Abstract
Key points
Cognitive performance is impaired by hypoxia despite global cerebral oxygen delivery and metabolism being maintained.
Using arterial spin labelled (ASL) magnetic resonance imaging, this is the first study to show regional reductions in cerebral blood flow (CBF) in response to decreased oxygen supply (hypoxia) at 2 h that increased in area and became more pronounced at 10 h.
Reductions in CBF were seen in brain regions typically associated with the ‘default mode’ or ‘task negative’ network.
Regional reductions in CBF, and associated vasoconstriction, within the default mode network in hypoxia is supported by increased vasodilatation in these regions to a subsequent hypercapnic (5% CO2) challenge.
These results suggest an anatomical mechanism through which hypoxia may cause previously reported deficits in cognitive performance.
Abstract
Hypoxia causes an increase in global cerebral blood flow, which maintains global cerebral oxygen delivery and metabolism. However, neurological deficits are abundant under hypoxic conditions. We investigated regional cerebral microvascular responses to acute (2 h) and prolonged (10 h) poikilocapnic normobaric hypoxia. We found that 2 h of hypoxia caused an expected increase in frontal cortical grey matter perfusion but unexpected perfusion decreases in regions of the brain normally associated with the ‘default mode’ or ‘task negative’ network. After 10 h in hypoxia, decreased blood flow to the major nodes of the default mode network became more pronounced and widespread. The use of a hypercapnic challenge (5% CO2) confirmed that these reductions in cerebral blood flow from hypoxia were related to vasoconstriction. Our findings demonstrate steady‐state deactivation of the default network under acute hypoxia, which become more pronounced over time. Moreover, these data provide a unique insight into the nuanced localized cerebrovascular response to hypoxia that is not attainable through traditional methods. The observation of reduced perfusion in the posterior cingulate and cuneal cortex, which are regions assumed to play a role in declarative and procedural memory, provides an anatomical mechanism through which hypoxia may cause deficits in working memory.
Keywords: Arterial Spin Label, ASL, CBF, cerebral blood flow, cerebral perfusion, default mode, DMN, hypoxia
Key points
Cognitive performance is impaired by hypoxia despite global cerebral oxygen delivery and metabolism being maintained.
Using arterial spin labelled (ASL) magnetic resonance imaging, this is the first study to show regional reductions in cerebral blood flow (CBF) in response to decreased oxygen supply (hypoxia) at 2 h that increased in area and became more pronounced at 10 h.
Reductions in CBF were seen in brain regions typically associated with the ‘default mode’ or ‘task negative’ network.
Regional reductions in CBF, and associated vasoconstriction, within the default mode network in hypoxia is supported by increased vasodilatation in these regions to a subsequent hypercapnic (5% CO2) challenge.
These results suggest an anatomical mechanism through which hypoxia may cause previously reported deficits in cognitive performance.
Abbreviations
- ASL
arterial spin labelled
- CBF
cerebral blood flow
- DMN
default mode network
- FSL
FMRIB Software Library
- MNI
Montreal Neurologic Institute
- MRI
magnetic resonance imaging
Introduction
Despite the common use of the term ‘resting state’, the human brain is never truly at rest. Demanding 15% of blood ejected from the heart and consuming almost 20% of total oxygen availability when at rest, the brain is always metabolically active (Shulman et al. 2004). Importantly, metabolic activity and thus oxygen consumption varies throughout the brain, which is paralleled by regional differences in blood flow and oxygen delivery, directly coupled to this metabolic activity (Fox et al. 1988; Shulman et al. 2004). However, this extremely high metabolic rate renders the human brain exquisitely sensitive to hypoxia.
The classical understanding of cerebral physiology under acute moderate (altitudes, 1500–3500 m; , ∼90%) and severe (altitudes, 3500 – 5500 m; , ∼80%) hypoxia is that global cerebral blood flow (CBF) increases such that global oxygen delivery remains constant (Ainslie et al. 2014) and global cerebral metabolic rate is unaffected (Kety et al. 1948; Cohen et al. 1967; Overgaard et al. 2012; Willie et al. 2015). Global cerebral metabolic rate is also assumed to be maintained during prolonged moderate hypoxia (9 h) (Bailey et al. 2009) and with acclimatization to high altitude (Möller et al. 2002). These data, however, are at odds with the suppression of cerebral metabolic activity seen in hypoxic tolerant animals (Hochachka, 1986; Hochachka et al. 1996; Boutilier, 2001) and reported in altitude adapted humans (Hochachka et al. 1994). Moreover, recently, several studies have calculated the cerebral metabolic rate non‐invasively using magnetic resonance imaging (MRI), where an unanticipated increase in cerebral metabolism was observed under poikilocapnic hypoxic conditions (Xu et al. 2012; Smith et al. 2013; Vestergaard et al. 2016). Under such poikilopcapnic hypoxic conditions, hyperventilatory‐induced hypocapnia will reduce cerebral tissue and extracellular pH with the potential to increase neuronal excitability via alterations in adenosine generation (Dulla et al. 2005). Indeed, using MRI, a further study has shown that prophylactic low‐dose acetazolamide, which may increase cerebral tissue , abolishes the increase in the cerebral metabolic rate of oxygen after 6 h of normobaric poikilocapnic hypoxia (Wang et al. 2015) Taken together, it is unclear exactly how cerebral metabolism is effected by acute or prolonged periods of poikilocapnic hypoxia.
Nevertheless, it is clear that hypoxia causes neurological deficits (Virues‐Ortega et al. 2004; Maiti et al. 2008; Wagner, 2010) at altitude. Moreover, clinical conditions that involve periods of hypoxaemia, such as sleep apnoea, chronic obstructive pulmonary disease, anaesthesia and stroke, all demonstrate concomitant cognitive declines. Thus, the model of increased global CBF maintaining global metabolism in hypoxia may not reflect the complexity of regional cerebral metabolic and blood flow changes that are truly present. For example, brain regions with exceptionally high metabolism, such as the precuneus and the posterior cingulate, may be particularly vulnerable to reduced oxygen availability (DeVolder, 1990; Laureys et al. 1999). Given this proposed regional susceptibility to hypoxia, regional measurements of CBF during prolonged periods of hypoxia under free‐living conditions are appropriate to fully understand the true response of the brain. Indeed, the concept of regional differences in across the brain despite maintained global cerebral oxygen delivery has been reviewed recently (Ainslie et al. 2016; Hoiland et al. 2016), adding further support that a finer grained examination of CBF in hypoxia is needed. Unfortunately, to the best of our knowledge, no such data exist for periods of hypoxia longer than 40 min (Buck et al. 1998; Binks et al. 2008; Pagani et al. 2011; Micarelli et al. 2013).
Consequently, the present study used arterial spin labelled (ASL) MRI to quantify regional changes in cerebral microvascular perfusion. Images were obtained in response to acute (2 h) and prolonged (10 h) exposure to poikilocapnic normobaric hypoxia (12% O2). It was hypothesized that regional measures of CBF during hypoxia (particularly when prolonged) would allow the identification of regions that showed increased CBF (most probably metabolically active areas of the brain; such as the cerebral cortex, grey matter), as well as regions that either did not show increases, or that may even show decreases. Determining the blood flow responses to these later regions may shed light on the discrepancies between current reports of maintained global cerebral metabolism despite cognitive deficits that are suggestive of regional impairment.
Methods
The present study and experimental design was approved by the ethics committee from the School of Sport Health and Exercise Sciences and the School of Psychology at Bangor University and conformed to the Declaration of Helsinki (2008). Informed consent was obtained in writing after each participant was given a verbal and written explanation of the experimental protocol and each fully understood the possible risks involved in taking part in the study. Procedures were carried out at the School of Sport, Health and Exercise Sciences (altitude = 20 m) or the Bangor Imaging Unit, in the School of Psychology (altitude = 50 m).
Participants
Thirteen males (mean ± SD: 26 ± 6 years; body mass = 77 ± 12 kg; height = 182 ± 9 cm) volunteered to take part. Exclusion criteria were any clinically diagnosed primary headache disorder and ascent to altitude above 1500 m in the previous 6 months.
Experimental design
The experimental design has been reported in detail elsewhere (Lawley et al. 2013; 2014 a; 2014 b) in studies investigating other imaging and physiological end‐points: water mobility and diffusion changes, as well as brain volumetric and intracranial pressure changes. The ASL data, presented for the first time in the present study, were collected at the same time as the other imaging end‐points, although this has not been reported previously except in abstract form. Participants reported to the laboratory at 07.00 h on two separate occasions separated by at least 5 days, having been asked to refrain from alcohol consumption and exhaustive exercise for a period of 48 h before each visit. To maintain adequate hydration participants were instructed to drink 40 ml kg‐1 of water in the 24 h prior to each visit, with an ad libitum diet, which was recorded and repeated in both trials. The controlled fluid and nutritional intake minimized the possibility of systemic dehydration effecting central fluid dynamics.
Trials consisted of either 10 h of normobaric normoxia (21% O2) or normobaric hypoxia (12% O2) in a temperature (23°C) and humidity (40%) controlled environmental chamber. Participants were blinded to the experimental conditions and were assigned to each exposure in a randomized order (http://www.randomization.com). MRI was performed after 2 and 10 h exposure in both trials. Importantly, the same oxic state (21% O2 or 12% O2) was maintained during transportation to and throughout the MRI via the use of appropriate gaseous mixtures through a facial mask fitted with a one‐way valve to minimize rebreathing of expired respiratory gases. MRI sequences were obtained after 10 min of supine rest. During the MRI acquisition, a short (5 min) hypercapnic (5% carbon dioxide) challenge was also introduced. Hypercapnia elevates the partial pressure of CO2 in arterial blood and is a potent cerebral vasodilator. Thus, comparing CBF before and during the hypercapnic challenge gave an indication of the cerebral microvascular capacity to dilate.
Cardiorespiratory variables
Oxygen saturation and heart rate were monitored continuously in the environmental chamber (TM‐2564GP; A&D Medical, San Jose, CA, USA). The use of the MRI system's in vivo monitoring equipment allowed pulse oximetry, heart rate and vector cardiograms to the obtained (Philips Medical, Eindhoven, The Netherlands). Haemoglobin was sampled from the ear‐lobe capillary at the end of each visit (HemoCue Photometer, Sheffield, UK) and arterial oxygen content was calculated as: haemoglobin × 1.39 × (oxygen saturation/100), which excludes oxygen dissolved in plasma. During the final minute of each MRI measurement, end‐tidal carbon dioxide was sampled and analysed using a fast responding gas analyser (IR gas analyser, PA404; Servomex, Crowborough, UK).
MRI acquisition and post‐processing
All MRI sequences were conducted on a 3 Tesla magnetic resonance imaging scanner (Achieva; Philips Medical) using a 16 channel head and neck coil. All imaging sequences were acquired with sensitivity encoding for fast magnetic resonance imaging.
Images
Acquisition
A high resolution T1 weighted image was acquired and used for registration of ASL data to a standard template. The T1 weighted image was acquired as 5 echo MP‐RAGE sequence (TE = 3.5, 5.1, 6.8, 8.5, 10.2 ms, TR = 12 ms, TI = 1150 ms, 3D acquisition, field‐of‐view = 240 × 220 × 130 mm, voxel dimensions = 0.7 × 0.7 × 0.7 mm).
Single‐phase arterial spin labelled images were acquired using the ASL package provided by Philips Medical. This is based on an echo planar acquisition using STAR (signal targeting with alternating radiofrequency) ASL labelling. Labelling of inflowing blood was achieved through a parallel slab applied 20 mm below the acquisition slices (slab thickness 100 mm, delay 1600 ms). ASL is limited with respect to the number of slices (and therefore coverage) that can be obtained in a single scan. Typically, inflowing blood is tagged using a radiofrequency pulse, then, after a short delay, images are taken of a region into which the tagged blood will have travelled during the delay. Imaging too many slices will lead to a situation where inflowing blood will have either left the lower regions, or not reached the upper regions, in the interval between the radiofrequency tag and image acquisition. As such, we were only able to cover a portion of the brain in this experiment. Specifically, 12 slices at 2 × 2 mm in plane resolution with 256 × 256 mm field‐of‐view and 6 mm slice thickness were acquired aligned to the AC–PC axis, with the bottom most slice covering the corpus callosum, providing coverage of the top half of the brain. Slices were acquired as 40 tagged and control pairs with TR of 3 s, and a TE of 15 ms, giving a total scan time of 4 min. ASL data were acquired during baseline normoxia/hypoxia and during a short (5 min) hypercapnic (5% CO2 added to inspired gas) challenge. The increase in blood flow that occurs with breathing CO2 enabled vascular reactivity to be assessed.
Analysis
Imaging data were exported from the scanner as dicom images before being converted to NIFTI format (dcm2nii; http://www.mccauslandcentre.sc.edu/mricro/mricron/dcm2nii.html). All image analysis was then run using the tools provided in the FMRIB Software Library (FSL) (Oxford, UK, http://fsl.fmrib.ox.ac.uk), version 5.0. T1 weighted images were first brain extracted using the FSL BET option (Smith, 2002) and then segmented using FAST (Zhang et al. 2001). ASL data were analysed using BASIL (Chappell et al. 2009), assuming a blood T1 of 1.66 s in normoxia (Lu et al. 2004) and a change to 1.61 s in hypoxia (Lu et al. 2004; Harris et al. 2013) as a result of the reduced O2 saturation. The CBF maps produced by BASIL were then registered to the T1 weighted structural images and smoothed with a Gaussian 4 mm kernel before being masked with the grey matter image from the T1 segmentation. The T1 weighted images were then registered to the Montreal Neurological Institute (MNI) T1 weighted average image supplied with FSL using the FLIRT (Jenkinson and Smith 2001, Jenkinson et al. 2002) toolbox and this registration applied to the grey matter masked CBF images. The grey matter CBF images were then compared using RANDOMISE (Winkler et al. 2014) with cluster based thresholding for correction of multiple comparisons using cluster mass at P < 0.01. MRI scanning was performed in normoxia and hypoxia at 2 and 10 h, giving four time points: normoxia–morning, normoxia–afternoon, hypoxia–morning and hypoxia–afternoon. Statistical comparisons were completed between conditions (normoxia vs. hypoxia) at 2 h and then again at 10 h, as well as within conditions (2 h vs. 10 h for normoxia and hypoxia). The images shown represent the change in blood flow between hypoxia and the respective normoxic time points, thus controlling for potential diurnal variations.
Vascular dilatation to the hypercapnic challenge was assessed by collecting ASL data as described above. Hypercapnia was induced by adding 5% CO2 to the atmosphere that participants were breathing (normoxic or hypoxic atmosphere). Subtracting the CBF maps for normal breathing from the hypercapnic breathing generated an image showing regions that exhibited increases in CBF, corresponding to vascular dilatation. A one sample t test was then run in RANDOMISE (Winkler et al. 2014) to compare normoxia to hypoxia for the 2 and 10 h time points with cluster based thresholding for correction of multiple comparisons using cluster mass at a P < 0.01.
Results
Acute cerebral blood flow responses to hypoxia
To determine the effect of hypoxia on regional CBF, human subjects were exposed to poikilocapnic normobaric hypoxia (12% O2) for 10 h, which caused significant haemoglobin desaturation (∼81%), reduced arterial oxygen content (∆–3 ml dl−1) and hypocapnia (∆–5 mmHg). We have previously reported that this protocol causes significant acute (2 h) increases in global CBF measured at the level of the external carotid and vertebral arteries (Lawley et al. 2014 a), such that global cerebral oxygen delivery remains normal. Resting steady‐state regional CBF was stable over the course of a day in normoxia, with no significant differences between 2 and 10 h.
As expected, regional CBF increased after 2 h in hypoxia, with significant clusters (cluster based correction: P < 0.01) in the frontal pole, middle frontal gyrus, anterior division of the cingulate gyrus, superior frontal gyrus and precentral gyrus, amongst others. Unexpectedly, small regional decreases in blood flow were also observed after 2 h of hypoxia, not only predominantly in the posterior cingulate gyrus, but also in the cuneal cortex and the supramarginal gyrus (cluster based corrections: P < 0.01), all regions normally associated with the default mode network (DMN) and sensitive to neural degeneration (Raichle et al. 2001; Buckner et al. 2005; Cole et al. 2014). Figure 1 shows the regions that exhibited increases (red) and decreases (blue) in CBF (uncorrected voxel‐wise: P < 0.05, improving the display of the regions exhibiting changes in CBF) after 2 h of hypoxia. MNI Co‐ordinates for the top 11 significant clusters are provided in Tables 1 and 2.
Figure 1. After 2 h of hypoxia, regional CBF is seen to vary compared to normoxia with increases (red) and decreases (blue) in CBF seen.
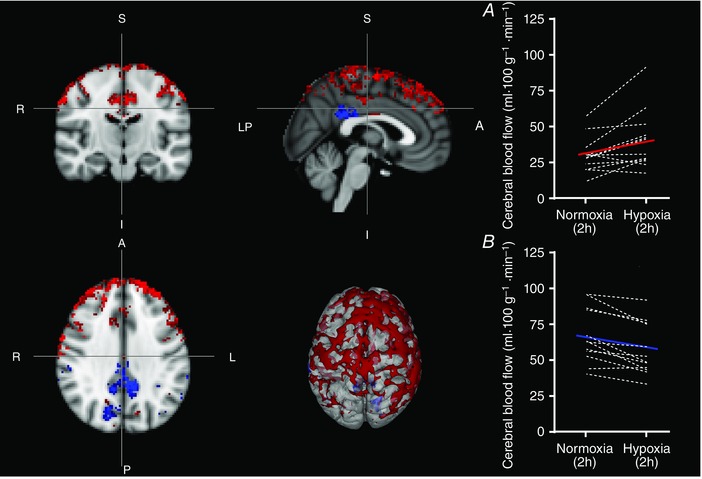
Significant clusters (cluster based correction: P < 0.01) in the frontal pole, middle frontal gyrus, anterior division of the cingulate gyrus, superior frontal gyrus and precentral gyrus, amongst others, showed expected increases in CBF (absolute CBF values in A), whereas unexpected decreases in regional CBF (absolute CBF values in B) were seen not only predominantly in the posterior cingulate gyrus, but also in the cuneal cortex and the supramarginal gyrus (uncorrected voxel‐wise: P < 0.05, improving the display of the regions exhibiting changes in CBF).
Table 1.
MNI co‐ordinates for 11 largest clusters showing increases in CBF after 2 h of hypoxia (clusterwise correction: P < 0.01)
| Cluster index | Number of voxels | MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) | COG X (mm) | COG Y (mm) | COG Z (mm) |
|---|---|---|---|---|---|---|---|---|
| 11 | 149 | 0.828 | 36 | 54 | 26 | 37.9 | 39.8 | 38.1 |
| 10 | 135 | 0.826 | −18 | 62 | 24 | −18.2 | 54.4 | 34.8 |
| 9 | 112 | 0.782 | −4 | −18 | 38 | −0.304 | −18.8 | 43.7 |
| 8 | 95 | 0.743 | 32 | −4 | 48 | 37.3 | −1.07 | 58.8 |
| 7 | 79 | 0.72 | −56 | −10 | 44 | −44.9 | −17.1 | 61.2 |
| 6 | 76 | 0.697 | 64 | −22 | 40 | 57.6 | −33.6 | 51.4 |
| 5 | 75 | 0.708 | 2 | 54 | 30 | 9.17 | 57.5 | 35.7 |
| 4 | 54 | 0.613 | −30 | 0 | 60 | −30.5 | 3.41 | 65.1 |
| 3 | 38 | 0.528 | 44 | 6 | 36 | 46.5 | 11.8 | 42 |
| 2 | 32 | 0.479 | 44 | −58 | 56 | 42.2 | −52.1 | 59.8 |
| 1 | 31 | 0.45 | 20 | 38 | 46 | 14 | 43.4 | 49.2 |
Table 2.
MNI co‐ordinates for 11 largest clusters showing decreases in CBF after 2 h of hypoxia (clusterwise correction: P < 0.01)
| Cluster index | Number of voxels | MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) | COG X (mm) | COG Y (mm) | COG Z (mm) |
|---|---|---|---|---|---|---|---|---|
| 11 | 183 | 0.865 | 2 | −44 | 24 | −0.481 | −42.5 | 32 |
| 10 | 56 | 0.645 | 20 | −74 | 24 | 16.7 | −72.4 | 28.6 |
| 9 | 27 | 0.449 | −66 | −28 | 24 | −64.4 | −31 | 29.2 |
| 8 | 21 | 0.383 | −44 | −64 | 38 | −46.3 | −62.6 | 39 |
| 7 | 19 | 0.337 | −24 | 36 | 24 | −27.7 | 35.9 | 27.7 |
| 6 | 17 | 0.314 | 58 | −32 | 30 | 54 | −31.1 | 32 |
| 5 | 12 | 0.217 | −42 | −38 | 38 | −35.8 | −40.3 | 41.3 |
| 4 | 11 | 0.189 | −10 | −78 | 24 | −13.1 | −75.6 | 24.2 |
| 3 | 10 | 0.177 | −62 | −46 | 24 | −64.6 | −44 | 25.4 |
| 2 | 9 | 0.142 | −26 | 12 | 46 | −26 | 13.3 | 48.2 |
| 1 | 8 | 0.152 | −42 | −68 | 24 | −42.8 | −67.5 | 25.8 |
CBF response to prolonged poikilocapnic hypoxia
Physiological adaptations to hypoxia are dynamic and tend to develop over time (Ainslie & Subudhi, 2014). For example, ventilation progressively increases over time, causing progressive hypocapnia (Smith et al. 2001). Thus, measuring regional CBF after 10 h of hypoxia provided the opportunity to assess the stability of these responses. After 10 h in hypoxia, cerebral regions that previously showed an increase in flow (after 2 h in hypoxia) had returned towards normoxic levels (10 h normoxia vs. 10 h hypoxia comparison). By contrast, the decreased cerebral blood flow that occurred within the DMN after 2 h in hypoxia was exacerbated after 10 h in hypoxia (10 h normoxia vs. 10 h hypoxia comparison). Again, decreases in CBF were not only predominantly in the posterior cingulate, but also extended to the superior division of the lateral occipital cortex, aspects of the anterior cingulate gyrus, the frontal pole, postcentral gyrus and the supramarginal gyrus, amongst others (cluster based corrections: P < 0.01). Figure 2 shows the regions that exhibited decreases in CBF (blue) after 10 h of hypoxia (uncorrected voxel‐wise: P < 0.01, improving the display of the regions exhibiting decreased CBF). Individual CBF in absolute units of ml 100 g−1 min−1 for those regions that showed a statistically significant difference between hypoxia and normoxia are also presented in Figs 1 and 2 for both 2 and 10 h. MNI co‐ordinates for the top 11 significant clusters are provided in Table 3.
Figure 2. Regional variations in CBF after 10 h of hypoxia saw a normalization of blood flow in cortical areas that had shown an increase at 2 h.
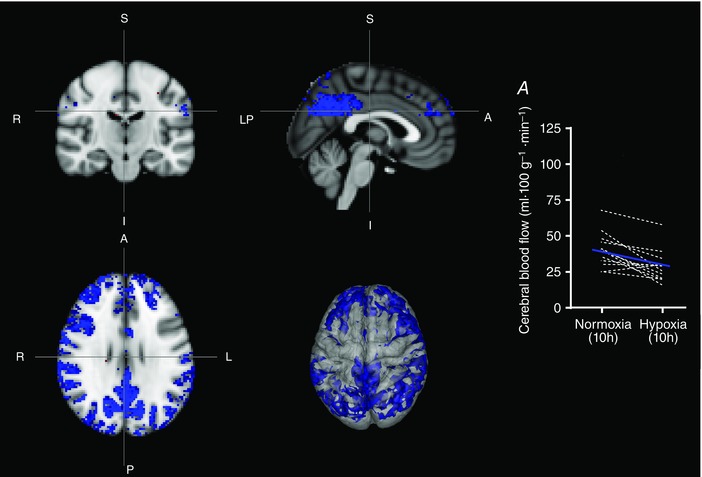
Regions of the DMN that had shown decreased CBF at 2 h, increased in extent at 10 h. Decreases in CBF were found not only predominantly in the posterior cingulate, but also extended to the superior division of the lateral occipital cortex, aspects of the anterior cingulate gyrus, frontal pole, postcentral gyrus and supramarginal gyrus, amongst others (cluster based corrections: P < 0.01). Regions that exhibited decreases in CBF are shown in blue (uncorrected voxel‐wise: P < 0.01, improving the display of the regions exhibiting decreased CBF) with the absolute changes shown in (A).
Table 3.
MNI co‐ordinates for 11 largest clusters showing decreases in CBF after 10 h of hypoxia (clusterwise correction: P < 0.01)
| Cluster index | Number of voxels | MAX | MAX X (mm) | MAX Y (mm) | MAX Z (mm) | COG X (mm) | COG Y (mm) | COG Z (mm) |
|---|---|---|---|---|---|---|---|---|
| 11 | 1485 | 0.968 | −16 | −78 | 24 | 0.935 | −54.5 | 34.3 |
| 10 | 599 | 0.928 | 4 | 28 | 24 | −16.1 | 39.5 | 36.1 |
| 9 | 332 | 0.876 | 62 | −6 | 24 | 50 | 13.5 | 31.6 |
| 8 | 271 | 0.85 | 50 | 34 | 24 | 30.8 | 48.8 | 28.4 |
| 7 | 174 | 0.792 | 60 | −34 | 24 | 62.3 | −30.9 | 31.2 |
| 6 | 129 | 0.754 | −60 | −52 | 24 | −61.2 | −40.4 | 28.4 |
| 5 | 111 | 0.726 | 34 | −50 | 36 | 39.5 | −52.7 | 45.8 |
| 4 | 96 | 0.702 | 62 | −48 | 24 | 56.3 | ‐47.7 | 31.9 |
| 3 | 74 | 0.636 | 32 | 32 | 30 | 32.4 | 34.5 | 36.8 |
| 2 | 55 | 0.576 | 30 | −74 | 36 | 33.6 | −70.8 | 40.4 |
| 1 | 45 | 0.536 | −42 | −60 | 24 | −45.8 | −62.5 | 35.7 |
Increased vascular tone in the default network during hypoxia revealed by a vasodilatory challenge
To confirm the presence of localized regions of vasoconstriction with the DMN, we exposed participants to 5% CO2 after 2 and 10 h in hypoxia. The hypercapnic challenge presented after acute hypoxia resulted in normalization of oxygen saturation ( = 96%) with increases in CBF at 2 h within posterior components of the DMN (i.e. regions that had previously shown reduced perfusion after 2 h in hypoxia) (Fig. 3). Consistent with the increase in regions showing reduced CBF from 2 to 10 h during steady‐state, regions of vasodilatation in response to the CO2 challenge increased after 10 h of hypoxia. Figure 3 shows areas of dilatation in response to hypercapnia after 2 h (blue) and 10 h (red) of exposure to hypoxia (cluster based correction: P < 0.01). Note that these vascular haemodynamics overlap with the steady‐state CBF decreases shown in Figs 1 and 2. Also, when comparing the vascular changes in response to a CO2 challenge in hypoxia with normoxia, the increase in CBF was greater during hypoxia at the 2 h time point (Fig. 3, yellow region, cluster corrected: P < 0.05) but not at 10 h.
Figure 3. Areas of vascular dilatation in response to hypercapnia after 2 h (blue) and 10 h (red) of exposure to hypoxia (cluster based correction: P < 0.01).

The hypercapnic challenge presented after acute hypoxia resulted in increases in CBF at 2 h within posterior components of the DMN (i.e. that had previously showed reduced perfusion after 2 h in hypoxia). Consistent with the increase in regions showing reduced CBF from 2 to 10 h during steady‐state, regions of vasodilatation in response to the CO2 challenge increased after 10 h of hypoxia. Note these vascular haemodynamics overlap with the steady‐state CBF decreases shown in Figs 1 and 2.
Discussion
Overview main findings
The classical understanding of cerebral physiology suggests that exposure to hypoxia causes both hypoxia‐induced vasodilatation and hypocapnia‐induced vasoconstriction (Ainslie & Subudhi, 2014). An increase in global CBF suggests a dominance of vasodilatation. The present study extends these classical findings by using advanced MRI techniques to quantify regional CBF. The present study demonstrated that acute (2 h) and prolonged (10 h) hypoxic exposure led to heterogeneous changes in resting cerebral microvascular perfusion. In particular, the results demonstrate profound and unexpected reductions in CBF within regions of the DMN at 2 h, which were exacerbated at 10 h. Further support for this finding of vasoconstriction within the DMN was obtained by CO2 inhalation. This experimental manipulation would predictably dilate constricted blood vessels and, indeed, resulted in greater vasodilatation across brain regions that almost perfectly overlapped the hypoxia induced vasoconstriction within the DMN.
Previous data on cerebral blood flow and hypoxia
Changes in cerebral perfusion under hypoxic conditions are extremely dynamic. Acutely, CBF increases (Binks et al. 2008; Imray et al. 2014; Lewis et al. 2014; Lawley et al. 2014 a), especially if is maintained via end‐tidal clamping procedures (Willie et al. 2012). However, regional changes in cerebral blood flow are heterogeneous acutely (Binks et al. 2008; Pagani et al. 2000; Buck et al. 1998; Pagani et al. 2011; Micarelli et al. 2013). Over the ensuing hours of poikilocapnic hypoxia (∼6 h) (Imray et al. 2014; Lewis et al. 2014), global cerebral perfusion begins to fall and even returns towards normoxic values after 10 h (Lawley et al. 2014 a) when measured at the level of the extra‐cranial arteries (Lawley et al. 2014 a). The current data obtained using an independent technique further suggests a decrease in blood flow in the cerebral capillaries. Under these or similar (12.9% O2) (Bailey et al. 2011) conditions, cerebral oxygen delivery is reduced. Given the tight metabolic‐flow coupling across the brain (Raichle et al. 2001), our findings of reduced localized perfusion strongly suggest the human brain down‐regulates regional energy turnover, specifically within areas of DMN that characteristically display high metabolic rates.
Mechanisms for decreased regional cerebral blood flow in hypoxia
Metabolism
Regional CBF parallels local neural activity (Raichle et al. 2001). Indeed, it is well established from neuroimaging studies that increased neural activity leads to elevated blood flow to meet the associated metabolic demands (Attwell & Iadecola, 2002; Peppiatt & Attwell, 2004; Shulman et al. 2004; Aubert et al. 2007). Conversely, decreases in neural activity produce concomitant reductions in CBF (Shmuel et al. 2002; Pasley et al. 2007). This suggests the intriguing proposition that regional decreases in CBF in response to hypoxia are a result of de‐activation (reduced metabolic activity) of those regions. That these reductions are found in the DMN further supports this proposition.
In the resting state, a default mode of increased brain function, neural activity and blood flow is seen within the medial prefrontal cortex, posterior cingulate cortex, retrosplenial cortex and inferior parietal lobe (the DMN). This has led to the suggestion that the increased activity of these regions at rest facilitates gathering of information from the external and/or internal environment (Gusnard & Raichle, 2001; Raichle et al. 2001) in a non‐directed fashion. When the brain is no longer at ‘rest’ and engaged in a task, activity in these regions is attenuated (Raichle et al. 2001; Buckner & Vincent, 2007; Raichle & Snyder, 2007). It is generally accepted that deactivation of the DMN during a given task reduces total metabolic load and mobilizes resources to support task associated behaviours. In traditional task‐based functional MRI studies, this is considered to be the result of an acute increase in the energy requirements of brain regions involved in the task. In the present study, however, participants were not engaged in a task and were lying quietly inside the MR scanner under steady‐state conditions. We therefore suggest that the steady‐state deactivation of the DMN that we report, as reflected by reduced CBF, is probably a result of the maintenance of homeostatic regulation (oxygen supply‐demand).
The homeostatic regulation interpretation is somewhat consistent with a classic model of hypoxia tolerance (Hochachka et al. 1996) and, although speculative, in line with measured reductions in glucose metabolism in lowlanders acclimatized to high altitude (Hochachka et al. 1999) and Quechua high altitude natives (Hochachka et al. 1994). However this finding is in contrast to the extensive literature reporting unchanged global cerebral metabolism (as measured directly by arterial–jugular cannulation) in humans under most hypoxic conditions (Cohen et al. 1967; Kety & Schmidt 1948; Overgaard et al. 2012; Ainslie et al. 2014; Möller et al. 2002, Willie et al. 2015). At present, the cause of this discrepancy is unclear, although at least three explanations are conceivable. First, global arterial–venous blood sampling across the brain does not possess the precision to detect subtle microvascular alterations as observed in the present study. Second, cerebral metabolism has predominantly been measured acutely (within minutes) or after successful acclimatization (days at altitude) when physiological adaptations, such as increased cerebral blood flow or haemoglobin concentration, maintain oxygen delivery to the brain (Kety et al. 1948; Cohen et al. 1967; Möller et al. 2002; Overgaard et al. 2012; Ainslie et al. 2014). Third, if cerebral metabolism is normal, our observations suggest regional reductions in CBF, possibly concomitant with increased cerebral arterial blood volume, could be a mechanism through which the brain defends against hypoxia by increasing mean capillary transit time and perfusion heterogeneity, as well as facilitating oxygen and glucose extraction (Jespersen & Ostergaard, 2012). A similar counterintuitive reduction in sublingual microcirculatory blood flow has been observed (Martin et al. 2010). The only study to measure global cerebral metabolism in healthy subjects using a similar protocol to that employed in the present study (9 h at 13% O2) noted increased cerebral oxygen extraction in the face of reduced oxygen delivery (Bailey et al. 2009). According to Raichle et al. (2001), such observations would actually suggest a decrease in global neural activity because of the tight coupling between CBF and oxygen consumption at rest.
Hypocapnia
Under free‐breathing conditions, hypoxia causes hypocapnia and respiratory alkalosis (Dempsey & Forster, 1982; Duong, 2007), which, together, are powerful cerebral vasoconstrictors and modulators of cerebral metabolism. Regional differences in cerebral vasoconstrictor response to acute hypocapnia have been observed previously (Ito et al. 2000; Schlunzen et al. 2010) but, importantly, without clear uniformity to the DMN. Moreover, although pH is a potential modulator of cerebral metabolism, hypocapnia and resultant alkalosis should theoretically increase cerebral metabolism (Chen & Pike, 2010; Jain et al. 2011), which is in contrast to the reductions in cerebral metabolism as inferred from the reductions in cerebral perfusion observed in the present study. It is also worth noting that the impact of pH on cerebral metabolism is not without controversy because reductions in global cerebral metabolism have been noted with mild hypocapnia (Kliefoth et al. 1979; Xu et al. 2011). In the present study, the magnitude of hypocapnia was mild, whereas recent data suggest that severe changes in cerebral pH are required to alter cerebral metabolism (Bain et al. 2016), at least with respect to hypercapnic hypoxia in elite apnoea divers. Taken together, it appears that mild hypocapnia may be responsible for the global fall in CBF between 2 and 10 h in hypoxia, although, at present, it probably does not explain regional hypoperfusion within the DMN.
Altitude symptoms
Prolonged hypoxia caused symptoms of altitude illness (headache, nausea, fatigue, etc.) [with a Lake Louise Score of 4(± 3) at 10 h in hypoxia] in the majority of this cohort, as reported previously (Lawley et al. 2014 b). Allocation of neural resources to the perception of pain and discomfort may have interrupted free‐thinking and caused deactivation of the DMN. However, very few individuals reported symptoms after 2 h in hypoxia despite clear evidence of regional hypoperfusion in the DMN. Moreover, no relationships were observed between symptoms of altitude illness at 10 h in hypoxia and regional reductions in cerebral blood flow. Collectively, these data suggest that there is no link between symptoms of altitude illness and decreases in cerebral blood flow within the DMN in hypoxia.
Implications of reduced default mode network perfusion
Cognition at altitude
Neurocognitive performance is substantially reduced with severe hypoxia (Subudhi et al. 2014), including memory functions (Huppert, 1982; Hornbein, 2001; Banderet & Shukitt‐Hale, 2002; Maiti et al. 2008; Wagner, 2010). However, preservation of global cerebral oxygen delivery and metabolism, both acutely (Kety et al. 1948; Cohen et al. 1967; Möller et al. 2002; Overgaard et al. 2012; Ainslie et al. 2014; Willie et al. 2015) and with acclimatization to hypoxia, make these observations difficult to explain (Möller et al. 2002). The posterior cingulate and cuneal cortex are assumed to play a role in declarative and procedural memory (Laureys et al. 1999); hence, the observed reductions in activity within these regions could conceivably explain decrements in memory function with hypoxia. However, we recognize that this is only speculation based on our data because we did not collect measures of cognitive function.
Hypoxia as a unifying theory of default network deactivation in cerebral disease
Normally, reduced activity in the DMN is associated with reductions in CBF. This is non‐deleterious because blood that does arrive is sufficiently oxygenated to provide a ‘luxury perfusion effect’ for basal metabolism. However, this may not be the case in conditions of environmental hypoxia (as in the present study) and in cerebral disease. Reductions in default network perfusion and/or metabolism have been observed in sleep apnoea (Binks et al. 2008; Santarnecchi et al. 2013; Shiota et al. 2013; Buratti et al. 2014; Osorio et al. 2014) and neurocognitive dysfunction is a hallmark feature of sleep apnoea (Findley et al. 1986; Gagnon et al. 2014; Vaessen et al. 2015), whereas treatment of nocturnal hypoxia in sleep apnoea patients improves DMN functional connectivity and task‐related deactivation of the DMN (Cooke et al. 2009; Prilipko et al. 2014; Troussière et al. 2014) and also enhances memory (Dalmases et al. 2015). A similar regional cerebral hypoperfusion (Smith et al. 1999; Nicolakakis & Hamel, 2011) and parenchyma hypoxia (Zlokovic, 2011) has also been observed in Alzheimer's disease that is associated with cognitive impairment and disease progression. These clinical observations, alongside the current findings, make a compelling (albeit indirect and speculative) case for future research to examine the hypothesis that intermittent and/or continuous hypoxia may lead to chronic deactivation of the DMN.
Limitations
A limitation of the present study is the lack of a concomitant assessment of neurocognition. Thus, any link between the observed reductions in cerebral blood flow in hypoxia and neurological deficits is highly speculative; yet anatomical parallels between brain regions with reduced blood flow and those known to be involved in memory are supportive of this proposition. Another minor limitation was our inability to control during 5% carbon dioxide breathing inside the MRI scanner. Breathing carbon dioxide increases ventilatation and normalizes oxygen saturation. Thus, the effect of hypoxia on the cerebral vasculature may have been removed, making it unclear as to which stimulus or combination thereof (increased – removal of hypoxia, or increased – hypercapnia) may be driving the measured changes in CBF. For clarity, the interpretation of a greater relative vasodilatation in the DMN during CO2 breathing in hypoxia could be confounded by the removal of hypoxic vasodilatation in other brain regions. However, after 10 h in hypoxia, there was no statistical evidence of vasodilatation and yet CO2 breathing still caused increased vasodilatation in the DMN. Thus, overall, our interpretation is the same: prolonged hypoxia leads to localized vasoconstriction, as well as reduced CBF within the DMN. Admittedly, it is not clear whether the hypercapnic challenge eliminated hypoxic dependent effects by increasing ventilatation and or caused a greater CO2 induced vasodilatation (or a combination thereof). Again, this does not change the fact that vasodilatation occurred, nor does it refute our primary finding of reduced CBF in hypoxia within these regions. To further aid this conclusion, comparisons between the hypercapnic response in normoxia with that in hypoxia showed a greater increase in CBF during hypoxia in these regions(Fig. 3). Thus, the greater vasodilatation in the DMN in hypoxia is probably not explained by inherently greater reactivity to CO2.
Conclusions
The results of the present study show that, in hypoxia, the human cerebral circulation alters regional perfusion, especially in areas that normally have high metabolic activity at rest. This suggests that the reduction of CBF reported in the present study is an attempt to adapt to metabolic demand in other regions of the brain. Usually, this demand is created by activation of other brain regions but, in hypoxia, it is created by the low oxygen environment. These results therefore provide exciting evidence of the microcirculatory effects of metabolic challenges and localized cerebral hypoxia in the normally healthy population, and suggest novel avenues of investigation in disease states with a hypoxic component.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
All experimental procedures were carried out at Bangor University. Hypoxic exposure was carried out in the Extremes Research Group Laboratory in the School for Sports Health and Exercise Sciences, whereas imaging was performed in the Bangor Imaging Unit within the School of Psychology. JSL, SJO, JHM and PGM concieved and designed the experiment. JSL and PGM acquired data. PGM analysed the arterial spin labelled images. All authors were involved in the interpretation of final results and the writing of the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
No funding was received for the present study.
Translational perspective.
Hypoxia causes decrements in cognitive performance, slowing simple and complex reaction times and impairing memory. To our knowledge, the present study is the first to show that, when oxygen is in short supply, the human brain responds by reducing blood flow to specific portions of the brain that typically consume large amounts of oxygen. Assuming that there is a consistency of metabolic‐flow coupling in hypoxia as in normoxia, these data indicate that, similar to many hypoxic tolerant animals, the human brain is capable of suppressing local neural activity in a bid to reduce overall metabolic demand. The finding that reduced perfusion is observed in brain regions governing memory and other cognitive processes is supportive of an anatomical mechanism through which hypoxia may cause deficits in cognitive performance.
References
- Ainslie PN & Subudhi AW (2014). Cerebral blood flow at high altitude. High Alt Med Biol 15, 133–140. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Hoiland RL & Bailey DM (2016). Lessons from the laboratory; integrated regulation of cerebral blood flow during hypoxia. Exp Physiol doi: 10.1113/EP085671. [DOI] [PubMed] [Google Scholar]
- Ainslie PN, Shaw AD, Smith KJ, Willie CK, Ikeda K, Graham J & MacLeod DB (2014). Stability of cerebral metabolism and substrate availability in humans during hypoxia and hyperoxia. Clin Sci 126, 661–670. [DOI] [PubMed] [Google Scholar]
- Attwell D & Iadecola C (2002). The neural basis of functional brain imaging signals. Trends Neurosci 25, 621–625. [DOI] [PubMed] [Google Scholar]
- Aubert A, Pellerin L, Magistretti PJ & Costalat R (2007). A coherent neurobiological framework for functional neuroimaging provided by a model integrating compartmentalized energy metabolism. Proc Natl Acad Sci USA 104, 4188–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Lundby C, McEneny J, Young IS, Evans KA, James PE, Shore A, Hullin DA, McCord JM, Pedersen BK & Möller K (2009). Increased cerebral output of free radicals during hypoxia: implications for acute mountain sickness? Am J Physiol Regul Integr Comp Physiol 297, R1283–R1292. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Taudorf S, Berg RMG, Lundby C, Pedersen BK, Rasmussen P & Möller K (2011). Cerebral formation of free radicals during hypoxia does not cause structural damage and is associated with a reduction in mitochondrial PO2; evidence of O2‐sensing in humans? J Cereb Blood Flow Metab 31, 1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain A, Ainslie P, Hoiland R, Barak O, Cavar M, Drvis I, Stembridge M, MacLeod D, Bailey D, Dujic Z & MacLeod D (2016). Cerebral oxidative metabolism is decreased with extreme apnea in humans; impact of acidosis. J Physiol doi: 10.1113/JP272404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banderet LE & Shukitt‐Hale B (2002). Cognitive performance, mood, and neurological status at high terrestrial elevations In Medical Aspects of Harsh Environments. eds. DE Lounsbury, RF Bellamy, and R Zajtchuk R, pp. 729–763. Office of the Surgeon General, Borden Institute, Washington, DC. [Google Scholar]
- Binks AP, Cunningham VJ, Adams L & Banzett RB (2008). Grey matter blood flow change is unevenly distributed during moderate isocapnic hypoxia in humans. J Appl Physiol 104, 212–217. [DOI] [PubMed] [Google Scholar]
- Boutilier RG (2001). Mechanisms of cell survival in hypoxia and hypothermia. J Exp Biol 204, 3171–3181. [DOI] [PubMed] [Google Scholar]
- Buck A, Schirlo C, Jasinksy V, Weber B, Burger C, von Schulthess GK, Koller EA & Pavlicek V (1998). Changes of cerebral blood flow during short‐term exposure to normobaric hypoxia. J Cereb Blood Flow Metab 18, 906–910. [DOI] [PubMed] [Google Scholar]
- Buckner RL & Vincent JL (2007). Unrest at rest: default activity and spontaneous network correlations. Neuroimage 37, 1091–1096. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC & Mintun MA (2005). Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratti L, Viticchi G, Falsetti L, Cagnetti C, Luzzi S, Bartolini M, Provinciali L & Silvestrini M (2014). Vascular impairment in Alzheimer's disease: the role of obstructive sleep apnea. J Alzheimer's Dis 38, 445–453. [DOI] [PubMed] [Google Scholar]
- Chappell MA, Groves AR & Whitcher B (2009). Variational Bayesian inference for a nonlinear forward model. IEEE Trans Sig Process 57, 223–236. [Google Scholar]
- Chen JJ & Pike GB (2010). Global cerebral oxidative metabolism during hypercapnia and hypocapnia in humans: implications for BOLD fMRI. J Cereb Blood Flow Metab 30, 1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PJ, Alexander SC, Smith TC, Reivich M & Wollman H (1967). Effects of hypoxia and normocarbia on cerebral blood flow and metabolism in conscious man. J Appl Physiol 23, 183–189. [DOI] [PubMed] [Google Scholar]
- Cole MW, Bassett DS, Power JD, Braver TS & Petersen SE (2014). Intrinsic and task‐evoked network architectures of the human brain. Neuron 83, 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JR, Ayalon L, Palmer BW & Loredo JS (2009). Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med 5, 305–309. [PMC free article] [PubMed] [Google Scholar]
- Dalmases M, Solé‐Padullés C, Torres M, Embid C, Nuñez MD, MÁ Martínez‐Garcia, R Farré, N Bargalló, Bartrés‐Faz D & Montserrat JM (2015). Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest 148, 1214–1223. [DOI] [PubMed] [Google Scholar]
- Dempsey JA & Forster HV (1982). Mediation of ventilatory adaptations. Physiol Rev 62, 262–346. [DOI] [PubMed] [Google Scholar]
- DeVolder AG (1990). Brain glucose metabolism in postanoxic syndrome. Arch Neurol 47, 197–199. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ & Masino SA (2005). Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron 48, 1011–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong TQ (2007). Cerebral blood flow and BOLD fMRI responses to hypoxia in awake and anaesthetized rats. Brain Res 1135, 186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findley LJ, Barth JT & Powers DC (1986). Cognitive impairment in patients with obstructive sleep apnea and associated hypoxemia. Chest 90, 686–690. [DOI] [PubMed] [Google Scholar]
- Fox P, Raichle M, Mintun M & Dence C (1988). Nonoxidative glucose consumption during focal physiologic neural activity. Science 241, 462–464. [DOI] [PubMed] [Google Scholar]
- Gagnon K, Baril AA, Gagnon JF, Fortin M, Décary A, Lafond C, Desautels A, Montplaisir J & Gosselin N (2014). Cognitive impairment in obstructive sleep apnea. Pathol Biol 62, 233–240. [DOI] [PubMed] [Google Scholar]
- Gusnard DA & Raichle ME (2001). Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2, 685–694. [DOI] [PubMed] [Google Scholar]
- Harris AD, Murphy K, Diaz CM, Saxena N, Hall JE, Liu TT & Wise RG (2013). Cerebral blood flow response to acute hypoxic hypoxia. NMR Biomed 26, 1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW (1986). Defense strategies against hypoxia and hypothermia. Science 231, 234–241. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ & Land SC (1996). Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA 93, 9493–9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Brown WD, Stanley C, Stone CK, Nickles RJ, Zhu GG, Allen PS & Holden JE (1994). The brain at high altitude: hypometabolism as a defense against chronic hypoxia? J Cereb Blood Flow Metab 14, 671–679. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Clark CM, Matheson GO, Brown WD, Stone CK, Nickles RJ & Holden JE (1999). Effects on regional brain metabolism of high‐altitude hypoxia: a study of six US marines. Am J Physiol Regul Integr Comp Physiol 277, R314–R319. [DOI] [PubMed] [Google Scholar]
- Hoiland RL, Bain AR, Rieger MG, Bailey DM & Ainslie PN (2016). Hypoxemia, oxygen content, and the regulation of cerebral blood flow. Am J Physiol Regul Integr Comp Physiol 310, R398–R413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbein TF (2001). The high‐altitude brain. J Exp Biol 204, 3129–3132. [DOI] [PubMed] [Google Scholar]
- Huppert FA (1982). Memory impairment associated with chronic hypoxia. Thorax 37, 858–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imray C, Chan C, Stubbings A, Rhodes H, Patey S, Wilson MH, Bailey DM, Wright AD, for the Birmingham Medical Research Expeditionary Society (2014). Time course variations in the mechanisms by which cerebral oxygen delivery is maintained on exposure to hypoxia/altitude. High Alt Med Biol 15, 21–27. [DOI] [PubMed] [Google Scholar]
- Ito H, Yokoyama I, Iida H, Kinoshita T, Hatazawa J, Shimosegawa E, Okudera T & Kanno I (2000). Regional differences in cerebral vascular response to PaCO2 changes in humans measured by positron emission tomography. J Cereb Blood Flow Metab 20, 1264–1270. [DOI] [PubMed] [Google Scholar]
- Jain V, Langham MC, Floyd TF, Jain G, Magland JF & Wehrli FW (2011). Rapid magnetic resonance measurement of global cerebral metabolic rate of oxygen consumption in humans during rest and hypercapnia. J Cereb Blood Flow Metab 31, 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis 5, 143–156. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17, 825–841. [DOI] [PubMed] [Google Scholar]
- Jespersen SN & Ostergaard L (2012). The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 32, 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS, Schmidt CF, Smith KJ, Willie CK, Ikeda K, MacLeod DB, Bailey DM, Rasmussen P, Ainslie PN, Möller K, Roach RC, Hochachka PW, Brown WD, Nickles RJ, Holden JE, Clark CM, Stanley C & Stone CK (1948). The effetcs of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27, 484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliefoth AB, Grurb RL & Raichle ME (1979). Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem 32, 661–663. [DOI] [PubMed] [Google Scholar]
- Laureys S, Lemaire C, Maquet P, Phillips C & Franck G (1999). Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry 67, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley JS, Alperin N, Bagci AM, Lee SH, Mullins PG, Oliver SJ & Macdonald JH (2014. a). Normobaric hypoxia and symptoms of acute mountain sickness: elevated brain volume and intracranial hypertension. Ann Neurol 75, 890–898. [DOI] [PubMed] [Google Scholar]
- Lawley JS, Oliver SJ, Mullins PG & Macdonald JH (2013). Investigation of whole‐brain white matter identifies altered water mobility in the pathogenesis of high‐altitude headache. J Cereb Blood Flow Metab 33, 1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawley JS, Oliver SJ, Mullins PG, Macdonald JH & Moore JP (2014. b). Prolonged (9 h) poikilocapnic hypoxia (12% O2) augments cutaneous thermal hyperaemia in healthy humans. Exp Physiol 99, 909–920. [DOI] [PubMed] [Google Scholar]
- Lewis NCS, Messinger L, Monteleone B & Ainslie PN (2014). Effect of acute hypoxia on regional cerebral blood flow: effect of sympathetic nerve activity. J Appl Physiol 116, 1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Clingman C, Golay X & van Zijl PCM (2004). Determining the longitudinal relaxation time (T1) of blood at 3.0 Tesla. Magn Reson Med 52, 679–682. [DOI] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Mallick B, Muthuraju S & Ilavazhagan G (2008). High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat 36, 227–238. [DOI] [PubMed] [Google Scholar]
- Martin DS, Goedhart P, Vercueil A, Ince C, Levett DZH & Grocott MPW (2010). Changes in sublingual microcirculatory flow index and vessel density on ascent to altitude. Exp Physiol 95, 880–891. [DOI] [PubMed] [Google Scholar]
- Micarelli A, Jacobsson H, Larsson SA, Jonsson C & Pagani M (2013). Neurobiological insight into hyperbaric hyperoxia. Acta Physiol 209, 69–76. [DOI] [PubMed] [Google Scholar]
- Möller K, Paulson OB, Hornbein TF, Colier WNJM, Paulson AS, Roach RC, Holm S & Knudsen GM (2002). Unchanged cerebral blood flow and oxidative metabolism after acclimatization to high altitude. J Cereb Blood Flow Metab 22, 118–126. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N & Hamel E (2011). Neurovascular function in Alzheimer's disease patients and experimental models. J Cereb Blood Flow Metab 31, 1354–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio RS, Ayappa I, Mantua J, Gumb T, Varga A, Mooney AM, Burschtin OE, Taxin Z, During E, Spector N, Biagioni M, Pirraglia E, Lau H, Zetterberg H, Blennow K, Lu S, Mosconi L, Glodzik L, Rapoport D & de Leon MJ (2014). The interaction between sleep‐disordered breathing and apolipoprotein E genotype on cerebrospinal fluid biomarkers for Alzheimer's disease in cognitively normal elderly individuals. Neurobiol Aging 35, 1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard M, Rasmussen P, Bohm AM, Seifert T, Brassard P, Zaar M, Homann P, Evans KA, Nielsen HB & Secher NH (2012). Hypoxia and exercise provoke both lactate release and lactate oxidation by the human brain. FASEB J 26, 3012–3020. [DOI] [PubMed] [Google Scholar]
- Pagani M, Salmaso D, Sidiras GG, Jonsson C, Jacobsson H, Larsson SA & Lind F (2011). Impact of acute hypobaric hypoxia on blood flow distribution in brain. Acta Physiol 202, 203–209. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Inglis Ben A & Freeman RD (2007). Analysis of oxygen metabolism implies a neural origin for the negative BOLD response in human visual cortex. Neuroimage 36, 269–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt C & Attwell D (2004). Neurobiology: feeding the brain. Nature 431, 137–138. [DOI] [PubMed] [Google Scholar]
- Prilipko O, Huynh N, Thomason ME, Kushida CA & Guilleminault C (2014). An fMRI study of cerebrovascular reactivity and perfusion in obstructive sleep apnea patients before and after CPAP treatment. Sleep Med 15, 892–898. [DOI] [PubMed] [Google Scholar]
- Raichle ME & Snyder AZ (2007). A default mode of brain function: A brief history of an evolving idea. Neuroimage 37, 1083–1090. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA & Shulman GL (2001). A default mode of brain function. Proc Natl Acad Sci USA 98, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarnecchi E, Sicilia I, Richiardi J, Vatti G, Polizzotto NR, Marino D, Rocchi R, Ville D & Rossi A (2013). Altered cortical and subcortical local coherence in obstructive sleep apnea: a functional magnetic resonance imaging study. J Sleep Res 22, 337–347. [DOI] [PubMed] [Google Scholar]
- Schlunzen L, Vafaee MS, Juul N & Cold GE (2010). Regional cerebral blood flow responses to hyperventilation during sevoflurane anaesthesia studied with PET. Acta Anaesthesiol Scand 54, 610–615. [DOI] [PubMed] [Google Scholar]
- Shiota S, Takekawa H, Matsumoto S‐E, Takeda K, Nurwidya F, Yoshioka Y, Takahashi F, Hattori N, Tabira T, Mochizuki H & Takahashi K (2013). Chronic intermittent hypoxia/reoxygenation facilitate amyloid‐β generation in mice. J Alzheimer's Dis 37, 325–333. [DOI] [PubMed] [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele P‐F, Adriany G, Hu X & Ugurbil K (2002). Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36, 1195–1210. [DOI] [PubMed] [Google Scholar]
- Shulman RG, Rothman DL, Behar KL & Hyder F (2004). Energetic basis of brain activity: implications for neuroimaging. Trends Neurosci 27, 489–495. [DOI] [PubMed] [Google Scholar]
- Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX & Avison MJ (1999). Altered brain activation in cognitively intact individuals at high risk for Alzheimer's disease. Neurology 53, 1391–1396. [DOI] [PubMed] [Google Scholar]
- Smith SM (2002). Fast robust automated brain extraction. Hum Brain Mapp 17, 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WDF, Poulin MJ, Paterson DH & Cunningham DA (2001). Dynamic ventilatory response to acute isocapnic hypoxia in septuagenarians. Exp Physiol 86, 117–126. [DOI] [PubMed] [Google Scholar]
- Smith ZM, Krizay E, Guo J, Shin DD, Scadeng M & Dubowitz DJ (2013). Sustained high‐altitude hypoxia increases cerebral oxygen metabolism. J Appl Physiol 114, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subudhi AW, Bourdillon N, Bucher J, Davis C, Elliott JE, Eutermoster M, Evero O, Fan J‐L, Houten SJ‐V, Julian CG, Kark J, Kark S, Kayser B, Kern JP, Kim SE, Lathan C, Laurie SS, Lovering AT, Paterson R, Polaner DM, Ryan BJ, Spira JL, Tsao JW, Wachsmuth NB & Roach RC (2014). AltitudeOmics: the integrative physiology of human acclimatization to hypobaric hypoxia and its retention upon reascent. PLoS ONE 9, e92191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troussière A‐C, Charley CM, Salleron J, Richard F, Delbeuck X, Derambure P, Pasquier F & Bombois S (2014). Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer's disease. J Neurol Neurosurg Psychiatry 85, 1405–1408. [DOI] [PubMed] [Google Scholar]
- Vaessen TJA, Overeem S & Sitskoorn MM (2015). Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 19, 51–58. [DOI] [PubMed] [Google Scholar]
- Vestergaard MB, Lindberg U, Aachmann‐Andersen NJ, Lisbjerg K, Christensen SJ, Law I, Rasmussen P, Olsen NV & Larsson HB (2016). Acute hypoxia increases the cerebral metabolic rate – a magnetic resonance imaging study. J Cereb Blood Flow Metab 36, 1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virues‐Ortega J, Buela‐Casal G, Garrido E & Alc zar B (2004). Neuropsychological functioning associated with high‐altitude exposure. Neuropsychol Rev 14, 197–224. [DOI] [PubMed] [Google Scholar]
- Wagner PD (2010). Operation Everest II. High Alt Med Biol 11, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Smith ZM, Buxton RB, Swenson ER & Dubowitz DJ (2015). Acetazolamide during acute hypoxia improves tissue oxygenation in the human brain. J Appl Physiol 119, 1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, Macleod DB, Shaw AD, Smith KJ, Tzeng YC, Eves ND, Ikeda K, Graham J, Lewis NC, Day TA & Ainslie PN (2012). Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 590, 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willie CK, MacLeod DB, Smith KJ, Lewis NC, Foster GE, Ikeda K, Hoiland RL & Ainslie PN (2015). The contribution of arterial blood gases in cerebral blood flow regulation and fuel utilization in man at high altitude. J Cereb Blood Flow Metab 35, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM & Nichols TE (2014). Permutation inference for the general linear model. Neuroimage 92, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Liu P, Pascual JM, Xiao G & Lu H (2012). Effect of hypoxia and hyperoxia on cerebral blood flow, blood oxygenation, and oxidative metabolism. J Cereb Blood Flow Metab 32, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Uh J, Brier MR, Hart J, Yezhuvath US, Gu H, Yang Y & Lu H (2011). The influence of carbon dioxide on brain activity and metabolism in conscious humans. J Cereb Blood Flow Metab 31, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brady M & Smith S (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation‐maximization algorithm. IEEE Trans Med Imaging 20, 45–57. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV (2011). Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12, 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]


