When it comes to cations that regulate neurotransmitter release, Ca2+, Na+ and K+ get all the attention. In a sense this is not surprising because the movement of these three cations across presynaptic plasma membranes (PMs) is vital for several aspects of neurotransmission. Whereas flux of Na+ and K+ establishes the resting membrane potential and ensures faithful propagation of action potentials, Ca2+ is arguably most critical for neurotransmitter release. Since an adequate elevation in presynaptic [Ca2+] is sufficient to trigger synaptic vesicle (SV) exocytosis, [Ca2+] is very tightly regulated in that compartment. This regulation involves the combined action of multiple proteins including voltage gated Ca2+ channels (VGCCs), which elevate presynaptic [Ca2+] and trigger SV exocytosis, and plasma membrane Ca2+‐ATPases (PMCAs), which extrude and restore presynaptic [Ca2+] to resting levels. An oft overlooked aspect of Ca2+ extrusion is that PMCAs are actually ATP‐driven Ca2+/H+ exchangers (Trapp et al. 1996). Thus, elevation of presynaptic [H+] is an integral byproduct of SV release. Since cytosolic acidification severely impacts cellular function, it stands to reason that mechanisms must exist to restore the pH balance in the presynaptic compartment during neurotransmission.
Multiple proteins have been documented to mediate the extrusion of presynaptic H+ including PM‐resident Na+/H+ exchangers (NHEs) and vesicular H+‐ATPases (vATPase) (Zhang et al. 2010). In this issue of The Journal of Physiology, Rossano et al. describe a novel role for the vesicular glutamate transporter (VGLUT) in extrusion of presynaptic H+ at the Drosophila neuromuscular junction (NMJ) (Rossano et al. 2017). In a study that is nothing short of a technical tour de force, the authors describe the development and in situ validation of a genetically encoded, cytosolic pH indicator called pHerry – a chimeric protein similar to ClopHensor (Arosio et al. 2010) that comprises the pH‐sensitive super‐eclipitic‐pHluorin fused to mCherry via a short linker (Fig. 1 A). As cytosolic pH in cells expressing pHerry is lowered, the fluorescence intensity of super‐eclipitic‐pHluorin (green channel) decreases, but that of mCherry (red channel) remains unchanged (Fig. 1 B). Thus, the ratio of green to red fluorescence is an elegant ratiometric approach to detect dynamic changes in cytosolic pH in situ in a manner similar to that of previously published work (Koivusalo et al. 2010) employing pHluorin–mCherry fusions in vitro (Fig. 1 B). When expressed in Drosophila motor neurons, this marker localized to the presynaptic compartment of the NMJ and displayed predicted responses to alterations in cytosolic pH. The authors used pHerry to confirm that neuronal activity causes presynaptic acidification proportional to the frequency of action potentials (APs), and noted that following rapid acidification, cytosolic pH gradually returned to baseline.
Figure 1. VGLUT mediates H+ efflux at presynaptic terminals.
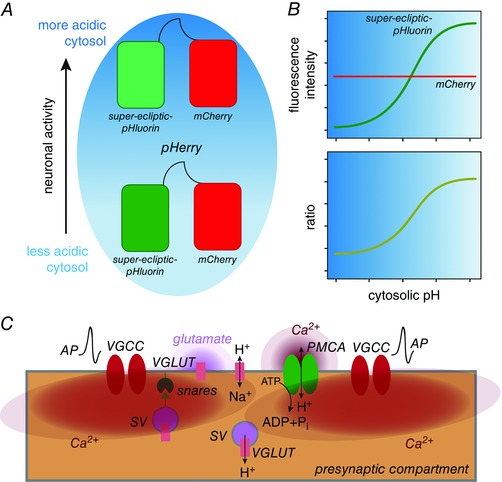
A and B, pHerry is a genetically encoded cytosolic pH indicator. C, action potential (AP)‐induced VGCC opening results in cytosolic Ca2+ (red) elevation, which triggers snare‐dependent SV exocytosis and glutamate (light purple) release. PMCA actively pumps out cytosolic Ca2+ while increasing cytosolic [H+]. The resulting cytosolic acidification is reversed by VGLUT inserted at the PM during SV exocytosis, which functions as a Na+/H+ exchanger to mediate H+ efflux.
Interestingly, the authors found that evoked release of SVs was required for re‐alkalinization, but not the initial acidification, of the cytosol. Whereas blocking SV exocytosis delayed the recovery of cytosolic pH, prolonged fusion of SVs led to precocious re‐alkalinization. These data prompted the authors to focus on the SV as the carrier of the protein(s) that mediate the extrusion of presynaptic H+. Using a pharmacogenetic approach, the authors show that VGLUT mediates the extrusion of presynaptic H+ in a manner that appears independent of glutamate transport. The shift from glutamate to Na+ transport exhibited by VGLUT in SVs and PM, is likely to be a function of the differences in concentrations of glutamate and Na+ in the presynaptic compartment versus the synaptic cleft. Importantly, the authors expressed Drosophila VGLUT in Xenopus oocytes and directly demonstrated that the protein possesses intrinsic Na+/H+ exchange activity, which explains its role in presynaptic H+ extrusion. Together, these studies establish that, at least in Drosophila, VGLUT is also a Na+/H+ exchanger involved in presynaptic re‐alkalinization after being inserted into the PM (Fig. 1 C). Since these findings are consistent with the notion that SVs bear the proteins involved in presynaptic re‐alkalinization, their elegant model explains how the presynaptic compartment scales its capacity for cytosolic re‐alkalinization with the strength of the initiating stimulus.
This study also raises some important questions for future investigation. For instance, it remains to be addressed whether mammalian VGLUTs are also Na+/H+ exchangers at the PM. The authors also found that pharmacological inhibition of both NHEs and vATPases altered the dynamics of cytosolic re‐alkalinization independent of VGLUT. However, these pharmacological findings might not be sufficient to completely distinguish involvement of NHEs or vATPases in intrinsic and activity‐enhanced presynaptic acid extrusion. Indeed, VGLUT, NHEs and vATPases could all mediate H+ extrusion in a context‐dependent manner that is constrained by the unique features of the synapse under observation. Further investigation will be needed to tease apart the functions of these proteins in presynaptic H+ extrusion. Finally, given that genetic studies in both Drosophila and mammals have described the role of the VGLUT proteins in synaptic plasticity as well as excitotoxicity (Daniels et al. 2011; Granseth et al. 2015), it would be worthwhile to examine whether VGLUT‐dependent regulation of presynaptic pH impacts synaptic plasticity and/or excitotoxicity‐induced neurodegeneration.
Additional information
Competing interests
None declared.
Funding
The author was supported by the NINDS grants, R01NS081301 and R21NS094860.
Linked articles This Perspective highlights an article by Rossano et al. To read this paper, visit http://dx.doi.org/10.1113/JP273105.
References
- Arosio D, Ricci F, Marchetti L, Gualdani R, Albertazzi L & Beltram F (2010). Simultaneous intracellular chloride and pH measurements using a GFP‐based sensor. Nat Methods 7, 516–518. [DOI] [PubMed] [Google Scholar]
- Daniels RW, Miller BR & DiAntonio A (2011). Increased vesicular glutamate transporter expression causes excitotoxic neurodegeneration. Neurobiol Dis 41, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granseth B, Andersson FK & Lindstrom SH (2015). The initial stage of reversal learning is impaired in mice hemizygous for the vesicular glutamate transporter (VGluT1). Genes Brain Behav 14, 477–485. [DOI] [PubMed] [Google Scholar]
- Koivusalo M, Welch C, Hayashi H, Scott CC, Kim M, Alexander T, Touret N, Hahn KM & Grinstein S (2010). Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol 188, 547–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossano AJ, Kato A, Minard KI, Romero MF & Meacleod GT (2017). Na+/H+‐exchange via the Drosophila vesicular glutamate transporter (DVGLUT) mediates activity‐induced acid efflux from presynaptic terminals. J Physiol 595, 805–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp S, Luckermann M, Kaila K & Ballanyi K (1996). Acidosis of hippocampal neurones mediated by a plasmalemmal Ca2+/H+ pump. Neuroreport 7, 2000–2004. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nguyen KT, Barrett EF & David G (2010). Vesicular ATPase inserted into the plasma membrane of motor terminals by exocytosis alkalinizes cytosolic pH and facilitates endocytosis. Neuron 68, 1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]


