Abstract
Background&Aims
c-Jun N-terminal kinase (JNK)1 and JNK2 are expressed in hepatocytes and have overlapping and distinct functions. JNK proteins are activated, via phosphorylation, in response to acetaminophen- or CCl4-induced liver damage; the level of activation correlates with the degree of injury. SP600125, a JNK inhibitor, has been reported to block acetaminophen-induced liver injury. We investigated the role of JNK in drug-induced liver injury (DILI) in liver tissues from patients and in mice with genetic deletion of JNK in hepatocytes.
Methods
We studied liver sections from patients with DILI (due to acetaminophen, phenprocoumon, non-steroidal anti-inflammatory drugs or autoimmune hepatitis), or patients without acute liver failure (controls), collected from a DILI Biobank in Germany. Levels of total and activated (phosphorylated) JNK were measured by immunohistochemistry and western blotting. Mice with hepatocyte-specific deletion of Jnk1 (Jnk1Δhepa) or combination of Jnk1 and Jnk2 (JnkΔhepa), as well as Jnk1- floxed C57BL/6 (control) mice, were given injections of CCl4 (to induce fibrosis) or acetaminophen (to induce toxic liver injury). We performed gene expression microarray, and phosphoproteomic analyses to determine mechanisms of JNK activity in hepatocytes.
Results
Liver samples from DILI patients contained more activated JNK, predominantly in nuclei of hepatocytes and in immune cells, than healthy tissue. Administration of acetaminophen to JnkΔhepa mice produced a greater level of liver injury than that observed in Jnk1Δhepa or control mice, based on levels of serum markers and microscopic and histologic analysis of liver tissues. Administration of CCl4 also induced stronger hepatic injury in JnkΔhepa mice, based on increased inflammation, cell proliferation, and fibrosis progression, compared to Jnk1Δhepa or control mice. Hepatocytes from JnkΔhepa mice given acetaminophen had an increased oxidative stress response, leading to decreased activation of AMPK, total protein AMPK levels, and pJunD and subsequent necrosis. Administration of SP600125 before or with acetaminophen protected JnkΔhepa and control mice from liver injury.
Conclusions
In hepatocytes, JNK1 and JNK2 appear to have combined effects in protecting mice from CCl4- and acetaminophen-induced liver injury. It is important to study the tissue-specific functions of both proteins, rather than just JNK1, in the onset of toxic liver injury. JNK inhibition with SP600125 shows off-target effects.
Keywords: APAP, mouse model, gene regulation, pharmacological treatment
Introduction
Acute and chronic liver injury is a growing worldwide problem despite the recent advances for the treatment of HBV and HCV infection. Especially, the frequency of toxic insults such as alcohol, drugs or obesity is even increasing in the Western World. In the liver, toxic injury triggers death signalling pathways, which may cause apoptosis, necrosis or pyroptosis of hepatocytes1, 2. However, the exact pathomolecular mechanisms determining the mode of cell death are not completely understood.
Liver injury of different aetiology activates JNK - members of the MAPK family. Whereas Jnk3 is exclusively expressed in the central nervous system, testis and heart, Jnk1 and Jnk2 are expressed in hepatocytes eliciting redundant but also distinct functions3–5. In order to characterise the compound functions of the JNK genes e.g. in hepatocytes, cell type-specific deletion of both Jnk1 and Jnk2 is essential. At present, most studies have been performed only using single knockout mice or JNK-specific inhibitors.
Toxic liver injury – acute or chronic – activates the oxidative stress response. Typical examples are acute liver damage after APAP intoxication or chronic liver injury by repetitive CCl4-injection. APAP-induced injury is related to the formation of highly reactive metabolites through CYP2E1. These toxic compounds are normally conjugated and inactivated by glutathione (GSH). In overdose conditions, the conjugation of the reactive metabolites leads to GSH depletion and thus enhances the generation of oxidative (ROS) and nitrosative species (RNS) triggering hepatocyte injury6. N-acetylcysteine (NAC) has been the standard antidote for APAP-induced liver intoxication7. NAC exerts its therapeutic effects by restoring depleted hepatic glutathione levels, and thus preventing the accumulation of oxidant species7.
Earlier results demonstrated that JNK is strongly activated by APAP correlating with the degree of liver injury8. Additionally, in vivo experiments evidenced that JNK inhibition blocked APAP-induced liver injury9. Thus JNK seems to play an essential role in APAP-induced hepatic damage, supporting the possibility of using JNK inhibitors as a therapeutic approach.
Kluwe et al10 first suggested that JNK is crucial for chronic CCl4-intoxication, associated with hepatocyte damage, necrosis, inflammation, and end-stage liver fibrosis. JNK activation is not only restricted to hepatocytes. Indeed, we found that Jnk1 is particularly important for trans-differentiation of hepatic stellate cells (HSCs) since Jnk1 deletion in HSCs reduces fibrogenesis after chronic CCl4-intoxication11.
In the present work we aimed to address specifically the yet unexplored dual role of Jnk1 and Jnk2 in hepatocytes in models of acute and chronic toxic liver injury in mice, and in patients with DILI. Based on the previous studies, we hypothesized that JNK1 and JNK2 in hepatocytes have redundant functions. For this purpose, we generated JnkΔhepa mice and studied the functional role of the JNK genes in APAP- and CCl4-induced toxic liver injury in vivo and in culture using primary hepatocytes.
Material and Methods
Generation of mice, animal experiments and human samples
Alb-Cre and Jnk2-deficient mice in a C57BL/6 background were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice with a floxed allele of Jnk1 were constructed by using homologous recombination in ES cells and backcrossed to the C57BL/6J strain as previously described12, 13. These mice were then crossed with Jnk2-deficient mice to create Jnk1LoxP/LoxP /Jnk2−/− mice. Genomic DNA was examined using PCR amplimers (5′-CTCAGGAAGAAAGGGCTTATTTC-3′ and 5′-GAACCACTGTTCCAATTTCCATCC-3′) to distinguish between the control floxed (Jnk1+ ,Jnk1f), and deleted (Jnk1Δ) alleles. Animal experiments were carried out according to the German legal requirements and animal protection law and approved by the authority for environment conservation and consumer protection of the state of North Rhine-Westfalia (LANUV, Germany). Induction of liver fibrosis was performed in 7–8 week-old age-matched male mice (n=9–10 per group) using CCl4-injection every 3 days for 4 weeks (0.6 mL/kg, i.p.). Control animals were injected with corn oil. The D-Galactosamine (GaIN)/Lipopolysaccharide (LPS) administration was performed in 7–8 weeks-old male control and JnkΔhepa male animals to induce acute hepatitis. 30 minutes prior to the LPS-injection (20 μg/kg, i.p.), GaIN (800 μg/kg, i.p.) was injected. Mice were sacrificed 8 h after LPS injection. We also performed the APAP-induced liver injury model. After fasting overnight, mice were injected with APAP (500 mg/kg), and sacrificed 8h after treatment. SP600125 was injected 2h (pre-treatment) or at the same time with APAP (co-treatment) at a dose of 30 mg/kg, i.p.).
Liver paraffin sections were obtained from patients with confirmed diagnosis of DILI (paracetamol, phenprocoumon, non-steroidal inflammatory drugs or autoimmune hepatitis) from the Department of Gastroenterology of the University Hospital Essen, Germany. Patients’ clinico-pathologic characteristics were analyzed, summarized and represented in Suppl. Table I.
Statistical analysis
All data are expressed as mean ± standard error of the mean. Statistical significance was determined by two-way analysis of variance (ANOVA) followed by a Student’s t test or by one-way ANOVA followed by a Newman-Keuls multicomparison test. P values less than 0.05 were considered to be significant.
Results
Expression of JNK in human acute liver failure
Drug-induced liver injury (DILI) is the most common cause of ALF14. First, we studied serum parameters of patients with different ALF etiologies. As indicated in Suppl. Table I, livers from patients suffering from paracetamol (1), phenprocoumon (2), non-steroidal anti-inflammatory drugs (NSAID) (3), and autoimmune hepatitis (AIH)-induced ALF (4) were investigated. We observed that the most prominent increase in transaminases was evident in patients with APAP- and AIH-induced ALF, while NSAID- and phenprocoumon-induced ALF patients showed less pronounced changes in serum markers. However, all serum samples showed impaired liver function as evidenced by changes in bilirubin and blood coagulation parameters (Suppl. Table I). Noticeably, the patient with APAP intoxication showed dramatically increased GLDH levels and blood coagulation parameters (Suppl. Table I).
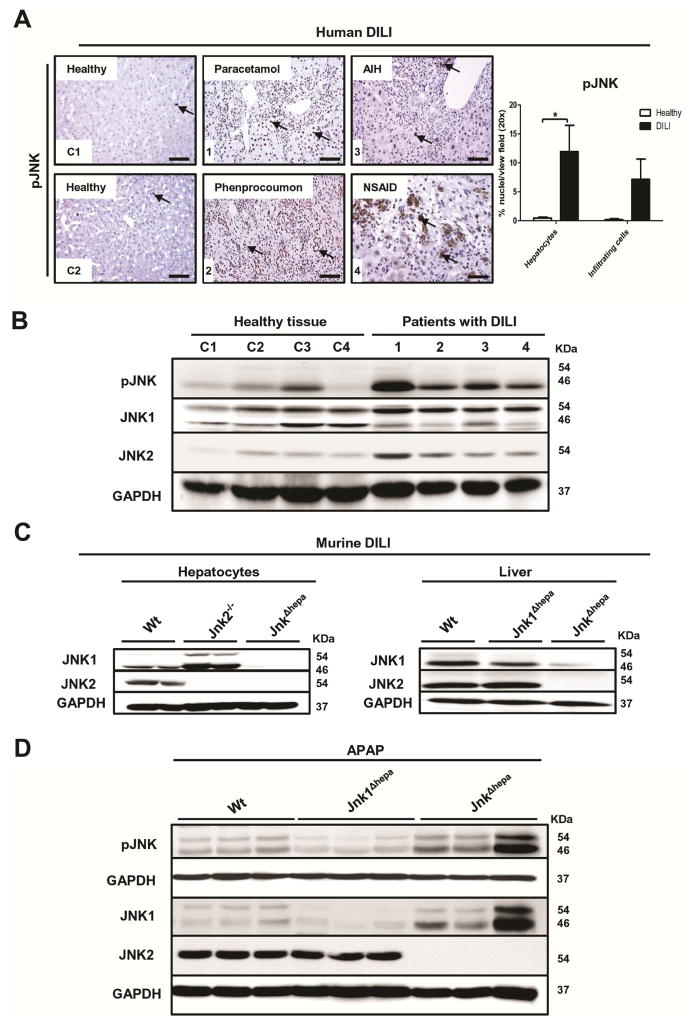
We next investigated the JNK activation pattern in these ALF liver samples (1–4) and normal healthy tissue as control (C1–C4) by performing pJNK staining and quantification (Figure 1A, Suppl. Fig. 1A). Absence or minimal activation of JNK was detectable in healthy tissue. Liver histology of the APAP patient in comparison with the other liver samples showed lower infiltration of immune cells and JNK phosphorylation mainly restricted to hepatocytes (Figure 1A). In contrast, liver samples obtained from other ALF subtypes displayed strong immune cell infiltration associated with pJNK positivity (Figure 1A). In the liver, two JNKs are expressed, JNK1 and JNK2. Thus, we performed Western Blot analysis of liver samples of ALF patients with anti-JNK1 and JNK2 antibodies (Figure 1B). Normal tissue displayed mild JNK phosphorylation, whereas ALF patients displayed higher JNK activation. Interestingly, we detected a differential pattern of JNK1 expression and increased levels of JNK2 in DILI-ALF patients (Figure 1B).
Figure 1. Expression of JNK during human and murine drug-induced liver injury (DILI).
(A) Activation of pJNK in liver samples of ALF patients with different aetiologies: normal liver tissue (C1, C2), paracetamol (1), phenprocoumon (2), autoimmune hepatitis (AIH) (3), and non-steroidal anti-inflammatory drug (NSAID) (4). Scale bars: 50μm. Phospho-JNK positive nuclei were quantified as the average number of hepatocytes or infiltrating cells with dark brown DAB signal per view field of liver sections from healthy and DILI patients. (B) Immunoblotting for pJNK, JNK1 and JNK2 was performed in normal liver tissue (C1–C4) and in liver samples of DILI patients (1–4). (C) Protein expression of JNK1 and JNK2 was assessed in primary hepatocytes (left panel) and livers (right panel) from 8 week-old control (Wt), Jnk2−/−, Jnk1Δhepa and JnkΔhepa mice. Total JNK1 and JNK2 protein levels were determined in whole liver extracts. GAPDH was used as loading control. (D) Immunoblotting for pJNK, JNK1 and JNK2 was performed in liver samples from control (Wt), Jnk1Δhepa and JnkΔhepa mice treated with APAP for 8h (n=6). GAPDH was used as loading control.
Deletion of JNK1 and JNK2 in hepatocytes
We next aimed to characterize the functional relevance of JNK activation during toxic liver injury. We generated mice with specific deletion of Jnk1 in hepatocytes (Jnk1Δhepa) and combined Jnk1 and Jnk2 knockout mice in hepatocytes (JnkΔhepa) (Figure 1C; Suppl. Fig. 1B+C). To exclude the possibility that JnkΔhepa mice exhibited a phenotype under basal conditions we carefully examined 6–8 weeks female and male Jnk1Δhepa and JnkΔhepa mice. Liver histology, serum parameters as well as body weight, and liver versus body weight ratio presented values of normal healthy mice (Suppl. Fig. 2A–F).
Since the human data demonstrated that JNK is strongly activated mainly in hepatocytes during APAP-induced ALF, we sought to translate and compare the human ALF results with APAP-derived liver injury in mice. Eight hours after injecting 500 mg/kg APAP, livers of Wt mice elicited JNK activation. Noticeably, mice with specific deletion of Jnk1 in hepatocytes (Jnk1Δhepa) displayed reduced pJNK, whereas JnkΔhepa mice showed strong JNK1 induction, suggesting that the infiltrating compartment is responsible for the activation of the MAPK in experimental murine APAP-derived injury (Figure 1D). Furthermore, we detected JNK1 and JNK2 protein expression after APAP in Wt, while the levels of JNK1 were strongly induced in JnkΔhepa mice. Expectedly, JNK2 expression was abrogated in mice lacking JNK in hepatocytes.
JnkΔhepa mice are sensitized towards acetaminophen-induced liver injury
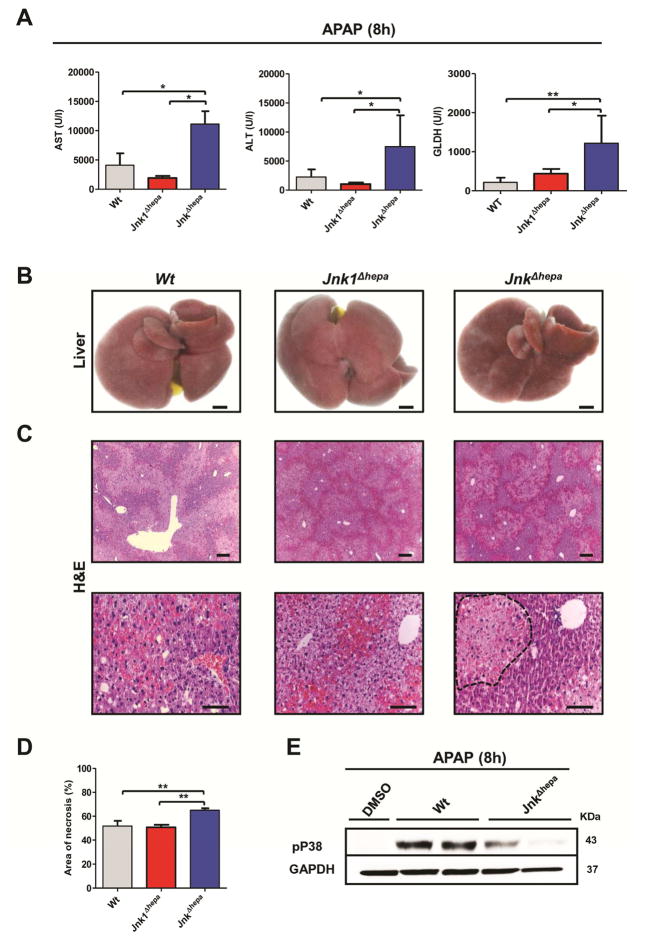
JnkΔhepa showed significantly exacerbated APAP-induced ALF as evidenced by significantly increased AST, ALT and GLDH levels (Figure 2A). No differences were found in liver versus body weight ratio (Suppl. Fig. 3A). Macroscopically, the surface of JnkΔhepa livers showed severe signs of haemorrhagic bleeding, and microscopically the liver was severely injured with presence of large necrotic foci (Figure 2B–D, respectively).
Figure 2. JnkΔhepa mice are sensitized towards acetaminophen-induced liver injury.
(A) Serum ALT (left), AST (center) and GLDH (right) levels were determined 8h after APAP challenge in control (Wt), Jnk1Δhepa and JnkΔhepa livers (n=10). (B) Representative macroscopic view of a liver from each group. Scale bars: 10mm. (C) Representative H&E staining of liver sections collected from mice sacrificed 8h after APAP challenge and examined by an experienced pathologist. Scale bars: 50μm (upper panel) and 100μm (lower panel), respectively. Dotted area represents a necrotic focus. (D) The area of necrosis was quantified in the same mice. (E) Protein levels of pP38 were determined by Western Blot in APAP-treated control (Wt) and JnkΔhepa livers. GAPDH was used as loading control. Data are expressed as mean±SEM (*p<0.05 and **p<0.01).
Acetaminophen is metabolized via CYP2E1 to the electrophilic reactive product N-acetyl-p benzoquinone imine (NAPQI)15. Interestingly, we detected a tendency towards in F2-isoprostanes - a direct marker of oxidative stress16 - and significantly reduced levels of p38 expression in JNK-depleted hepatocytes after APAP treatment (Figure 2E, Suppl. Fig. 3B+C).
These striking results in APAP-induced DILI prompted us to investigate whether the function of JNK1 and JNK2 is universal or dependent on the context of hepatic injury. Thus, we treated JnkΔhepa mice with D-GalN combined with endotoxin (LPS). Unexpectedly, no differences were observed after D-GalN/LPS treatment in survival, serum markers of liver injury, tissue injury or liver versus body weight ratio between both control groups and JnkΔhepa animals. These data suggest that JNK1 and JNK2 in hepatocytes play a prominent protective role especially during toxic liver injury (Suppl. Fig. 4A–E).
CCl4–induced chronic liver injury is worsened in mice with combined deletion of Jnk1 and Jnk2 in hepatocytes
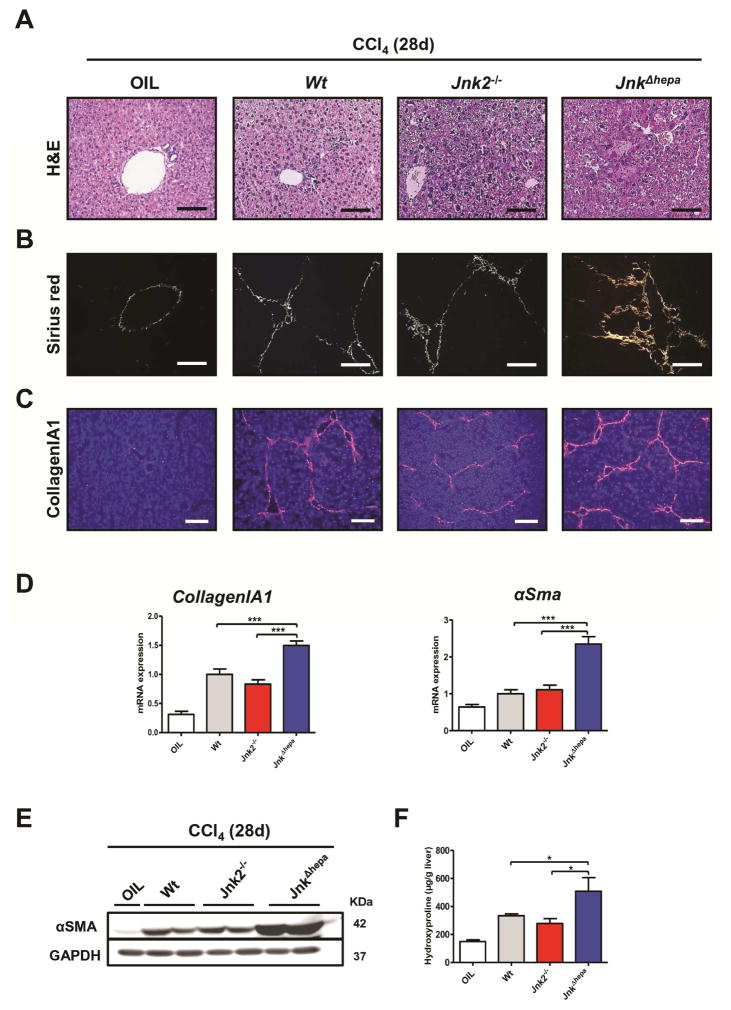
Since we previously showed that JNK1 in hepatocytes has no impact on the progression of CCl4-induced toxic liver injury11, we questioned whether compound function of JNK1 and JNK2 in hepatocytes is implicated in chronic toxic liver injury. 28 days after repetitive CCl4 treatment, JnkΔhepa livers showed larger numbers and size of necrotic areas compared with Jnk2−/− or Wt control mice (Figure 3A, Suppl. Fig. 5A). JnkΔhepa livers showed increased collagen deposition as evidenced by Sirius red staining, CollagenIA1 and α-smooth muscle actin (αSMA) protein and mRNA expression (Figure 3B–E, Suppl. Fig. 5B–E). Additional liver fibrosis markers such as hydroxyproline quantification, Timp1 and Mmp2 (Figure 3F, Suppl. Fig. 5F) suggested a protective role of combined JNK1 and JNK2 activation in hepatocytes during chronic toxic CCl4–induced liver injury.
Figure 3. Liver fibrogenesis is aggravated in JnkΔhepa after chronic CCl4 treatment.
(A) Representative H&E staining of liver sections of control (Wt), Jnk2−/− and JnkΔhepa livers, 28 days after repeated injections of CCl4. Corn-oil injections were used as controls. Scale bars: 100μm. (B) Representative Sirius red staining of paraffin sections from the same livers. Scale bars: 100μm. (C) A representative Collagen IA1 staining of frozen sections from the same mice is shown. Scale bars: 200μm. (D) In addition, mRNA levels for Collagen IA1 and αSMA were determined by qRT-PCR. (E) Protein levels of αSMA were determined. GAPDH was used as a loading control. (F) Hydroxyproline contents were assessed in the same livers. Data are expressed as mean±SEM (*p<0.05 and ***p<0.001).
Aggravated cell death and compensatory proliferation in JnkΔhepa liver after repetitive CCl4–injection
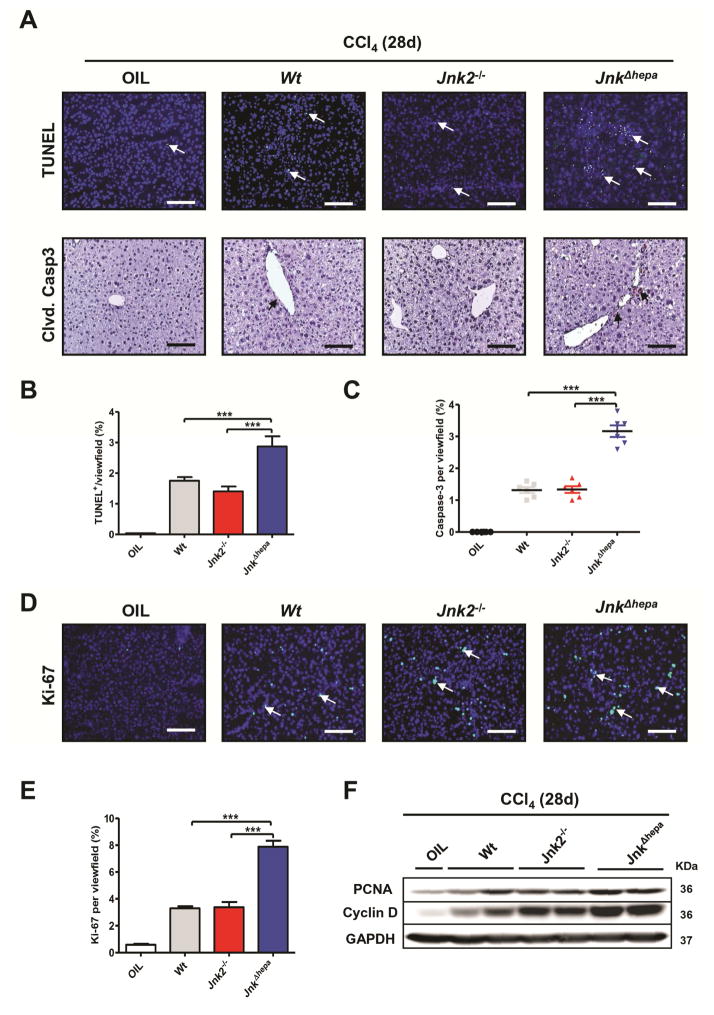
To better characterize our findings, we analyzed parameters of cell death and proliferation. TUNEL- and cleaved Caspase3-positive nuclei were significantly enhanced 28 days after CCl4 treatment in JnkΔhepa livers compared with Jnk2−/− or control mice (Figure 4A–C). Concomitantly, compensatory proliferation examined by Ki-67 staining was significantly increased in JnkΔhepa livers (Figure 4D+E). These results were strengthened by higher mRNA expression of cell cycle markers such as Pcna (mRNA) and up-regulation of PCNA and CyclinD protein levels in JnkΔhepa compared with Jnk2−/− or control livers (Figure 4F, Suppl. Fig. 6A+B). Thus loss of JNK1 and JNK2 in hepatocytes resulted in stronger cell death accompanied by an increased proliferative response in the liver.
Figure 4. Cell death and compensatory proliferation are exacerbated after combined deletion of JNK1 and JNK2 in hepatocytes.
(A) Representative TUNEL staining performed on frozen liver sections (upper; Scale bars: 100μm) and cleaved CASPASE3 immunohistochemistry in paraffin sections (lower panel; Scale bars: 100μm) of control (Wt), Jnk2−/− and JnkΔhepa livers after 4 weeks of repeated CCl4 injections are shown. Quantification of TUNEL- (left) (B) and Caspase3 enzyme activity (C) was analyzed in 4- week CCl4 treated control (Wt), Jnk2−/− and JnkΔhepa livers. (D) Representative Ki-67 staining performed on frozen liver sections of the same livers. Scale bars: 100μm. (E) Quantification of Ki-67 positive cells per view field is shown. (F) Protein levels of PCNA and CyclinD were determined by Western Blot in the same samples. GAPDH was used as a loading control. Data are expressed as mean±SEM (***p<0.001). Arrows denote positive cells.
The inflammatory response is increased in JnkΔhepa livers
Chronic inflammation triggers progression of liver fibrosis17. After 28 days of CCl4-injection, we found a significant increase in CD11b- and F4/80-positive cells, putative macrophages/Kupffer cells, in JnkΔhepa compared with Jnk2−/− and control livers (Suppl. Fig. 7A+B). Additionally, JnkΔhepa livers displayed stronger expression of inflammatory markers such as IL1α, I L1β, Mcp1 and Tnfα (Suppl. Fig. 7C–F). Hence, loss of JNK1 and JNK2 in hepatocytes caused severe inflammatory liver injury and fibrosis.
Gene and protein profile of JnkΔhepa livers and hepatocytes in basal conditions
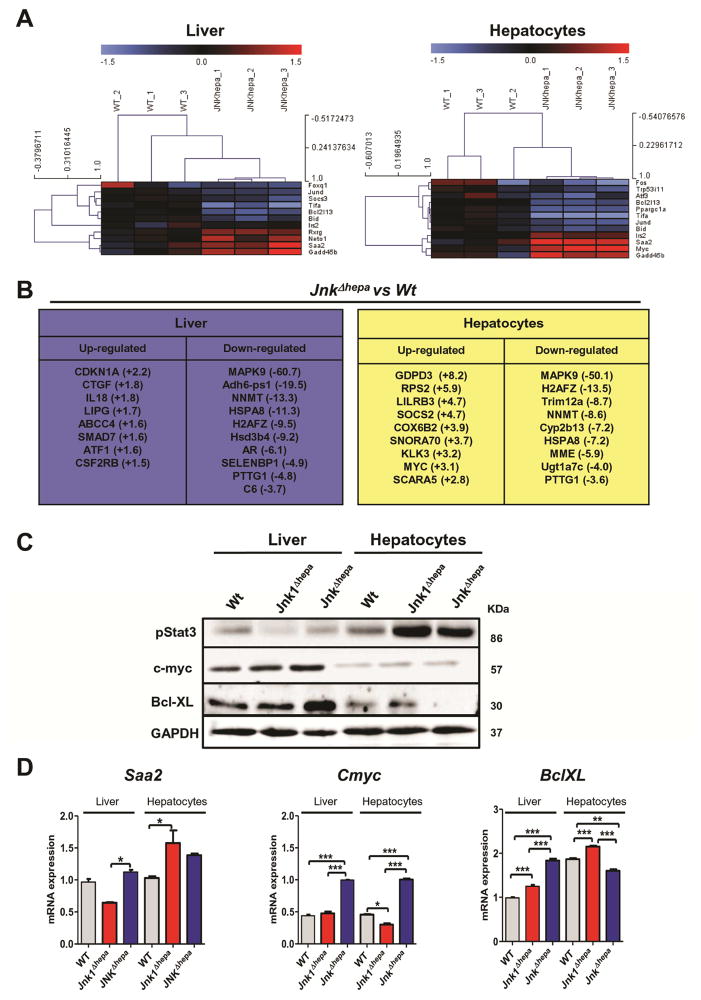
To study the impact of Jnk deletion on gene expression in liver and hepatocytes, we performed Affymetrix GeneChip microarray analysis of liver and primary hepatocytes isolated from 8 week-old control and JnkΔhepa mice. This comparison revealed significant changes (− 1.5<FC>1.5) in the transcript expression of 355 genes which were up-regulated (197 in liver and 158 in hepatocytes) and 448 down-regulated (171 in liver and 277 hepatocytes) in JnkΔhepa mice (Suppl. Fig. 8A+B). We first performed hierarchical clustering of the JNK substrates commonly up- or down-regulated in both livers and hepatocytes of JnkΔhepa mice. Interestingly, we found significantly reduced transcript levels of JNK target genes including transcription factors Atf, JunD or Fos and apoptotic markers (Bcl2l13 and Bid), and up-regulation of Gadd45b, Saa2 and Cmyc (Figure 5A). Moreover, Ingenuity Pathway Analysis™ (IPA) demonstrated that combined deletion of JNK1 and JNK2 in hepatocytes affected the expression of genes associated with cell death and proliferation (Cdkn1a, Atf, myc), inflammation (Smad7, IL18, Ctgf, Cox6b2) as well as metabolism (ABCC4, CYP2B13) (Figure 5B).
Figure 5. Microarray analysis of JnkΔhepa livers and hepatocytes manifest dysregulation in cell proliferation, apoptosis and inflammation at basal levels.
(A) Gene array analysis was performed in 8 week-old control (Wt) and JnkΔhepa liver and primary isolated hepatocytes. Correlation of the fold induction of genes in hepatocytes and liver is shown. Log2 expression values of the individual mice were divided by the mean of the sham-operated mice. Log ratios were saved in a txt file and analyzed with the Multiple Experiment Viewer. Top up- and down-regulated JNK-target substrates are shown (Red: Up-regulated; Blue: Down-regulated, n=3, −1.5<FC>1.5). (B) Ingenuity Pathway Analysis™ (IPA) was performed in the same samples and the expression values (in brackets) of the top up- and down-regulated genes in liver (left panel) and primary isolated hepatocytes (right panel) are represented. (C) Liver and hepatocytic protein extracts of untreated control (Wt), Jnk1Δhepa and JnkΔhepa were prepared. The protein expression levels of pStat3, c-myc and Bcl-XL were determined by Western Blot. GAPDH was used as loading control. (D) mRNA expression was determined in liver tissue and primary hepatocytes of untreated 8 week-old control (Wt), Jnk1Δhepa and JnkΔhepa mice. The mRNA expression levels of Saa2, Cmyc and BclXL are shown (n=3–5; *p<0.05; **p<0.01;***p<0.001).
To validate the main findings of the microarray analysis, protein and mRNA expression was analyzed in both livers and freshly isolated primary hepatocytes from 8 week-old Wt, Jnk1Δhepa and JnkΔhepa mice revealing enhanced expression of pSTAT3, Saa2 and c-myc (cell cycle), and down-regulation of BclXL and Bad (apoptosis). As expected, lack of Mapk9/Jnk2 expression was found in JnkΔhepa mice (Figure 5C, Suppl. Fig. 8C+D).
Morphological changes and mitochondrial damage are aggravated in APAP-treated JnkΔhepa primary hepatocytes
The acute and chronic toxic models suggested that JNK1 and JNK2 have synergistic functions for hepatocyte protection. We aimed to better define the molecular mechanisms explaining our findings in vivo, in primary control (Wt) and JnkΔhepa hepatocytes. Liver cells were cultured for up to 48h without treatment (Suppl. Fig. 9A). Lack of Jnk expression did not affect viability compared with WT hepatocytes (Suppl. Fig. 9B). However, basal cell cycle activity was reduced in JnkΔhepa compared to control hepatocytes (Suppl. Fig. 9C+D).
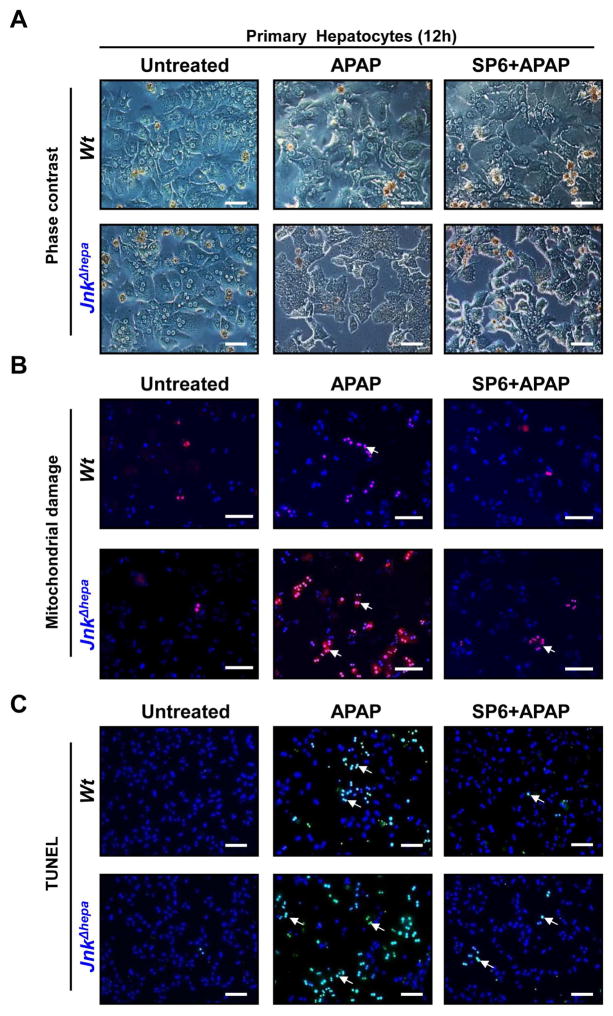
Next, we investigated the mechanism underlying the strong phenotype induced by APAP [10 mM] in JnkΔhepa hepatocytes for up to 48h. 12h after APAP treatment JnkΔhepa hepatocytes evidenced cytoplasmic projections, loss of cell-cell contact and detachment in contrast to polyhedral rounded shaped control hepatocytes (Figure 6A). Up to 24h upon treatment, nuclear condensation or fragmentation was significantly stronger in JnkΔhepa compared to control hepatocytes (not shown).
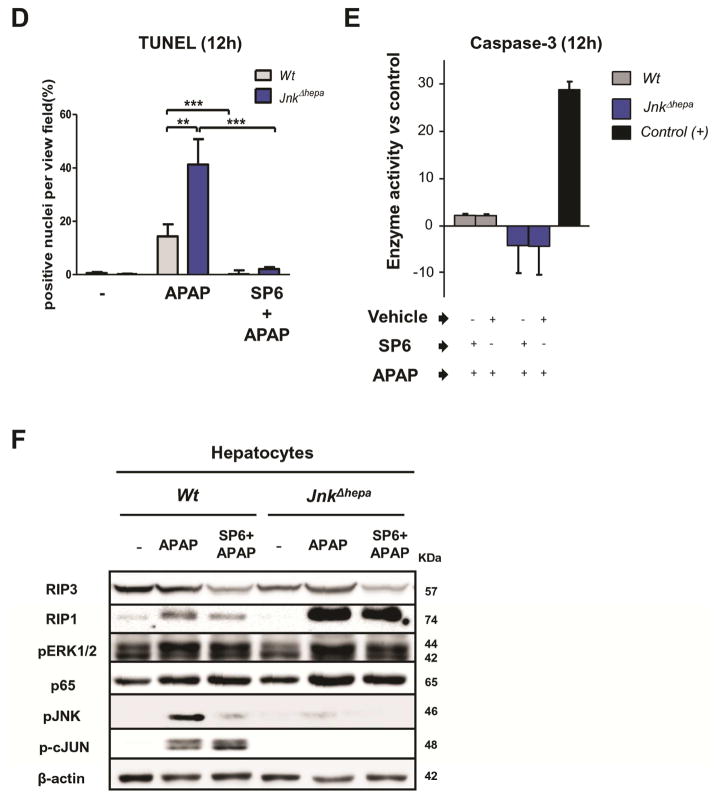
Figure 6. APAP modifies the morphology and exacerbates mitochondrial damage and necrotic cell death in primary JnkΔhepa hepatocytes.
(A) Primary hepatocytes were isolated from control (Wt) and JnkΔhepa mice. A total number of 500.000 cells were seeded in 6-well plates and cultivated for up to 12h. Visible light microphotographs were taken in presence or absence of APAP and/or SP600125. Scale bars: 200μm. (B) At the same time we performed Mitosox® staining to assess mitochondrial damage (red –arrows–, counter- stained with DAPI, blue). Microphotographs were taken (scale bars: 100μm). (C) TUNEL staining was performed. Scale bars: 200μm. TUNEL-positive cells were evaluated in the same mice and represented in percentage per view field. (E) The enzyme activity of Caspase3 was measured in frozen tissue. (F) Hepatocytic protein extracts were collected and treated with APAP for 8h in culture in presence or absence of APAP and/or SP600125. The protein expression levels of RIP3, RIP1, p65, pJNK, p-cJUN and pERK were determined by Western Blot. β-actin was used as a loading control.
SP600125, an anthrapyrazolone specific JNK inhibitor, has been shown to protect against APAP-induced ALF in mice18. Unexpectedly, control and JnkΔhepa-APAP treated hepatocytes were protected to the same extent by co-administration of SP600125+APAP (Figure 6A). APAP mediates its toxic effect by forming highly reactive metabolites. Concomitant with the morphological changes, we detected a significant increase in mitochondrial ROS in APAP-treated JnkΔhepa compared with control hepatocytes. Additionally, SP600125 significantly reduced the changes in mitochondrial membrane potential in both control and JnkΔhepa APAP-treated hepatocytes (Figure 6B, Suppl. Fig. 10A).
APAP exacerbates necrotic cell death in JnkΔhepa-primary hepatocytes
TUNEL staining evidenced significant lower cell survival of APAP-treated JnkΔhepa compared with control hepatocytes 12h after treatment (Figure 6C). Cell death was significantly reduced after SP600125±APAP co-administration in control and JnkΔhepa hepatocytes (Figure 6C+D). We next sought to define the mode of cell death in APAP-treated hepatocytes. We first tested Caspase3 activity before and after APAP administration including SP600125. APAP- treatment in any combination and at different time points did not significantly change Caspase3 activity (Figure 6E). This result was further confirmed by Caspase3 immunostaining (not shown). To detect the relevance of necrotic cell death, we performed Annexin V/Ethidium Homodimer III staining. The amount of double positive (i.e. necrotic) hepatocytes were significantly higher in JnkΔhepa compared with control hepatocytes, while SP600125 blocked necrosis in both control and JnkΔhepa hepatocytes (Suppl. Fig. 10B+C).
Receptor interacting protein 3 (RIP3) plays an essential role in mediating necrotic cell death. We studied RIP3 and RIP1 expression in WT and JnkΔhepa hepatocytes before and after APAP±SP600125 co-treatment (Figure 6F). APAP treatment had no effect on RIP3, but stimulated RIP1 expression in both control and was strongly induced in JnkΔhepa hepatocytes. SP600125+APAP co-treatment strongly reduced RIP3 expression to comparable levels in control and JnkΔhepa hepatocytes, while the decreasing effect on the RIP1 protein was more prominent in control hepatocytes (Figure 6F).
To characterize the specificity of our findings, we included pERK and p65 expression in our analysis. APAP treatment stimulated both pERK and p65 expression independent of SP600125 co-treatment, suggesting that both pathways are of minor relevance in explaining the effect on necrosis (Figure 6F). To further confirm this result we tested pJNK and p-cJUN expression before and after treatment. As shown in Figure 6F, APAP- induced pJNK and p-cJUN activation in control hepatocytes, while SP600125 blocked JNK, but not cJUN phosphorylation. In contrast, no significant pJNK and p-cJUN expression in either treatment conditions was evident in JnkΔhepa hepatocytes.
In vivo treatment with SP600125 suppresses APAP-induced liver injury in mice with compound deletion of JNK1 and JNK2 in hepatocytes
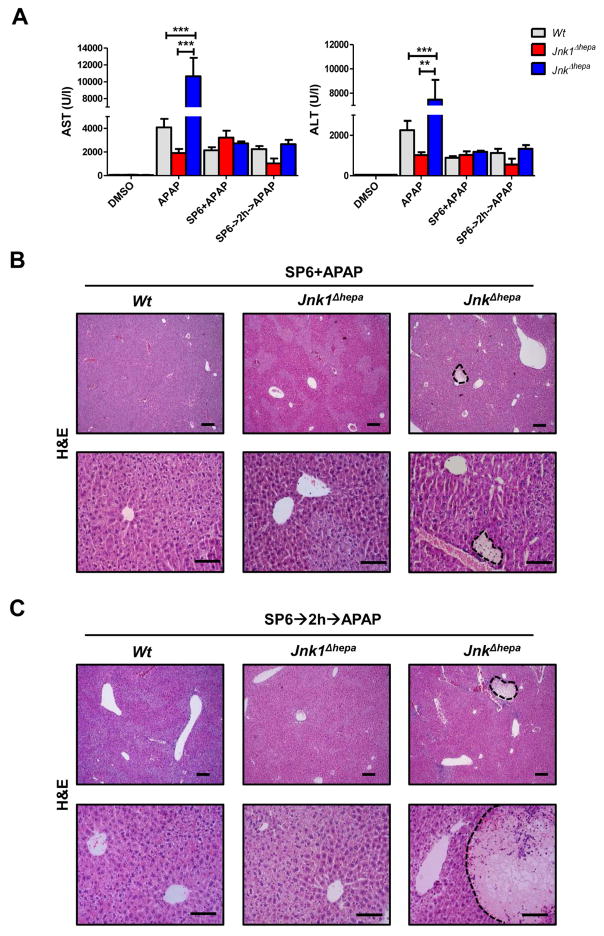
To validate the in vitro findings, we studied SP600125 co- and pre-administration with APAP in JnkΔhepa animals in vivo. Co-treatment with the JNK inhibitor provided protection in control and Jnk1Δhepa but also in JnkΔhepa mice against APAP-induced liver injury (Figure 7A–C). To exclude the possibility that SP600125 affects APAP metabolism, we pre-administered SP600125 to control, Jnk1Δhepa and JnkΔhepa mice, and two hours later we injected APAP (Figure 7A–C). However, pre-administration did not affect the protective effect. Noticeably, the number of necrotic foci was largely reduced in liver parenchyma of SP600125 co- or pre-treated JnkΔhepa mice (Figure 7B+C).
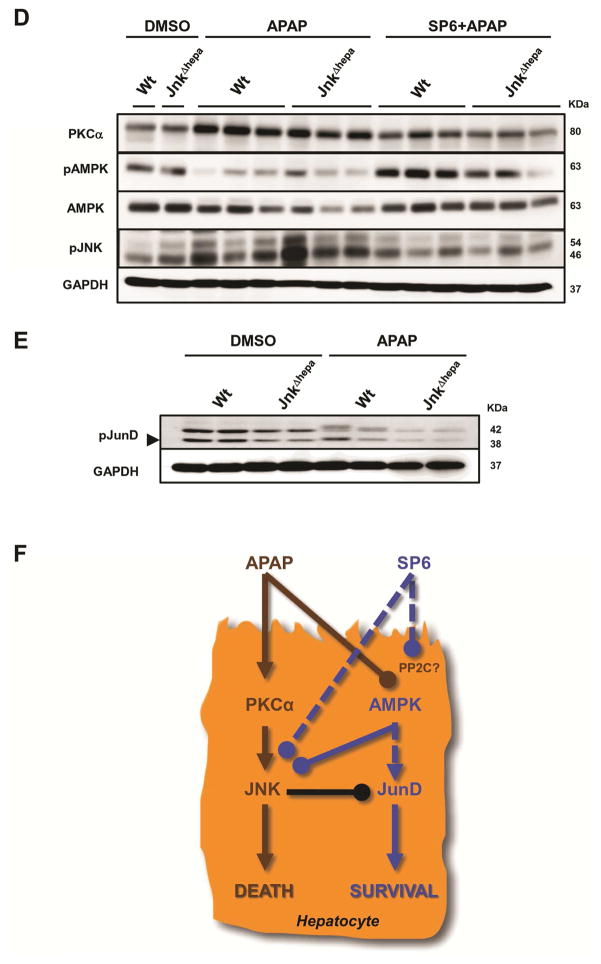
Figure 7. In vivo treatment with SP600125 suppresses APAP-induced liver injury in mice with combined JNK1 and JNK2 deletion in hepatocytes.
(A) Serum AST (left) and ALT (right) levels were determined in control (Wt), Jnk1Δhepa and JnkΔhepa mice injected with SP600125 at the same time as APAP or pre-treated with SP600125, 2h before APAP challenge. Comparison with DMSO and APAP-treated mice is shown (n=10). (B+C) Representative H&E staining of liver sections collected from the same mice sacrificed 8h after treatment and examined by an experienced pathologist. Scale bars: 50 (upper panels) and 200μm (lower panels), respectively. Dotted areas represent necrotic foci. (D) Liver extracts were collected and immunoblotting was performed, and the protein levels of PKCα, pAMPK, AMPK and pJNK determined by Western Blot. (E) Phosphorylation of JunD was performed in hepatocytic extracts. GAPDH was used as loading control. (F) APAP triggers higher PKCα, and lower AMPK – a survival pathway- expression inducing JNK phosphorylation and cell death. The JNK classical inhibitor SP600125 exerts an off- target effect by facilitating phosphorylation of AMPK and preventing JNK phosphorylation in hepatocytes.
Since our results suggested that SP600125 protects against APAP-induced liver injury via JNK-independent mechanisms, we explored other pathways associated with APAP-toxicity. Sabery and colleagues19 recently showed that the interplay between PKCα and JNK mediates APAP-induced liver injury. PKCα activation increased after APAP treatment and was attenuated by SP600125 co-administration in Wt livers (Figure 7D). Moreover, AMPK activity – protective against APAP hepatotoxicity – was abrogated after APAP challenge in control and JnkΔhepa animals. Total AMPK levels were also affected by APAP treatment. Noticeably, APAP treatment caused a decline in total AMPK levels in Wt and dramatically in JnkΔhepa livers. In contrast, co-treatment with the classical JNK inhibitor, SP600125, reversed the APAP-mediated effect on pAMPK and AMPK levels in both Wt and JnkΔhepa animals.
Next, we examined whether lower AMPK levels correlated with JNK activity. APAP treatment increased JNK in Wt and, to a higher extent, in JnkΔhepa livers, an effect blocked by co-treatment with SP600125 (Figure 7D). Collectively, these data suggest that; (1) SP600125 exerts an off-target effect on AMPK, and; (2) the protective effect of SP600125 occurs in liver parenchymal cells.
Lack of JunD activation in APAP-treated JnkΔhepa murine hepatocytes
We performed a set of quantitative phosphoproteomics experiments to characterize proteins which could be differentially phosphorylated after APAP treatment in control and JnkΔhepa hepatocytes (Suppl. Fig. 11A). Amongst several differentially phosphorylated proteins, which we could not validate by western blot due to lack of commercially available phospho-antibodies, our screening approach identified JunD – a known JNK nuclear substrate – to be substantially less phosphorylated in APAP-treated JnkΔhepa hepatocytes (Figure 7E). In addition, we investigated overall phosphorylation of MAPKs, which are known substrates of JNK, using a MAPK-substrate specific antibody (Suppl. Fig. 11B). This approach revealed at least nine proteins with reduced phosphorylation in APAP-treated JnkΔhepa hepatocytes. The identity of these proteins was estimated by comparison of their appropriate molecular weights with known JNK substrates (Suppl. Fig. 11B). In summary, these results demonstrate substantially attenuated phosphorylation of JunD and other proteins in APAP-treated JnkΔhepa hepatocytes, which need to be identified in future studies. These findings indicate that a JNK-JunD dependent mechanism might be involved in protecting against APAP-induced liver injury.
Discussion
Acetaminophen- and CCl4-induced liver injury have been extensively studied since APAP overdose is the leading cause of DILI in the United States and accidental CCl4 ingestion still occurs14. An overwhelming amount of evidence demonstrated that JNK activation plays a major role in toxic liver injury20. Our first investigations using human DILI liver samples showed activation of JNK (pJNK) predominantly in nuclei of hepatocytes and in infiltrating cells. These results are in line with observations that JNK activation is not only limited to hepatocytes but also found in pro-fibrotic and inflammatory cells during progression of liver disease in humans and mice10, 11, 21.
Noticeably, our results suggest differential pattern of JNK1 expression, and increased JNK2 levels in human DILI-ALF liver biopsies compared with normal tissue. Furthermore, JNK activation was a common feature of murine APAP-induced liver injury not only in Wt but also especially in JnkΔhepa mice. Noticeably, the intense JNK phosphorylation as well as increased JNK1 expression in JnkΔhepa livers after APAP correlated with the dramatic increase in transaminases. These results suggest that JNK1 phosphorylation in infiltrating cells and non-parenchymal cells correlates with the degree of liver injury. However, the functional role of these cell compartments during APAP-induced liver injury needs to be further addressed.
As found in human samples, JNK1 and JNK2 protein expression was also dysregulated after APAP challenge in mice, indicating an analogous mechanism between human and murine DILI. Altogether our results indicated that (1) JNK is strongly activated in all forms of human DILI-induced ALF, (2) etiology-dependent not only hepatocytes but also immune infiltrating cells are pJNK positive and (3) hence, a dysregulation of JNK1 and JNK2 protein expression is characteristic of both human and murine DILI.
Additionally, our array analysis led us to the working hypothesis that JNK1 and JNK2 might have distinct and shared essential functions in hepatocytes. In the current study, we found that combined JNK1 and JNK2 deletion in hepatocytes triggers more severe liver injury, inflammation and progression after repetitive CCl4 injection. Interestingly, our previous publication revealed that JNK1 is involved in HSCs trans- differentiation into collagen-producing myofibroblasts11, associated with reduced liver injury. Consequently, JNK2 expression is sufficient to rescue the loss of JNK1 in hepatocytes and protects from CCl4-mediated cell death.
The prominent role of necrotic cell death in APAP-dependent liver injury has been shown in several studies22–26. Previous reports by the lab of Kaplowitz27 suggested that disruption of JNK2, but not of JNK1, partially prevented APAP-induced liver injury, indicating that targeting JNK2 could be a promising therapeutic approach. In contrast, Henderson and collaborators found no differences in APAP- induced liver injury between Jnk1−/− and Jnk2−/− mice28. Our data demonstrate that lack of JNK1 and JNK2 expression in hepatocytes caused extensive necrosis within 8h after APAP injection. Concomitant with these findings, we observed a dramatic oxidative stress response in the liver of JnkΔhepa mice, associated with necrotic cell death22, 23. We excluded that apoptotic cell death plays a significant role in APAP-treated JnkΔhepa hepatocytes. Instead, we demonstrate that combined JNK1 and JNK2 activities are protective against APAP-induced necrotic cell death of hepatocytes by controlling the oxidative stress response.
The observations found in APAP-induced liver injury were specific for this form of ALF, since we observed no differences in the acute hepatitis-D-GalN/LPS model between JnkΔhepa and control animals. This is in agreement with a recent study indicating that combined deletion of JNK1 and JNK2 in hepatocytes did not alter ConA- or LPS-induced liver injury29. However, hematopoietic deficiency in JNK1 and JNK2 prevented ConA-induced liver injury by suppressing TNF production29. These findings clearly suggest that, under certain conditions (e.g. aetiology of liver disease), JNK activation in infiltrating cells rather than in hepatocytes is critical. Altogether these results suggest that the context-specific activation of JNK in different cell-types during ALF is essential, and needs to be further defined.
To better assess these results, we included the classical JNK inhibitor, SP600125, in our analysis. This compound has been reported to target the highly conserved ATP-binding sites of JNK and protects from APAP-induced liver injury in vivo30. Surprisingly, SP600125 was found to confer protection not only to control but also to Jnk-deleted hepatocytes in vivo and in vitro. Moreover, pre-administration of SP600125 did not affect APAP metabolism. These results suggested that the beneficial effect of SP600125 on APAP-induced liver injury likely results through off-target effects, e.g., on AMPK. Concomitant with previous reports19, we found decreased levels of pAMPK and a further decline of total AMPK levels – protective against APAP hepatotoxicity– in JnkΔhepa livers, indicating that survival pathways are reduced in animals with JNK deficiency in hepatocytes. Thus SP600125 might act on protein phosphatases such as 2C (PP2C) which negative regulate AMPK signalling, preventing the dephosphorylation of AMPK (Figure 7F).
Additionally, we employed functional proteomics to map differential phosphorylation caused by APAP treatment in JNK-deficient hepatocytes. Our findings suggest repression of JunD activation, another protective element and part of the cell stress response, was further decreased in the absence of JNK in hepatocytes. Hence, the role of other kinases involved in necrotic cell death such as RIP3, RIP1 or MLKL are currently under intensive investigation24, 31.
In summary, we demonstrate that combined JNK1 and JNK2 activation is essential to protect hepatocytes from acute and chronic toxic liver injury in vivo and in vitro. Our results show that JNK inhibition is a questionable treatment option for APAP-induced liver injury as the protecting effect of SP600125, the classical JNK inhibitor, is mediated by off-target effects. Hence our results show that the cell-type specific function of JNK is a potential therapeutic target. However, the context-specific function needs to be defined before these options can be employed in the clinic.
Supplementary Material
Acknowledgments
Grant support: This work was supported by the IZKF (UKA, RWTH Aachen), the SFB/TRR57, the START-Program of the Faculty of Medicine (#691405, RWTH Aachen), the DFG (BE#3967/3-1), the DFG TR 285/10-1 and the DFG-EI/ZUK2/I3TM/SF_15_5_17.
We would like to thank Prof. Neil Kaplowitz for helpful discussion, and Dr. Svenja Sydor, Dr. Henning W. Zimmermann and Johanna Reissing for the samples from patients with ALF-DILI. The Proteomics Core Facility is supported by a grant from the Interdisciplinary Centre for Clinical Research within the faculty of Medicine at the RWTH Aachen University. F.J.C. is a Ramón y Cajal Researcher (RYC-2014-15242).
Abbreviations (in alphabetical order)
- ANOVA
Analysis of variance
- AIH
Autoimmune hepatitis
- ALF
Acute liver failure
- ALT
Alanine aminotransferase
- AMPK
adenosine monophosphate-activated protein kinase
- AP
Alkaline phosphatase
- APAP
Acetaminophen
- AST
Aspartate aminotransferase
- BMT
Bone marrow transplantation
- CCl4
Carbon tetrachloride
- ConA
Concanavalin A
- CYP2E1
Cytochrome P450, isoform 2E1
- DILI
Drug-induced liver injury
- ECM
Extracellular matrix
- ES
Embryonic stem cells
- GSH
Glutathione
- HBV
Hepatitis B virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C virus
- H&E
Hematoxylin and eosin
- HRP
Horseradish peroxidase
- HSCs
Hepatic stellate cells
- JNK
c-Jun N-terminal kinases
- JnkΔhepa
Dual deletion of Jnk1 and Jnk2 in hepatocytes
- Jnk1Δhepa
Hepatocyte-specific deletion of Jnk1
- Jnk2−/−
Mice carrying constitutive deletion of Jnk2
- KC
Kupffer cells
- MAPK
Mitogen-activated protein kinases
- MMP2
Matrix metalloproteinase-2
- NAC
N-acetylcysteine
- NAPQI
N-acetyl-p benzoquinone imine
- NSAID
non-steroidal anti-inflammatory drug
- PCNA
Proliferating cell nuclear antigen
- PCR
Polymerase chain reaction
- PDGFβ
Platelet derived growth factor-beta
- PFA
Paraformaldehyde
- PKCα
Protein kinase C-alpha
- qPCR
Quantitative real-time PCR analysis
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- αSMA
α-smooth muscle actin
- TIMP1
Tissue inhibitor of metalloproteinase-1
- TNFα
Tumor necrosis factor-α
- TUNEL
TdT-mediated dUTP-biotin nick end labeling
Footnotes
Disclosures: The authors declare that they have no competing personal or financial conflicts of interests.
Transcript Profiling: Affymetrix microarray data have been deposited with the NCBI Gene Expression Omnibus under accession number GSE59602 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE59602).
Authors contributions: F.J.C. generated and acquired data, interpreted the results, analyzed the microarray, and wrote the manuscript. M.E.Z., W.H. and G.Z. generated the data. J.P. performed immunostainings and immunoblottings of liver samples with acute liver failure. Y.A.N. acquired and analyzed protein expression. M.A.M performed qPCRs, and RT-PCRs, serum analysis and protein extracts as well as technical assistance in general. L.P.B. and A.E.C. provided the human samples. M.V.B. and M.M. performed the microarray. C.P., M.E.Z. and F.J.C. performed the proteomics analysis and validation. N.G. provided his expertise in pathology. R.J.D. created and provided the Jnk1-floxed mice. T.L. and C.L. provided material and critically reviewed the manuscript. C.T. conceived, supervised, co-wrote the manuscript and provided funds for the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fink SL, Cookson BT. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun. 2005;73:1907–16. doi: 10.1128/IAI.73.4.1907-1916.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guicciardi ME, Malhi H, Mott JL, et al. Apoptosis and necrosis in the liver. Compr Physiol. 2013;3:977–1010. doi: 10.1002/cphy.c120020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conze D, Krahl T, Kennedy N, et al. c-Jun NH(2)-terminal kinase (JNK)1 and JNK2 have distinct roles in CD8(+) T cell activation. J Exp Med. 2002;195:811–23. doi: 10.1084/jem.20011508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–52. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 5.Jaeschke A, Rincon M, Doran B, et al. Disruption of the Jnk2 (Mapk9) gene reduces destructive insulitis and diabetes in a mouse model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102:6931–5. doi: 10.1073/pnas.0502143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid AB, Kurten RC, McCullough SS, et al. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J Pharmacol Exp Ther. 2005;312:509–16. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- 7.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–92. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsumaru K, Ji C, Kaplowitz N. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. Hepatology. 2003;37:1425–34. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 9.Hanawa N, Shinohara M, Saberi B, et al. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluwe J, Pradere JP, Gwak GY, et al. Modulation of hepatic fibrosis by c-Jun-N-terminal kinase inhibition. Gastroenterology. 2010;138:347–59. doi: 10.1053/j.gastro.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao G, Hatting M, Nevzorova YA, et al. Jnk1 in murine hepatic stellate cells is a crucial mediator of liver fibrogenesis. Gut. 2014;63:1159–72. doi: 10.1136/gutjnl-2013-305507. [DOI] [PubMed] [Google Scholar]
- 12.Das M, Jiang F, Sluss HK, et al. Suppression of p53-dependent senescence by the JNK signal transduction pathway. Proc Natl Acad Sci U S A. 2007;104:15759–64. doi: 10.1073/pnas.0707782104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das M, Sabio G, Jiang F, et al. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–60. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 15.Lee SS, Buters JT, Pineau T, et al. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem. 1996;271:12063–7. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- 16.Bansal S, Liu CP, Sepuri NB, et al. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. J Biol Chem. 2010;285:24609–19. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunami Y, Leithauser F, Gul S, et al. Hepatic activation of IKK/NF-kappaB signaling induces liver fibrosis via macrophage-mediated chronic inflammation. Hepatology. 2012 doi: 10.1002/hep.25711. [DOI] [PubMed] [Google Scholar]
- 18.Xu JJ, Hendriks BS, Zhao J, et al. Multiple effects of acetaminophen and p38 inhibitors: towards pathway toxicology. FEBS Lett. 2008;582:1276–82. doi: 10.1016/j.febslet.2008.01.063. [DOI] [PubMed] [Google Scholar]
- 19.Saberi B, Ybanez MD, Johnson HS, et al. Protein kinase C (PKC) participates in acetaminophen hepatotoxicity through c-jun-N-terminal kinase (JNK)-dependent and -independent signaling pathways. Hepatology. 2014;59:1543–54. doi: 10.1002/hep.26625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seki E, Brenner DA, Karin M. A liver full of JNK: signaling in regulation of cell function and disease pathogenesis, and clinical approaches. Gastroenterology. 2012;143:307–20. doi: 10.1053/j.gastro.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toivola DM, Ku NO, Resurreccion EZ, et al. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology. 2004;40:459–66. doi: 10.1002/hep.20277. [DOI] [PubMed] [Google Scholar]
- 22.Kon K, Kim JS, Jaeschke H, et al. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 23.Ni HM, Bockus A, Boggess N, et al. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–32. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramachandran A, McGill MR, Xie Y, et al. Receptor interacting protein kinase 3 is a critical early mediator of acetaminophen-induced hepatocyte necrosis in mice. Hepatology. 2013;58:2099–108. doi: 10.1002/hep.26547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antoine DJ, Jenkins RE, Dear JW, et al. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J Hepatol. 2012;56:1070–9. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.McGill MR, Sharpe MR, Williams CD, et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunawan BK, Liu ZX, Han D, et al. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–78. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 28.Henderson NC, Pollock KJ, Frew J, et al. Critical role of c-jun (NH2) terminal kinase in paracetamol- induced acute liver failure. Gut. 2007;56:982–90. doi: 10.1136/gut.2006.104372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Das M, Garlick DS, Greiner DL, et al. The role of JNK in the development of hepatocellular carcinoma. Genes Dev. 2011;25:634–45. doi: 10.1101/gad.1989311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii M, Suzuki Y, Takeshita K, et al. Inhibition of c-Jun NH2-terminal kinase activity improves ischemia/reperfusion injury in rat lungs. J Immunol. 2004;172:2569–77. doi: 10.4049/jimmunol.172.4.2569. [DOI] [PubMed] [Google Scholar]
- 31.Dara L, Johnson H, Suda J, et al. Receptor interacting protein kinase 1 mediates murine acetaminophen toxicity independent of the necrosome and not through necroptosis. Hepatology. 2015 doi: 10.1002/hep.27939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.