Abstract
Key points
Cold water immersion and active recovery are common post‐exercise recovery treatments. A key assumption about the benefits of cold water immersion is that it reduces inflammation in skeletal muscle. However, no data are available from humans to support this notion.
We compared the effects of cold water immersion and active recovery on inflammatory and cellular stress responses in skeletal muscle from exercise‐trained men 2, 24 and 48 h during recovery after acute resistance exercise.
Exercise led to the infiltration of inflammatory cells, with increased mRNA expression of pro‐inflammatory cytokines and neurotrophins, and the subcellular translocation of heat shock proteins in muscle. These responses did not differ significantly between cold water immersion and active recovery.
Our results suggest that cold water immersion is no more effective than active recovery for minimizing the inflammatory and stress responses in muscle after resistance exercise.
Abstract
Cold water immersion and active recovery are common post‐exercise recovery treatments. However, little is known about whether these treatments influence inflammation and cellular stress in human skeletal muscle after exercise. We compared the effects of cold water immersion versus active recovery on inflammatory cells, pro‐inflammatory cytokines, neurotrophins and heat shock proteins (HSPs) in skeletal muscle after intense resistance exercise. Nine active men performed unilateral lower‐body resistance exercise on separate days, at least 1 week apart. On one day, they immersed their lower body in cold water (10°C) for 10 min after exercise. On the other day, they cycled at a low intensity for 10 min after exercise. Muscle biopsies were collected from the exercised leg before, 2, 24 and 48 h after exercise in both trials. Exercise increased intramuscular neutrophil and macrophage counts, MAC1 and CD163 mRNA expression (P < 0.05). Exercise also increased IL1β, TNF, IL6, CCL2, CCL4, CXCL2, IL8 and LIF mRNA expression (P < 0.05). As evidence of hyperalgesia, the expression of NGF and GDNF mRNA increased after exercise (P < 0.05). The cytosolic protein content of αB‐crystallin and HSP70 decreased after exercise (P < 0.05). This response was accompanied by increases in the cytoskeletal protein content of αB‐crystallin and the percentage of type II fibres stained for αB‐crystallin. Changes in inflammatory cells, cytokines, neurotrophins and HSPs did not differ significantly between the recovery treatments. These findings indicate that cold water immersion is no more effective than active recovery for reducing inflammation or cellular stress in muscle after a bout of resistance exercise.
Keywords: cryotherapy, cytokines, inflammation, macrophages, neutrophils, recovery
Key points
Cold water immersion and active recovery are common post‐exercise recovery treatments. A key assumption about the benefits of cold water immersion is that it reduces inflammation in skeletal muscle. However, no data are available from humans to support this notion.
We compared the effects of cold water immersion and active recovery on inflammatory and cellular stress responses in skeletal muscle from exercise‐trained men 2, 24 and 48 h during recovery after acute resistance exercise.
Exercise led to the infiltration of inflammatory cells, with increased mRNA expression of pro‐inflammatory cytokines and neurotrophins, and the subcellular translocation of heat shock proteins in muscle. These responses did not differ significantly between cold water immersion and active recovery.
Our results suggest that cold water immersion is no more effective than active recovery for minimizing the inflammatory and stress responses in muscle after resistance exercise.
Abbreviations
- BSA
bovine serum albumin
- CCL2
monocyte chemotactic protein 1
- CCL4
macrophage inflammatory protein 1β
- CCL5
regulated on activation, normal T cell expressed and secreted (RANTES)
- CXCL2
macrophage inflammatory protein 2α
- GDNF
glial cell derived neurotrophic factor
- HSP
heat shock protein
- IGF
insulin‐like growth factor
- IL
interleukin
- LIF
leukaemia inhibitory factor
- MAC
macrophage integrin
- mTOR
mammalian target of rapamycin
- NGF
nerve growth factor
- TBST
Tris‐buffered saline–Tween 20
- TGF
transforming growth factor
- TNF
tumour necrosis factor
Introduction
Our group has previously reported that, compared with active recovery, regular application of cold water immersion after exercise reduces gains in muscle mass and strength following 3 months of resistance training (Roberts et al. 2015 b). Cold water immersion may have attenuated long‐term adaptive responses to resistance exercise by modulating inflammation and cellular stress. There exists a long‐standing belief that by reducing temperature and blood flow in skeletal muscle, cryotherapy such as icing or cold water immersion reduces the metabolic rate of and/or inflammation in tissues within and around the injured site in skeletal muscle. This supposedly protects neighbouring cells against ischaemia after injury, which is thought to reduce the risk of secondary cell injury or death (Bleakley et al. 2010). Animal studies demonstrate the effectiveness of ice massage (Puntel et al. 2011; Takagi et al. 2011; Vieira Ramos et al. 2016) or local infusion of cold saline (Lee et al. 2005; Schaser et al. 2007) for reducing inflammation in muscle following injury. However, no research has examined whether cold water immersion reduces local inflammation in human skeletal muscle after resistance exercise.
Understanding the effects of treatments such as cold water immersion and active recovery on inflammation within skeletal muscle after exercise is important. Cold water immersion is a widespread practice among various sports, and a growing body of evidence suggests that this strategy may affect muscle recovery from strenuous exercise. Repair of skeletal muscle tissue following injury is complex. It involves interactions between inflammatory cells, satellite cells, fibroblasts and endothelial cells, and a range of soluble factors secreted by these cells (Chazaud, 2016). Reducing inflammation in muscle after injury often impedes muscle repair (Urso, 2013). The notion that the anti‐inflammatory effects of cryotherapy such as icing or cold water immersion is beneficial for muscle repair has underpinned sports medicine practice for many years (Meeusen & Lievens, 1986). However, research directly supporting this notion in humans is currently lacking.
The aim of the current study was to investigate whether cold water immersion reduces local inflammation in muscle following exercise compared with active recovery. To conduct this analysis, we used muscle samples that we collected as part of a large study (Roberts et al. 2015 b). In this large study, we compared cold water immersion with active recovery for two reasons. First, active recovery in the form of a low‐intensity ‘warm down’ is also a common strategy that athletes use to recover after exercise (Reilly & Ekblom, 2005) in the belief that it helps to reduce soreness and remove metabolic by‐products in muscle after exercise. Second, compared with remaining sedentary, active recovery after exercise increases cardiac output and muscle blood flow, and reduces total peripheral resistance (Bangsbo et al. 1994; Journeay et al. 2005). We have also previously demonstrated that active recovery and cold water immersion cause divergent changes in cardiac output, temperature, and microvascular blood flow in muscle after exercise (Roberts et al. 2015 a). In the current study, we measured the following: neutrophil and macrophage infiltration in muscle, because these cells are important mediators of inflammation during muscle repair (Tidball & Villalta, 2010); intramuscular gene expression of cytokines and chemokines, because they recruit inflammatory cells to damaged muscle tissue (Peterson et al. 2006; Shireman et al. 2007; Kohno et al. 2011; Zhang et al. 2013) and regulate muscle repair (Broussard et al. 2004; Chen et al. 2007; Serrano et al. 2008; Yahiaoui et al. 2008; Zhang et al. 2013); intramuscular gene expression of nerve growth factor (NGF) and glial cell derived neurotrophic factor (GDNF), because they mediate pain and nociceptor activity in muscle (Murase et al. 2010; Murase et al. 2013); and the heat shock proteins HSP70 and αB‐crystallin, because they have a cytoprotective role, prevent aggregation of denatured proteins, and stabilize the cytoskeleton in cells (Morton et al. 2009). We hypothesized that compared with active recovery, cold water immersion would attenuate leucocyte infiltration and the expression of pro‐inflammatory cytokines, neurotrophins as mediators of muscle soreness, and heat shock proteins as mediators of cellular stress in muscle after exercise.
Methods
Ethical approval
Before providing their written informed consent, all participants were informed of the requirements and potential risks of the study. The experimental procedures adhered to the standards set by the latest revision of the Declaration of Helsinki, and were approved by the Human Research Ethics Committee of The University of Queensland (project number 2012000662).
Experimental design
Nine physically active young men (mean ± SD age 22.1 ± 2.2 years, height 1.80 ± 0.06 m, body mass 83.9 ± 15.9 kg) completed one bout of single‐leg resistance exercise on two separate days (using alternate legs). Each of the sessions was followed by either cold water immersion or active recovery. Muscle biopsies were collected from the vastus lateralis of the exercised leg before and after each training session. The order of the two trials was randomized and counterbalanced to minimize any series order effects. Six of the nine men completed the two trials 1 week apart, and the other three men completed their trials 4 weeks apart. This variation in the timing of the trials was unavoidable, unfortunately, because the investigator who performed the muscle biopsies (T.R.) was not available to perform the biopsies on all of the men at 1‐week intervals. All participants had at least 12 months of experience in resistance training ≥ 3 times per week, and were familiar with all exercise aspects of the study. The data presented herein are part of a large study, from which we have previously published two papers containing separate findings, which are described above (Roberts et al. 2015 b; Figueiredo et al. 2016).
Resistance exercises
The resistance training sessions for the two experimental trials were identical and involved single‐leg exercises such as 45 deg leg press (six sets of 8–12 repetitions), single‐leg squats (three sets of 12 repetitions), knee extensions (six sets of 8–12 repetitions), and walking lunges (three sets of 12 repetitions). The total duration of the session was ∼45 min. All resistance training was supervised and performed at normal room temperature (23–25°C).
Recovery therapies
Cold water immersion was initiated 5 min after the training session. For the cold water immersion treatment, the participants sat in an inflatable bath (iCool iBody; iCool, Miami, Queensland, Australia) for 10 min with both legs immersed in water up to the waist. Water was circulated continuously and maintained at 10.3 ± 0.5°C using a circulatory cooling unit (iCool LITE). For the active recovery treatment, the participants performed 10 min of active recovery at a self‐selected low intensity on a stationary cycle ergometer (Wattbike, Nottingham, UK). The mean power output during active recovery was 36.6 ± 13.8 W. The participants minimized any rewarming following cold water immersion or cooling following active recovery by not showering or bathing for at least 2 h after the recovery therapies. We have previously demonstrated that these recovery therapies stimulate robust and distinct changes in muscle soreness and limb girth (Roberts et al. 2014), cardiac output, muscle temperature and microvascular perfusion (Roberts et al. 2015 a).
Blood and muscle tissue collection. Blood samples were collected before exercise, immediately after exercise, immediately after the recovery therapies (i.e. 15 min after exercise) and 30 min, 1, 2, 24, and 48 h after exercise. The blood samples were collected from an antecubital vein into a serum separation tube (BD, Franklin Lakes, NJ, USA). Serum tubes were left to clot at room temperature for 30 min before centrifugation at 4°C at 3000 g for 10 min to separate the serum, which was then stored at −80°C until the day of analysis. Muscle biopsies were collected from the midportion of the vastus lateralis while the participants were in a fed state before exercise and again at 2, 24 and 48 h after exercise. Pre‐exercise and 2 h post‐exercise biopsies were collected from the same incision. The pre‐exercise biopsy was collected with the needle inserted in a distal direction, and the 2 h biopsy was collected with the needle inserted in a proximal direction. Biopsies at 24 and 48 h were collected from separate incisions, each ∼3 cm proximal from the previous incision, with a proximal needle insertion. This method ensured that all biopsy sites were separated by at least 3 cm to minimize any artefact related to inflammation resulting from multiple biopsies. The same muscle tissue that was analysed in the acute study section of our previous reports (Roberts et al. 2015 b; Figueiredo et al. 2016) was used for the current analyses.
Control procedures
We attempted to minimize potential variation in training responses by providing standardized nutrition before and after each training session and by instructing the participants to avoid performing any extra exercise for 72 h before and for 48 h after each trial. On the morning of each trial, the participants consumed the same meal 2 h before the pre‐exercise muscle biopsy and a 30 g serve of a whey protein isolate drink after exercise before each recovery treatment. They were then allowed to drink only water until the 2 h biopsy was collected, at which time they were provided with another 30 g of whey protein isolate to drink. The participants were instructed to consume their habitual diet for 2 days before each experimental trial and until the 48 h muscle biopsy. The participants were instructed to avoid consuming any additional supplements of any kind between 4 days before each pre‐exercise biopsy and the 48 h post‐exercise muscle biopsy. Dietary intake before and during the first experimental trial was recorded in a food diary and replicated for the second experimental trial.
Blood and muscle tissue analysis
Creatine kinase
Serum creatine kinase activity was measured using a spectrophotometric assay on an automated analyser (Model 7450, Hitachi, Japan).
Plasma cytokines
Plasma cytokine concentrations were measured using commercial enzyme‐linked immunosorbent assays for interleukin (IL) 6, IL‐10 and IL‐1ra. These particular cytokines were selected because they consistently show the greatest increase following exercise; the plasma concentrations of IL‐1β, tumour necrosis factor (TNF) α and monocyte chemotactic protein 1 (CCL2; MCP‐1) do not increase to the same extent (Peake et al. 2015). IL‐6 was measured using a Quantikine High‐Sensitivity Colorimetric Sandwich ELISA (SS600B) from R&D Systems Inc. (Minneapolis, MN, USA). IL‐10 was measured using an OptEIA ELISA Kit II (BD‐550613) from BD Biosciences (San Diego, CA, USA). IL‐1ra was measured using a Quantikine Colorimetric Sandwich ELISA (SRA00B) from R&D Systems, Inc. Measurements were made using a microplate reader (VERSAmax, Molecular Devices, Sunnyvale, CA, USA).
RT‐PCR
Total RNA was extracted from ∼20 mg of muscle tissue using the AllPrep DNA/RNA/miRNA Universal Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. cDNA was synthesized using the High‐Capacity RNA‐to‐cDNA kit (Life Technologies, Carlsbad, CA, USA). mRNA expression was then measured using RT‐PCR on a LightCycler 480 II (Roche Applied Science, Penzberg, Germany) using SYBR Green I Master Mix (Roche Applied Science). The sequences for the primers used in this study are shown in Table 1. The geometric mean of three housekeeping genes (i.e. chromosome 1 open reading frame 43, charged multivesicular body protein 2A, and endoplasmic reticulum membrane protein complex subunit 7) was used for normalization (Vandesompele et al. 2002). Standard and melting curves were obtained for each target to establish primer efficiency and single product amplification.
Table 1.
mRNA primer sequences
| Primer | Forward | Reverse |
|---|---|---|
| MAC1 (CD11b) | TCAGGTGGTGAAAGGCAAGG | ATCTGTCCTTCTCTTAGCCGA |
| CD163 | GCGGCTTGCAGTTTCCTCAA | CTGAAATCAGCTGACTCATGGGA |
| NGF | GAGCGCAGCGAGTTTTGG | TGGCCAGGATAGAAAGCTGC |
| GDNF | GAACTCTTGCCCCTGACCTG | GCGGCACCTCGGATCG |
| HSP70 | TGTTCCGTTTCCAGCCCCCAA | GGGCTTGTCTCCGTCGTTGAT |
| IL6 | TCAATGAGGAGACTTGCCTGG | GGGTCAGGGGTGGTTATTGC |
| IL1β | TTCGAGGCACAAGGCACAA | TGGCTGCTTCAGACACTTGAG |
| IL8 | ACCGGAAGGAACCATCTCAC | GGCAAAACTGCACCTTCACAC |
| LIF | TGAAAACTGCCGGCATCTGA | CACAACTCCTGCCGCCAA |
| CCL2 | GCAATCAATGCCCCAGTCAC | CTTGAAGATCACAGCTTCTTTGGG |
| CCL4 | CTCCCAGCCAGCTGTGGTATTC | CCAGGATTCACTGGGATCAGC |
| CXCL2 | GAAAGCTTGTCTCAACCCCG | TGGTCAGTTGGATTTGCCATTTT |
| TNF | AGCCCATGTTGTAGCAAACC | TGAGGTACAGGCCCTCTGAT |
| EMC7 | GGGCTGGACAGACTTTCTAATG | CTCCATTTCCCGTCTCATGTCAG |
| CHMP2A | CGCTATGTGCGCAAGTTTGT | GGGGCAACTTCAGCTGTCTG |
| C1orf43 | CTATGGGACAGGGGTCTTTGG | TTTGGCTGCTGACTGGTGAT |
Western blotting
Pieces of muscle tissue weighing 45−55 mg were homogenized and fractionated into cytosolic and cytoskeletal fractions using a commercial fractionation kit (ProteoExtract Subcellular Proteome Extraction Kit, cat. no. 539790, Calbiochem, EMD Biosciences, Germany) according to the manufacturer's procedures. The purity of the fractions was confirmed by specific markers for the respective fractions (glyceraldehyde 3‐phosphate dehydrogenase (GAPDH; cytosol and nuclear), poly (ADP‐ribose) polymerase (PARP; nuclear), cyclooxygenase 2 (COX2; membrane) and desmin (cytoskeletal)). Protein concentration was measured in triplicate using a commercial kit (DC Protein Microplate assay, cat. no. 0113, 0114 and 0115, Bio‐Rad, Hercules, CA, USA), a filter photometer (Expert 96, ASYS Hitech, UK), and the software provided (Kim, ver. 5.45.0.1, Daniel Kittrich, Prague, Czech Republic).
Equal amounts of protein were loaded per well (16−50 μg) and were separated by 4−12% SDS‐PAGE under denaturizing conditions for 35−45 min at 200 V in cold Mes running buffer (NuPAGE MES SDS Running Buffer, Invitrogen, Carlsbad, CA, USA). All samples were run in duplicate. After gel electrophoresis, the proteins were transferred onto a polyvinylidene fluoride membrane for 90 min at 30 V using an XCell II Blot Module (Thermo Fisher Scientific, Hemel Hempstead, UK) and NuPAGE transfer buffer (Invitrogen). Membranes were blocked at room temperature for 2 h in 5% fat‐free skimmed milk and 0.1% Tris‐buffered saline with Tween 20 (TBST) (cat. no. 170‐6435, Bio‐Rad; Tween‐20, cat. no. 437082Q, VWR International, Radnor, PA, USA; skim milk, cat. no. 1.15363, Merck, Darmstadt, Germany). Blocked membranes were incubated overnight at 4°C with a primary monoclonal antibody against αB‐crystallin (mouse anti‐αB‐crystallin, cat. no. ADI‐SPA‐222, Enzo Life Sciences, Farmingdale, NY, USA) diluted 1:4000. After incubation, membranes were washed and incubated with a secondary antibody at room temperature for 1 h. The membranes for αB‐crystallin immunoblotting were incubated with a secondary antibody diluted 1:30 000 (goat anti‐mouse, cat. no. 31430, Thermo Fisher Scientific/Pierce Biotechnology, Rockford, IL, USA).
Membranes used for HSP70 quantification were incubated initially with another primary antibody (anti‐FoxO3a; data not shown) and secondary antibody. The primary and secondary antibodies were then stripped from the membranes using Restore Western Blot Stripping Buffer (cat. no. 21059, Thermo Fisher Scientific), blocked for 2 h at room temperature, and incubated with the primary polyclonal antibody to HSP70 (rabbit anti‐HSP70, cat. no. ADI‐SPA‐812, Enzo Life Sciences) diluted 1:4000 at 4°C overnight. The membranes were then incubated with a secondary antibody (anti‐rabbit IgG, horseradish peroxidase (HRP)‐linked antibody, cat. no. 7074, Cell Signaling Technology, Danvers, MA, USA). All antibodies were diluted in 1% fat‐free skimmed milk and 0.1% TBST solution. Between stages, membranes were washed in 0.1% TBST. Bands were visualized using an HRP detection system (Super Signal West Dura Extended Duration Substrate, cat. no. 34076, Thermo Fisher Scientific/Pierce Biotechnology). Chemiluminescence was measured using a ChemiDoc MP System (Bio‐Rad Laboratories), and band intensities were calculated with molecular imaging software (Image Lab, Bio‐Rad Laboratories). All samples were analysed in duplicate, and mean values were used for statistical analyses.
Immunohistochemistry
Cross‐sections of muscle tissue, 800 μm thick, were cut using a microtome at −20°C (CM3050, Leica Biosystems, GmbH), mounted on microscope slides (Superfrost Plus, Thermo Scientific, Boston, MA, USA), air‐dried and stored at −80°C. Muscle sections from each subject obtained at all time points before and after both trials were mounted on the same microscope slide. Before immunostaining, frozen sections were air‐dried and blocked in 1% bovine serum albumin (BSA) in phosphate‐buffered saline (PBS) for 30 min. Sections were then incubated with primary antibodies (listed in Table 2) in 1% BSA overnight at 4°C. The following primary antibodies were used: anti‐laminin to stain the inner surface of myofibres (cat. no. Z009701‐2; DakoCytomation, Glostrup, Denmark; dilution 1:1000); CD66b to stain granulocytes (cat. no. M1594; clone CLB‐B13.9, Sanquin Reagents, Amsterdam, The Netherlands; dilution 1:500); CD68 to stain macrophages and cells with bilobed nuclei (cat. no. M0718; clone EBM‐11, DakoCytomation; dilution 1:300); anti‐αB‐crystallin to stain αB‐crystallin bound to cytoskeletal/myofibrillar structures (cat. no. ADI‐SPA‐222, Enzo Life Sciences; dilution 1:200) and SC‐71 to quantify type IIa and IIx fibres (cat. no. SC‐71, Developmental Studies Hybridoma Bank, Iowa City, IA, USA; dilution 1:500).
Table 2.
Plasma cytokine concentrations
| Pre | Post | Rec | 0.5 h | 1 h | 2 h | 24 h | 48 h | |
|---|---|---|---|---|---|---|---|---|
| IL‐6 (pg ml–1) | ||||||||
| CWI | 1.1 (0.5) | 2.2 (1.2)* | 3.0 (1.2)* | 3.0 (1.3)* | 3.6 (1.7)* | 2.0 (1.8) | 1.5 (0.5) | 1.9 (1.7) |
| ACT | 1.2 (0.6) | 2.3 (0.8)* | 3.0 (1.3)* | 3.3 (1.6)* | 2.7 (1.1)* | 2.7 (1.4)* | 1.3 (0.7) | 1.2 (0.3) |
| IL‐10 (pg ml–1) | ||||||||
| CWI | 8.4 (9.0) | 33.0 (62.0) | 10.0 (12.8) | 9.1 (8.2) | 18.7 (25.3) | 15.2 (18.0) | 8.8 (11.3) | 7.7 (8.4) |
| ACT | 11.5 (16.2) | 8.9 (11.0) | 9.1 (9.2) | 5.4 (2.2) | 9.8 (11.3) | 11.6 (10.2) | 7.8 (10.8) | 8.1 (8.5) |
| IL‐1ra (pg ml–1) | ||||||||
| CWI | 243 (145) | 343 (240) | 203 (158) | 243 (148) | 293 (197) | 269 (167) | 230 (138) | 262 (165) |
| ACT | 263 (203) | 348 (234) | 282 (217) | 425 (379) | 312 (216) | 313 (242) | 281 (166) | 246 (138) |
Data are mean (SD). n = 9. * P < 0.05 versus pre‐exercise. ACT, active recovery; CWI, cold water immersion; Pre, pre‐exercise; Post, immediately post‐exercise; Rec, immediately after recovery therapies.
After overnight incubation, the slides were washed three times in PBS for 10 min. Sections were then incubated for 1 h with secondary antibodies diluted 1:200 in 1% BSA at room temperature. The secondary antibodies used were Alexa Fluor 594 F(ab′)2 fragment of goat anti‐rabbit IgG (cat. no. A‐11072, Invitrogen/Life Technologies), Alexa Fluor 488 anti‐mouse IgG (cat. no. A‐11029, Invitrogen), CF488A goat anti‐mouse IgG (cat. no. 20010, Biotium, Hayward, CA, USA), and CF594 goat anti‐rabbit IgG (cat. no. 20112, Biotium). The fluorochrome‐stained sections were washed three times in PBS for 10 min. After the last wash, the sections were mounted with ProLong Gold Antifade reagent with 4′,6‐diamidino‐2‐phenylindole (DAPI; Invitrogen).
Muscle sections were visualized using a high‐resolution camera (DP72, Olympus, Japan) mounted on a microscope (BX61, Olympus) with a fluorescent light source (X‐Cite 120PCQ, EXFO, Canada). For leucocyte analysis, the numbers of CD66b‐ and CD68‐positive cells, and the total number of muscle fibres from the area examined were counted (see Figs 2 and 3). The data are presented as the number of CD66b‐ or CD68‐positively stained cells per 100 skeletal muscle fibres. For the αB‐crystallin analysis, the numbers of αB‐crystallin‐positive and ‐negative fibres were counted. A fibre was considered positive if the staining inside the fibre was scattered and uneven, and negative if the staining was homogeneous. The data are presented as the percentage of αB‐crystallin‐positive fibres. Areas of sections that contained freeze damage or were folded during the cutting procedure were not included in the analyses.
Figure 2. Representative image of immunofluorescence staining for CD66b+ neutrophils.
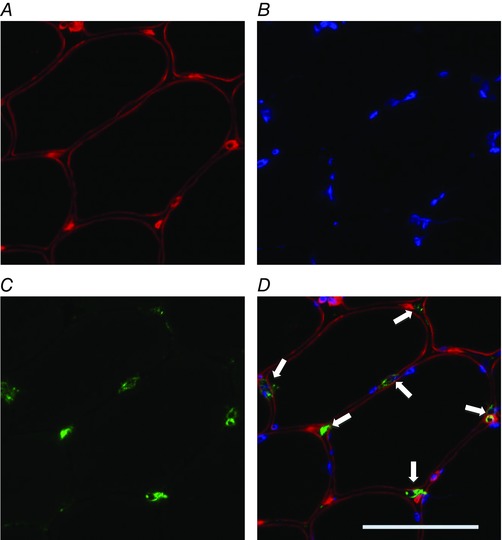
A, red laminin staining of the sarcolemma; B, blue DAPI staining of nuclei; C, green staining for CD66b; D, merged images. Arrows indicate CD66b+ neutrophils. Scale bar represents 50 μm. n = 9. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3. Representative image of immunofluorescence staining for CD68+ macrophages.
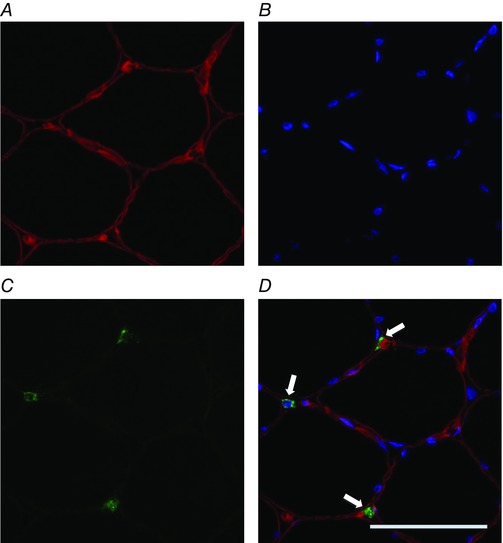
A, red laminin staining of the sarcolemma; B, blue DAPI staining of nuclei; C, green staining for CD68; D, merged images. Arrows indicate CD68+ macrophages. Scale bar represents 50 μm. n = 9. [Colour figure can be viewed at wileyonlinelibrary.com]
Statistical analysis
Before statistical analysis, all data were checked to determine if they were normally distributed. Log transformations were applied to data that were not normally distributed (i.e. macrophage cell counts; MAC1, TNF and NGF mRNA; serum creatine kinase activity). Normally distributed data (i.e. GDNF mRNA, HSP70, αB‐crystallin and plasma cytokine concentrations) were analysed using a 2 × 3 repeated‐measures ANOVA to calculate the main effects of time and time × trial interaction. When a significant main effect was evident (P < 0.05), a post hoc Student's paired t test was used to compare changes over time and differences between the trials. Normally distributed data are presented as the mean ± SD, and log‐transformed data are presented as the geometric mean ± 95% confidence interval of the geometric mean. Data that were not normally distributed (i.e. neutrophil cell counts and CD163, IL1β, IL‐6, CCL2 and HSP70 mRNA) were analysed using Friedman's test, followed by Wilcoxon's signed‐ranked tests to compare changes over time and differences between the trials. Non‐normally distributed data are presented as the median ± interquartile range. The false discovery rate was used to correct for multiple comparisons.
Results
Inflammatory cell infiltration
Exercise induced a strong and sustained inflammatory response in muscle (Fig. 1). The number of CD66b+ neutrophils in muscle was higher than the pre‐exercise number at 2 h after active recovery (9‐fold difference; P = 0.015) and tended to be higher at 2 h after cold water immersion (3‐fold difference; P = 0.086). mRNA expression of macrophage cell surface receptors increased in muscle after exercise. As a general marker of pro‐inflammatory cells, MAC1 expression was higher than the pre‐exercise expression at 24 h (1.2‐fold; P = 0.020) and 48 h (2.4‐fold; P = 0.010) after active recovery, and 48 h after cold water immersion (1.8‐fold; P = 0.036). The number of CD68+ macrophages in muscle was higher than before exercise at 48 h after active recovery (1.5‐fold P = 0.008) and tended to be higher 48 h after cold water immersion (1.7‐fold; P = 0.071). As a marker of anti‐inflammatory macrophages, CD163 expression was higher than the pre‐exercise expression at 24 h (6.7‐fold; P = 0.008) and 48 h (3.2‐fold; P = 0.011) after active recovery, and at 24 h after cold water immersion (3.2‐fold; P = 0.008). MAC1 and CD163 mRNA expression and neutrophil and macrophage counts in muscle did not differ significantly between the trials. Representative images of staining for CD66b+ neutrophils and CD68+ macrophages are shown in Figs 2 and 3.
Figure 1. Post‐exercise changes in CD66b+ neutrophil infiltration, CD68+ macrophage infiltration, and MAC1 and CD163 mRNA expression.
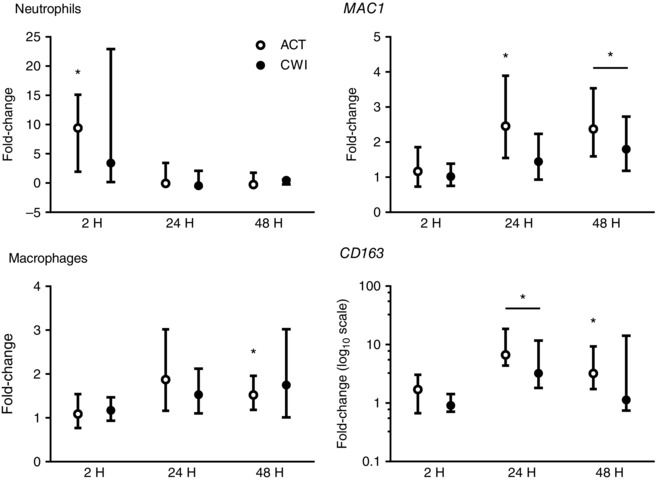
Data are presented as the change in the median ± interquartile range for neutrophils and CD163 mRNA, and the geometric mean ± 95% confidence interval for macrophages and MAC1 mRNA. ACT, active recovery; CWI, cold water immersion. n = 9. * P < 0.05 versus pre‐exercise value.
Cytokines and chemokines
Exercise induced the expression of several pro‐inflammatory cytokine and chemokine genes in muscle (Figs 4 and 5). IL1β expression was higher than before exercise at 2 h after active recovery (9‐fold; P = 0.011) and at 2 h after cold water immersion (27‐fold; P = 0.021). TNF expression was higher than before exercise at 2 h (2.6‐fold; P = 0.004) and 24 h (2.9‐fold; P = 0.005) after active recovery, and at 2 h after cold water immersion (2.7‐fold; P = 0.026). IL6 expression was higher than before exercise at 2 h after active recovery (11‐fold; P = 0.004) and at 2 h (8.6‐fold; P = 0.008), 24 h (1.7‐fold; P = 0.021), and 48 h (2.2‐fold; P = 0.015) after cold water immersion. Monocyte chemotactic protein 1 (CCL2) expression was higher than before exercise at 2 h after active recovery (21‐fold; P = 0.008) and cold water immersion (30‐fold; P = 0.008), and it remained higher at 24 h and 48 h after both trials. Macrophage inflammatory protein 1β (CCL4) expression was higher than before exercise at 24 h after active recovery (2.8‐fold; P = 0.019), and tended to be higher than before exercise at 24 h after cold water immersion (1.7‐fold; P = 0.068). CCL5 expression showed a similar pattern of changes to CCL4 (data not shown). Macrophage inflammatory protein 2α (CXCL2) expression was higher than before exercise at 2 h after active recovery (9.4‐fold; P < 0.001) and cold water immersion (17‐fold; P < 0.001). It also tended to be higher than before exercise at 24 h after active recovery (1.8‐fold; P = 0.065) and was higher 24 h after cold water immersion (1.6‐fold; P = 0.017). IL8 expression was higher than before exercise at 2 h after active recovery (125‐fold; P < 0.001) and cold water immersion (272‐fold; P < 0.001). It was also higher than before exercise at 24 h after active recovery (8.9‐fold; P = 0.030) and tended to be higher 24 h after cold water immersion (5.3‐fold; P = 0.052). Leukaemia inhibitory factor (LIF) expression was higher than before exercise at 2 h after active recovery (32‐fold; P < 0.001) and cold water immersion (37‐fold; P < 0.001). It also tended to be higher than before exercise at 24 h after active recovery (2.5‐fold; P = 0.065) and was higher 24 h after cold water immersion (2.2‐fold; P = 0.037). Cytokine and chemokine mRNA expression in muscle did not differ significantly between the trials.
Figure 4. Post‐exercise changes in expression of IL1β, TNF, IL6 and CCL2 mRNA.
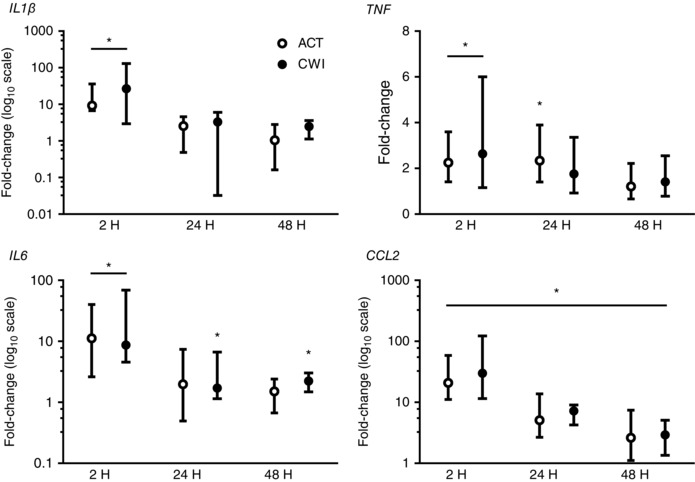
Data are presented as changes in the median ± interquartile range for IL1β, IL6 and CCL2 expression, and the geometric mean ± 95% confidence interval for TNF expression. n = 9. * P < 0.05 versus pre‐exercise value.
Figure 5. Post‐exercise changes in expression of CCL4, CXCL2, IL8 and LIF mRNA.
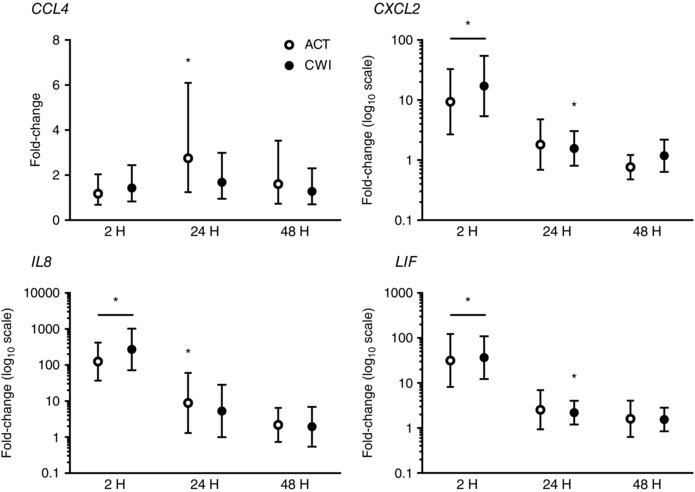
Data are presented as changes in the geometric mean ± 95% confidence interval. n = 9. * P < 0.05 versus pre‐exercise value.
Neurotrophins
Exercise stimulated the expression of two neurotrophins associated with muscle soreness in muscle (Fig. 6). GDNF and NGF expression increased in muscle after exercise. GDNF expression was higher than before exercise at 2 h after active recovery (3.7‐fold; P = 0.001) and cold water immersion (4.3‐fold; P < 0.001). NGF expression was higher than before exercise at 24 h after active recovery (2.0‐fold; P = 0.040), and at 2 h (1.2‐fold; P = 0.040), 24 h (2.1‐fold; P = 0.010) and 48 h (1.5‐fold; P = 0.010) after cold water immersion. GDNF or NGF expression in muscle did not differ significantly between the trials.
Figure 6. Post‐exercise changes in expression of GDNF and NGF mRNA.
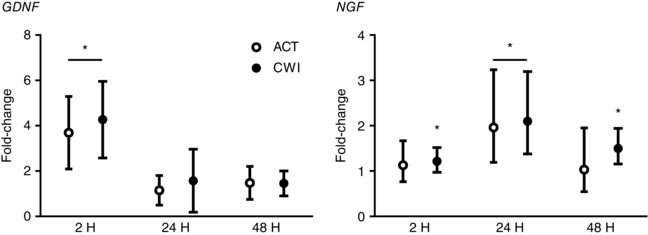
Data are presented as changes in the mean ± SD for GDNF and the geometric mean ± 95% confidence interval for NGF. n = 9. * P < 0.05 versus pre‐exercise value.
HSPs
HSP70 mRNA expression was higher than before exercise at 2 h after active recovery (2.1‐fold; P = 0.013) and cold water immersion (2.0‐fold; P = 0.028) (Fig. 7). The protein content of HSP70 in the cytosol fraction of muscle homogenates was lower than before exercise at 2 h (14%; P = 0.032) and 48 h (15%; P = 0.034) after active recovery, and at 2 h after cold water immersion (18%; P = 0.044) (Fig. 8). The protein content of HSP70 in the cytoskeletal fraction was unchanged after both trials. The protein content of αB‐crystallin in the cytosol fraction of muscle homogenates was lower than before exercise at 2 h after both active recovery (−33%; P = 0.001) and cold water immersion (−36%; P = 0.003) (Fig. 8). It remained lower than the pre‐exercise value for therest of the post‐exercise recovery period in both trials. Conversely, the protein content of αB‐crystallin in the cytoskeletal fraction of muscle homogenates showed a strong trend toward an increase after exercise (P = 0.052). This response was accompanied by an increase in the percentage of αB‐crystallin‐positive fibres (Fig. 9). The median percentage of αB‐crystallin‐positive fibres was 26% (interquartile range 3−77%) at 2 h after active recovery and 19% (interquartile range 2−43%) at 2 h after cold water immersion. Staining for αB‐crystallin was scattered and evident mainly in type II fibres (Fig. 9). The percentage of αB‐crystallin‐positive fibres did not differ significantly between the trials.
Figure 7. Post‐exercise changes in expression of HSP70 mRNA.
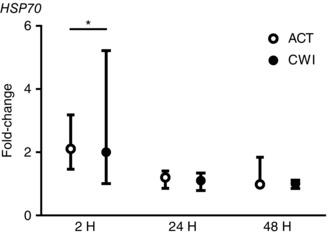
Data are presented as the change in the median ± interquartile range. n = 9. * P < 0.05 versus pre‐exercise value.
Figure 8. Representative immunoblots and post‐exercise changes in the protein content of HSP70 and αB‐crystallin in the cytosol and cytoskeletal fraction of muscle homogenates.
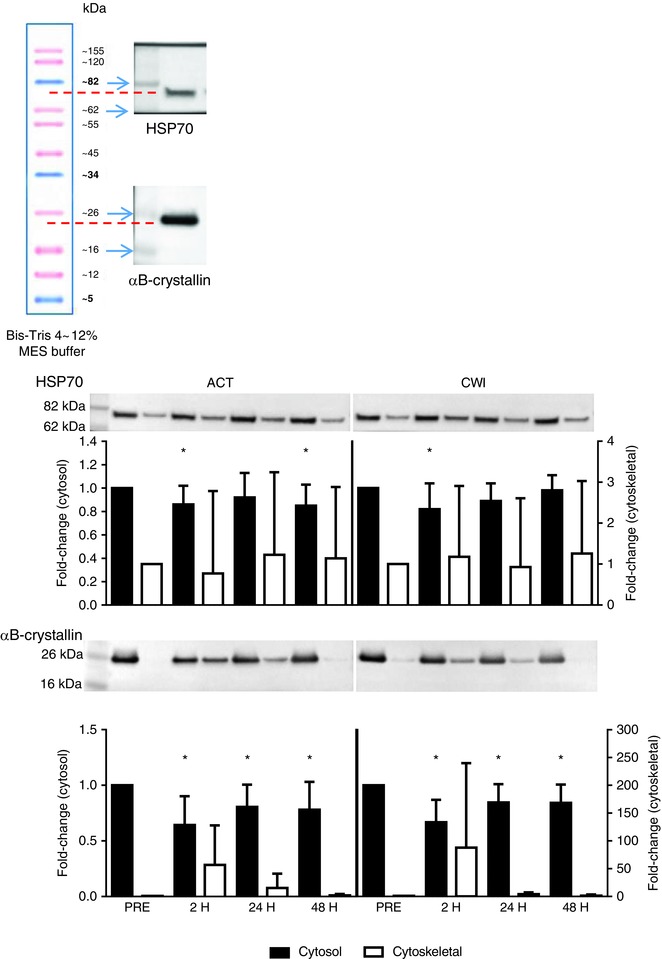
Data are presented as the mean ± SD. n = 9. * P < 0.05 versus pre‐exercise value. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 9. Intramuscular localisation of αB‐crystallin.
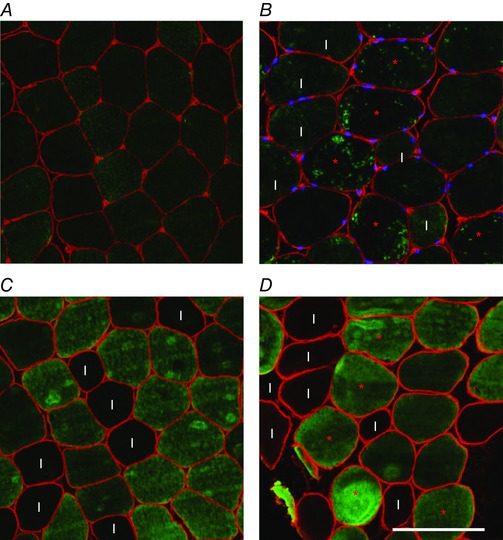
Upper panels show immunohistochemistry staining for αB‐crystallin in muscle fibres before exercise (A) and at 2 h after exercise (B). A fibre was considered positive if the staining inside the fibre was scattered and uneven (marked with red asterisks). Fibres were considered negative if the staining was homogeneous (all fibres in the left image). Lower panels show immunohistochemistry staining for myosin heavy chain IIA and IIX (SC71 antibody) in neighbouring sections. Before exercise, there was more αB‐crystallin protein present in type I fibres (marked ‘I’ in panel B, C and D), whereas after exercise, the scattered αB‐crystallin staining was found mainly in type II fibres (panel D). Scale bar represents 100 μm. n = 9. [Colour figure can be viewed at wileyonlinelibrary.com]
Creatine kinase and cytokines
A systemic indirect marker of muscle damage, serum creatine kinase activity, increased moderately after both exercise trials (P < 0.05) (Fig. 10). It remained elevated up to 48 h after active recovery (P < 0.05). Plasma IL‐6 concentration also increased moderately after both exercise trials, and remained elevated up to 2 h after exercise (Table 2). By contrast, the plasma concentrations of IL‐10 (P = 0.40) and IL‐1ra (P = 0.24) did not change after either trial (Table 2). The magnitude of the changes in creatine kinase and cytokines was consistent with the intermittent nature and limited muscle mass used for the single‐leg resistance exercise. There were no significant differences in serum creatine kinase activity or plasma cytokine concentrations between the trials.
Figure 10. Post‐exercise changes in serum creatine kinase activity.
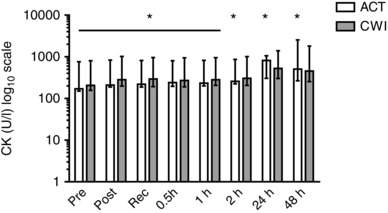
Data are presented as the geometric mean ± 95% confidence interval. n = 9. * P < 0.05 versus pre‐exercise value.
Discussion
To our knowledge, this is the first study to compare the effects of cold water immersion versus active recovery on inflammation, neurotrophins and HSPs within skeletal muscle following exercise in humans. Exercise stimulated intramuscular inflammation, as demonstrated by increased mRNA expression of MAC1 and CD163, and increased the numbers of neutrophils and macrophages. Intramuscular gene expression of cytokines and neurotrophins also increased, and HSPs translocated from the cytosol to cytoskeletal structures in muscle after exercise. Contrary to our hypothesis, these responses did not differ substantially between cold water immersion and active recovery. These findings provide evidence against the traditional notion that cryotherapy such as cold water immersion helps to restrict inflammation and cellular stress responses in muscle following exercise. Taking into account our previous observation that regular application of cold water immersion attenuated long‐term muscle adaptation compared with active recovery (Roberts et al. 2015 b), the present findings suggest that this response was not due to a reduction in inflammation and/or cellular stress after cold water immersion.
Animal studies have demonstrated that icing (Puntel et al. 2011) or infusing cold saline (Lee et al. 2005; Schaser et al. 2007) into injured muscle of rats reduces leucocyte rolling and adhesion, and neutrophil infiltration and activation. By contrast, another study found that cold water immersion did not reduce leucocyte counts in muscle of rats after exercise (Camargo et al. 2012). Icing reduces and/or delays macrophage infiltration in rat muscle after muscle injury (Takagi et al. 2011; Vieira Ramos et al. 2016). In the present study, there were no significant differences in the numbers of neutrophils and macrophages, or mRNA expression of the cell surface receptors MAC1 and CD163 between cold water immersion and active recovery (Fig. 1).
Compared with research into the effects of cryotherapy on cell infiltration into muscle, less is known about its effects on the intramuscular expression of cytokines. In the present study, we focused on changes in the gene expression of IL‐1β, TNF‐α, IL‐6, CCL2 (MCP‐1), CCL4 (MIP‐1β), CXCL2 (MIP‐2α), IL‐8 and LIF in muscle after exercise because these cytokines are responsive to mechanical loading associated with exercise (Peake et al. 2015). They also play important roles in recruiting inflammatory cells to damaged muscle tissue (Peterson et al. 2006; Shireman et al. 2007; Kohno et al. 2011; Zhang et al. 2013) and regulating muscle repair (Broussard et al. 2004; Chen et al. 2007; Serrano et al. 2008; Yahiaoui et al. 2008; Zhang et al. 2013). Two animal studies have reported that icing reduces the expression of TGFβ and TNF in the days following muscle injury (Takagi et al. 2011; Vieira Ramos et al. 2016). In the present study, IL1β, IL6, TNF, CCL2, CXCL2, IL8 and LIF mRNA increased in skeletal muscle after both cold water immersion and active recovery (Figs 4 and 5). However, cytokine expression did not differ significantly between the cold water immersion and active recovery trials. The effects of ice massage (Tseng et al. 2013), cold water immersion (Vaile et al. 2008; Pointon et al. 2012; Gonzalez et al. 2014; Roberts et al. 2014), or exposure to −30°C air (Pournot et al. 2011; Guilhem et al. 2013) on systemic inflammatory responses to intense eccentric exercise or resistance exercise are variable and are relatively minor. We discovered that although plasma IL‐6 concentration increased after exercise, there was no significant difference between the two trials. Collectively, these findings suggest that cryotherapy does not substantially alter local or systemic inflammatory responses to exercise‐induced muscle damage.
Several factors could (theoretically) account for the differences between the present study and the animal studies described above. First, data from animal studies tend to indicate that icing (Puntel et al. 2011; Takagi et al. 2011; Vieira Ramos et al. 2016) is more effective than cold water immersion (Camargo et al. 2012) for reducing inflammation in muscle. This difference could be related to difference in the temperature of ice compared with cold water, which is in the range of 9−10°C. Second, we (Roberts et al. 2015 a) and others (Ihsan et al. 2013) have demonstrated that cold water immersion reduces muscle temperature at a depth of 3 cm and microvascular perfusion at a depth of 1−2 cm. Because animal muscles are smaller than human muscles, icing or cold water immersion may produce more extensive changes in muscle temperature and blood flow throughout animal muscles compared with human muscles. This may partly explain the greater anti‐inflammatory effects of icing in animal muscle (Puntel et al. 2011; Takagi et al. 2011; Vieira Ramos et al. 2016). Third, the animal studies described above induced muscle injury through blunt impact trauma (Lee et al. 2005; Schaser et al. 2007; Puntel et al. 2011), freeze injury (Vieira Ramos et al. 2016), or by crushing muscle with forceps (Takagi et al. 2011). No research has directly compared these injury models with exercise. Yet it seems reasonable to suggest that tissue injury and inflammation may be more severe and prolonged after blunt impact trauma or freeze or crush injury compared with exercise (Gayraud‐Morel et al. 2009). These differences may influence the efficacy of treatments for muscle injury and inflammation. Finally, these studies compared the effects of cryotherapy with no treatment, as opposed to active recovery.
Less muscle soreness after intense exercise may be the most consistent effect of cold water immersion (Leeder et al. 2011; Versey et al. 2013). We did not assess muscle soreness in the present study. However, we have previously demonstrated that the same cold water immersion protocol (i.e. 10 min of cold water immersion at 10°C) significantly reduced muscle soreness after intense resistance exercise (Roberts et al. 2014). The mechanisms through which cold water immersion reduces muscle soreness after exercise are unknown. At rest (i.e. without prior exercise), topical icing of the ankle reduces nerve conduction velocity, and increases pain threshold and pain tolerance (Algafly & George, 2007). These findings suggest that cryotherapy may influence the activity of nociceptors in soft tissues. Pain and nociceptor activity in muscle are mediated, in part, by pro‐inflammatory cytokines (Schafers et al. 2003; Hoheisel et al. 2005), bradykinin, and the neurotrophins NGF and GDNF (Murase et al. 2010; Murase et al. 2013). NGF and GDNF mRNA expression increases in skeletal muscle following lengthening (eccentric) muscle contractions in rats (Murase et al. 2010, 2013) and 60 min dynamic knee extension exercise in humans (Romero et al. 2016). Consistent with these responses, we found that GDNF expression peaked at 2 h after exercise, whereas NGF expression peaked at 24 h after exercise (Fig. 6). NGF and GDNF expression did not differ significantly after cold water immersion and active recovery. Therefore, these findings suggest that the analgesic effects of cold water immersion after exercise do not involve changes in the expression of these neurotrophins.
HSPs including HSP70 and αB‐crystallin play important roles in cytoprotection and as molecular chaperones to prevent aggregation of denatured proteins. They also regulate the refolding of proteins and stabilize the cytoskeleton in cells (Morton et al. 2009). We observed that HSP70 mRNA expression increased (Fig. 7), whereas the cytosolic protein content of HSP70 (Fig. 8) and αB‐crystallin (Fig. 8) in muscle decreased acutely after both cold water immersion and active recovery, and did not differ significantly between the trials. The increase in HSP70 mRNA expression is consistent with the findings of other studies (Paulsen et al. 2007). Previous studies have reported a delayed increase (Paulsen et al. 2007, 2009) or no change (Cumming et al. 2014) in cytosolic HSP70 content and an acute decrease (Paulsen et al. 2009; Cumming et al. 2014) in cytosolic αB‐crystallin content. The acute decrease in the cytosolic content of HSPs after exercise reflects their mobilization to cytoskeletal structures, which was confirmed by the increased αB‐crystallin content in the cytoskeletal fraction, where they may help to stabilize and protect stressed myofibrillar proteins (Paulsen et al. 2007, 2009).
The increase in the number of αB‐crystallin‐positive fibres (fibres with scattered and uneven αB‐crystallin staining) is further evidence that this stress protein binds to damaged cytoskeletal or myofibrillar structures. We have previously reported a similar staining pattern after high‐force eccentric exercise, and more detailed observations with immunogold staining and electron microscopy revealed accumulation of αB‐crystallin in Z‐disks connected to disrupted sarcomeres (Paulsen et al. 2009). Consistent with other reports of the accumulation of another small HSP (HSP27) in type II fibres after resistance exercise (Folkesson et al. 2008), the scattered αB‐crystallin staining was evident mainly in type II fibres in the present study. This finding suggests that the mechanical strain on myofibrillar structures was more pronounced in type II fibres. Collectively, the current findings suggest that cold water immersion did not mitigate the stress‐related signals that stimulate cellular movement of HSPs in skeletal muscle after exercise. This may also partly explain why cold water immersion did not significantly alter the infiltration of inflammatory cells or cytokine gene expression in skeletal muscle following exercise.
Several methodological considerations relating to the present study warrant brief discussion. First, several studies have reported that exercise with one leg induces adaptation in the contralateral leg (Howatson & van Someren, 2007; Starbuck & Eston, 2012; Xin et al. 2014). To address this issue, we analysed the changes in cellular infiltration and the expression of cytokine mRNA, neurotrophin mRNA and heat shock proteins between the first and second bout of exercise that each participant performed (independent of cold water immersion or active recovery treatments). Indeed, there were no statistically significant differences (P > 0.05) between the first and second bouts of exercise for any of these variables, which suggests that no adaptation occurred in the contralateral leg between the first and second bouts of exercise.
Second, some studies have reported that repeated muscle biopsies can cause injury and inflammation in muscle (Guerra et al. 2011; Van Thienen et al. 2014). By contrast, we and others have found that repeated muscle biopsies do not alter the expression of a wide array of genes (Lundby et al. 2005) or the infiltration of inflammatory cells in muscle (Paulsen et al. 2010). We aimed to minimize injury and inflammation arising from the muscle biopsies in two ways: (1) for the pre‐exercise and 2 h post‐exercise biopsy, we inserted the biopsy needle in opposite directions; (2) for the 24 h and 48 h biopsies, we inserted the biopsy needle at two separate sites, 3 cm and 6 cm distal (respectively) from the previous incisions.
Third, we acknowledge that comparing cold water immersion with inactive recovery may have been optimal for true experimental purposes. However, in reality, athletes are unlikely to remain completely sedentary after exercise (Reilly & Ekblom, 2005). We contend that our comparison between cold water immersion and active recovery is more reflective of typical athletic practice. We also believe that the effects of active recovery itself were relatively minor, because other research has demonstrated little or no difference in plasma creatine kinase activity (Saxton & Donnelly, 1995) or circulating cytokines (including IL‐6 and IL‐10) (Andersson et al. 2010) between active recovery and inactive/sedentary recovery after exercise. Notwithstanding possible differences between systemic versus intramuscular markers of tissue damage/inflammation, the findings from these studies tend to suggest that our results would have been similar if we had included inactive/sedentary recovery rather than active recovery. Last, we did not include women in our study to minimize variation arising from fluctuations in oestrogen as part of the menstrual cycle. Oestrogen is known to influence inflammatory responses in muscle after exercise (Tiidus, 2003). We acknowledge that our results may not be applicable to women.
In conclusion, contrary to popular anecdotal belief and the findings from preclinical studies on cryotherapy treatments for muscle injury, we found that compared with active recovery, cold water immersion did not significantly reduce inflammation or cellular stress within muscle after exercise. It is important to consider the implications of these findings within the broader context of understanding the factors that regulate inflammatory responses in muscle after exercise, and managing athletic conditioning and recovery. Considering the large differences in cardiac output, temperature and microvascular blood flow in muscle that occur after cold water immersion versus active recovery (Roberts et al. 2015 a), the present findings suggest that these physiological factors are not major determinants of local inflammation and cellular stress in human muscle after exercise. Cold water immersion consistently improves perceptions of fatigue and muscle soreness (Stanley et al. 2012) and enhances recovery of muscle function/performance following exercise (Leeder et al. 2011; Versey et al. 2013; Roberts et al. 2014). It also reduces clinical signs of inflammation such as limb swelling/oedema after exercise (Yanagisawa et al. 2003; Yanagisawa et al. 2004; Roberts et al. 2014). Therefore, it would appear that cold water immersion may still confer some short‐term clinical and/or functional benefits for athletes, without any changes in local inflammatory reactions within skeletal muscle during recovery from exercise. Periodic use of cold water immersion may assist athletes when they need to recovery quickly between training sessions or competitive events. However, in the long term, regular cold water immersion appears to be detrimental for developing muscle strength and hypertrophy.
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
J.M.P, L.A.R, V.C.F, J.F.M, J.C.S, D.C.‐S. and T.R. contributed to the conception and design of the research; L.A.R, V.C.F, I.E, S.K, S.N.A, K.S. and J.F.M performed the experiments; J.M.P analysed the data; J.M.P, V.C.F, S.K., K.S., J.F.M. and T.R. interpreted the results of the experiments; J.M.P, L.A.R I.E., S.K., S.N.A. and T.R. prepared the figures; J.M.P drafted the manuscript; J.M.P., L.A.R., V.C.F., I.E., S.K., S.N.A., K.S., J.F.M., J.S.C., D‐C.‐S. and T.R. edited and revised the manuscript. All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This study was funded by research grants from the American College of Sports Medicine Research Foundation and Exercise and Sport Science Australia awarded to L.A.R, J.C. and J.P.; and a grant from Queensland University of Technology awarded to J.P. L.A.R. was supported by an International Postgraduate Research Scholarship from The University of Queensland, and a top‐up scholarship from the Queensland Academy of Sport.
Acknowledgements
We thank the subjects for their dedication, time, and effort. We also thank Dr Masaki Takahashi and Dr Miki Tomari for the assistance with the study, and Body Science International Pty Ltd for providing nutritional supplements. The SC‐71 antibody was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Linked articles This article is highlighted by a Perspective by McPhee & Lightfoot. To read this Perspective, visit http://dx.doi.org/10.1113/JP273503.
This is an Editor's Choice article from the 1 February 2017 issue.
References
- Algafly AA & George KP (2007). The effect of cryotherapy on nerve conduction velocity, pain threshold and pain tolerance. Br J Sports Med 41, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson H, Bohn SK, Raastad T, Paulsen G, Blomhoff R & Kadi F (2010). Differences in the inflammatory plasma cytokine response following two elite female soccer games separated by a 72‐h recovery. Scand J Med Sci Sports 20, 740–747. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Graham T, Johansen L & Saltin B (1994). Muscle lactate metabolism in recovery from intense exhaustive exercise: impact of light exercise. J Appl Physiol 77, 1890–1895. [DOI] [PubMed] [Google Scholar]
- Bleakley C, Glasgow P, Phillips N, Hanna L, Callaghan M, Davison G, Hopkins T & Delahunt E (2010). Management of Acute Soft Tissue Injury Using Protection Rest Ice Compression and Elevation: Recommendations from the Association of Chartered Physiotherapists in Sports and Exercise Medicine (ACPSM) Association of Chartered Physiotherapists in Sports and Exercise Medicine, Sheffield. http://www.physiosinsport.org/media/wysiwyg/ACPSM_Physio_Price_A4.pdf.
- Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, Dantzer R & Kelley KW (2004). IL‐1β impairs insulin‐like growth factor I‐induced differentiation and downstream activation signals of the insulin‐like growth factor I receptor in myoblasts. J Immunol 172, 7713–7720. [DOI] [PubMed] [Google Scholar]
- Camargo MZ, Siqueira CP, Preti MC, Nakamura FY, de Lima FM, Dias IF, Toginho Filho Dde O & Ramos Sde P (2012). Effects of light emitting diode (LED) therapy and cold water immersion therapy on exercise‐induced muscle damage in rats. Lasers Med Sci 27, 1051–1058. [DOI] [PubMed] [Google Scholar]
- Chazaud B (2016). Inflammation during skeletal muscle regeneration and tissue remodeling: application to exercise‐induced muscle damage management. Immunol Cell Biol 94, 140–145. [DOI] [PubMed] [Google Scholar]
- Chen SE, Jin B & Li YP (2007). TNF‐α regulates myogenesis and muscle regeneration by activating p38 MAPK. Am J Physiol Cell Physiol 292, C1660–C1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming K, Paulsen G, Wernbom M, Ugelstad I & Raastad T (2014). Acute response and subcellular movement of HSP27, αB‐crystallin and HSP70 in human skeletal muscle after blood‐flow‐restricted low‐load resistance exercise. Acta Physiol (Oxf) 211, 634–646. [DOI] [PubMed] [Google Scholar]
- Figueiredo VC, Roberts LA, Markworth JF, Barnett MP, Coombes JS, Raastad T, Peake JM & Cameron‐Smith D (2016). Impact of resistance exercise on ribosome biogenesis is acutely regulated by post‐exercise recovery strategies. Physiol Rep 4, e12670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkesson M, Mackey AL, Holm L, Kjaer M, Paulsen G, Raastad T, Henriksson J & Kadi F (2008). Immunohistochemical changes in the expression of HSP27 in exercised human vastus lateralis muscle. Acta Physiol (Oxf) 194, 215–222. [DOI] [PubMed] [Google Scholar]
- Gayraud‐Morel B, Chretien F & Tajbakhsh S (2009). Skeletal muscle as a paradigm for regenerative biology and medicine. Regen Med 4, 293–319. [DOI] [PubMed] [Google Scholar]
- Gonzalez AM, Fragala MS, Jajtner AR, Townsend JR, Wells AJ, Beyer KS, Boone CH, Pruna GJ, Mangine GT, Bohner JD, Fukuda DH, Stout JR & Hoffman JR (2014). Effects of β‐hydroxy‐β‐methylbutyrate free acid and cold water immersion on expression of CR3 and MIP‐1β following resistance exercise. Am J Physiol Regul Integr Comp Physiol 306, R483–R489. [DOI] [PubMed] [Google Scholar]
- Guerra B, Gomez‐Cabrera MC, Ponce‐Gonzalez JG, Martinez‐Bello VE, Guadalupe‐Grau A, Santana A, Sebastia V, Vina J & Calbet JA (2011). Repeated muscle biopsies through a single skin incision do not elicit muscle signaling, but IL‐6 mRNA and STAT3 phosphorylation increase in injured muscle. J Appl Physiol 110, 1708–1715. [DOI] [PubMed] [Google Scholar]
- Guilhem G, Hug F, Couturier A, Regnault S, Bournat L, Filliard JR & Dorel S (2013). Effects of air‐pulsed cryotherapy on neuromuscular recovery subsequent to exercise‐induced muscle damage. Am J Sports Med 41, 1942–1951. [DOI] [PubMed] [Google Scholar]
- Hoheisel U, Unger T & Mense S (2005). Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain 114, 168–176. [DOI] [PubMed] [Google Scholar]
- Howatson G & van Someren KA (2007). Evidence of a contralateral repeated bout effect after maximal eccentric contractions. Eur J Appl Physiol 101, 207–214. [DOI] [PubMed] [Google Scholar]
- Ihsan M, Watson G, Lipski M & Abbiss CR (2013). Influence of post exercise cooling on muscle oxygenation and blood volume changes. Med Sci Sports Exerc 45, 876–882. [DOI] [PubMed] [Google Scholar]
- Journeay WS, Reardon FD, McInnis NH & Kenny GP (2005). Nonthermoregulatory control of cutaneous vascular conductance and sweating during recovery from dynamic exercise in women. J Appl Physiol 99, 1816–1821. [DOI] [PubMed] [Google Scholar]
- Kohno S, Ueji T, Abe T, Nakao R, Hirasaka K, Oarada M, Harada‐Sukeno A, Ohno A, Higashibata A, Mukai R, Terao J, Okumura Y & Nikawa T (2011). Rantes secreted from macrophages disturbs skeletal muscle regeneration after cardiotoxin injection in Cbl‐b‐deficient mice. Muscle Nerve 43, 223–229. [DOI] [PubMed] [Google Scholar]
- Lee H, Natsui H, Akimoto T, Yanagi K, Oshshima N & Kono I (2005). Effects of cryotherapy after contusion using real‐time intravital microscopy. Med Sci Sports Exerc 37, 1093–1098. [DOI] [PubMed] [Google Scholar]
- Leeder J, Gissane C, Van Someren KA, Gregson W & Howatson G (2011). Cold water immersion and recovery from strenuous exercise: a meta‐analysis. Br J Sports Med 46, 233–240. [DOI] [PubMed] [Google Scholar]
- Lundby C, Nordsborg N, Kusuhara K, Kristensen KM, Neufer PD & Pilegaard H (2005). Gene expression in human skeletal muscle: alternative normalization method and effect of repeated biopsies. Eur J Appl Physiol 95, 351–360. [DOI] [PubMed] [Google Scholar]
- Meeusen R & Lievens P (1986). The use of cryotherapy in sports injuries. Sports Med 3, 398–414. [DOI] [PubMed] [Google Scholar]
- Morton J, Kayani A, McArdle A & Drust B (2009). The exercise‐induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med 39, 643–642. [DOI] [PubMed] [Google Scholar]
- Murase S, Terazawa E, Hirate K, Yamanaka H, Kanda H, Noguchi K, Ota H, Queme F, Taguchi T & Mizumura K (2013). Upregulated glial cell line‐derived neurotrophic factor through cyclooxygenase‐2 activation in the muscle is required for mechanical hyperalgesia after exercise in rats. J Physiol 591, 3035–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase S, Terazawa E, Queme F, Ota H, Matsuda T, Hirate K, Kozaki Y, Katanosaka K, Taguchi T, Urai H & Mizumura K (2010). Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed‐onset muscle soreness). J Neurosci 30, 3752–3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen G, Crameri R, Benestad HB, Fjeld JG, Morkrid L, Hallen J & Raastad T (2010). Time course of leukocyte accumulation in human muscle after eccentric exercise. Med Sci Sports Exerc 42, 75–85. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Lauritzen F, Bayer M, Kalhovde J, Ugelstad I, Owe S, Hallén J, Bergersen L & Raastad T (2009). Subcellular movement and expression of HSP27, αB‐crystallin, and HSP70 after two bouts of eccentric exercise in humans. J Appl Physiol 107, 570–582. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Vissing K, Kalhovde J, Ugelstad I, Bayer M, Kadi F, Schjerling P, Hallén J & Raastad T (2007). Maximal eccentric exercise induces a rapid accumulation of small heat shock proteins on myofibrils and a delayed HSP70 response in humans. Am J Physiol Regul Integr Comp Physiol 293, R844–R853. [DOI] [PubMed] [Google Scholar]
- Peake JM, Della Gatta P, Suzuki K & Nieman DC (2015). Cytokine expression and secretion by skeletal muscle cells: regulatory mechanisms and exercise effects. Exerc Immunol Rev 21, 8–25. [PubMed] [Google Scholar]
- Peterson J, Feeback K, Baas J & Pizza F (2006). Tumor necrosis factor‐α promotes the accumulation of neutrophils and macrophages in skeletal muscle. J Appl Physiol 101, 1394–1399. [DOI] [PubMed] [Google Scholar]
- Pointon M, Duffield R, Cannon J & Marino FE (2012). Cold water immersion recovery following intermittent‐sprint exercise in the heat. Eur J Appl Physiol 112, 2483–2494. [DOI] [PubMed] [Google Scholar]
- Pournot H, Bieuzen F, Louis J, Mounier R, Fillard JR, Barbiche E & Hausswirth C (2011). Time‐course of changes in inflammatory response after whole‐body cryotherapy multi exposures following severe exercise. PLoS One 6, e22748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel GO, Carvalho NR, Amaral GP, Lobato LD, Silveira SO, Daubermann MF, Barbosa NV, Rocha JB & Soares FA (2011). Therapeutic cold: An effective kind to modulate the oxidative damage resulting of a skeletal muscle contusion. Free Radic Res 45, 125–138. [DOI] [PubMed] [Google Scholar]
- Reilly T & Ekblom B (2005). The use of recovery methods post‐exercise. J Sports Sci 23, 619–627. [DOI] [PubMed] [Google Scholar]
- Roberts L, Muthalib M, Stanley J, Lichtwark G, Nosaka K, Coombes J & Peake J (2015. a). Effects of cold water immersion and active recovery on hemodynamics and recovery of muscle strength following resistance exercise. Am J Physiol Regul Integr Comp Physiol 309, 389–398. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Nosaka K, Coombes JS & Peake JM (2014). Cold water immersion enhances recovery of submaximal muscle function after resistance exercise. Am J Physiol Regul Integr Comp Physiol 307, R998–R1008. [DOI] [PubMed] [Google Scholar]
- Roberts LA, Raastad T, Markworth JF, Figueiredo VC, Egner IM, Shield A, Cameron‐Smith D, Coombes JS & Peake JM (2015. b). Post‐exercise cold water immersion attenuates acute anabolic signalling and long‐term adaptations in muscle to strength training. J Physiol 593, 4285–4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero SA, Hocker AD, Mangum JE, Luttrell MJ, Turnbull DW, Struck AJ, Ely MR, Sieck DC, Dreyer HC & Halliwill JR (2016). Evidence of a broad histamine footprint on the human exercise transcriptome. J Physiol 594, 5009–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton JM & Donnelly AE (1995). Light concentric exercise during recovery from exercise‐induced muscle damage. Int J Sports Med 16, 347–351. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sorkin LS & Sommer C (2003). Intramuscular injection of tumor necrosis factor‐alpha induces muscle hyperalgesia in rats. Pain 104, 579–588. [DOI] [PubMed] [Google Scholar]
- Schaser KD, Disch AC, Stover JF, Lauffer A, Bail HJ & Mittlmeier T (2007). Prolonged superficial local cryotherapy attenuates microcirculatory impairment, regional inflammation, and muscle necrosis after closed soft tissue injury in rats. Am J Sports Med 35, 93–102. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Baeza‐Raja B, Perdiguero E, Jardi M & Munoz‐Canoves P (2008). Interleukin‐6 is an essential regulator of satellite cell‐mediated skeletal muscle hypertrophy. Cell Metab 7, 33–44. [DOI] [PubMed] [Google Scholar]
- Shireman P, Contreras‐Shannon V, Ochoa O, Karia B, Michalek J & McManus L (2007). MCP‐1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81, 775–785. [DOI] [PubMed] [Google Scholar]
- Stanley J, Buchheit M & Peake JM (2012). The effect of post‐exercise hydrotherapy on subsequent exercise performance and heart rate variability. Eur J Appl Physiol 112, 951–961. [DOI] [PubMed] [Google Scholar]
- Starbuck C & Eston RG (2012). Exercise‐induced muscle damage and the repeated bout effect: evidence for cross transfer. Eur J Appl Physiol 112, 1005–1013. [DOI] [PubMed] [Google Scholar]
- Takagi R, Fujita N, Arakawa T, Kawada S, Ishii N & Miki A (2011). Influence of icing on muscle regeneration after crush injury to skeletal muscles in rats. J Appl Physiol 110, 382–388. [DOI] [PubMed] [Google Scholar]
- Tidball JG & Villalta SA (2010). Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol Regul Integr Comp Physiol 298, R1173–R1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiidus PM (2003). Influence of estrogen on skeletal muscle damage, inflammation, and repair. Exerc Sport Sci Rev 31, 40–44. [DOI] [PubMed] [Google Scholar]
- Tseng CY, Lee JP, Tsai YS, Lee SD, Kao CL, Liu TC, Lai C, Harris MB & Kuo CH (2013). Topical cooling (icing) delays recovery from eccentric exercise‐induced muscle damage. J Strength Cond Res 27, 1354–1361. [DOI] [PubMed] [Google Scholar]
- Urso ML (2013). Anti‐inflammatory interventions and skeletal muscle injury: benefit or detriment? J Appl Physiol 115, 920–928. [DOI] [PubMed] [Google Scholar]
- Vaile J, Halson S, Gill N & Dawson B (2008). Effect of hydrotherapy on the signs and symptoms of delayed onset muscle soreness. Eur J Appl Physiol 102, 447–455. [DOI] [PubMed] [Google Scholar]
- Van Thienen R, D'Hulst G, Deldicque L & Hespel P (2014). Biochemical artifacts in experiments involving repeated biopsies in the same muscle. Physiol Rep 2, e00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A & Speleman F (2002). Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol 3, research0034.1–research0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versey NG, Halson SL & Dawson BT (2013). Water immersion recovery for athletes: effect on exercise performance and practical recommendations. Sports Med 43, 1101–1130. [DOI] [PubMed] [Google Scholar]
- Vieira Ramos G, Pinheiro C, Messa S, Delfino G, de Cassia Marqueti R, de Fatima Salvini T & Durigan J (2016). Cryotherapy reduces inflammatory response without altering muscle regeneration process and extracellular matrix remodeling of rat muscle. Sci Rep 6, 18525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Hyldahl RD, Chipkin SR & Clarkson PM (2014). A contralateral repeated bout effect attenuates induction of NF‐κB DNA binding following eccentric exercise. J Appl Physiol 116, 1473–1480. [DOI] [PubMed] [Google Scholar]
- Yahiaoui L, Gvozdic D, Danialou G, Mack M & Petrof BJ (2008). CC chemokines directly regulate myoblast responses to skeletal muscle injury. J Physiol 586, 3991–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa O, Kudo H, Takahashi N & Yoshioka H (2004). Magnetic resonance imaging evaluation of cooling on blood flow and oedema in skeletal muscles after exercise. Eur J Appl Physiol 91, 737–740. [DOI] [PubMed] [Google Scholar]
- Yanagisawa O, Niitsu M, Takahashi H, Goto K & Itai Y (2003). Evaluations of cooling exercised muscle with MR imaging and 31P MR spectroscopy. Med Sci Sports Exerc 35, 1517–1523. [DOI] [PubMed] [Google Scholar]
- Zhang C, Li Y, Wu Y, Wang L, Wang X & Du J (2013). Interleukin‐6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem 288, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]


