Abstract
Aims and Objectives:
Imbalances between the oxidant-antioxidant status have been implicated in the pathogenesis of several diseases, including oral cancer. Mostly, all oral cancer lesions are preceded by a stage of premalignancy. The present study aims to evaluate lipid peroxidation and antioxidant status in the venous blood of patients with different clinicopathologic stages of leukoplakia.
Materials and Methods:
A case control study was designed with the inclusion of 20 new cases of histopathologically proven leukoplakia of various clinical stages along with an equal number of positive and negative control individuals. The concentrations of thiobarbituric acid reactive substances and the activities of the antioxidant enzymes, namely superoxide dismutase, reduced glutathione, glutathione peroxidase, and catalase, were estimated in plasma using spectrophotometric methods. The data are expressed as mean ± SD. The statistical comparisons between and within the study groups were performed by one-way analysis of variance followed by post hoc analysis. Karl Pearson correlation was performed for the biochemical parameters within the group and between the groups. For statistically significant correlations, simple linear regression was performed.
Results:
Significant enhanced lipid peroxidation (P < 0.001) with a decrease in antioxidants (P < 0.001) was observed in the venous blood of leukoplakia patients compared with positive as well as negative controls. Accordingly, significant (P < 0.001) pattern of progression in thiobarbituric acid reactive substances levels was observed at various clinical stages among patients of both control groups. Among enzymes, glutathione showed significant (P < 0.001) reduction along the stages on comparison with two control groups.
Conclusion:
Enhanced lipid peroxidation and compromised antioxidant defense in plasma indicate the development of oxidative stress. Among the antioxidant enzymes, reduced glutathione and glutathione Pperoxidase may play a decisive role in malignant transformation.
Key words: Antioxidant enzymes, free radicals, lipid peroxidation, oral precancer, oxidative stress
INTRODUCTION
Free radicals are formed in both physiological and pathological conditions in mammalian tissues.[1] Compounds that dispose, scavenge, and suppress the formation of free radicals or oppose their actions are called antioxidants. Under normal physiological limits, a balance exists between the two. An uncontrolled production of free radicals with a concurrent depletion of antioxidants is termed as oxidative stress.[2] It is considered to be an important factor in the cell destruction caused by damage to various cellular micro and macromolecules. Damage of the abundant and thus most susceptible polyunsaturated fatty acid (PUFA) is termed as lipid peroxidation.[3]
Alterations in the oxidant-antioxidant profile were extensively studied in conditions such as gastrointestinal ulcerogenesis, rheumatoid arthritis, cardiovascular diseases, neurodegenerative disease, and cancer.[4] Oral conditions such as lichen planus and chronic periodontitis have also reported involvement of oxidative stress during their clinical course.[5,6]
Previously, we reported changes in lipid peroxidation and antioxidants in plasma as well as tissue sample of oral cancer patients and succeeded in establishing the role of oxidative damage in oral cancer.[7,8] It is seen that almost all cases of oral cancer are preceded by a variety of potentially malignant oral disorders (PMOD), of which leukoplakia appears to be most common. Moreover, high survival rate and low morbidity associated with leukoplakia, makes their detection of utmost importance.[9] In the light of the facts given above, it will be essential to evaluate oxidative stress levels in PMOD. To the best of our knowledge, very limited number of studies[10,11] has been cited in the literature, especially in the context of the South Indian rural population. Because we initially observed and reported reduced lipid peroxidation along with raised levels of reduced glutathione (GSH) and glutathione peroxidase (GPx) in tissue samples of leukoplakia,[12] the current study aims to evaluate the pattern of the same parameters in plasma samples of leukoplakia. The present study also outscores from the previous investigators in terms of comparing parameters such as thiobarbituric acid reactive substances (TBARS), superoxide dismutase (SOD), GSH, GPx, and catalase (CAT) in various clinicopathological stages of Leukoplakia. This study was undertaken in an attempt to evaluate the relationship between the parameters and dysplasia, which in turn will reflect their impact on malignant transformation. The usage of statistical tools also appears to be limited in similar studies.
The purpose of the study was to address the role of free radical damage in the progression of disease in patients with premalignant disorder when compared with a control group. The study hypothesizes that the burden caused by oxidative damage and the compromised antioxidant enzymes keeps on increasing as the disease progresses through the stages.
MATERIALS AND METHODS
Study sample and its characteristics
The study participants (sample size, n = 60) were selected from a population of patients who presented to the outpatient department of Oral medicine and Radiology for evaluation and management of oral diseases. These individuals were divided into three groups. The group I/Leukoplakia consisted of 20 randomly selected, newly diagnosed patients with histopathologically confirmed diagnosis of leukoplakia. The exclusion criteria for enrollment were previously treated cases of leukoplakia, patients with a history of diabetes, hypertension, jaundice, liver or kidney disorders, or history of other systemic diseases. Group II/(positive control) consisted of 20 age and sex-matched healthy volunteers with tobacco chewing history. Twenty age and sex-matched healthy volunteers with no history of tobacco chewing were recruited as the normal control (Negative) group (Group III). The study was approved by the institutional ethical committee.
Data collection management
Written informed consent was obtained from all the participants of the study after being explained the purpose of the study. A detailed case history of the patients with emphasis on their habits (chewing betel nut and/or tobacco, smoking, and alcohol) was taken and recorded on a standard Performa along with thorough clinical examination. Clinicopathological oral leukoplakia staging system (OLEP) was adopted to categorize group I (Leukoplakia) patients with stages I/II/III/IV.
Blood sample collection
Blood samples (5 ml) were collected from both the study and control group patients by venous arm puncture under aseptic precautions and transferred into a presterilized ethylenediaminetetraacetic (EDTA) vial. The collected samples were then subjected to centrifugation at 3000 rpm for 10 min to segregate plasma and erythrocytes. After plasma separation, the Buffy coat was removed and the packed cells were washed three times with normal physiological saline. A known volume of erythrocytes was lysed with hypotonic phosphate buffer (pH 7.4). The hemolysate was separated by centrifugation at 3500 rpm for 15 min at 2°C. TBARS and antioxidant enzyme estimation were carried in plasma and erythrocyte lysate.
Procedure for estimation
Lipid peroxidation was estimated as evidenced by the formation of TBARS. It was analyzed in plasma by the method described by ohkawa et al. In this method, Malonialdehyde and TBARS react with thiobarbituric acid in an acidic condition to generate a pink color chromophore which was read at 532 nm.
GSH was estimated by the method of Ellman. This method is based on the development of a yellow color, read at 412 nm spectrophotometrically, when 5, 5'-dithiobis (2-nitrobenzoic acid) (DTNB) was added to compounds containing sulphydryl groups. SOD was assayed by the method described by Kakkar et al. The assay is based on the inhibition of the formation of NADH-phenazine methosulphate nitroblue tetrazolium (NBT) formazon at 520 nm. The activity of CAT was assayed by the method of Sinha based on the utilization of H2 O2 by the enzyme. The color developed was read at 620 nm. GPx activity was estimated by the method of Rotruck et al., with some modifications. A known amount of enzyme preparation was incubated with H2 O2 in the presence of GSH for a specified time period.
Statistical analysis
All quantitative data were expressed as mean ± standard deviation (SD), whereas qualitative data were expressed in numbers and percentiles. All the variables of the study were statistically analyzed for the mean values, SD and P value. The statistical comparison of biochemical parameters between case and control groups was performed by analysis of variance (ANOVA) and post hoc analysis. Linear regression analysis followed by Pearson's correlation was performed to evaluate the association between the parameters. The data were analyzed using the SPSS (Statistical Package for Social Sciences) Version 13 software package.
RESULTS
Table 1 shows the clinicopathological characteristics of leukoplakia patients and controls who participated in the study. In our study, the average age of patients with leukoplakia was 46.20 ± 11.08 years, with a male predominance (75%). Our findings are consistent with age, gender, and site distribution reported in the literature.[13] Because a history of tobacco with or without additives was a consistent finding among all participants, our study also suggests that the cause of leukoplakia is closely related to the usage of tobacco, as well-documented in the literature.[14]
Table 1.
Clinicopathological characteristics of case group – leukoplakia patients (Group I) and control groups (Group II and Group III)
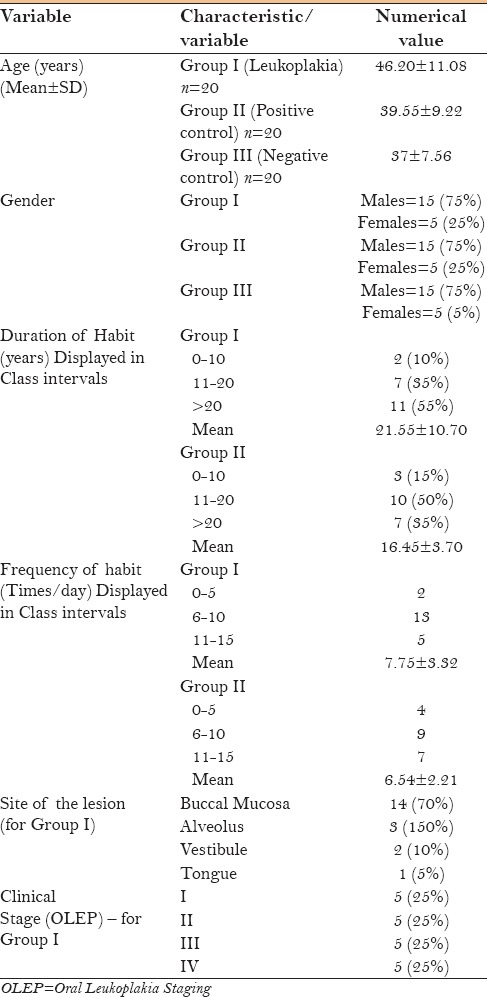
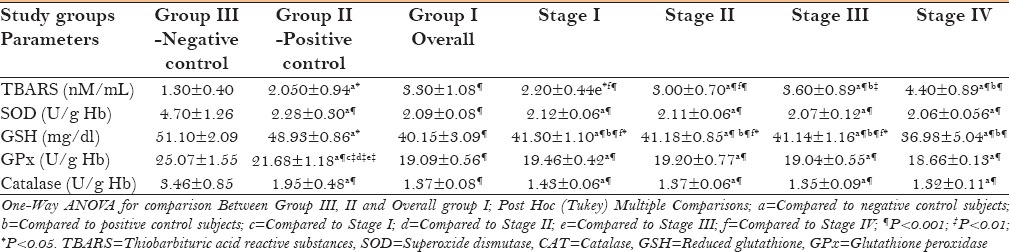
Table 2 shows a comparison of lipid peroxidation end products (TBARS) and various antioxidant enzyme profiles between leukoplakia and controls. Lipid peroxidation was found to be significantly (P < 0.001) increased whereas all antioxidant enzymes were significantly decreased (P < 0.001) in the case group on comparison with the control groups. A significant increase in the mean values of TBARS was seen with each progressing stage of leukoplakia when compared with positive as well as negative control group. Stage IV has shown significant (P < 0.001) elevated levels of TBARS on comparison with stages I and II. Unlike TBARS, all antioxidant enzymes displayed significant (P < 0.001) decreasing mean values along the stages on comparison with negative controls alone. With respect to GSH, Stage IV showed significant decrease on comparison with both control groups (P < 0.001) as well as with the rest of the stages (P < 0.05 and P < 0.001).
Table 2.
Comparison of TBARS, SOD, GSH, GPx, and Catalase among control groups and group I as well as among clinical stages of leukoplakia group with control groups (All values are expressed in mean±SD)
On analyzing the various biochemical parameters using Pearson's correlation, it was observed that GSH and GPx showed a significant (P < 0.001) negative correlation with TBARS, whereas a significant positive correlation with SOD (P < 0.05) and catalase (P < 0.001) [Table 3].
Table 3.
Correlation and regression analysis between TBARS and antioxidant enzymes in leukoplakia group
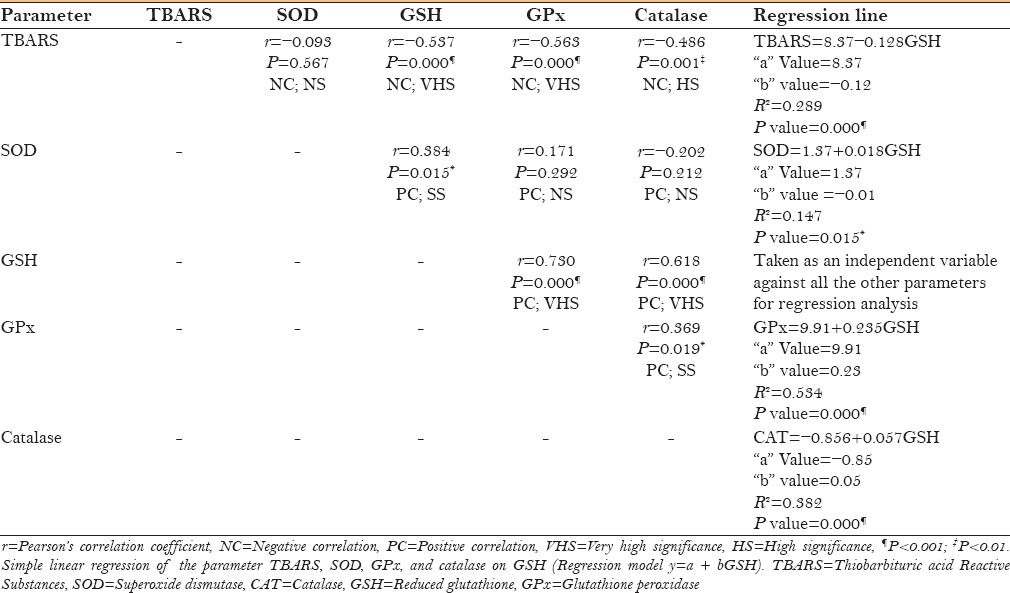
On performing linear regression analysis with regard to GSH, it was found that if it is presumed to be an independent variable, then it can reliably predict the dependent variables like GPx, Catalase, TBARS (P < 0.001), and SOD (P < 0.05). A significant proportion of variance (R2 =0. 53; 53%) in the GPx is thought to be caused by GSH. Regression equation (GPx = 9. 91 + 0.235 GSH) will be able to predict the value of GPx for a unit change in GSH [Table 3].
DISCUSSION
Leukoplakia is a clinical term indicating a white patch or a plaque in the oral mucosa that cannot be rubbed off and cannot be characterized clinically as any other disease. They constitute the most common precursor of oral cancer (85% of all precancerous lesions).[15] Leukoplakia that histologically displays severe dysplasia or carcinoma in situ is often asymptomatic and clinically appears completely harmless. Later, these lesions undergo malignant transformation and develop as oral cancer. Such lesions in their early stages are considered to be reversible and curable with insignificant morbidity and mortality.[16] Clinicians face a major challenge in the early recognition of such innocuous lesions. Early detection methods and biomarkers have become increasingly important for various ongoing researches.
ROS and their deleterious consequences such as lipid peroxidation have already been implicated in the pathogenesis of oral cancer.[7,17] The continuum of the disease process from normal mucosa to leukoplakia to oral cancer opens an important avenue for research implications of ROS in premalignant lesions.
Free radicals are very unstable entities, making it impossible to quantify. Therefore, byproduct of lipid peroxidation (TBARS) serves as an index of free radical activity, thereby estimating them indirectly.[18] We observed significantly increased (P < 0.001) plasma levels of TBARS in patients with leukoplakia as compared to control individuals [Table 2]. Control participants without the habit of tobacco consumption have shown the least peroxidation activity as compared with the controls with tobacco habits. A similar result was reported by Chole et al.,[19] D'souza et al.,[20] and Subapriya et al.[10] (P < 0.05) in serum samples; whereas Khanna et al.[11] reported contradicting results. Comparatively larger sample size (n = 20), and more importantly, heterogeneous patients with respect to clinicopathological stages, are the probable reasons for the significant results of the present study. Erythrocytes in plasma are highly susceptible to oxygen free radical-induced lipid peroxidation due to continuous exposure to oxygen tension and high content of PUFA.[10] Thus, from the abovementioned result, it can be understood that there is a definite excess load of free radicals in leukoplakia patients.
We also found a statistically non-significant increasing trend of TBARS while comparing among clinicopathological stages of leukoplakia except in stages I and II [Table 2]. Limitation of the present study in terms of sample size (five patients per stage) could be the reason for this result.
In the present study, the levels of GSH, GPx, Catalase, and SOD (P < 0.001) were significantly decreased in leukoplakia patients compared to both control groups [Table 2]. Control participants without a habit of tobacco consumption showed the maximum levels of enzymes among the compared groups. Similar results were reported by a previous investigator.[10] Uncontrolled growth of the tumor tissue is also facilitated by the influx of these antioxidant enzymes from the plasma.[21] Thus, depleting antioxidant defensive enzymes, along with the overpopulation of free radicals in plasma, prepare the ground for oxidative stress in leukoplakia patients.
The current study showed significant decline (P < 0.001) of all antioxidant enzymes along the progressing stages of leukoplakia when compared with negative controls [Table 2]. Stage IV for GSH needs a special mention because it was found to be significantly low when compared with individual stages as well as with positive as well as negative control group. Two interesting inferences can be made from the above observations. First, despite the small sample size in each category of stages (5 in each), GSH showed very high significant (P < 0.001) difference in all stages in comparison with both the control groups. This proposes that these enzymes have a serious impact in the development of oxidative stress. Second, the abovementioned result regarding the stage IV indicates the influencing role of GSH on severe dysplasia and points out its potential role in malignant transformation. To the best of our literature search, no study has performed such a comparative analysis along the stages of leukoplakia.
Based on the results so far, the role of TBARS, GSH, and GPx were found to be most promising in the development of oxidative stress. To further understand interactions among them, correlation and regression statistical tools were used. As expected, TBARS showed very high significant (P < 0.001) negative correlation with both the enzymes [Table 3]. Hence, confirming the antagonistic relationship between TBARS and antioxidant enzymes, as clearly documented in the literature.[22] Among the enzymes, GSH showed significant (for SOD = P < 0.05; for GPx and CAT = P <0.001) positive correlation with the rest of the enzymes [Table 3].
Because GSH showed promising results in correlation analysis, it was decided to further probe its role via linear regression analysis considering it to be an independent variable against the rest of the parameters. This showed a very high significance (P < 0.001) for TBARS, GPx, and Catalase. Further, considering the R2 value, the contribution of GSH in the variance seen in GPx was found to be 53%. Derived regression equations will help us to predict the levels of other parameters, provided that GSH levels are known [Table 3]. According to our results, GSH emerged as the most influencing independent parameter. Literature explains in great detail about the multiple roles played by glutathione against oxidative stress. Glutathione's three major roles in the body are as an antioxidant, an immune booster, and a detoxifier. In its reduced form (GSH), glutathione is an important antioxidant (radical scavenger), which reacts directly with free radicals.[1] It also bears the capability to regenerate the most important antioxidants, vitamins C and E, back to their active forms. 22 The capacity of glutathione to regenerate the most important antioxidants is linked with the redox state of the glutathione disulphide-glutathione couple (GSSG/2GSH), and therefore, the intracellular “redox homeostasis” or “redox buffering” capacity is substantiated primarily by GSH.[1] Our results contribute in strengthening the facts presented above.
Thus, with the present study, the role of oxidative stress in leukoplakia and its progression is made evident. Further, the relationship of GSH and the development of dysplasia are well emphasized. Even with the small sample size, the current study projects a highly significant association between GSH and GPx.
Further studies of large samples are warranted to delineate the exact role of GSH in malignant transformation. Moreover, if the threshold level of GSH can be deduced, it will become a breakthrough in the field of early detection.
CONCLUSION
The assay of plasma oxidant-antioxidant parameters has brought oxidative stress into the pathogenesis and evolution of leukoplakia. Further studies of larger sample are needed in the assessment of these parameters, especially GSH in evaluating the disease activity and severity in terms of dysplasia and malignant transformation.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Katakwar P, Metgud R, Naik S, Mittal R. Oxidative stress marker in oral cancer: A review. J Can Res Ther. 2016;12:438–46. doi: 10.4103/0973-1482.151935. [DOI] [PubMed] [Google Scholar]
- 2.Kattappagari KK, Ravi Teja CS, Kommalapati RK, Poosarla C, Gontu SR, Reddy BV. Role of antioxidants in facilitating the body functions: A review. J Orofac Sci. 2015;7:71–5. [Google Scholar]
- 3.Niki E. Antioxidants: Basic principles, emerging concepts, and problems. Biomed J. 2014;37:106–11. doi: 10.4103/2319-4170.128727. [DOI] [PubMed] [Google Scholar]
- 4.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Ergun S, Trosala SC, Warnakulasuriya S, Ozel S, Onal AE, Ofluoglu D, et al. Evaluation of oxidative stress and antioxidant profile in patients with oral lichen planus. J Oral Pathol Med. 2011;40:286–93. doi: 10.1111/j.1600-0714.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahiya P, Kamal R, Gupta R, Saini H. Evaluation of the serum antioxidant status in patients with chronic periodontitis. Indian J Multidiscip Dent. 2016;6:3–6. [Google Scholar]
- 7.Srivastava KC, Austin RD, Shrivastava D, Sethupathy S, Rajesh S. A Case control study to evaluate oxidative stress in plasma samples of oral malignancy. Contemp Clin Dent. 2012;3:271–6. doi: 10.4103/0976-237X.103617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srivastava KC, Austin RD, Shrivastava D. Evaluation of Oxidant-antioxidant status in tissue samples in oral cancer: A case control study. Dent Res J. 2016;13:181–7. doi: 10.4103/1735-3327.178210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van der Waal I, Schepman KP, Van der Meij EH, Smeele LE. Oral Leukoplakia: A Clinicopathological Review. Oral Oncol. 1997;33:291–301. doi: 10.1016/s1368-8375(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 10.Subapriya R, Kumaraguruparan R, Nagini S, Thangavelu A. Oxidant-Antioxidant Status in Oral Precancer and Oral Cancer Patients. Toxicol Mech Methods. 2003;13:77–81. doi: 10.1080/15376510309825. [DOI] [PubMed] [Google Scholar]
- 11.Khanna R, Thapa PB, Khanna HD, Khanna S, Khanna AK, Shukla HS. Lipid peroxidation and antioxidant enzyme status in oral carcinoma patients. Kathmandu Univ Med J (KUMJ) 2005;3:334–9. [PubMed] [Google Scholar]
- 12.Srivastava KC, Austin RD, Shrivastava D, Pranavadhyani G. Oxidant-antioxidant status in tissue samples of oral leukoplakia. Dent Res J. 2014;11:180–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Napier SS, Speight PM. Natural history of potentially malignant oral lesions and conditions: An overview of the literature. J Oral Pathol Med. 2008;37:1–10. doi: 10.1111/j.1600-0714.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 14.Yang YH, Ho PS, Lu HM, Huang IY, Chen CH. Comparing dose-response measurement of oral habits on oral Leukoplakia and oral submucous fibrosis from a community screening program. J Oral Pathol Med. 2010;39:306–12. doi: 10.1111/j.1600-0714.2009.00820.x. [DOI] [PubMed] [Google Scholar]
- 15.Warnakulasuriya S, Johnson NW, Van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med. 2007;36:575–80. doi: 10.1111/j.1600-0714.2007.00582.x. [DOI] [PubMed] [Google Scholar]
- 16.Warnakulasuriya S, Reibel J, Bouquot J, Dabelsteen E. Oral Epithelial dysplasia Classification Systems: Predictive value, utility, weaknesses and scope for improvement. J Oral Pathol Med. 2008;37:127–33. doi: 10.1111/j.1600-0714.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 17.Manoharan S, Kolanjiappan K, Suresh K, Panjamurthy K. Lipid peroxidation and antioxidants status in patients with oral squamous cell carcinoma. Indian J Med Res. 2005;122:529–34. [PubMed] [Google Scholar]
- 18.Klaunig JE, Xu Y, Isenberg JS, Bachowski S, Kolaja KL, Jiang J, et al. The Role of Oxidative Stress in Chemical Carcinogenesis. Environ Health Perspect. 1998;106:289–95. doi: 10.1289/ehp.98106s1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chole RH, Patil RN, Basak A, Palandurkar K, Bhowate R. Estimation of serum malondialdehyde in oral cancer and precancer and its association with healthy individuals, gender, alcohol, and tobacco abuse. J Can Res Ther. 2010;6:487–91. doi: 10.4103/0973-1482.77106. [DOI] [PubMed] [Google Scholar]
- 20.D’souza D, Babu GS, Shetty SR, Balan P. Estimation of serum malondialdehyde in potentially malignant disorders and post-antioxidant treated patients: A biochemical study. Contemp Clin Dent. 2012;3:448–51. doi: 10.4103/0976-237X.107438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative Stress and Oxidative Damage in Carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- 22.Battino M, Bullon P, Wilson M. Oxidative injury and inflammatory periodontal diseases: The challenge of antioxidants to free radicals and reactive oxygen species. Crit Rev Oral Biol Med. 1999;10:458–76. doi: 10.1177/10454411990100040301. [DOI] [PubMed] [Google Scholar]


