Abstract
Infants with complex congenital heart disease are at high risk for poor neurodevelopmental outcomes. However, implementation of dedicated congenital heart disease follow-up programs presents important infrastructure, personnel, and resource challenges. We present the development, implementation, and retrospective review of 1- and 2-year outcomes of a Complex Congenital Heart Defect Neurodevelopmental Follow-Up program. This program was a synergistic approach between the Pediatric Cardiology, Cardiothoracic Surgery, Pediatric Intensive Care, and Neonatal Intensive Care Unit Follow-Up teams to provide a feasible and responsible utilization of existing infrastructure and personnel, to develop and implement a program dedicated to children with congenital heart disease. Trained developmental testers administered the Ages and Stages Questionnaire-3 over the phone to the parents of all referred children at least once between 6 and 12 months’ corrected age. At 18 months’ corrected age, all children were scheduled in the Neonatal Intensive-Care Unit Follow-Up Clinic for a visit with standardized neurological exams, Bayley III, multidisciplinary therapy evaluations and continued follow-up. Of the 132 patients identified in the Cardiothoracic Surgery database and at discharge from the hospital, a total number of 106 infants were reviewed. A genetic syndrome was identified in 23.4% of the population. Neuroimaging abnormalities were identified in 21.7% of the cohort with 12.8% having visibly severe insults. As a result, 23 (26.7%) received first-time referrals for early intervention services, 16 (13.8%) received referrals for new services in addition to their existing ones. We concluded that utilization of existing resources in collaboration with established programs can ensure targeted neurodevelopmental follow-up for all children with complex congenital heart disease.
Keywords: heart defects, congenital, intensive care units, neonatal, neuroimaging, neurologic examination, referral, consultation
Neurodevelopmental delays and impairments are common in children with complex congenital heart disease (CHD). In 2012, the American Heart Association (AHA) identified a population of infants with congenital heart defects at high risk for neurodevelopmental delays and impairments and recommended the institution of dedicated cardiac neurodevelopmental programs.1 The purpose of such programs is to promote the early identification of developmental problems to “leverage resources, such as early intervention programs and other therapies, so patients with identified deficits can achieve their highest potential”.2
Although these recommendations emphasize the professional concerns of practitioners caring for young patients with CHD, the establishment of complex neurodevelopmental and educational programs is often challenging, because of limited resources and infrastructure.
This challenge was met for the past 30 years by existing programs of neurodevelopmental surveillance, diagnosis, and interventions, but refined for Neonatal Intensive Care Unit (NICU) graduates. NICU Follow-Up Clinics across the nation have been providing programmatic approaches to their high-risk infants for clinical care and research purposes.3–8 Because of this long history, they often have the infrastructure, developmental personnel, and resources, as well as regional and national networks to provide this type of family-centered specialty care.
Therefore, in cases of limited resources, the strong sense of responsibility that cardiologists, cardiothoracic surgeons, and intensivists feel toward infants with CHD can drive cooperation with existing NICU Follow-Up Programs.
Goals and Vision of the Joint CHD-NICU Follow-Up Program
The vision of this program was to create a synergistic approach between the Pediatric Cardiology, Cardiothoracic Surgery, Pediatric Intensive Care, and NICU Follow-Up teams to provide a feasible and responsible utilization of existing infrastructure and personnel, to rapidly develop and implement a tailored program dedicated to all children with CHD as recommended by the AHA. The 1-year results of this approach are presented in this article, in support of this highly CHD-specific and cooperative paradigm.
Local Challenges in Implementation
Limited Resources
The costs of neurodevelopmental and educational specialists for the program were prohibitive when considering a de novo program for ≈120 patients per year. Full-time psychologists, developmental providers, occupational, speech and physical therapists were required along with clinic space, schedulers, care coordinators, and equipment. A decision was made by the Pediatric Cardiology team to leverage existing resources and supplement them, rather than to create a program in isolation.
Potential Duplication of Existing Resources
Existing programs, care pathways, personnel, and space made duplication of resources a real concern leading to inefficient use of the medical system and increased parental frustration. Three areas of overlap were identified:
The Pediatric Cardiology division had a team of outstanding discharge planners/resource managers who ensured complete and excellent follow-up and continuity of care after discharge from intensive care units or the pediatric floor.
The Division of Neonatology had a thriving multidisciplinary neurodevelopmental follow-up program established in the early 1980s that provided standardized assessments and state-of-the-art care from birth until age 3.
The Division of Developmental Medicine had a team of expert developmental pediatricians and psychologists, who in concert with various specialized program across the medical campus (autism, behavioral, parenting, deaf-blind education, and other) provided excellent assessment and care for children from 3 years until adulthood.
A decision was made to combine these 3 existing assets and focus on design of tailored care and communication pathways for CHD infants. Support was allocated for a coordinator to ensure seamless neurodevelopmental care transition from birth to childhood.
Access Challenges
The CHD population concerned presented 2 distinct concerns. (1) The catchment area for the CHD population at our large tertiary care center (with advanced cardiothoracic surgery specialists) spanned a radius of 100 miles. Transportation and time for a visit had the potential to be costly or difficult to implement for some families. (2) Infants with CHD often were hospitalized in the first year making early scheduled visits impossible; after discharge from the hospital, parents were then justifiably reluctant to expose their recovering infants to communicable illnesses for nonemergent reasons, such as a neurodevelopmental visit. To improve flexibility of access to care, visits were coordinated at times parents were coming for any tertiary care visit, cardiology, or other. The electronic medical record was used to communicate between developmental and cardiology providers to ensure concerted care. Transportation vouchers were provided through the Tennessee Title V Maternal and Child Health Block Grant9 to help defray the cost of lengthy developmental visits at extended distances.
Design of the Initiative
Before the start of this initiative infants with CHD were not being evaluated unless they happened to meet the referral criteria for the NICU Follow-Up Clinic (<32 weeks of estimated gestational age, brain injury on magnetic resonance imaging [MRI], seizures, congenital anomalies, extracorporeal membrane oxygenation, Trisomy 21—the clinic performed neurodevelopmental testing at the request of the Down Syndrome Clinic, or birth asphyxia) or the Neurology/Stroke clinic (seizures, MRI evidence of infarct, or thrombosis).
The Pediatric Cardiology team provided explicit guidance on the unique challenges of outpatient care of CHD infants, including numerous visits to cardiology and other medical subspecialty providers, distance to referral center, frequent procedures or surgery in the first 2 years of life, and the difficulty of the care-by-parent model used for less ill infants in intensive care. The Cardiothoracic Surgery team helped identify and narrow qualifying infants who might meet the AHA recommendations using a comprehensive and detailed database. The database included data from any child who had a cardiothoracic procedure along with their confirmed diagnoses and procedures. The diagnoses were cross-referenced with Table 3 of the Marino et al1 published in Circulation article on AHA recommendations for neurodevelopmental follow-up. This process facilitated the referral of all infants already discharged from the hospital but still <2 years of age, who might still benefit from an early developmental follow-up. The Cardiology team discharge planners’ knowledge of the referral pathway (from the hospital and from the database) was critical to consistent referral of patients to the CHD program, in addition to establishing home-based early intervention services before discharge. They also followed the guidelines found in the AHA Statement Paper: to perform surveillance, elicit and attend to the parents concerns, maintain a developmental history, make accurate and informed observations of the child, identify the presence of risk and protective factors, document the process and findings, make referrals for early intervention and formal developmental and medical evaluation.1
Table 3.
Neurodevelopmental Outcomes Stratified Using Congenital Heart Surgery Mortality Categories
| STAT 1–2, n (%) |
STAT 3–4, n (%) |
STAT 5, n (%) | |
|---|---|---|---|
| At least 1 ASQ-3 (n=96) | 23 | 42 | 31 |
| ASQ-3 scores at high-risk | |||
| Communication | 2 (8.7) | 8 (19.0) | 4 (12.9) |
| Gross motor | 11 (47.8) | 26 (61.9) | 16 (51.6) |
| Fine motor | 6 (26.0) | 12 (28.6) | 10 (32.2) |
| Problem solving | 3 (13.0) | 18 (42.8) | 8 (25.8) |
| Personal-social | 4 (17.4) | 12 (28.6) | 5 (16.1) |
| All 5 | 1 (4.3) | 3 (7.1) | 1 (3.2) |
| Tested with BSID-III (N=38) |
STAT 1–2, median (IQR), n=10 |
STAT 3–4, median (IQR), n=14 |
STAT 5, median (IQR), n=14 |
| Cognitive composite | 92 (85, 101) | 90 (77, 91) | 85 (84, 100) |
| Language composite | 87 (79, 94) | 86 (76, 94) | 87 (79, 97.7) |
| Receptive language | 7 (6, 9) | 7 (5, 9) | 8 (8, 9) |
| Expressive language | 7 (6, 10) | 7.5 (5, 9) | 7.5 (5, 10.2) |
| Motor composite | 92 (82, 99) | 91 (63, 97) | 91 (79, 97) |
| Fine motor | 10 (8, 11.5) | 9 (5.5, 10.5) | 9 (7.5, 11.2) |
| Gross motor | 8 (6, 8.2) | 6.5 (2.7, 9) | 7.5 (4.7, 8.2) |
| BSID scores <85 | |||
| Cognitive composite | 1 | 5 | 3 |
| Language composite | 4 | 6 | 6 |
| Motor composite | 4 | 6 | 5 |
ASQ-3 indicates Ages and Stages-3 Questionnaires; BSID-III, Bayley Scales of Infant Development-III; IQR, interquartile range (25th, 75th); and STAT, Society of Thoracic Surgeons- European Association for Cardio-thoracic Surgery Congenital Heart Surgery Mortality Categories.2
Implementation of the Initiative
We first took a deliberate approach with departmental leadership, to educate both the Pediatric Cardiology team and the Follow-Up Clinic Team about the benefits and feasibility of the program implementation. Then, in collaboration with the Cardiology team, program description materials were created, approved, and distributed to the clinic team and displayed in the clinic. These materials included information about the program and the importance of follow-up for children with CHD. The electronic chart system documentation forms were updated to include fields applicable to the population. Efforts were made to educate parents and caregivers about the importance of follow-up for patients with CHD and services that were available to them.
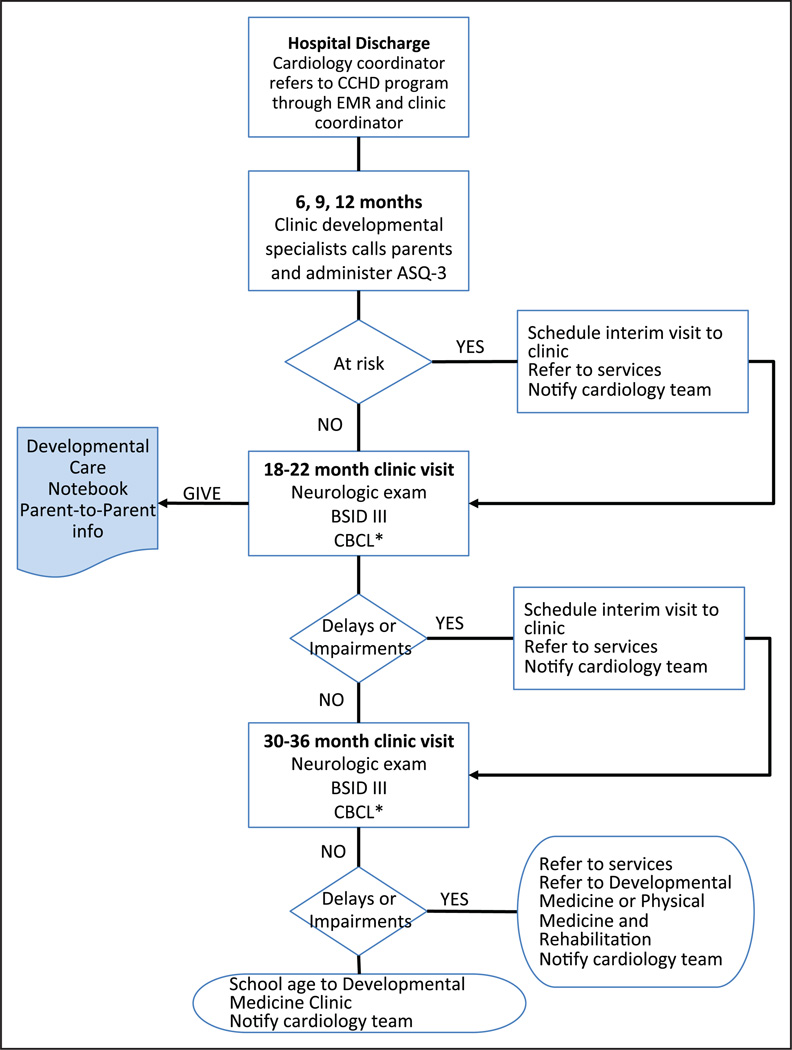
The flow process for the CHD Follow-Up Program can be visualized in Figure 1. The discharge planners for the Cardiology Team made referrals and spoke with the NICU Follow-Up Clinic coordinator to ensure that referrals to State Early Intervention services were made for infants at the highest risk for delays, at discharge from the hospital. In the NICU Follow-Up Clinic, early screening, identification of developmental service needs and referrals were made within the first year of life. To accomplish this, trained developmental testers (occupational therapists/physical therapists/pediatric nurse practitioners) administered the Ages and Stages Questionnaire-3 (ASQ-3)10 over the phone to the parents of all referred children at least once between 6 and 12 months’ corrected age, with at-risk infants receiving a second assessment 3 months later, and with some flexibility to allow for the child being hospitalized during the first year.
Figure 1.
Flow process for Congenital Heart Disease (CHD) Follow-Up Pilot Program. ASQ-3 indicates Ages and Stages-3 Questionnaires; BSID-III, Bayley Scales of Infant Development-III; CBCL, Child Behavior Checklist; EMR, electronic medical record. *If provider or parental concern.
This approach is not standard of care for all neurodevelopmental follow-up in the United States, although most State Early Intervention services and care coordinators make phone evaluations a routine part of their care. However, in Europe, developmental specialists have questioned the need for in person follow-up for large-scale outcome studies. Others have validated the parent report tools such as The Parent Report of Children's Abilities- Revised, as an assessment tool with comparable validity to the in person Bayley Scales of Infant Development (BSID).11,12
At 18 months’ corrected age all children were scheduled in the NICU Follow-Up Clinic for a visit. This multidisciplinary clinic includes occupational and physical therapists, orthotists, and a psychologist and developmental specialists. The visit included a full developmental history, neurological examination, and standardized assessment with a BSID-III,13 and if suspicion of a behavioral challenge was identified, a Child Behavior Checklist.1,14 In addition, parents were given a developmental care notebook (Heads & Hearts, Impact of Complex Congenital Heart Conditions on Children’s Neurodevelopment, Malley and Henry Fund, OK) as well as information on the state parent-to-parent support network. Parent-based organizations are extremely useful to both families and providers, both during the hospitalization and afterward. The cardiology team asked the developmental team to review the written materials provided by this organization before engaging them, to ensure a strong evidence base. The parent-to-parent partners also identified a need for education in the hospital, and the follow-up coordinator was scheduled in the rotation of talks available to parents of infants with CHD.
Any necessary referrals were made at the time of the visits, and the next assessment visits were scheduled 6 to 12 months later depending on whether a concern was identified.
Our clinic agreed to test infants with Trisomy 21 to assist the Down Syndrome program in their service provision. Although these infants have unique neurodevelopmental challenges and access to many services, we included infants with both Trisomy 21 and CHD because of their worse neurodevelopmental outcomes in the first years compared to those without CHD (even if school-age differences are less apparent).15 Furthermore, many Down Syndrome programs do not perform a standardized 3-domain test such as the BSID-III, at both the 18- to 24-month age and the 36-month age. This was of particular interest to parents as it provides a developmental trajectory using the same assessment at 2 time points.
After 3 years of age, patients were referred to Developmental Medicine or Physical Medicine and Rehabilitation Clinics for continuing care as needed. All records of assessments and interventions were directly visible to the cardiology team through the electronic medical record with frequent communication through electronic mail baskets.
Study Design
We conducted a retrospective review of data on all patients referred to the CHD Program of the NICU Developmental Follow-Up Clinic at the Monroe Carrell Junior Children’s Hospital between January 1, 2014, and January 1, 2015. Referred infants too young to have completed any neurodevelopmental follow-up assessments at the time of study (<6 months’ corrected age) were excluded. Demographic data, cardiac diagnoses, surgical procedures, genetic syndromes (as determined by complete testing by the Pediatric Genetics team), most severe radiographic cranial imaging (severe injury was defined as intraventricular hemorrhage grades III or IV, periventricular leukomalacia, or ischemia), auditory brain stem response testing results and feeding status at discharge (all oral versus any gastrostomy feedings) were collected from the electronic medical record (Tables 1 and 2).
Table 1.
Characteristics of Follow-Up Cohort
| n | % | |
|---|---|---|
| EGA, wk, median (IQR) | 39 (37, 39) | |
| <34 wk EGA | 4 | 3.0 |
| 34 0/7–36 6/7 wk EGA | 18 | 13.6 |
| 37 0/7–39 6/7 wk EGA | 90 | 68.2 |
| ≥40 0/7 | 20 | 15.2 |
| Birth weight, g, median (IQR) | 3110 (2730, 3510) | |
| Discharge age, mo, median (IQR) | 1 (1, 2) | |
| Sex (% female) | 57 | 43.1 |
| Race | ||
| White | 115 | 87.1 |
| Black | 13 | 9.8 |
| Hispanic | 12 | 9.0 |
| Other | 4 | 3.0 |
| Genetic syndrome | 31 | 23.4 |
| Trisomy 21 | 14 | 10.6 |
| Failed ABR | 8 | 7.0 |
| Seizures on EEG | 2 | 1.5 |
| Discharge on full oral feeds | 62 | 46.9 |
| Neuroimaging | ||
| Not performed | 23 | 17.4 |
| Read as normal | 56 | 42.4 |
| Read as severe neural injury* | 17 | 12.9 |
| Other types of neuroimaging findings† | 36 | 27.3 |
| Cardiac diagnosis | ||
| Hypoplastic left heart | 25 | 18.9 |
| Tetralogy of Fallot | 14 | 10.6 |
| Truncus arteriosus | 3 | 2.3 |
| Tricuspid atresia | 6 | 4.5 |
| Transposition of the great vessels | 11 | 8.3 |
| Total anomalous pulmonary venous return | 5 | 3.8 |
| Pulmonary artery, critical pulmonary stenosis | 5 | 3.8 |
| Aortic stenosis, atresia, coarctation | 34 | 25.8 |
| Atrioventicular septal defect | 16 | 12.1 |
| Other | 13 | 9.8 |
ABR indicates auditory brainstem response; EEG, electroencephalogram; EGA, estimated gestational age at birth; IQR, interquartile range (25th, 75th).
Severe neural injury defined as intraventricular hemorrhage grades III or intraventricular hemorrhage, periventricular leukomalacia, diffuse ischemia; all severe findings documented on magnetic resonance imaging or computed tomography.
Intraventricular hemorrhage grades I or II, partial agenesis of the corpus callosum, mineralizing vasculopathy of noninfectious origin, small subdural hematomas.
Table 2.
Outcomes After 1 and 2 Years
| n (%) Total | |
|---|---|
| Referrals from discharge | 106 |
| Received at least 1 ASQ-3 | 96 (90.6) |
| Received 2 or more ASQ-3s | 51 (48.1) |
| ASQ-3 scores at high-risk* | |
| Communication | 14 (14.4) |
| Gross motor | 53 (54.6) |
| Fine motor | 28 (28.9) |
| Problem solving | 29 (29.9) |
| Personal-social | 21 (21.7) |
| All 5 | 5 (5.1) |
| Infants with existing referrals at ASQ-3 | 39 (40.6) |
| Infants with new referrals at ASQ-3 | 6 (6.2) |
| 18–24-mo follow-up from discharge referrals and database (n=38) | |
| BSID-III scores, median (IQR) | |
| Cognitive composite | 90 (84,96) |
| Language composite | 86 (79, 94) |
| Receptive language | 8 (6,9) |
| Expressive language | 7 (5.7, 10) |
| Motor composite | 91 (78, 97) |
| Fine motor | 9.5 (7, 11) |
| Gross motor | 7.5 (4, 8.2) |
| Infants with BSID scores <85, n (%) | |
| Cognitive composite | 9 (23.1) |
| Language composite | 16 (41.0) |
| Motor composite | 15 (38.5) |
| Infants already receiving services, n (%) | |
| Physical therapy | 13 (33.3) |
| Occupational therapy | 6 (15.4) |
| Speech therapy | 11 (28.2) |
| Feeding interventions | 14 (35.9) |
| New referrals made at visit, n (%) | |
| Physical therapy | 3 (7.7) |
| Occupational therapy | 4 (10.3) |
| Speech therapy | 13 (33.3) |
| Feeding interventions | 17 (43.6) |
ASQ-3: Ages and Stages-3 Questionnaires;, BSID-III: Bayley Scales of Infant Development-III; and IQR, interquartile range (25th, 75th).
Hearing impairments were determined by pediatric audiologists utilizing the auditory brain stem response, and otoacoustic emissions or tympanometry were utilized as needed. Vision impairments were diagnosed in Pediatric Opthalmology clinic. The presence of neurological abnormalities or cerebral palsy was determined according to published algorithms and definitions.2,16,17 For the BSID-III, the normative population mean of BSID-III is 100 and scores >1 SD (15 points) below the mean represent a delay or impairment and prompted referral for specialized evaluations and services. Data were maintained in a deidentified repository database approved by the Institutional Review Board at Vanderbilt University. The Institutional Review Board waiver of consent was approved for completion of this study (Institutional Review Board 150440).
Success of the Initiative
Results
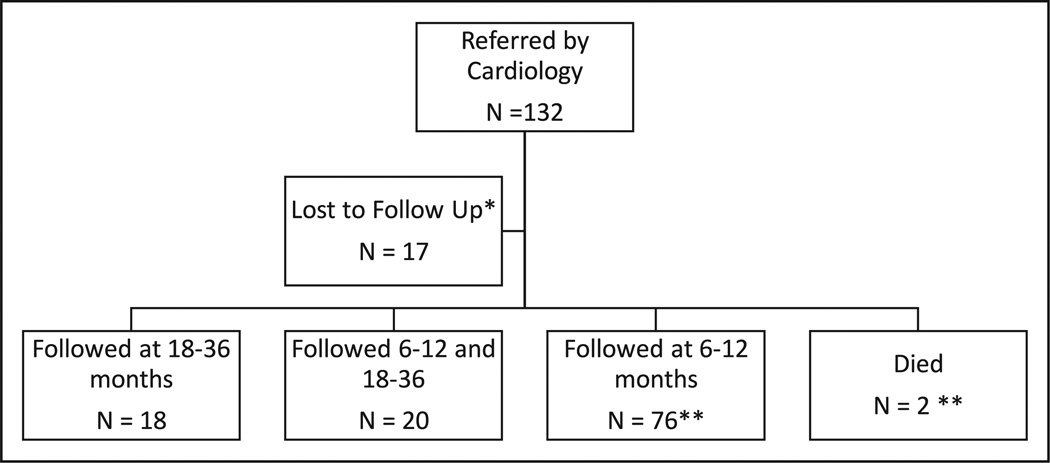
Of the 132 patients identified in the Cardiothoracic Surgery database and at discharge from the hospital, 17 were lost to follow-up (7/25 for the database versus 10/107 referred by discharge planners; Figure 2).
Figure 2.
Pilot program patient referral and follow-up. *1 parent refused, 10 no-showed to clinic, and 7 unreachable. **1 patient died before any follow-up, the other after 9-month follow-up.
Fifty-seven percent of the 132 were women and 31% of the patients were born preterm (<38 weeks’ gestation age) with median birth weight 3110 g (interquartile range, 2730–3510). Preterm infants had a median estimated gestational age of 36 with an interquartile range of 35 to 37. Two (n=2) infants were <32 weeks (25 and 27 weeks’ gestation age). A genetic syndrome was identified in 23.4% of the population, including 14 Trisomy 21 (10.6%), 7 DiGeorge, 5 Turner, 1 Shwachman-Diamond, 1 Greig cephalopolysyndactyly, and 3 other chromosomal mutations or translocations associated with cardiac anomalies (2p12; GDF1 and t(2;11)(p23;q21)). The most common cardiac diagnoses were coarctation of the aorta (or critical aortic stenosis) 25.8%, hypoplastic left heart syndrome (19%), and Tetralogy of Fallot (10.6%).
Neuroimaging abnormalities were identified in 21.7% of the cohort with only 12.8% having visibly severe insults. Infants had cranial ultrasounds with an MRI if a suspicious finding was identified, a head computed tomography or an MRI alone. Of the entire cohort, 23 had no documented imaging (Table 2).
With regard to seizures, of 13 infants with electroencephalograms because of suspect clinical findings, 2 infants had documented seizures and were discharged on antiepileptics. These infants had evidence of severe neural insults on MRI.
At follow-up, 69.2% of the patients had undergone a cardiac surgery between discharge to home and the first clinic visit, with the most common procedure being a bidirectional Glenn operation (20.5%). Minor hearing impairments were identified in 3.8% of the population (conductive and auditory neuropathy, unilateral), and 4.5% had minor vision impairments (strabismus, myopia). No patient had a confirmed diagnosis of cerebral palsy at the time of article preparation.
Developmental screening showed that 53% of the population scored below the cutoff on ASQ-3 in the gross motor domain, followed by 29% in the problem-solving domain. The phone assessments with a developmental specialist and the ASQ-3 resulted in 6% additional referrals to early intervention services and 4% referrals for in person visits. The 6 infants who needed additional referrals had not originally been referred to the State Early Intervention program, but were found to need services based on their risk cutoff as well as parental concerns evaluated by the developmental specialists.
Only 5% of the population scored below cutoff in all 5-domain categories. BSID-III scores below <85, indicating the presence of at least a mild developmental delay, were identified in 23% to 41% of the population. Of the 116 patients who completed ≥1 follow-up assessment, 23 (26.7%) were already receiving early intervention services.
As a result of CHD Program follow-up, 23 (26.7%) received first-time referrals for early intervention services, whereas 16 (13.8%) received referrals for new services in addition to their existing ones.
Our evaluation of the program and analysis was not designed or powered to compare associations between severity of CHD and outcomes. Descriptively (Table 3), infants in STAT1/2 categories seemed to have higher mean developmental scores on the BSID-III and were less often identified as at risk on the ASQ-3 than infants in STAT categories 3 to 5 (Table 2).18,19
Similarly, a stratification by severity of findings on neuroimaging (see Methods section of this article for description) was limited by samples size and by current practice utilizing cranial ultrasounds as initial screening methodology (Table 4). MRI and computed tomography were utilized in the ICUs to confirm any abnormal imaging findings on cranial ultrasound. As expected, infants with severe abnormalities on neuroimaging had a higher proportion of high-risk scores on the ASQ-3 than those with no or mild abnormalities. However, more than half of infants with no reported abnormalities on neuroimaging had high-risk scores in the gross motor domain. Parents and medical providers reported a high degree of satisfaction with availability of this combination of phone and in-person follow-up because of the unique challenges of families of children with CHD.
Table 4.
Neurodevelopmental Outcomes Stratified by Risk of Neurological Findings (Neuroimaging and Electrophysiology)
| No Reported Abnormalities, n (%) |
Mild* Abnormalities, n (%) |
Severe† Abnormalities, n (%) |
|
|---|---|---|---|
| At least 1 ASQ-3 (n=91‡) |
70 | 10 | 11 |
| ASQ-3 scores at high risk | |||
| Communication | 6 (8.6) | 2 (20.0) | 4 (36.4) |
| Gross motor | 36 (51.4) | 2 (20.0) | 9 (81.8) |
| Fine motor | 20 (28.6) | 1 (10.0) | 3 (27.3) |
| Problem solving | 23 (32.8) | 1 (10.0) | 3 (27.3)` |
| Personal-social | 13 (18.6) | 0 (0) | 5 (36.4) |
| All 5 | 0 (0) | 1 (10.0) | 3 (27.3) |
| Tested with BSID-III (n=33‡) |
Median (IQR), n=22 |
Median (IQR), n=7 |
Median (IQR), n=4 |
| Cognitive composite |
90 (84, 100) | 85 (75,90) | 85 (75, 90) |
| Language composite |
87 (79, 94) | 83 (77,94) | 91 (86, 99) |
| Receptive language |
8 (7, 8) | 8 (5,9) | 9 (7.5, 9.7) |
| Expressive language |
7.5 (5, 10) | 7 (5,7) | 8.5 (6.5, 10.5) |
| Motor composite | 91 (78, 97) | 82 (73,97) | 92 (77, 96) |
| Fine motor | 9 (6.7, 11) | 8 (6,11) | 10.5 (7.5, 12) |
| Gross motor | 8 (4.7, 9) | 6 (4,8) | 6.5 (4.5, 7.7) |
| BSID scores <85 | |||
| Cognitive composite |
6 | 2 | 0 |
| Language composite |
12 | 2 | 0 |
| Motor composite | 10 | 3 | 1 |
ASQ-3 indicates Ages and Stages-3 Questionnaires; BSID-III, Bayley Scales of Infant Development-III; and IQR, interquartile range (25th, 75th).
Mild neural injury defined as intraventricular hemorrhage grades I or II, partial agenesis of the corpus callosum, mineralizing vasculopathy of noninfectious origin, small subdural hematomas.
Severe neural injury defined as intraventricular hemorrhage grades III or IVH, periventricular leukomalacia, diffuse ischemia; all severe findings documented on MRI or CT.
Twenty-three patients with no known neuroimaging or electrophysiology evaluations were excluded from this table. The 2 patients with seizures on electroencephalogram also had neuroimaging findings consistent with severe neural insults.
The ASQ-3, in addition to providing risk cutoffs also has many open-ended questions allowing parents to voice their concerns. Subsequently, the BSID-III at ≈2 and 3 years provides good assessment of developmental delays and has adequate predictive validity for long-term developmental outcomes.20 Follow-up at 18 to 22 months was also more feasible for patients and caregivers, because a majority of patients receive additional surgical procedures between 3 and 16 months.
Summary of the Experience, Future Directions, and Challenges
The retrospective review of this program presents the first evidence for the feasibility for a concerted approach to complex CHD neurodevelopmental follow-up by cardiologists, cardiothoracic surgeons, and the NICU Follow-Up Clinic. Consistent with previous reports of children with specific CHD types,1,21–23 the infants in this program had a high prevalence of developmental delays and impairments, mostly in the mild to moderate range. Disability seemed more severe depending on the complexity of the CHD, need for continued surgical interventions, and presence of genetic findings.
Because of their later gestational age at birth (35–37 weeks), most of the preterm infants included in the CHD Program would not have been followed in NICU Follow-Up Clinics across United States. However, these late preterm/early term infants are at increased risk for neurodevelopmental problems; the AHA recommendations are therefore particularly important in ensuring follow-up of children who may otherwise be overlooked.24
The CHD program’s benchmark for success was the referral of children requiring essential early intervention services. Consistent with known problems in this population feeding therapy had the highest rate of referrals,23 but physical, occupational, and speech therapy were often needed as well.25
Many infants were not receiving services at the time of the 6-month ASQ-3 but were being evaluated or referred to services by early intervention. The role of the ASQ-3 check was then to ensure that parents and State Early Intervention specialists understood the real need for these services. The open-ended questions on the ASQ-3 focused on strengths and concerns, and most parents had comments in this section. Only 1 parent refused to take the phone assessment, saying their child did not need it. We can only speculate that parents of infants with CHD have a uniquely intense experience during the first year of their child’s life and may therefore be particularly aware of daily changes in their children’s bodies and social or environmental interactions.
The phone assessment is limited in the fact that it does not replace a full, standardized infant neurological examination that must occur in person. We chose 18 months as an in-person visit age for infants who were not already referred to Pediatric Neurologists because of seizures and stroke. This choice can certainly be questioned or modified according to a program’s imperatives and we offer it only as a successful example. Our rationale was guided by our surgeons and cardiologists knowledge of medical follow-up and surgical procedure schedules.
Under-diagnosis is always a possibility in any developmental evaluation, whether over the phone or in person. Developmental specialists across disciplines acknowledge that the snapshot of a patient’s development evaluated in a stressful clinic visit may not represent the full spectrum of an infant’s function or impairments. Overall, we found that parents of infants with CHD were extremely knowledgeable about their infant’s current development and eager to learn about developmental milestones from the developmental specialists over the phone.
Limitations of our program’s evaluation include its retrospective nature and relatively short time span, precluding the demonstration of long-term developmental trajectories, usually the hallmark of NICU Follow-Up Clinic research. Additionally, environmental and social aspects of patient’s development are routinely assessed in the clinic but were not included in this small feasibility evaluation. With regard to the program model, over-the-phone parental questionnaires are not ideal as the sole form of follow-up care of high-risk infants. However, in combination with the interview of a developmental specialist, they allow for effective contact with caregivers and assessment of interim service needs. Strengths of this study and program are its all-inclusive nature, allowing any patient meeting AHA criteria rather than specific diagnoses to receive neurodevelopmental follow-up.
Our evaluation of the program’s effectiveness also suffers from a strong skew toward survivors. Out of the 106 infants referred after discharge from the pediatric intensive care unit postop, 96 received ASQ-3 and 2 had died in the time of phone follow-up. These infants had by definition survived a first surgical intervention and thus do represent a skewed population. The population of children followed from the cardiothoracic surgery database was also skewed as we contacted those infants who had survived to 18 months and were <30 months to bring them back to the clinic. First, these infants were by definition survivors; second, these infants had families who were concerned enough to bring their children to the clinic even though their cardiologist had not previously encouraged them to do so.
Also, although we had good rates of follow-up (90.6%) when referrals were made from discharge, these rates could certainly be improved in the future to ensure the best possible service to our families.
Future directions include refinement of the early follow-up period, including possible in-hospital assessments by clinic personnel using standardized neurological examination and assessments, improved communication with developmental specialists in the hospital and in early intervention programs throughout the state and continuing education of parents about the importance of follow-up while their child is still hospitalized.
Resources can be a limiting factor at any institution. One recommendation for our site and others would be to leverage telemedicine resources if available. The developmental specialist could, in addition to ASQ-3 administration, visualize the child and address some of the areas of concern. This may improve accuracy of the early referral process. In addition, establishing closer ties with the State Early Intervention Program may improve the early referral process by raising awareness and concern levels in this high-risk population. This may prevent the referred at discharge patients still being evaluated for needed feeding services 6 months later.
Speech-language pathologists, physical and occupational therapists are essential in the first 2 to 3 years. The ideal solution would be to craft a program with a strong focus on evidence-based feeding interventions and to provide families with needed home-based services to alleviate the stresses of frequent outpatient visits or unavailability of therapy services in remote or underserved areas.
As remarked on by others, school-age needs required a different set of providers such as behavioral pediatricians, education specialists, psychologists, and neuropsychiatrists. Therefore, we recommend additional engagement of the Developmental Pediatrics and Psychology Departments/Divisions in designing an efficient transition process after the age of 3 years.
Conclusions
Advances of diagnostic and surgical techniques as well as postoperative management strategies have led to increased survival rates of infants with CHD.26 The need of these children for systematic assessments and coordination of neurodevelopmental developmental services is frequently unmet despite examples of successful programs and high rates of patient and family utilization.27
A synergistic approach between the Pediatric Cardiology, Cardiothoracic Surgery, and the NICU Follow Up clinic teams provides a feasible and responsible utilization of existing resources to provide a unique neurodevelopmental program for all children with complex CHD. To maximize the developmental potential of these children, other such cooperative approaches could be encouraged.
Footnotes
Disclosures
None.
References
- 1.Marino BS, Lipkin PH, Newburger JW, Peacock G, Gerdes M, Gaynor JW, Mussatto KA, Uzark K, Goldberg CS, Johnson WH, Jr, Li J, Smith SE, Bellinger DC, Mahle WT. American Heart Association Congenital Heart Defects Committee, Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Stroke Council. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126:1143–1172. doi: 10.1161/CIR.0b013e318265ee8a. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Lu M, Ohye RG, William Gaynor J, Goldberg CS, Sleeper LA, Pemberton VL, Mussatto KA, Williams IA, Sood E, Krawczeski CD, Lewis A, Mirarchi N, Scheurer M, Pasquali SK, Pinto N, Jacobs JP, McCrindle BW, Newburger JW. A predictive model for neurodevelopmental outcome after the Norwood procedure. Pediatr Cardiol. 2013;34:327–333. doi: 10.1007/s00246-012-0450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papile LA, Munsick-Bruno G, Schaefer A. Relationship of cerebral intraventricular hemorrhage and early childhood neurologic handicaps. J Pediatr. 1983;103:273–277. doi: 10.1016/s0022-3476(83)80366-7. [DOI] [PubMed] [Google Scholar]
- 4.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27:281–287. doi: 10.1016/s0146-0005(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 5.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 6.Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115:997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- 7.Saigal S. Follow-up of very low birthweight babies to adolescence. Semin Neonatol. 2000;5:107–118. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- 8.Kuppala VS, Tabangin M, Haberman B, Steichen J, Yolton K. Current state of high-risk infant follow-up care in the United States: results of a national survey of academic follow-up programs. J Perinatol. 2012;32:293–298. doi: 10.1038/jp.2011.97. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed July 1, 2013];Maternal & Child Health Block Grant [Internet] 2015 [Google Scholar]
- 10.Squires J, Bricker D. Ages & Stages Questionnaires®, Third Edition (ASQ-3(TM)): A Parent-Completed Child Monitoring System. 3rd. Baltimore, MD: Brookes Publishing; 2009. [Google Scholar]
- 11.Marlow N. Is survival and neurodevelopmental impairment at 2 years of age the gold standard outcome for neonatal studies? Arch Dis Child Fetal Neonatal Ed. 2015;100:F82–F84. doi: 10.1136/archdischild-2014-306191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson S, Wolke D, Marlow N. Preterm Infant Parenting Study Group. Developmental assessment of preterm infants at 2 years: validity of parent reports. Dev Med Child Neurol. 2008;50:58–62. doi: 10.1111/j.1469-8749.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 13.Bayley N. Bayley scales of infant and toddler development. 3rd. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 14.Achenbach TM, Rescorla LA. ASEBA Preschool Forms & Profiles. Aseba. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2000. [Google Scholar]
- 15.Alsaied T, Marino BS, Esbensen AJ, Anixt JS, Epstein JN, Cnota JF. Does Congenital Heart Disease Affect Neurodevelopmental Outcomes in Children with Down Syndrome? Congenit Heart Dis. 2016;11:26–33. doi: 10.1111/chd.12322. [DOI] [PubMed] [Google Scholar]
- 16.Kuban KC, Allred EN, O’Shea M, Paneth N, Pagano M, Leviton A. ELGAN Study Cerebral Palsy-Algorithm Group. An algorithm for identifying and classifying cerebral palsy in young children. J Pediatr. 2008;153:466–472. doi: 10.1016/j.jpeds.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris C. Definition and classification of cerebral palsy: a historical perspective. Dev Med Child Neurol Suppl. 2007;109:3–7. doi: 10.1111/j.1469-8749.2007.tb12609.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs JP, O’Brien SM, Pasquali SK, Jacobs ML, Lacour-Gayet FG, Tchervenkov CI, Austin EH, 3rd, Pizarro C, Pourmoghadam KK, Scholl FG, Welke KF, Gaynor JW, Clarke DR, Mayer JE, Jr, Mavroudis C. Variation in outcomes for risk-stratified pediatric cardiac surgical operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94:564–571. doi: 10.1016/j.athoracsur.2012.01.105. discussion 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kansy A, Maruszewski B, Jacobs J, Maruszewski P. Application of four complexity stratification tools (Aristotle Basic Score, RACHS-1, STAT, Morality Score, and STAT Mortality Categories) to evaluate early congenital heart surgery outcomes over 16 years at a single institution. Kardiochir Torakochir Pol. 2013;1:115–119. [Google Scholar]
- 20.Bode MM, D’Eugenio DB, Mettelman BB, Gross SJ. Predictive validity of the Bayley, Third Edition at 2 years for intelligence quotient at 4 years in preterm infants. J Dev Behav Pediatr. 2014;35:570–575. doi: 10.1097/DBP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 21.Chock V, Lee HC. Neurodevelopmental Outcomes for Infants Born With Congenital Heart Disease. NeoReviews. 2014;15:e344–e353. [Google Scholar]
- 22.Rollins CK, Newburger JW. Cardiology patient page. Neurodevelopmental outcomes in congenital heart disease. Circulation. 2014;130:e124–e126. doi: 10.1161/CIRCULATIONAHA.114.008556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mussatto KA, Hoffmann RG, Hoffman GM, Tweddell JS, Bear L, Cao Y, Brosig C. Risk and prevalence of developmental delay in young children with congenital heart disease. Pediatrics. 2014;133:e570–e577. doi: 10.1542/peds.2013-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40:739–751. doi: 10.1016/j.clp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, Goldberg CS, Hövels-Gürich H, Ichida F, Jacobs JP, Justo R, Latal B, Li JS, Mahle WT, McQuillen PS, Menon SC, Pemberton VL, Pike NA, Pizarro C, Shekerdemian LS, Synnes A, Williams I, Bellinger DC, Newburger JW International Cardiac Collaborative on Neurodevelopment (ICCON) Investigators. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135:816–825. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tweddell JS, Hoffman GM, Mussatto KA, Fedderly RT, Berger S, Jaquiss RD, Ghanayem NS, Frisbee SJ, Litwin SB. Improved survival of patients undergoing palliation of hypoplastic left heart syndrome: lessons learned from 115 consecutive patients. Circulation. 2002;106(12 suppl 1):I82–I89. [PubMed] [Google Scholar]
- 27.Soto CB, Olude O, Hoffmann RG, Bear L, Chin A, Dasgupta M, Mussatto K. Implementation of a routine developmental follow-up program for children with congenital heart disease: early results. Congenit Heart Dis. 2011;6:451–460. doi: 10.1111/j.1747-0803.2011.00546.x. [DOI] [PubMed] [Google Scholar]