Lipid, whether stored or consumed, can be utilized as a readily available substrate during times of increased energy demand through highly regulated processes. For ATP to be synthesized from stored lipid, triglyceride must first be liberated by hormone‐sensitive lipase to yield long chain fatty acids (LCFAs). The LCFAs must then be converted to acyl coenzyme A (acyl‐CoA) by an acyl‐CoA synthetase (ACSL) on the outer mitochondrial membrane for transfer into the inner mitochondrial membrane by the rate‐limiting enzyme carnitine palmitoyltransferase‐1 (CPT‐1) for fat oxidation. The regulation of skeletal muscle acyl‐CoA synthesis has received much attention, given its proximity with the mitochondria and associative role in excess lipid accumulation in insulin‐resistant skeletal muscle (Soupene & Kuypers, 2008). Skeletal muscle resistance to anabolic stimuli, such as nutrients, insulin or resistance exercise, is a major concern for the obese, aged and other conditions manifested by insulin resistance. Thus, the role of elevated intramyocellular lipid concentrations contributing to the induction of insulin resistance remains to be clarified.
There are currently five established ACSL genes (1, 3, 4, 5, 6) that are expressed in various tissues and cellular locations, depending upon the functional role. For the purposes of this short perspective, we will primarily focus on ACSL6. Much of the limited research on ACSL6 has focused on brain and neural development and red blood cell formation. ACSL6 is responsible for the promotion of lipid synthesis in brain/neural tissue (Soupene & Kuypers, 2008). ACSL6 is also responsible for the maintenance of phospholipid membrane composition in red blood cells, which lack de novo lipid synthesis (Soupene & Kuypers, 2008). Interestingly, work examining human isoforms of the acsl6 gene described three distinct isoforms that can form dimeric complexes with similar or dissimilar isoforms (Soupene et al. 2010). However, little has been established on the role of ACSL6 in skeletal muscle lipid metabolism, let alone its modulation by diet or obesity in human skeletal muscle.
In this issue of The Journal of Physiology, Teodoro and colleagues (Teodoro et al. 2017) present findings from complementary translational approaches in cell culture, animal, or human models to ascertain the function of ACSL6 on ‘lipid partitioning’ in skeletal muscle. One of the major findings of this work was that the enhanced expression of the long chain acyl‐CoA synthetase ACSL6 drives acyl‐CoA towards lipid synthesis (see Figure 1). First, the authors show that acute high fat diet consumption promotes an increase in ACSL6 mRNA expression in rat and human skeletal muscle. Similarly, ACSL6 overexpression in human skeletal muscle myotube cultures promoted sphingomyelin and phosphatidylcholine ion intensity, while reducing palmitate oxidation. Interestingly, skeletal muscle ACSL6 expression appears to be lower under conditions of diet‐induced obesity in rats and obese humans compared with low‐fat‐fed conditions. This finding may suggest a protective mechanism in place to limit excessive lipid accumulation in skeletal muscle. Consistent with this finding, recent work in mice that lack ACSL5 exhibit reduced adiposity, increased energy expenditure, and delayed triglyceride absorption when compared to ACSL5 wild‐type mice (Bowman et al. 2016). Previous work suggests that treatment of rat cardiomyocytes with insulin promotes ACSL6 mRNA expression (Durgan et al. 2006). Moreover, a cardiac‐specific loss of the insulin receptor in mice reduced ACSL6 mRNA expression in the heart. However, in cardiac tissue, high‐fat feeding for 4 weeks had no significant effects on the expression of any ACSL isoforms. Thus, the mechanisms responsible for directing fatty acids towards oxidation or storage remain to be completely elucidated in the heart.
Figure 1. Regulation of skeletal muscle lipid synthesis during altered states of metabolism.
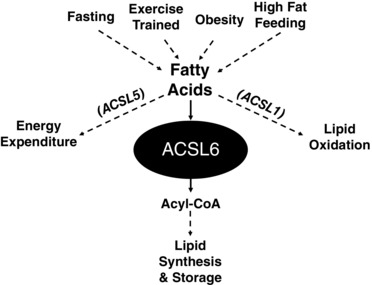
Acyl‐CoA synthetase (ACSL) 6 drives acyl coenzyme A (acyl‐CoA) toward lipid synthesis, while other acyl‐CoA‐synthetases (ACSL 1 or 5) drive lipid oxidation or energy expenditure, respectively. Specifically, acute high fat feeding promotes ACSL6 expression, whereas fasting or chronic aerobic exercise training downregulates gene expression of ACSL6 and markers of lipid synthesis.
Conversely, the results of Teodoro et al. (2017) collectively show that a reduction of ACSL6 promotes lipid oxidation and enhanced mitochondrial respiration. In support of this, the authors report that acute overnight fasting decreased ACSL6 (and SREBP1c) in skeletal muscle. Using a more mechanistic approach, the authors silenced ACSL6 expression in skeletal muscle cultures. There were large reductions in lipid droplet formation and enhanced palmitate oxidation observed that were associated with increased mitochondrial respiratory capacity and oxygen consumption. Extending these findings, Teodoro and colleagues reported that a reduction of ACSL6 in myotube cultures promoted the phosphorylation of 5′‐AMPK‐activated protein kinase (AMPK) and the expression of the transcriptional co‐activator PGC‐1α, both of which are associated with mitochondrial biogenesis and oxidative metabolism. This finding highlights an important aspect of the work that provides a link between ACSL6 and mitochondrial biogenesis. Extending this to a long term stimulus known to promote lipid oxidation (as well as AMPK activation and PGC‐1α expression), skeletal muscle from aerobic exercise trained rats exhibited reduced ACSL6 mRNA expression compared with non‐exercised rats. Interestingly, loss of another acyl‐CoA synthetase, ACSL1, limits the endurance of the knockout mice versus their wild‐type counterparts (Li et al. 2015). This finding was associated with reduced skeletal muscle fatty acid oxidation, coupled with enhanced glucose use that resulted in systemic hypoglycaemia, demonstrating distinct lipid regulatory roles for different ACSL proteins.
The findings of this work by Teodoro and colleagues (Teodoro et al. 2017) provided complementary cell, animal and human approaches to examine the role of ACSL6 in lipid metabolism. These translational findings show that ACSL6 has a distinct role from other acyl‐CoA synthetases in that it ‘drives’ acyl‐CoA towards lipid synthesis, while a reduction in ACSL6 favours mitochondrial respiration and lipid oxidation. Understanding the mechanisms through which intracellular lipid is partitioned between oxidation and storage will be advantageous moving forward towards the development of novel targets to combat obesity or other insulin‐resistant disease states.
Additional information
Competing interests
None declared.
Linked articles This Perspective highlights an article by Teodoro et al. To read this paper, visit http://dx.doi.org/10.1113/JP272962.
References
- Bowman TA, O'Keeffe KR, D'Aquila T, Yan QW, Griffin JD, Killion EA, Salter DM, Mashek DG, Buhman KK & Greenberg AS (2016). Acyl CoA synthetase 5 (ACSL5) ablation in mice increases energy expenditure and insulin sensitivity and delays fat absorption. Mol Metab 5, 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durgan DJ, Smith JK, Hotze MA, Egbejimi O, Cuthbert KD, Zaha VG, Dyck JR, Abel ED & Young ME (2006). Distinct transcriptional regulation of long‐chain acyl‐CoA synthetase isoforms and cytosolic thioesterase 1 in the rodent heart by fatty acids and insulin. Am J Physiol Heart Circ Physiol 290, H2480–H2497. [DOI] [PubMed] [Google Scholar]
- Li LO, Grevengoed TJ, Paul DS, Ilkayeva O, Koves TR, Pascual F, Newgard CB, Muoio DM & Coleman RA (2015). Compartmentalized acyl‐CoA metabolism in skeletal muscle regulates systemic glucose homeostasis. Diabetes 64, 23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E, Dinh NP, Siliakus M & Kuypers FA (2010). Activity of the acyl‐CoA synthetase ACSL6 isoforms: role of the fatty acid Gate‐domains. BMC Biochem 11, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupene E & Kuypers FA (2008). Mammalian long‐chain acyl‐CoA synthetases. Exp Biol Med (Maywood) 233, 507–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teodoro BG, Sampaio IH, Bomfim LHM, Queiroz AL, Silveira LR, Souza AO, Fernandes AMAP, Eberlin MN, Huang T‐Y, Zheng D, Neufer PD, Cortright RN & Alberici LC (2017). Long‐chain acyl‐CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle. J Physiol 595, 677–693. [DOI] [PMC free article] [PubMed] [Google Scholar]


