Abstract
OBJECTIVE
This study examined the association between increased early oxidative stress, measured by F2-isoprostanes (IsoPs), and respiratory morbidity at term equivalent age and neurological impairment at 12 months of corrected age (CA).
STUDY DESIGN
Plasma samples were collected from 136 premature infants on days 14 and 28 after birth. All participants were infants born at ≤28 weeks of gestational age enrolled into the Prematurity and Respiratory Outcomes Program (PROP) study. Respiratory morbidity was determined at 40 weeks of postmenstrual age (PMA) by the Respiratory Severity Index (RSI), a composite measure of oxygen and pressure support. Neurodevelopmental assessment was performed using the Developmental Assessment of Young Children (DAYC) at 12 months of CA. Multivariable logistic regression models estimated associations between IsoP change, RSI and DAYC scores. Mediation analysis was performed to determine the relationship between IsoPs and later outcomes.
RESULTS
Developmental data were available for 121 patients (90% of enrolled) at 12 months. For each 50-unit increase in IsoPs, regression modeling predicted decreases in cognitive, communication and motor scores of − 1.9, − 1.2 and − 2.4 points, respectively (P<0.001). IsoP increase was also associated with increased RSI at 40 weeks of PMA (odds ratio = 1.23; P = 0.01). RSI mediated 25% of the IsoP effect on DAYC motor scores (P = 0.02) and had no significant impact on cognitive or communication scores.
CONCLUSIONS
In the first month after birth, increases in plasma IsoPs identify preterm infants at risk for respiratory morbidity at term equivalent age and worse developmental outcomes at 12 months of CA. Poor neurodevelopment is largely independent of respiratory morbidity.
INTRODUCTION
Respiratory morbidity affects a substantial proportion of infants born at extremely low birth weight, irrespective of whether or not they develop bronchopulmonary dysplasia (BPD). Many infants with BPD also subsequently demonstrate significant neurodevelopmental impairments.1–4 Although some reports imply that the burden of respiratory disease lies in the causative pathway to poor neurodevelopment,1–3,5–7 a causal relationship remains unproven, and poor neurodevelopmental outcomes can develop in the absence of BPD.8
An alternative explanation is that early insults simultaneously affect the development of the brain and lungs, leading to poor long-term outcomes for both systems. For example, oxidative stress (OS) has been linked to both pulmonary and neural injury in preterm infants and may be one of several common antecedents to poor long-term outcome.9–13 OS results from the unopposed breakdown of cell membranes and cellular components by free radicals generated during normal and pathological processes.14 F2-isoprostanes (IsoPs) are prostaglandin-like compounds formed from the free radical oxidation of arachidonic acid.15–17 IsoPs can be reliably quantified in plasma using mass spectrometry and are considered to be a reliable biomarker of endogenous OS-related lipid peroxidation.18 Although absolute levels of IsoPs in preterm infants have not been consistently established, increases in IsoPs can reflect increased OS at birth and in response to oxygen during post-resuscitation care.19,20
Using plasma IsoP measurements in the first month after birth as a biomarker of OS, we performed a prospective observational study to test the hypothesis that increased early OS in very preterm infants is associated with both worse respiratory outcomes at term postmenstrual age (PMA) and worse neurodevelopmental outcomes at 1 year of age. We tested the hypothesis that respiratory morbidity at term PMA mediates only a small proportion of the effects of OS on neurodevelopmental outcomes.
METHODS
Population
Subjects were enrolled in the Vanderbilt University Medical Center cohort of the multicenter Prematurity and Respiratory Outcomes Program (PROP: NIH 1U01HL101456), a prospective observational study of preterm infants from September 2011 through December 2013. Eligible infants were ≤28 weeks of gestation at birth, and delivered at or transferred to Vanderbilt University Medical Center before 8 days of age. Infants with congenital heart disease, other than a patent ductus arteriosus or a hemodynamically insignificant ventricular or atrial septal defect, or with structural anomalies of the upper airway, lungs or chest wall were not eligible. Infants with congenital malformations or syndromes that could affect life expectancy or cardiopulmonary development were also excluded. Written informed consent was obtained from a parent of all participants. The Vanderbilt University Medical Center institutional review board approved the study.
Study design
Clinical data were collected daily by trained research specialists during the initial newborn intensive care unit hospitalization until 40 weeks of PMA or discharge. If an infant was transferred to another hospital or discharged before 40 weeks of PMA, a health-care provider or parent was contacted for necessary clinical information.
Analysis of F2-IsoPs
Blood samples were collected on days 14 ± 2 and 28 ± 2 of age into tubes containing EDTA (lavender caps). Samples were kept cool and spun to collect plasma within 30 min of collection and transferred immediately to a cryovial for storage at − 80 °C until analysis. IsoPs can form ex vivo if the sample is stored at warmer temperatures or thawed before IsoP analysis, but this oxidation process does not occur at temperatures < − 70 °C.21,22 We measured plasma IsoPs using gas chromatography-mass spectrometry on thawed samples according to previously described methodology.22–24 The lower limit of detection of IsoPs is 4 pg using an internal standard with a blank of 3 parts per 1000. The precision of this assay in biological fluids is ± 6% and the accuracy 94%.
Use of respiratory support and supplemental oxygen was recorded at 36 weeks and 40 weeks of PMA. A room air challenge was performed for clinically stable infants who remained on respiratory support at 36 weeks of PMA (definition of BPD).6 To examine respiratory morbidity at term equivalent age, we used the Respiratory Severity Index (RSI), a modification of the Respiratory Severity Score25,26 calculated at 40 weeks of PMA. The Respiratory Severity Score is the product of the fractional inspired oxygen received (FiO2) and mean airway pressure. To account for infants on noninvasive respiratory support, we modified this measurement to reflect the amount of flow delivered by a high flow nasal cannula. In this study, RSI = FiO2 × flow. Severe abnormalities on neuroimaging were defined as periventricular or cortical echogenicity or echolucencies persisting on cranial ultrasounds or magnetic resonance imagings beyond the first 30 days after birth.27 These are equivalent to the spectrum of severe encephalopathy of prematurity, including but not limited to Grade III and IV intraventricular hemorrhage, periventricular leukomalacia and severe hydrocephalus.28
Outcomes
Patients discharged on supplemental oxygen or other respiratory home equipment were followed in the Bronchopulmonary Dysplasia Clinic by a trained research specialist and pediatric pulmonologist. The participants were also evaluated at 12 months of corrected age (CA; ± 1 month) in the newborn intensive care unit follow-up clinic. At that visit, trained examiners performed the Developmental Assessment of Young Children (DAYC).29 Although a second edition of the DAYC became available during the study, for consistency, we report composite standardized scores using the first edition.
Statistical analysis
The primary outcome of our study was DAYC score at 12 months of CA. We estimated that 90% of surviving infants from the first 150 infants enrolled in PROP at Vanderbilt would be evaluated at 12 months, based on historical compliance with follow-up visits. We also accounted for 10 deaths before discharge given the low gestational age of the infants, resulting in 126 infants available for analysis. With a s.d. of 15 points on the DAYC scores, we calculated 90% power to detect a 4.3-point change in DAYC scores per 1 s.d. change in IsoP slope at P<0.05.
Multivariable regression models were used to estimate the association between the change in plasma IsoP concentration between day 14 and day 28 (ΔIsoP), log (RSI) and DAYC scores, adjusted for gestational age, maternal education and presence of severe abnormalities on neuroimaging. Next, we performed a mediation analysis. A mediation model attempts to identify the mechanism that underlies the association between a predictor variable and the outcome. It estimates the proportion of the association that goes through a third variable, the mediator, and the proportion of the association that goes directly between the predictor and the outcome controlling for the mediator. We used a linear regression framework to determine what proportion of the association between ΔIsoP (per 50-unit increase) and DAYC score is mediated by RSI at term equivalent (average causal mediation effects). The effect of ΔIsoP directly on the DAYC score (average direct effects), without any contribution from respiratory status, was determined as well as the combined effect (total) of ΔIsoP and RSI on the DAYC score. For the analyses, variables examined were DAYC scores and RSI as continuous outcomes, ΔIsoP as a continuous variable predictor and RSI as a continuous mediator. Gestational age, maternal education and presence of severe abnormalities on neuroimaging (as defined above) were covariates. Analyses were conducted using version 3.1.3 of the R statistical package (Frederiksberg, Denmark).
RESULTS
Of the 150 PROP infants eligible for inclusion in the study, 143 were enrolled, 8 died before 36 weeks of PMA and neurodevelopmental follow-up data were available for 121 (90%) infants at 12 months of CA. Clinical characteristics, maternal education and outcomes of infants are reported in Table 1. More than half of infants had BPD using the physiologic definition. Median DAYC for cognitive, communication and motor scores at 12 months of CA were generally average but encompassed a wide range.
Table 1.
Patient characteristics
| N | ||
|---|---|---|
| Gestation age, median (IQR) | 135 | 26 (25–28) |
| Birth weight, median (IQR) | 135 | 880 (760–1040) |
| Mother received ANS | 135 | 121 (89.6) |
| Infant had supplemental oxygen in DR | 135 | 96 (71.1) |
| Infant received surfactant in the DR | 135 | 111 (82.2) |
| Maternal education >HS,a N (%) | 128 | 47 (36.7) |
| Severe findings on neuroimaging,b N (%) | 135 | 6 (4.4) |
| Failed RAC at 36 weeks,c n (%) | 133 | 73 (55) |
| 40-Week respiratory support | ||
| On any support, n (%) | 135 | 56 (41) |
| FiO2 in %, mean (range) | 135 | 42.7 (21–100) |
| Flow in L, mean (range) | 135 | 0.53 (0–9) |
| RSI, mean (range) | 135 | 20.4 (0–312) |
| DAYC scores at 12 months, median (IQR), range | ||
| Cognitive | 121 | 107 (98.5–112.5), 68–132 |
| Communication | 121 | 104 (98–110), 73–127 |
| Motor | 121 | 100 (94–107), 58–117 |
Abbreviations: ANS, antenatal steroid; DAYC, Developmental Assessment of Young Children; DR, delivery room; HS, high school; IQR, interquartile range (25th–75th); RAC, room air challenge; RSI, Respiratory Severity Index (fractional inspired oxygen received (FiO2) × mean airway pressure (MAP) or flow).
Educational status was divided into high school education or less vs partial college or more.
Persistent periventricular leukomalacia, severe intraventricular hemorrhage (Grade III or IV) or cerebellar hemorrhage.
Two infants transferred before obtaining data. This is the most recent definition of bronchopulmonary dysplasia (BPD).
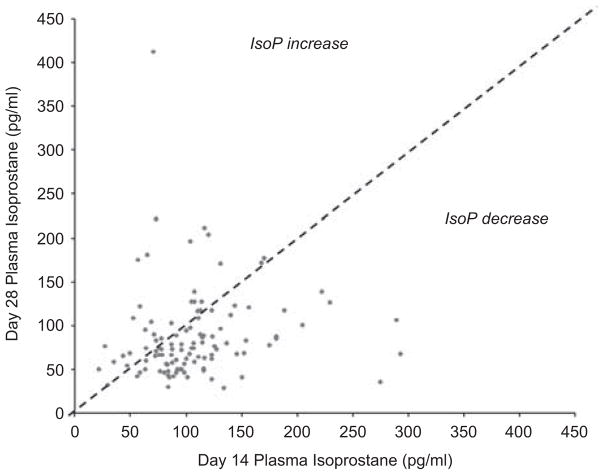
Median plasma IsoPs at 14 and 28 days were 100 pg ml−1 (interquartile range 76 to 123) and 74 pg ml−1 (interquartile range 57.5 to 107.5) respectively. Median ΔIsoP was − 24.5 pg ml−1 with a range of − 239 to 756 pg ml−1. The negative median ΔIsoP indicates an overall decrease in IsoP plasma levels between the time points. The range demonstrated a high degree of variability and IsoPs increased in some infants between the two time points (Figure 1).
Figure 1.
Isoprostane (IsoP) levels at 14 and 28 days. Each patient is represented by a single point. Patients on the diagonal had no change in IsoP levels between days 14 and 28, whereas those below the diagonal demonstrated a decrease in IsoP levels and those above an increase in IsoP levels.
RSI scores of 0 corresponded to infants on room air without support, whereas scores of >0 and ≤10 grossly corresponded to infants on varying levels of low flow nasal cannula with FiO2 = 1.0; scores of >10 corresponded to increasing amounts of flow, airway pressure and oxygen concentration, whereas infants on ventilators typically had an RSI of >100. Increased ΔIsoP was associated with increased RSI at 40 weeks of PMA (odds ratio 1.23 (1.04 to 1.44); P = 0.01).
After adjustments for gestational age, maternal education and severe abnormalities on neuroimaging, linear regression models predicted decreases in cognitive, communication and motor scores for each 50-unit increase in IsoPs between day 14 to day 28 (Table 2). ΔIsoP accounted for greater variation in DAYC scores (R2 = 0.1, 0.05 and 0.14 for cognitive, communication and motor scores, respectively) than maternal education or gestational age alone. Mediation analysis demonstrated the proportion of ΔIsoP effect on DAYC scores that was explained by RSI. RSI mediated 25% of IsoP effect on DAYC motor scores (P = 0.02) but did not significantly mediate cognitive or communication scores (Table 3).
Table 2.
Increase in IsoPs between 14 and 28 days is associated with worse neurodevelopmental scores at 12 months of corrected age
| Outcome | Model | OR | 95% Cl | P-value |
|---|---|---|---|---|
| (a) ORs for higher scores per 50-unit increase in IsoP | ||||
| Cognition | Unadjusted | 0.74 | (0.62 to 0.88) | <0.001 |
| Communication | Unadjusted | 0.81 | (0.69 to 0.95) | 0.010 |
| Motor | Unadjusted | 0.75 | (0.64 to 0.88) | <0.001 |
| Cognition | Adjusted | 0.72 | (0.59 to 0.88) | 0.001 |
| Communication | Adjusted | 0.81 | (0.68 to 0.97) | 0.026 |
| Motor | Adjusted | 0.69 | (0.57 to 0.84) | <0.001 |
| Outcome | Model | Score change in points | Cl | P-value |
|
| ||||
| (b) Predicted change in DAYC scores per 50-unit increase in IsoP | ||||
| Cognition | Unadjusted | − 1.81 | (−2.77 to − 0.85) | <0.001 |
| Communication | Unadjusted | − 1.05 | (−1.93 to − 0.16) | 0.010 |
| Motor | Unadjusted | − 2.18 | (−3.20 to − 1.17) | <0.001 |
| Cognition | Adjusted | − 1.87 | (−2.95 to − 0.79) | 0.001 |
| Communication | Adjusted | − 1.05 | (−1.05 to − 0.07) | 0.026 |
| Motor | Adjusted | − 2.30 | (−3.43 to − 1.17) | <0.001 |
Abbreviations: CI, confidence interval; DAYC, Developmental Assessment of Young Children; IsoP, F2-isoprostane; OR, odds ratio.
Table 3.
Mediation analysis
| Outcome (DAYC) | Direct IsoP effect (ADE) | 95% Cl | RSI-mediated effect (ACME) | 95% Cl | Proportion of IsoP effect mediated by RSI |
|---|---|---|---|---|---|
| Cognitive | − 1.5 | (−3.1 to − 0.5)** | − 0.3 | (−0.7 to 0.04) | 0.16 |
| Communication | − 0.06 | (−1.6 to 0.5) | − 0.4 | (−0.9 to − 0.05)* | 0.4 |
| Motor | − 1.7 | (−3.1 to − 0.4)* | − 0.58 | (−1.2 to − 0.09)* | 0.25* |
Abbreviations: ACME average causal mediation effect; ADE, average direct effect; CI, confidence interval; DAYC, Developmental Assessment of Young Children; IQR, interquartile range (25th–75th); IsoP effect, effect of the change in F2-isoprostane levels between 14 and 28 days; RSI, Respiratory Severity Index (fractional inspired oxygen received (FiO2) × mean airway pressure (MAP) or flow).
P ≤0.05;
P ≤0.01.
DISCUSSION
We have shown that increased OS, as evidenced by an increase in plasma IsoPs in the first month after birth, is associated with increased receipt of respiratory support at term equivalent age and worse neurodevelopmental outcomes at 1 year in extremely preterm infants. In addition, we found that the relationship between increased IsoPs and worse neurodevelopmental scores is largely independent of respiratory morbidity at term equivalent, when controlling for known factors such as gestational age, severe injury on neuroimaging and maternal education. Our novel findings support the possibility of OS as one of the several common antecedents to respiratory and neural injury, rather than a direct causal pathway from respiratory disease to neurodevelopmental delays or impairments.
IsoPs are a validated and reliable biomarker of lipid peroxidation, and have been studied in a variety of human conditions including respiratory and neurologic disease.30 Because OS reflects a balance between free radical generation and antioxidant mechanisms, an absolute ‘reference’ value for IsoP would be difficult to establish in preterm infants as their organs and cells are developing in the context of both increased free radical generation and decreased antioxidant capacity.10,18 Others have shown that cord blood plasma IsoPs reflect birth events and that changes in IsoPs in the first week after birth reflect resuscitation management.31,32 After 2 weeks, plasma IsoPs reflect the balance between oxidative influences such as hyperoxia or inflammation and antioxidant capacity, derived either endogenously or nutritionally.32,33 In order to evaluate OS reflective of experiences in the first month of neonatal intensive care we measured the change in plasma IsoPs from day 14 to day 28.
We found a corresponding decrease in DAYC scores at 12 months of CA for each 50-unit increase in IsoPs between days 14 and 28 of age, after adjusting for known confounders. The premature brain is particularly vulnerable to injury from OS, with oligodendrocyte progenitor cells and pre-oligodendrocytes being the most susceptible to oxidative damage.28 Loss of pre-oligodendrocytes characterizes white matter injury of prematurity that may lead to delays or defects in neurodevelopment.34 It is also possible that the increase in IsoPs reflects microstructural white matter injury in the very premature brain and therefore identifies infants at risk for worse neurodevelopment. The fact that all three domains of development were associated with increased ΔIsoP appears to support this.
Increased ΔIsoP was also associated with increased respiratory support at 40 weeks of PMA, as measured by the RSI. However, as we hypothesized, overt respiratory morbidity at term equivalent age only mediated 25% of the effects of OS on DAYC motor scores and did not mediate the effects of IsoP on cognitive and communication scores. The small effect on motor scores could be explained by the fact that motor developmental scores are less influenced by maternal education, which we adjusted for in this model, compared with cognitive and language development.35,36 In addition, infants with more severe respiratory disease require prolonged use of respiratory equipment, resulting in longer periods of restricted movement that may limit early motor development.37,38
Reports documenting outcomes of children who developed BPD in the newborn intensive care unit frequently imply that respiratory disease of prematurity leads to poor neurodevelopmental outcomes.1–3,6,7,39 Although BPD may play an important role in the long-term outcomes of preterm infants, our findings show that increased IsoPs between days 14 and 28 are associated with both worse respiratory disease at term equivalent age and with worse neurodevelopmental scores in the first year. OS, therefore, may be a common antecedent to both outcomes instead of early respiratory status directly affecting neurodevelopmental outcomes.
Our study has several limitations, including our choice of IsoP as a primary marker of OS, as there are many other products of lipid peroxidation. Measurement of urinary isofurans, another product of non-enzymatic arachidonic acid oxidation, in the first 4 days after birth has shown promise as a potential marker of hyperoxic lung injury.40 Early urine measurements could be combined with our later assessments to improve their predictive value and to further elucidate the mechanisms leading to respiratory and neural injury in preterm infants. Neuroprostanes and neurofurans are analogous products to IsoPs and isofurans and are generated from the non-enzymatic oxidation of docosahexaenoic acid. New methodologies to measure neurofurans and neuroprostanes in urine indirectly suggest that future studies could examine these promising biomarkers.40,41
In addition, we did not study the antioxidant component of the oxidative balance. It is likely that in extremely preterm infants the antioxidant enzymes, superoxide dismutase, glutathione peroxidase and reductase, glutathione-S-transferase and the antioxidant activity of vitamins E and C are all decreased.33,42 In contrast to our study, other studies have used cord blood to measure OS biomarkers in preterm infants at birth.33 Although this would have presented an interesting link between perinatal events and later morbidities, cord blood was not included in the initial data collection of the larger PROP study from which our patients were enrolled. We attempted to increase the precision of our classifications of respiratory morbidity by using a term equivalent age instead of a 36-week time point, but both RSI and current classifications of BPD use respiratory support as a surrogate for respiratory disease.43,44 In fact, very preterm infants who do not meet current definitions of BPD still have increased risks for poor pulmonary function, for susceptibility to viral illnesses and for morbidity throughout childhood.45,46 We anticipate that individual studies currently underway as part of the PROP will result in definitions of respiratory disease of prematurity based on lung function, molecular underpinnings and long-term pulmonary outcomes.47
In conclusion, early measurement of plasma IsoP levels, in combination with clinical factors and neuroimaging findings, may help identify patients at risk for poor long-term outcomes. This timely recognition may guide clinical practice in the future and provide a valuable marker of responses to therapeutic interventions aimed at reducing OS. Future studies will investigate the role of antioxidant mechanisms in the association between early OS and both respiratory disease and neurodevelopment, potentially uncovering novel therapeutic targets.
Acknowledgments
We thank Dr Lynn Taussig and the PROP Scholars program for supporting this work through NHLBI subaward U01 HL101794 to NL Maitre. This study was also supported by NHLBI U01 HL101456 to JL Aschner and NICHD 1K23HD074736-01A1 to NL Maitre. REDCap database was utilized for data collection and analysis through CTSA award UL1 TR000445 from NCATS/NIH. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Each author contributed to the manuscript and approved the final manuscript as submitted.
References
- 1.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:227–232. doi: 10.1053/j.semperi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Natarajan G, Pappas A, Shankaran S, Kendrick DE, Das A, Higgins RD, et al. Outcomes of extremely low birth weight infants with bronchopulmonary dysplasia: impact of the physiologic definition. Early Hum Dev. 2012;88:509–515. doi: 10.1016/j.earlhumdev.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, et al. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics. 2003;112:e359. doi: 10.1542/peds.112.5.e359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, Sauve RS, Whitfield MF, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289:1124–1129. doi: 10.1001/jama.289.9.1124. [DOI] [PubMed] [Google Scholar]
- 5.Doyle LW, Anderson PJ. Long-term outcomes of bronchopulmonary dysplasia. Semin Fetal Neonatal Med. 2009;14:391–395. doi: 10.1016/j.siny.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Kobaly K, Schluchter M, Minich N, Friedman H, Taylor HG, Wilson-Costello D, et al. Outcomes of extremely low birth weight (<1 kg) and extremely low gestational age (<28 weeks) infants with bronchopulmonary dysplasia: effects of practice changes in 2000 to 2003. Pediatrics. 2008;121:73–81. doi: 10.1542/peds.2007-1444. [DOI] [PubMed] [Google Scholar]
- 7.Trittmann JK, Nelin LD, Klebanoff MA. Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. Eur J Pediatr. 2013;172:1173–1180. doi: 10.1007/s00431-013-2016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 9.Back SA, Luo NL, Mallinson RA, O’Malley JP, Wallen LD, Frei B, et al. Selective vulnerability of preterm white matter to oxidative damage defined by F2-isoprostanes. Ann Neurol. 2005;58:108–120. doi: 10.1002/ana.20530. [DOI] [PubMed] [Google Scholar]
- 10.Saugstad OD. Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol. 2003;8:39–49. doi: 10.1016/s1084-2756(02)00194-x. [DOI] [PubMed] [Google Scholar]
- 11.Perrone S, Tataranno LM, Stazzoni G, Ramenghi L, Buonocore G. Brain susceptibility to oxidative stress in the perinatal period. J Matern Fetal Neonatal Med. 2015;28(Suppl 1):2291–2295. doi: 10.3109/14767058.2013.796170. [DOI] [PubMed] [Google Scholar]
- 12.Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C. Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res. 2002;52:213–218. doi: 10.1203/00006450-200208000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Perrone S, Tataranno ML, Buonocore G. Oxidative stress and bronchopulmonary dysplasia. J Clin Neonatol. 2012;1:109–114. doi: 10.4103/2249-4847.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15:191–195. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Montuschi P, Barnes PJ, Roberts LJ. Isoprostanes: markers and mediators of oxidative stress. FASEB J. 2004;18:1791–1800. doi: 10.1096/fj.04-2330rev. [DOI] [PubMed] [Google Scholar]
- 16.Cháfer-Pericás C, Rahkonen L, Sánchez-Illana A, Kuligowski J, Torres-Cuevas I, Cernada M, et al. Ultra high performance liquid chromatography coupled to tandem mass spectrometry determination of lipid peroxidation biomarkers in newborn serum samples. Anal Chim Acta. 2015;886:214–220. doi: 10.1016/j.aca.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Song W-L, Lawson JA, Reilly D, Rokach J, Chang C-T, Giasson B, et al. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J Biol Chem. 2008;283:6–16. doi: 10.1074/jbc.M706124200. [DOI] [PubMed] [Google Scholar]
- 18.Perrone S, Negro S, Tataranno ML, Buonocore G. Oxidative stress and antioxidant strategies in newborns. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):63–65. doi: 10.3109/14767058.2010.509940. [DOI] [PubMed] [Google Scholar]
- 19.Tataranno ML, Oei JL, Perrone S, Wright IM, Smyth JP, Lui K, et al. Resuscitating preterm infants with 100% oxygen is associated with higher oxidative stress than room air. Acta Paediatr. 2015;104:759–765. doi: 10.1111/apa.13039. [DOI] [PubMed] [Google Scholar]
- 20.Buonocore G, Perrone S, Longini M, Vezzosi P, Marzocchi B, Paffetti P, et al. Oxidative stress in preterm neonates at birth and on the seventh day of life. Pediatr Res. 2002;52:46–49. doi: 10.1203/00006450-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Morrow JD, Roberts LJ. Mass spectrometric quantification of F 2-isoprostanes in biological fluids and tissues as measure of oxidant stress. Meth Enzymol. 1999;300:3–12. doi: 10.1016/s0076-6879(99)00106-8. [DOI] [PubMed] [Google Scholar]
- 22.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2:221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 23.Milne GL, Yin H, Hardy KD, Davies SS, Roberts LJ. Isoprostane generation and function. Chem Rev. 2011;111:5973–5996. doi: 10.1021/cr200160h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milne GL, Gao B, Terry ES, Zackert WE, Sanchez SC. Measurement of F2- isoprostanes and isofurans using gas chromatography-mass spectrometry. Free Radic Biol Med. 2013;59:36–44. doi: 10.1016/j.freeradbiomed.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merrill JD, Ballard RA, Cnaan A, Hibbs AM, Godinez RI, Godinez MH, et al. Dysfunction of pulmonary surfactant in chronically ventilated premature infants. Pediatr Res. 2004;56:918–926. doi: 10.1203/01.PDR.0000145565.45490.D9. [DOI] [PubMed] [Google Scholar]
- 26.Iyer NP, Mhanna MJ. Non-invasively derived respiratory severity score and oxygenation index in ventilated newborn infants. Pediatr Pulmonol. 2013;48:364–369. doi: 10.1002/ppul.22607. [DOI] [PubMed] [Google Scholar]
- 27.O’Shea TM, Kuban KCK, Allred EN, Paneth N, Pagano M, Dammann O, et al. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–e669. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpe JJ. The encephalopathy of prematurity--brain injury and impaired brain development inextricably intertwined. Semin Pediatr Neurol. 2009;16:167–178. doi: 10.1016/j.spen.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev. 2013;89:781–786. doi: 10.1016/j.earlhumdev.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne GL, Musiek ES, Morrow JD. F2-isoprostanes as markers of oxidative stress in vivo: an overview. Biomarkers. 2005;10(Suppl 1):S10–S23. doi: 10.1080/13547500500216546. [DOI] [PubMed] [Google Scholar]
- 31.Buonocore G, Perrone S, Longini M, Terzuoli L, Bracci R. Total hydroperoxide and advanced oxidation protein products in preterm hypoxic babies. Pediatr Res. 2000;47:221–224. doi: 10.1203/00006450-200002000-00012. [DOI] [PubMed] [Google Scholar]
- 32.Vento M, Moro M, Escrig R, Arruza L, Villar G, Izquierdo I, et al. Preterm resuscitation with low oxygen causes less oxidative stress, inflammation, and chronic lung disease. Pediatrics. 2009;124:e439–e449. doi: 10.1542/peds.2009-0434. [DOI] [PubMed] [Google Scholar]
- 33.Perrone S, Tataranno ML, Negro S, Cornacchione S, Longini M, Proietti F, et al. May oxidative stress biomarkers in cord blood predict the occurrence of necrotizing enterocolitis in preterm infants? J Matern Fetal Neonatal Med. 2012;25(Suppl 1):128–131. doi: 10.3109/14767058.2012.663197. [DOI] [PubMed] [Google Scholar]
- 34.Khwaja O, Volpe JJ. Pathogenesis of cerebral white matter injury of prematurity. Arch Dis Child Fetal Neonatal Ed. 2008;93:F153–F161. doi: 10.1136/adc.2006.108837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bacharach VR, Baumeister AA. Effects of maternal intelligence, marital status, income, and home environment on cognitive development of low birthweight infants. J Pediatr Psychol. 1998;23:197–205. doi: 10.1093/jpepsy/23.3.197. [DOI] [PubMed] [Google Scholar]
- 36.Tong S, Baghurst P, Vimpani G, McMichael A. Socioeconomic position, maternal IQ, home environment, and cognitive development. J Pediatr. 2007;151:284–288. e1. doi: 10.1016/j.jpeds.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Vignochi CM, Miura E, Canani LH. Effects of motor physical therapy on bone mineralization in premature infants: a randomized controlled study. J Perinatol. 2008;28:624–631. doi: 10.1038/jp.2008.60. [DOI] [PubMed] [Google Scholar]
- 38.Miller ME. The bone disease of preterm birth: a biomechanical perspective. Pediatr Res. 2003;53:10–15. doi: 10.1203/00006450-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Newman JB, Debastos AG, Batton D, Raz S. Neonatal respiratory dysfunction and neuropsychological performance at the preschool age: a study of very preterm infants with bronchopulmonary dysplasia. Neuropsychology. 2011;25:666–678. doi: 10.1037/a0023895. [DOI] [PubMed] [Google Scholar]
- 40.Kuligowski J, Aguar M, Rook D, Lliso I, Torres-Cuevas I, Escobar J, et al. Urinary lipid peroxidation byproducts: are they relevant for predicting neonatal morbidity in preterm infants? Antioxid Redox Signal. 2015;23:178–184. doi: 10.1089/ars.2015.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solberg R, Longini M, Proietti F, Vezzosi P, Saugstad OD, Buonocore G. Resuscitation with supplementary oxygen induces oxidative injury in the cerebral cortex. Free Radic Biol Med. 2012;53:1061–1067. doi: 10.1016/j.freeradbiomed.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Buonocore G, Groenendaal F. Anti-oxidant strategies. Semin Fetal Neonatal Med. 2007;12:287–295. doi: 10.1016/j.siny.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 43.Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12:1822–1830. doi: 10.1513/AnnalsATS.201504-218OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poindexter BB, Jobe AH. The diagnostic conundrum of bronchopulmonary dysplasia. J Pediatr. 2015;167:517–518. doi: 10.1016/j.jpeds.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 45.Pryhuber GS. Postnatal infections and immunology affecting chronic lung disease of prematurity. Clin Perinatol. 2015;42:697–718. doi: 10.1016/j.clp.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maitre NL, Ballard RA, Ellenberg JH, Davis SD, Greenberg JM, Hamvas A, et al. Respiratory consequences of prematurity: evolution of a diagnosis and development of a comprehensive approach. J Perinatol. 2015;35:313–321. doi: 10.1038/jp.2015.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pryhuber GS, Maitre NL, Ballard RA, Cifelli D, Davis SD, Ellenberg JH, et al. Prematurity and respiratory outcomes program (PROP): study protocol of a prospective multicenter study of respiratory outcomes of preterm infants in the United States. BMC Pediatr. 2015;15:37. doi: 10.1186/s12887-015-0346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]