek P, Hébert PC, Heyland DK, Robinson BG. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108–1118. doi: 10.1056/NEJMoa1204942. http://dx.doi.org/10.1056/NEJMoa1204942. [DOI] [PubMed] [Google Scholar]
- 5.Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. http://dx.doi.org/10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 6.Macrae D, Grieve R, Allen E, Sadique Z, Morris K, Pappachan J, Parslow R, Tasker RC, Elbourne D. A randomized trial of hyperglycemic control in pediatric intensive care. N Engl J Med. 2014;370:107–118. doi: 10.1056/NEJMoa1302564. http://dx.doi.org/10.1056/NEJMoa1302564. [DOI] [PubMed] [Google Scholar]
- 7.Agus MSD, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, Alexander JL, Scoppettuolo LA, Pigula FA, Charpie JR, Ohye RG, Gaies MG. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med. 2012;367:1208–1219. doi: 10.1056/NEJMoa1206044. http://dx.doi.org/10.1056/NEJMoa1206044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeschke MG, Kulp GA, Kraft R, Finnerty CC, Mlcak R, Lee JO, Herndon DN. Intensive insulin therapy in severely burned pediatric patients: a prospective randomized trial. Am J Respir Crit Care Med. 2010;182:351–359. doi: 10.1164/rccm.201002-0190OC. http://dx.doi.org/10.1164/rccm.201002-0190OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirshberg EL, Sward KA, Faustino EVS, Nadkarni VM, Agus MSD, Morris AH, Lacroix J. Clinical equipoise regarding glycemic control: a survey of pediatric intensivist perceptions. Pediatr Crit Care Med. 2013;14:123–129. doi: 10.1097/PCC.0b013e31826049b3. http://dx.doi.org/10.1097/PCC.0b013e31826049b3. [DOI] [PubMed] [Google Scholar]
- 10.Ruttimann UE, Pollack MM. Variability in duration of stay in pediatric intensive care units: a multiinstitutional study. J Pediatr. 1996;128:35–44. doi: 10.1016/s0022-3476(96)70425-0. http://dx.doi.org/10.1016/S0022-3476(96)70425-0. [DOI] [PubMed] [Google Scholar]
- 11.Slater A, Shann F, Pearson G. PIM2: a revised version of the paediatric index of mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. http://dx.doi.org/10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 12.Steil GM, Deiss D, Shih J, Buckingham B, Weinzimer S, Agus MSD. Intensive care unit insulin delivery algorithms: why so many? How to choose? J Diabetes Sci Technol. 2009;3:125–140. doi: 10.1177/193229680900300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piper HG, Alexander JL, Shukla A, Pigula F, Costello JM, Laussen PC, Jaksic T, Agus MSD. Real-time continuous glucose monitoring in pediatric patients during and after cardiac surgery. Pediatrics. 2006;118:1176–1184. doi: 10.1542/peds.2006-0347. http://dx.doi.org/10.1542/peds.2006-0347. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Balo A. The accuracy and efficacy of the Dexcom G4 platinum continuous glucose monitoring system. J Diabetes Sci Technol. 2015;9:1021–1026. doi: 10.1177/1932296815577812. http://dx.doi.org/10.1177/1932296815577812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintergerst K, Deiss D, Buckingham B, Cantwell M, Kache S, Agarwal S, Wilson DM, Steil G. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007;9:211–222. doi: 10.1089/dia.2006.0031. http://dx.doi.org/10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 16.Bier DM, Leake RD, Haymond MW, Arnold KJ, Gruenke LD, Sperling MA, Kipnis DM. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes. 1977;26:1016–1023. doi: 10.2337/diab.26.11.1016. http://dx.doi.org/10.2337/diab.26.11.1016. [DOI] [PubMed] [Google Scholar]
- 17.Steil GM, Langer M, Jaeger K, Alexander J, Gaies M, Agus MS. Value of continuous glucose monitoring for minimizing severe hypoglycemia during tight glycemic control. Pediatr Crit Care Med. 2011;12:643–648. doi: 10.1097/PCC.0b013e31821926a5. http://dx.doi.org/10.1097/PCC.0b013e31821926a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. http://dx.doi.org/10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 19.Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, Iapichino G, Leverve X, Nitenberg G, Singer P, Wernerman J, Joannidis M, Stecher A, Chioléro R. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–1748. doi: 10.1007/s00134-009-1585-2. http://dx.doi.org/10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 20.Arabi YM, Dabbagh OC, Tamim HM, Al-Shimemeri AA, Memish ZA, Haddad SH, Syed SJ, Giridhar HR, Rishu AH, Al-Daker MO, Kahoul SH, Britts RJ, Sakkijha MH. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. http://dx.doi.org/10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 21.De La Rosa GDC, Donado JH, Restrepo AH, Quintero AM, Gonzalez LG, Saldarriaga NE, Bedoya M, Toro JM, Velasquez JB, Valencia JC, Arango CM, Aleman PH, Vasquez EM, Chavarriaga JC, Yepes A, Pulido W, Cadavid CA. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12:R120. doi: 10.1186/cc7017. http://dx.doi.org/10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. doi: 10.1097/PCC.0b013e318172d401. http://dx.doi.org/10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 23.Polito A, Thiagarajan RR, Laussen PC, Gauvreau K, Agus MS, Scheurer MA, Pigula FA, Costello JM. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118:2235–2242. doi: 10.1161/CIRCULATIONAHA.108.804286. CIRCULATIONAHA.108.804286 [pii]\r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leteurtre S, Martinot A, Duhamel A, Proulx F, Grandbastien B, Cotting J, Gottesman R, Joffe A, Pfenninger J, Hubert P, Lacroix J, Leclerc F. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362:192–197. doi: 10.1016/S0140-6736(03)13908-6. http://dx.doi.org/10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 25.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the pediatric logistic organ dysfunction score. Crit Care Med. 2013;41:1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. http://dx.doi.org/10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix J, Cotting J. Severity of illness and organ dysfunction scoring in children. Pediatr Crit Care Med. 2005;6:S126–S134. doi: 10.1097/01.PCC.0000161287.61028.D4. http://dx.doi.org/10.1097/01.PCC.0000161287.61028.D4. [DOI] [PubMed] [Google Scholar]
- 27.Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Hubert P, Lacroix J. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171:348–353. doi: 10.1164/rccm.200405-630OC. http://dx.doi.org/10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 28.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30:1772–1777. doi: 10.1097/00003246-200208000-00016. http://dx.doi.org/10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Pediatric Acute Lung Injury Consensus Conference Group, T.P.A.L.I.C.C. Group. Pediatric Acute Respiratory Distress Syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16:428–439. doi: 10.1097/PCC.0000000000000350. http://dx.doi.org/10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. http://dx.doi.org/10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Sparrow SS, Cicchetti DV. Diagnostic uses of the vineland adaptive behavior scales. J Pediatr Psychol. 1985;10:215–225. doi: 10.1093/jpepsy/10.2.215. http://dx.doi.org/10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- 32.Achenbach TM. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. 1991. [Google Scholar]
- 33.Varni JW, Seid M, Kurtin PS. PedsQL (TM) 4.0: reliability and validity of the pediatric quality of life Inventory (TM) Version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–812. doi: 10.1097/00005650-200108000-00006. http://dx.doi.org/10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults. JAMA. 2008;300:933–944. doi: 10.1001/jama.300.8.933. http://dx.doi.org/10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]
- 35.Aragon D. Evaluation of nursing work effort and perceptions about blood glucose testing in tight glycemic control. Am J Crit Care. 2006;15:370–377. [PubMed] [Google Scholar]
- 36.Reid GB, Nygren TE. The subjective workload assessment technique: a scaling procedure for measuring mental workload. Adv Psychol. 1988;52:185–218. http://dx.doi.org/10.1016/S0166-4115(08)62387-0. [Google Scholar]
- 37.Hart SG, Staveland LE. Development of NASA-TLX (Task Load Index): results of empirical and theoretical research. Adv Psychol. 1988;52:139–183. http://dx.doi.org/10.1016/S0166-4115(08)62386-9. [Google Scholar]
- 38.Thompson BT, Orme JF, Zheng H, Luckett PM, Truwit JD, Willson DF, Duncan Hite R, Brower RG, Bernard GR, Curley MAQ, Steingrub JS, Sorenson DK, Sward K, Hirshberg E, Morris AH Reengineering Critical Care Clinical Research. Multicenter validation of a computer-based clinical decision support tool for glucose control in adult and pediatric intensive care units. J Diabetes Sci Technol. 2008;2:357–368. doi: 10.1177/193229680800200304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miranda DR, Moreno R, Iapichino G. Nine equivalents of nursing manpower use score (NEMS) Intensive Care Med. 1997;23:760–765. doi: 10.1007/s001340050406. http://dx.doi.org/10.1007/s001340050406. [DOI] [PubMed] [Google Scholar]
- 40.Ashley A, Draper E, Fleming T, Lamming C, McKinney P, McShane P, Parslow R, Skinner S, Thiru K. Paediatric Intensive Care Audit Network National Report 2006–2008. Leeds, United Kingdom: 2009. http://www.picanet.org.uk/Audit/Annual-Reporting/Annual-Report-Archive/PICANet_National_Report_2009.pdf. [Google Scholar]
- 41.Draper E, Fleming T, McKinney P, Parslow R, Thiru K, Willshaw A. Pediatric Intensive Care Audit Network National Report 2004–2006. Leeds, United Kingdom: 2007. http://www.picanet.org.uk/Audit/Annual-Reporting/Annual-Report-Archive/PICANet_National_Report_2007.pdf. [Google Scholar]
- 42.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. http://dx.doi.org/10.1097/01.PCC.0000128607.68261.7C. [DOI] [PubMed] [Google Scholar]
- 43.Faustino EV, Apkon M. Persistent hyperglycemia in critically ill children. J Pediatr. 2005;146:30–34. doi: 10.1016/j.jpeds.2004.08.076. http://dx.doi.org/10.1016/j.jpeds.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 44.Preissig CM, Rigby MR. Pediatric critical illness hyperglycemia: risk factors associated with development and severity of hyperglycemia in critically ill children. J Pediatr. 2009;155:734–739. doi: 10.1016/j.jpeds.2009.05.007. http://dx.doi.org/10.1016/j.jpeds.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Srinivasan V, Agus MSD. Tight glucose control in critically ill children—a systematic review and meta-analysis. Pediatr Diabetes. 2014;15:75–83. doi: 10.1111/pedi.12134. http://dx.doi.org/10.1111/pedi.12134. [DOI] [PubMed] [Google Scholar]
- 46.Jacobi J, Nicholas B, Krinsley J, Agus M, Braithwaite SS, Deutschmann C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251–3276. doi: 10.1097/CCM.0b013e3182653269. http://dx.doi.org/10.1097/CCM.0b013e3182653269. [DOI] [PubMed] [Google Scholar]
- 47.Moghissi ES, Korytkowski MT, DiNardo M, Einhorn D, Hellman R, Hirsch IB, Inzucchi SE, Ismail-Beigi F, Kirkman MS, Umpierrez GE. American Association of Clinical Endocrinologists and American Diabetes Association Consensus Statement on Inpatient Glycemic Control. Diabetes Care. 2009;32:1119–1131. doi: 10.2337/dc09-9029. http://dx.doi.org/10.2337/dc09-9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qaseem A, Chou R, Humphrey LL, Shekelle P. Inpatient glycemic control: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Am J Med Qual. 2013;29:95–98. doi: 10.1177/1062860613489339. http://dx.doi.org/10.1177/1062860613489339. [DOI] [PubMed] [Google Scholar]
- 49.Cornblath M, Hawdon J, Williams A, Aynsley-Green A, Ward-Platt M, Schwartz R, Kalhan S. Controversies regarding definition of neonatal hypoglycemia: suggested operational thresholds. Pediatrics. 2000;105:1141–1145. doi: 10.1542/peds.105.5.1141. http://dx.doi.org/10.1542/peds.105.5.1141. [DOI] [PubMed] [Google Scholar]
- 50.Faustino EVS, Bogue CW. Relationship between hypoglycemia and mortality in critically ill children. Pediatr Crit Care Med. 2010;11:690–698. doi: 10.1097/PCC.0b013e3181e8f502. http://dx.doi.org/10.1097/PCC.0b013e3181e8f502. [DOI] [PubMed] [Google Scholar]
- 51.Sadhwani A, Asaro LA, Goldberg C, Ware J, Butcher J, Gaies M, Smith C, Alexander JL, Wypij D, Agus MSD. Impact of tight glycemic control on neurodevelopmental outcomes at 1 year of age for children with congenital heart disease: a randomized controlled trial. J Pediatr. 2015;174:193–198. doi: 10.1016/j.jpeds.2016.03.048. http://dx.doi.org/10.1016/j.jpeds.2016.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]