Abstract
Key points
Oxytocin release from the posterior pituitary gland stimulates uterine contraction during birth but the central mechanisms that activate oxytocin neurones for birth are not well characterized.
We found that that kisspeptin fibre density around oxytocin neurones increases in late‐pregnant rats.
These kisspeptin fibres originated from hypothalamic periventricular nucleus neurones that upregulated kisspeptin expression in late pregnancy.
Oxytocin neurones were excited by central kisspeptin administration in late‐pregnant rats but not in non‐pregnant rats or early‐ to mid‐pregnant rats.
Our results reveal the emergence of a new excitatory kisspeptin projection to the oxytocin system in late pregnancy that might contribute to oxytocin neurone activation for birth.
Abstract
The hormone oxytocin promotes uterine contraction during parturition. Oxytocin is synthesized by magnocellular neurones in the hypothalamic supraoptic and paraventricular nuclei and is released into the circulation from the posterior pituitary gland in response to action potential firing. Systemic kisspeptin administration increases oxytocin neurone activity to elevate plasma oxytocin levels. Here, immunohistochemistry revealed that rats on the expected day of parturition (day 21 of gestation) had a higher density of kisspeptin‐positive fibres in the perinuclear zone surrounding the supraoptic nucleus (which provides dense glutamatergic and GABAergic innervation to the supraoptic nucleus) than was evident in non‐pregnant rats. Retrograde tracing showed the kisspeptin projections to the perinuclear zone originated from the hypothalamic periventricular nucleus. Quantitative RT‐PCR showed that kisspeptin receptor mRNA, Kiss1R mRNA, was expressed in the perinuclear zone–supraoptic nucleus and that the relative Kiss1R mRNA expression does not change over the course of pregnancy. Finally, intracerebroventricular administration of kisspeptin increased the firing rate of oxytocin neurones in anaesthetized late‐pregnant rats (days 18–21 of gestation) but not in non‐pregnant rats, or in early‐ or mid‐pregnant rats. Taken together, these results suggest that kisspeptin expression is upregulated in the periventricular nucleus projection to the perinuclear zone of the supraoptic nucleus towards the end of pregnancy. Hence, this input might activate oxytocin neurones during parturition.
Keywords: kisspeptin, oxytocin, pregnancy, supraoptic nucleus, vasopressin
Key points
Oxytocin release from the posterior pituitary gland stimulates uterine contraction during birth but the central mechanisms that activate oxytocin neurones for birth are not well characterized.
We found that that kisspeptin fibre density around oxytocin neurones increases in late‐pregnant rats.
These kisspeptin fibres originated from hypothalamic periventricular nucleus neurones that upregulated kisspeptin expression in late pregnancy.
Oxytocin neurones were excited by central kisspeptin administration in late‐pregnant rats but not in non‐pregnant rats or early‐ to mid‐pregnant rats.
Our results reveal the emergence of a new excitatory kisspeptin projection to the oxytocin system in late pregnancy that might contribute to oxytocin neurone activation for birth.
Abbreviations
- 3V
third ventricle
- AVPV
anteroventral periventricular nucleus
- CCK8S
cholecystokinin‐8S
- G
gestation
- GnRH
gonadotropin releasing hormone
- GPR54
kisspeptin receptor
- Kiss1R
kisspeptin receptor
- KP
kisspeptin
- NKB
neurokinin B
- NP
non‐pregnant
- NPFF1/2R
neuropeptide FF 1 and 2 receptor
- OC
optic chiasm
- PNZ
perinuclear zone
- RP3V
rostral periventricular region of the third ventricle
- SFO
subfornical organ
- SON
supraoptic nucleus
- TBS
tris‐buffered saline
Introduction
Oxytocin is required for normal delivery of the offspring during parturition because oxytocin receptor antagonists delay the initiation of parturition and prolong parturition once initiated (Antonijevic et al. 1995). Oxytocin is synthesized in magnocellular neurones of the hypothalamic supraoptic and paraventricular nuclei. Magnocellular neurones send a single axon to the posterior pituitary gland where they release oxytocin (or vasopressin) into the circulation from axonal endings in response to action potential firing (Brown et al. 2013). However, the mechanisms that drive oxytocin neurone activity during parturition are not well characterized.
Kisspeptin is the protein product of the Kiss1 gene and the endogenous ligand for the kisspeptin receptor (Kiss1R, GPR54) (Kotani et al. 2001). Kisspeptin strongly excites gonadotropin releasing hormone (GnRH) neurones (Han et al. 2005) and, while there is some evidence of fertility in the absence of kisspeptin neurons (Mayer & Boehm, 2011), its action on GnRH neurones is widely accepted as being essential for normal fertility (Kirilov et al. 2013). However, kisspeptin neurones project to a wide variety of brain regions, including the supraoptic nucleus (Desroziers et al. 2010), suggesting a potential role for regulation of oxytocin neurone activity. Indeed, intravenous (i.v.) kisspeptin administration increases oxytocin neurone firing rate (Scott & Brown, 2011, 2013) and consequently increases circulating oxytocin concentrations in female rats (Kotani et al. 2001). However, the excitation of oxytocin neurones by i.v. kisspeptin is not direct, but is mediated by activation of vagal afferent inputs (Scott & Brown, 2011). Our preliminary observations suggest that central kisspeptin might excite oxytocin neurones in late‐pregnant rats but not in non‐pregnant rats (Scott & Brown, 2013). Here, we aimed to determine whether central kisspeptin excitation of oxytocin neurones emerges during pregnancy and to identify the afferent input that mediates the excitation.
In rodents, kisspeptin cell bodies are found in two major anatomical locations: the arcuate nucleus and the rostral periventricular region of the third ventricle (RP3V), which comprises the anteroventral periventricular nucleus (AVPV) and the periventricular nucleus (Herbison, 2008; Clarkson et al. 2009). We found that a kisspeptin projection to the oxytocin system emerged in late pregnancy from a sub‐population of kisspeptin neurones in the periventricular nucleus and that intracerebroventricular (i.c.v.) kisspeptin administration increased the firing rate of oxytocin neurones in late‐pregnant rats but not in non‐pregnant, early‐pregnant or mid‐pregnant rats. Hence, this kisspeptin projection might be important for activation of oxytocin neurones during parturition.
Methods
Ethical approval
All experimental procedures were approved by the University of Otago Animal Ethics Committee and were carried out in accordance with the New Zealand Animal Welfare Act (1999) and associated guidelines.
Animals
Female Sprague‐Dawley rats were group‐housed in the University of Otago animal facility under controlled conditions (12 h light, 12 h dark cycle; lights on at 06.00 h, 22 ± 1ºC) with free access to food and water. The oestrous cycle was monitored daily using vaginal cytology to examine the appearance of the epithelial cells. On pro‐oestrus, females were placed overnight in a cage with a male. Mating was considered to be successful when the vaginal smear showed presence of sperm on the following morning and this was recorded as day 0 of gestation (G0).
Immunohistochemistry
Adult female rats that were either cycling or pregnant were anaesthetized with sodium pentobarbitone (60 mg kg−1, intraperitoneal (i.p.)) and transcardially perfused with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.6). Brains were then removed and post‐fixed overnight in paraformaldehyde solution then cryoprotected in 30% sucrose in 0.1 m phosphate buffer solution for 72 h at 4ºC. Brains were cut on a freezing microtome into three sets of 30 μm thick coronal sections.
We used a subset of eight to twelve sections through the supraoptic nucleus from non‐pregnant rats that were perfused on dioestrus, and rats perfused on day 7 (G7; n = 6), 14 (G14; n = 6) or 21 (G21; n = 7) of gestation. Endogenous aldehydes were blocked in a solution of 0.1% sodium borohydride for 20 min and then washed three times in tris‐buffered saline (TBS; 10 min per wash). Sections were then incubated in a cocktail of polyclonal rabbit anti‐kisspeptin‐10 (AC 564, gift from Alan Caraty; 1:25,000) and monoclonal mouse anti‐oxytocin antiserum (MAB5296, Millipore, Billerica, MA, USA; 1:5000) in TBS with 0.3% Triton X‐100 and 0.25% bovine serum albumin (pH 7.6; herein referred to as incubation solution) plus 2% normal goat serum for 48 h at 4ºC. Sections were washed in TBS and incubated in a cocktail of Alexa Fluor goat anti‐rabbit 568 (A11036, Molecular Probes, Eugene, OR, USA; 1:200) and Alexa Fluor goat anti‐mouse 488 (A11029, Molecular Probes; 1:200) in incubation solution for 90 min at room temperature. Following subsequent washes, sections were mounted on gelatin‐coated glass slides and coverslipped using Vectashield HardSet mounting medium (H‐1400, Vector Laboratories, Burlingame, CA, USA).
The majority of kisspeptin neurones in the arcuate nucleus, but not the RP3V, co‐express neurokinin B (NKB) and dynorphin (Burke et al. 2006; Navarro et al. 2009). Therefore, in a second experiment we used a subset of eight to twelve sections through either the arcuate nucleus, RP3V or supraoptic nucleus from non‐pregnant rats on dioestrus (n = 5), and G21 rats (n = 5). Procedures were as above, except sections were double‐labelled for kisspeptin and NKB. NKB labelling was performed using guinea‐pig anti‐NKB (IS‐3/63, gift from Philippe Ciofi; 1:4000) followed by Alexa Fluor goat anti‐guinea‐pig 488 (A11073, Molecular Probes; 1:200).
Negative controls were run with omission of primary antibodies and showed no non‐specific staining for kisspeptin, NKB or oxytocin. The anti‐kisspeptin antibody is commercially available (Millipore AB9754) and specificity of kisspeptin staining has previously been confirmed in rats by pre‐adsorption tests conducted with rat kisspeptin at 0.1, 1 and 10 μm, which abolished all staining throughout the brain (Desroziers et al. 2010).
Retrograde tracing surgery
To determine the origin of kisspeptin fibres in the perinuclear zone–supraoptic nucleus, we performed retrograde tracing along with fluorescent immunohistochemistry for kisspeptin. Surgery was performed on non‐pregnant rats and rats on day 17 of gestation. Rats were anaesthetized with isofluorane (2% with 1 l O2 min−1) and given 5 mg sub‐cutaneous carprofen (Norbrook, Newry, Northern Ireland, UK) as an analgesic. Rats were placed in a stereotaxic headframe and an injection of 200 nl of the retrograde tracer: fluorescent green conjugated latex microspheres (Lumafluor Inc, New York City, NY, USA) was stereotaxically injected into the right supraoptic nucleus (co‐ordinates: 0.9 mm caudal to bregma; 1.7 mm lateral; 9.6 mm below the ventral surface of the skull) over 5 min with the needle left in place for an additional 5 min. Rats were allowed to recover in single cages and monitored daily. Non‐pregnant rats (n = 6) were perfused between 4 and 8 days following surgery, on the day of dioestrus, and pregnant rats were perfused at G21 (n = 4). A subset of eight to twelve sections through the arcuate nucleus, AVPV or periventricular nucleus were labelled for kisspeptin as described above.
Immunohistochemical analysis
To determine whether the density of kisspeptin fibres in the perinuclear zone–supraoptic nucleus is different between non‐pregnant rats and G21 rats, images were acquired using a Zeiss 710 Confocal Laser Scanning Microscope (Carl Zeiss Ag, Oberkochen, Germany) using excitation at 488 nm and 543 nm. A Z‐series of 8–12 images was obtained using a ×40 objective with a pinhole of 4 μm and a slice interval of 2 μm. Fibre density was measured as previously reported (Polston & Simerly, 2003; Patisaul et al. 2008). Briefly, using Image J (NIH), a substack of images was taken to control for section thickness. A region of interest covering a portion of the supraoptic nucleus or an equal sized portion of the perinuclear zone (example shown in Fig. 1 A) was selected and the sections were binarized to a threshold that allowed the best signal‐to‐noise ratio. Images were skeletonized to make fibres one pixel thick, thus controlling for the fibre thickness. The voxel counter plugin (NIH) was then used to count the number of voxels and the number in the substack was averaged. The fraction of signal‐containing voxels to total voxels in the region of interest was calculated and the numbers from cycling rats normalized to 1. Relative fibre densities were compared between different reproductive groups. To calculate co‐expression of kisspeptin and NKB in supraoptic nucleus sections, the fraction of NKB voxels to kisspeptin voxels was calculated to give the percentage of kisspeptin‐fibres that co‐localize NKB.
Figure 1. Kisspeptin fibre density in the perinuclear zone of non‐pregnant and pregnant rats.
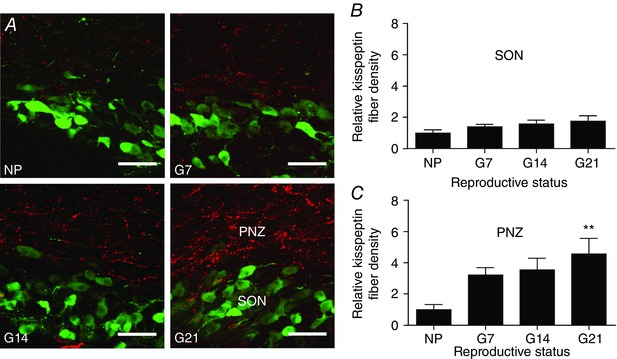
A, representative dual‐label confocal images showing kisspeptin immunoreactive fibres (red) and oxytocin immunoreactive neurones (green) in the supraoptic nucleus (SON) and perinuclear zone (PNZ) of a non‐pregnant rat (NP), an early‐pregnant rat (G7), a mid‐pregnant rat (G14) and a late‐pregnant rat (G21). Scale bars = 50 μm. B, relative kisspeptin fibre density within the supraoptic nucleus. One‐way ANOVA showed no significant effect of reproductive status (F 3,43 = 1.46, P = 0.24). C, relative kisspeptin fibre density within the perinuclear zone. One‐way ANOVA showed a significant effect of reproductive status (F 3,43 = 3.95, P = 0.01); ** P ≤ 0.01 compared to non‐pregnant rats, Bonferroni's post hoc test. [Colour figure can be viewed at wileyonlinelibrary.com]
To determine whether the number of kisspeptin neurones present was different in non‐pregnant rats and pregnant rats, we counted kisspeptin‐positive cell bodies, using an Olympus BX51 microscope with fluorescent filters. The number of clearly discernible cell bodies was counted on both sides of the nuclei in four representative sections through the RP3V or arcuate nucleus by a researcher blinded to experimental groups. The mean number of cell bodies per section was compared between groups. When counting cells from rats that had retrograde tracer injected, only cells in the ipsilateral nucleus to the injection site were counted because retrograde tracer‐positive cell bodies were mainly labelled on this side.
Quantitative real time‐PCR (qRT‐PCR)
Non‐pregnant rats on dioestrus (n = 8), or G7 (n = 5), G14 (n = 7) and G21 (n = 7) rats were killed by decapitation while conscious. Brains were rapidly removed and frozen on dry ice. Coronal brains slices were prepared at a 300 μm thickness, the supraoptic nucleus was microdissected and total RNA was extracted as previously described (Augustine et al. 2016).
Primer pairs (Table 1) were designed for use in quantitative PCR using Primer‐BLAST on the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) and OligoAnalyzer on Integrated DNA Technologies websites (http://sg.idtdna.com/calc/analyzer) and were the same as previously reported for Kiss1R mRNA (Wyatt et al. 2013).
Table 1.
Oligonucleotide sequences for β‐actin and Kiss1R
| Gene symbol | Primers/probes | Oligonucleotide sequence (5’–3’) |
|---|---|---|
| β‐actin | Forward | AGATGACCCAGATCATGTTTGAGA |
| Reverse | ACCAGAGGCATACAGGGACAA | |
| Kiss1R | Forward | GCTGGGAGACTTCATGTGCAA |
| Reverse | AGCGGGAACACAGTCACATACC |
Oligonucleotide sequences used to quantify Kiss1R mRNA expression in the perinuclear zone–supraoptic nucleus of non‐pregnant and pregnant rats by qRT‐PCR (Fig. 4).
Quantitative RT‐PCR was carried out as previously described (Augustine et al. 2016) using SensiFAST SYBR No‐ROX mix (included SYBR Green I dye; Bioline, Eveleigh NSW, Australia) and a LightCycler 480. The Cq of each individual reaction was characterized as the PCR cycle at which fluorescence rose above the background fluorescence and was analysed using the second derivate maximum method available with the LightCycler 480 software. The mean Cq between the triplicate reactions was calculated and values entered into Excel (Microsoft Corp., Redmond, WA, USA). The relative quantification of gene expression was calculated using the comparative Cq method, with normalization of the target gene to the endogenous reference gene, β‐actin. The expression of the reference gene was not significantly different between groups (P = 0.25, one‐way ANOVA). The relative expression of Kiss1R mRNA in each experimental group was normalized to the expression in the non‐pregnant group using an induction factor = 2−ΔΔCq, where ΔΔCq = ((Cq gene of interest (experimental group) – Cq reference gene (experimental group)) – (Cq gene of interest (control) – Cq ref gene (control))) and assuming that the efficiency of the PCR reaction was close to two.
In vivo electrophysiology
Female rats were used that were either non‐pregnant, early‐pregnant (G1–G11), mid‐pregnant (G12–G17) or late‐pregnant (G18–G21). Rats were anaesthetized with 1.25 g kg−1 i.p. urethane (ethyl carbamate, Sigma, St Louis, MO, USA). Upon cessation of the pedal withdrawal reflex, an intravenous (i.v.) catheter was inserted into the left femoral vein for i.v. injection of cholecystokinin‐8S (CCK8S) or kisspeptin. A 28‐gauge guide cannula was then placed in the left cerebral ventricle (1.3 mm lateral to bregma, 3 mm below the surface of the skull) and fixed with dental cement bonded to stainless steel screws, for i.c.v. injection of kisspeptin. The pituitary stalk and right supraoptic nucleus were exposed using transpharyngeal surgery (Brown et al. 2014). A glass recording electrode (15–40 MΩ) was filled with 0.9% saline and inserted into the supraoptic nucleus to record electrical activity in magnocellular neurones. A side‐by‐side SNEX‐200 stimulating electrode (Science Products GmbH, Hofheim, Germany) was inserted onto the pituitary stalk to evoke antidromic action potentials recorded at the supraoptic nucleus to confirm that recordings were from magnocellular neurones.
Recordings were made using Spike2 software (Cambridge Electronic Design). Neurones that fired less than one spike every 10 s were classed as silent and were not analysed. Oxytocin and vasopressin neurones were distinguished by their response to i.v. CCK8S (20 μg kg−1, 0.5 ml kg−1 in 0.9% saline; Sigma). Oxytocin neurones showed a transient excitation to i.v. CCK8S (Brown et al. 1996), while vasopressin neurones showed a transient inhibition, or were unaffected (Sabatier et al. 2004). i.c.v. injections of kisspeptin were given at 2 μg in 2 μl of artificial cerebrospinal fluid (in mm: 150 NaCl, 3 KCl, 1.2 MgCl2, 26 NaHCO2, 2.5 CaCl2, 10 glucose) through an infusion cannula that protruded 2 mm below the tip of the guide cannula. i.v. kisspeptin injections (100 μg in 0.9% saline) were given through the i.v. catheter. At the end of experiments, rats were killed with an anaesthetic overdose. While the full‐length peptide is kisspeptin‐54, we used kisspeptin‐10 (Metastin 44–54; Calbiochem, San Diego, CA, USA), which has comparable, if not greater, potency at Kiss1R than kisspeptin‐54 (Muir et al. 2001; Ohtaki et al. 2001).
Effects of kisspeptin were analysed by comparing the difference between the mean firing rate in the 2 min following administration and 2 min prior to administration. To determine whether vasopressin neurones showed burst firing in response to i.v. kisspeptin, the firing rate in the 10 s following kisspeptin had to be higher than two standard deviations above the mean of the 10 s period prior to kisspeptin administration for three consecutive 2 s bins.
Statistical analysis
All data was analysed using Graphpad Prism (Version 6.00). Values were expressed as means ± standard error of the mean (SEM). Unpaired two‐tailed Student's t tests were used to compare two groups and one‐way ANOVA with Bonferroni's post hoc tests were used to compare three or more groups; P ≤ 0.05 was considered significant.
Results
Kisspeptin fibre density increases in the perinuclear zone during pregnancy
Kisspeptin fibres and oxytocin neurones were labelled using immunohistochemistry in coronal sections containing the supraoptic nucleus from non‐pregnant rats (n = 5), G7 rats (n = 6), G14 rats (n = 6) and G21 rats (n = 7). The majority of kisspeptin fibres did not appear to be distributed near oxytocin neurones within the supraoptic nucleus itself, but were more evident in the perinuclear zone, immediately dorsal to the supraoptic nucleus (Fig. 1 A). Kisspeptin fibre density was measured and fibre density from non‐pregnant rats was normalized to 1. Relative fibre densities were not different between the supraoptic nuclei of non‐pregnant rats (1.00 ± 0.21), G7 rats (1.40 ± 0.17), G14 rats (1.59 ± 0.24) and G21 rats (1.77 ± 0.36; F 3,43 = 1.45, P = 0.24, one‐way ANOVA; Fig. 1 B). Relative kisspeptin fibre density in the perinuclear zone was 1.00 ± 0.33 for non‐pregnant rats, 3.24 ± 0.47 for G7 rats, 3.57 ± 0.74 for G14 rats and 4.58 ± 1.01 for G21 rats and there was a significant effect of reproductive status for kisspeptin fibre density within the perinuclear zone (F 3,43 = 3.95, P = 0.01, one‐way ANOVA; Fig. 1 C).
Kisspeptin fibres in the perinuclear zone of the supraoptic nucleus do not express neurokinin B
To determine whether the changes in kisspeptin fibre density observed in the perinuclear zone was associated with increased kisspeptin expression in specific populations of neurones, kisspeptin cell bodies were quantified (Fig. 2). Most kisspeptin cell bodies were not visible in the RP3V of non‐pregnant rats (Fig. 2 A) but were easily visualized in the RP3V of G21 rats (Fig. 2 B). The mean number of kisspeptin‐positive cell bodies per section was significantly higher (P < 0.001, Student's t test) in G21 rats (11.9 ± 1.6; n = 4) than in non‐pregnant rats (0.7 ± 0.34, n = 6; Fig. 2 C). Conversely, kisspeptin‐positive cell bodies were easily visualized in the arcuate nucleus of both non‐pregnant rats (Fig. 2 D) and G21 rats (Fig. 2 D) and the mean number of kisspeptin‐positive cell bodies per section was significantly lower in G21 rats (5.9 ± 0.43, n = 6) than in non‐pregnant rats (15.8 ± 2.5, n = 6; P = 0.003, Student's t test; Fig. 2 F). Kisspeptin cell bodies were not observed in other areas where they have previously been reported (data not shown), including the medial amygdala and bed nucleus of the stria terminalis (Kim et al. 2011; Xu et al. 2012).
Figure 2. Kisspeptin and neurokinin B expression in non‐pregnant and pregnant rats.
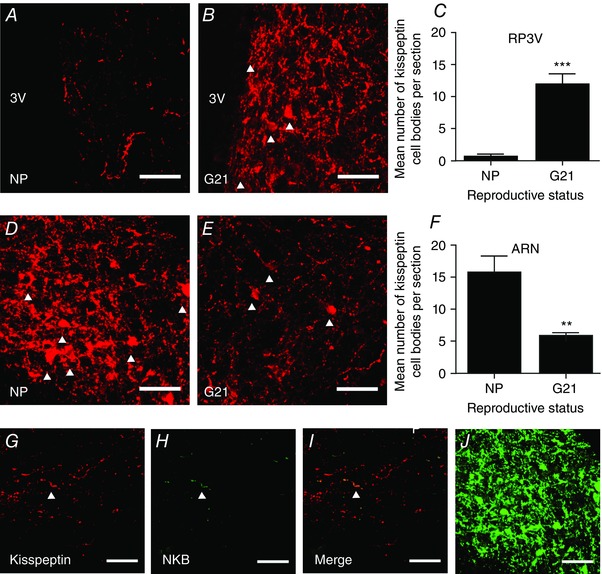
A and B, representative confocal images showing kisspeptin expression in the rostral periventricular area of the third ventricle (RP3V) from a non‐pregnant rat (NP; A) and a late‐pregnant rat (G21; B). White arrowheads indicate labelled cell bodies. C, number of kisspeptin‐positive cell bodies in the RP3V of non‐pregnant rats and late‐pregnant rats; *** P < 0.001, Student's t test. D and E, representative confocal images showing kisspeptin expression in the arcuate nucleus (ARN) of a non‐pregnant rat (D) and a late‐pregnant rat (E). F, number of kisspeptin‐positive cell bodies in the arcuate nucleus of non‐pregnant rats and late‐pregnant rats; ** P < 0.01, Student's t test. G–I, representative confocal images showing limited co‐expression of neurokinin B (NKB) with kisspeptin in supraoptic nucleus/perinuclear zone sections from a late‐pregnant rat. White arrowhead indicates a co‐labelled fibre. J, NKB labelling of cell bodies and fibres in the arcuate nucleus of a non‐pregnant rat. Scale bars in all images = 50 μm. [Colour figure can be viewed at wileyonlinelibrary.com]
Kisspeptin cells in the arcuate nucleus, but not the RP3V, co‐express NKB and dynorphin. Therefore, co‐expression of kisspeptin and NKB in perinuclear zone fibres would identify projections from the arcuate nucleus. We double‐labelled slices containing the perinuclear zone from non‐pregnant rats and G21 rats with kisspeptin and NKB but found NKB fibre staining to be sparsely distributed in both groups (Fig. 2 G–I). We calculated the percentage co‐expression of NKB‐positive voxels in kisspeptin‐positive voxels and found only 1.9 ± 0.7% of kisspeptin‐positive voxels in non‐pregnant rats (n = 5) and 1.1 ± 0.3% of kisspeptin‐positive voxels in G21 rats (n = 5) co‐expressed NKB with no significant difference between groups (P = 0.26, Student's t test), despite clear NKB labelling in the arcuate nucleus (Fig. 2 J).
Kisspeptin fibres of the perinuclear zone originate from the periventricular nucleus
To determine the origin of kisspeptin fibres, we injected green fluorescent microspheres into the perinuclear zone–supraoptic nucleus and labelled sections containing the arcuate nucleus, AVPV and periventricular nucleus for kisspeptin. We confirmed that the injection site encompassed the left perinuclear zone–supraoptic nucleus in each rat (Fig. 3 A). As an additional control for accurate targeting of the perinuclear zone–supraoptic nucleus, we visualized retrograde label in the subfornical organ (SFO; Fig. 3 B), an area that is known to send projections to the supraoptic nucleus (Weiss & Hatton, 1990). Only rats that had an injection site in the perinuclear zone–supraoptic nucleus and tracer‐positive cells in the SFO were included in the analysis (6 of 21 virgin rats and 4 of 11 pregnant rats). While there was clear co‐localization of kisspeptin and tracer in cell bodies within the periventricular nucleus (Fig. 3 C and D), there were very few double‐labelled cell bodies in the AVPV (Fig. 3 E) and none in the arcuate nucleus (Fig. 3 F). In the periventricular nucleus, a similar number of cells were retrogradely labelled from the perinuclear zone–supraoptic nucleus in non‐pregnant rats (18.4 ± 3.9 per section; n = 6) and G21 rats (16.6 ± 2.6 per section; n = 4; P = 0.74, Student's t test; Fig. 3 G) but more cells expressed kisspeptin in G21 rats (9.4 ± 2.7) than in non‐pregnant rats (2.4 ± 0.69 per section; P = 0.01, Student's t test; Fig. 3 H), with a larger proportion of the retrogradely labelled cells co‐expressing kisspeptin in G21 rats (8.73 ± 1.24%) than in non‐pregnant rats (1.62 ± 0.75%; P = 0.0008, Student's t test; Fig. 3 I).
Figure 3. Retrograde labelling of kisspeptin neurones in the periventricular nucleus from the supraoptic nucleus/perinuclear zone.
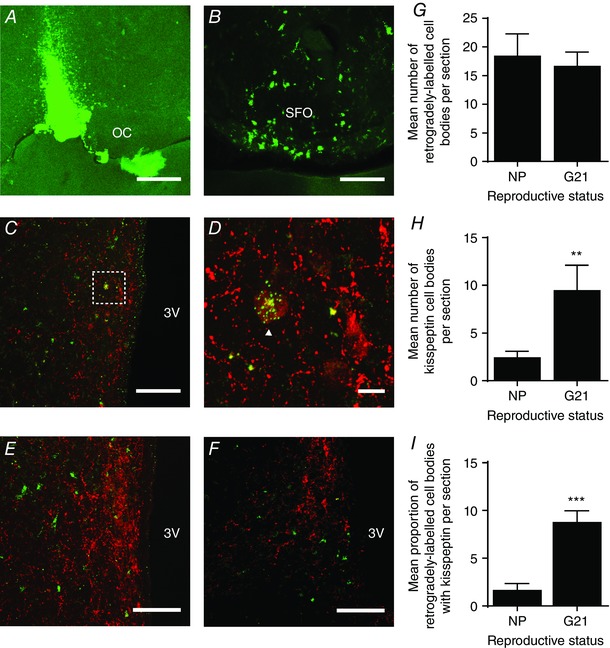
A, confocal image showing a positive injection site in the perinuclear zone–supraoptic nucleus, immediately lateral to the optic chiasm (OC). Scale bar = 500 μm. B, confocal image showing retrograde labelling of cell bodies in the subfornical organ (SFO), an area known to project to the supraoptic nucleus. Scale bar = 50 μm. C, confocal image showing kisspeptin and tracer expression in the periventricular nucleus, lateral to the third ventricle (3V). Scale bar = 100 μm. D, high power image of the area bounded by the box in C, showing a kisspeptin‐positive neurone showing punctate tracer labelling (white arrowhead). Scale bar = 10 μm. E and F, representative confocal images showing no retrograde labelling of kisspeptin neurones in the anteroventral periventricular nucleus (E) and arcuate nucleus (F). Scale bars = 100 μm. G, the mean number of retrogradely labelled neurones per section in the periventricular nucleus of non‐pregnant rats (NP) and late‐pregnant rats (G21). H, the mean number of kisspeptin‐positive neurones per section in the periventricular nucleus of non‐pregnant rats and late‐pregnant rats. I, the percentage of retrogradely labelled neurones that were kisspeptin‐positive in the periventricular nucleus of non‐pregnant rats and late‐pregnant rats. *** P < 0.0001 versus non‐pregnant rats, Student's t test. [Colour figure can be viewed at wileyonlinelibrary.com]
Kiss1R mRNA expression is present in the perinuclear zone–supraoptic nucleus
We used qRT‐PCR to determine whether the expression of Kiss1R mRNA changes in the perinuclear zone–supraoptic nucleus over the course of pregnancy. Kiss1R mRNA was present in perinuclear zone–supraoptic nucleus punches and there was no difference between expression levels relative to non‐pregnant rats (1.00 ± 0.21; n = 8), G7 rats (0.99 ± 0.06; n = 5), G14 rats (0.76 ± 0.19; n = 7) and G21 rats (1.36 ± 0.26; n = 7; F 3,23 = 1.43, P = 0.26, one‐way ANOVA; Fig. 4).
Figure 4. Kiss1R mRNA expression in the supraoptic nucleus/perinuclear zone.
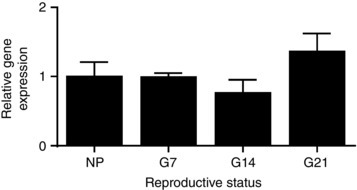
Kiss1R mRNA expression in the supraoptic nucleus/perinuclear zone non‐pregnant rats and of early‐ (G7) mid‐ (G14) and late‐ (G21) pregnant rats, relative to non‐pregnant rats. There was no significant effect of reproductive status (P = 0.25, one‐way ANOVA).
In vivo electrophysiology: basal firing rates
In vivo electrophysiological recordings were made from 75 magnocellular neurones in 58 rats: 41 oxytocin neurones and 34 vasopressin neurones. There was no difference between basal firing rates of oxytocin neurones from non‐pregnant rats (4.8 ± 0.7 spikes s−1, n = 13), early‐ to mid‐pregnant rats (5.3 ± 0.6 spikes s−1, n = 14) and late‐pregnant rats (3.8 ± 0.5 spikes s−1, n = 14; F 2,38 = 1.66, P = 0.20, one‐way ANOVA). Similarly, there was no difference between basal firing rates of vasopressin neurones from non‐pregnant rats (5.5 ± 0.6 spikes s−1, n = 11), early‐ to mid‐pregnant rats (5.2 ± 0.7 spikes s−1, n = 12) and late‐pregnant rats (7.2 ± 0.6 spikes s−1, n = 11; F 2,31 = 2.68, P = 0.081, one‐way ANOVA).
Effects of central kisspeptin administration on oxytocin neurone activity in vivo
Previously, we have shown that i.c.v. kisspeptin does not affect oxytocin neurone firing rate in non‐pregnant rats (Scott & Brown, 2011). Given the emergence of a kisspeptin projection from the periventricular nucleus to the perinuclear zone–supraoptic nucleus, we determined whether i.c.v. kisspeptin changes oxytocin neurone firing rate in pregnant rats. One‐way ANOVA revealed a significant effect of reproductive status on the change in firing rate after i.c.v. kisspeptin administration (F 5,37 = 8.60; P < 0.001). i.c.v. kisspeptin had no effect on oxytocin neurone firing rate in non‐pregnant rats (+0.1 ± 0.3 spikes s−1 change, n = 5; e.g. Fig. 5 A), early‐pregnant rats (+0.6 ± 0.3 spikes s−1 change, n = 8; e.g. Fig. 5 B) or mid‐pregnant rats (+0.4 ± 0.6 spikes s−1, n = 6; e.g. Fig. 5 C). By contrast, i.c.v. kisspeptin increased the firing rate of oxytocin neurones in late‐pregnant rats (+2.6 ± 0.6 spikes s−1 change, n = 8; P = 0.004, Bonferroni's post hoc test; Fig. 5 D and E).
Figure 5. i.c.v. kisspeptin excites oxytocin neurones only in late‐pregnant rats.
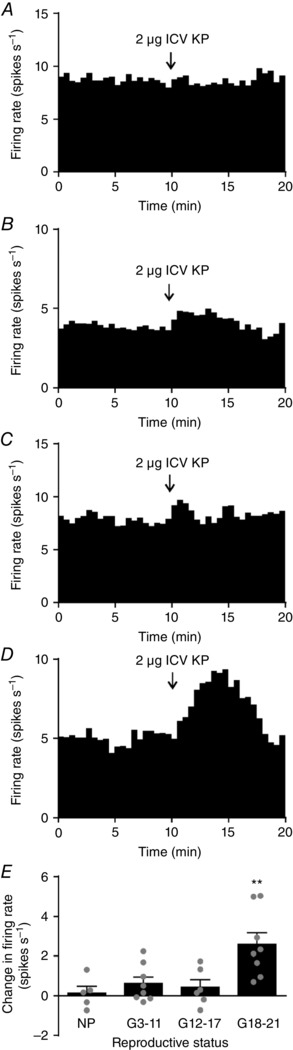
A–D, representative ratemeter records (in 30 s bins) showing the effect of intracerebroventricular (i.c.v.) kisspeptin (KP; 2 μg) on the firing rate of oxytocin neurones from a non‐pregnant rat (A; NP), early‐pregnant rat (B; G3–11), mid‐pregnant rat (C; G12–17) and late‐pregnant rat (D; G18–21). E, change in firing rate 2 min before and after i.c.v. kisspeptin administration. One‐way ANOVA showed a significant effect of reproductive status (F 5,37 = 8.60, P < 0.001); ** P < 0.01 compared to non‐pregnant rats, Bonferroni's post hoc test).
Effects of systemic kisspeptin administration on oxytocin neurone activity in vivo
We have previously shown that i.v. kisspeptin transiently excites oxytocin neurones in non‐pregnant rats (Scott & Brown, 2011). To determine whether this excitatory effect is changed in pregnancy, we compared the change in firing rate response to kisspeptin in neurones from non‐pregnant rats (n = 15; Fig. 6 A), early‐ to mid‐pregnant rats (n = 6) and late‐pregnant rats (n = 11; Fig. 6 B). i.v. kisspeptin caused a similar increase in oxytocin neurone firing rate in all groups (F 2,34 = 1.47, P = 0.24, one‐way ANOVA).
Figure 6. i.v. kisspeptin excites oxytocin neurones.
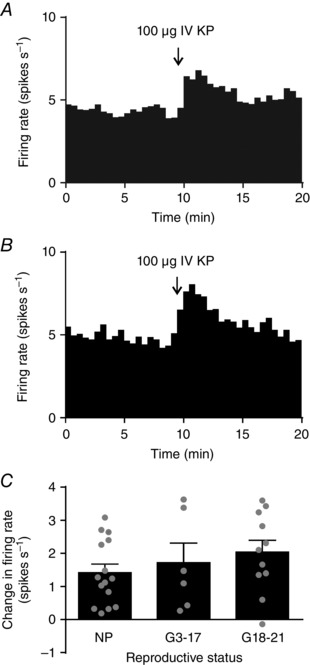
A and B, representative ratemeter records (in 30 s bins) showing intravenous (i.v.) kisspeptin (KP; 100 μg) excitation of oxytocin neurones from a non‐pregnant rat (A) and a late‐pregnant rat (B). C, change in firing rate 2 min before and after i.v. kisspeptin administration in early‐ or mid‐pregnant rats (G3–17) and late‐pregnant rats (G18–21). One‐way ANOVA showed no significant effect of reproductive status (F 2,34 = 1.47, P = 0.24).
Effects of kisspeptin on vasopressin neurone activity in vivo
We have previously reported that i.c.v. kisspeptin does not affect vasopressin neurone firing rate, and that i.v. kisspeptin causes short high frequency bursts in some vasopressin neurones (Scott & Brown, 2011). Similarly to our previous observations, i.c.v. kisspeptin did not change the firing rate of vasopressin neurones in non‐pregnant rats (n = 9; Fig. 7 A), early‐ to mid‐pregnant rats (n = 10) or late‐pregnant rats (n = 7; F 2,30 = 0.42, P = 0.66, one‐way ANOVA; Fig. 7 B and C). i.v. kisspeptin did not change the firing rate of vasopressin neurones in non‐pregnant rats (n = 5), early‐ to mid‐pregnant rats (n = 8) or late‐pregnant rats (n = 6) when measured over 2 min before and after administration (F 3,29 = 0.52, P = 0.68, one‐way ANOVA; Fig. 7 D). However, there was a clear increase in firing rate in some vasopressin neurones after i.v. kisspeptin that lasted a few seconds in each instance (Fig. 7 E–G). When firing rate was analysed in 2 s bins for 10 s before and after i.v. kisspeptin administration, 1 of 5 neurones from non‐pregnant rats were excited, 3 of 8 neurones from early‐ to mid‐pregnant rats were excited and 2 of 6 neurones from late‐pregnant rats were excited.
Figure 7. Effects of kisspeptin on vasopressin neurones.
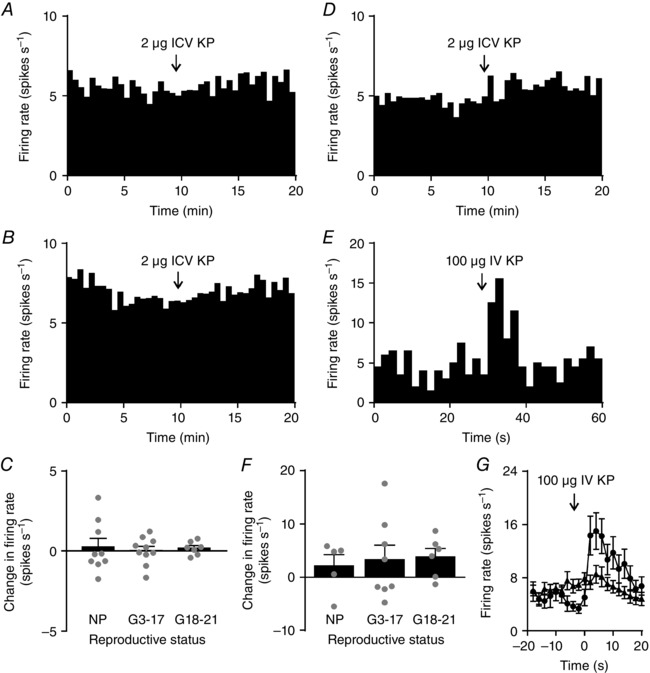
A and B, representative ratemeter records (in 30 s bins) showing no effect of intracerebroventricular (i.c.v.) kisspeptin (KP; 2 μg) on vasopressin neurone activity in a non‐pregnant rat (A) and a late‐pregnant rat (B). C, Change in firing rate 2 min before and after i.c.v. kisspeptin administration in early‐ or mid‐pregnant rats (G3–17) and late‐pregnant rats (G18–21). One‐way ANOVA showed no significant effect of reproductive status (F 2,30 = 0.42, P = 0.66). D, representative ratemeter record (in 30 s bins) from a late‐pregnant rat, showing no sustained effect of intravenous (i.v.) kisspeptin (100 μg) on vasopressin neurone firing rate. E, ratemeter record from the same rat in D in 2 s bins showing a transient increase in vasopressin neurone firing rate after i.v. kisspeptin. F, change in firing rate of vasopressin neurones 2 min before and after i.v. kisspeptin in early‐ or mid‐pregnant rats (G3–17) and late‐pregnant rats (G18–21). One‐way ANOVA showed no significant effect of reproductive status (F 2,26 = 1.76, P = 0.19). G, mean firing rate of the vasopressin neurones shown in F for 20 s before and after i.v. kisspeptin (in 2 s bins); circles show vasopressin neurones that responded to kisspeptin with a short burst of firing, and triangles show vasopressin neurones that were unaffected by i.v. kisspeptin.
Discussion
Here, we show for the first time that central kisspeptin excitation of oxytocin neurones emerges in late pregnancy and that this excitation is likely to be mediated by upregulation of kisspeptin expression in a subpopulation of periventricular nucleus neurones that project to the perinuclear zone that surrounds the supraoptic nucleus. Given the timing of the emergence of this excitatory kisspeptin input, it appears likely it will increase the excitability of oxytocin neurones for parturition.
Kisspeptin expression in periventricular nucleus projections to the perinuclear zone surrounding the supraoptic nucleus
While some kisspeptin fibres were evident within the supraoptic nucleus, the densest labelling was in the surrounding perinuclear zone, which contains glutamatergic and GABAergic neurones that principally project to the supraoptic nucleus (Armstrong & Stern, 1997). Hence, kisspeptin excitation of oxytocin neurones might be mediated indirectly via glutamatergic perinuclear zone inputs, as has been shown for noradrenergic inputs (Boudaba et al. 2003), which are activated during birth (Meddle et al. 2000). Perinuclear zone stimulation induces multiple excitatory postsynaptic currents in supraoptic nucleus neurones (Boudaba et al. 1997). Hence, relaying upstream signals through the perinuclear zone amplifies the signal, which might be important for sustaining high frequency bursts in oxytocin neurones that are necessary for normal delivery. In addition to projections into the supraoptic nucleus, perinuclear zone neurones also project elsewhere in the brain (Armstrong & Stern, 1997), including the hypothalamic paraventricular nucleus (Boudaba & Tasker, 2006). Hence, while we also observed increased kisspeptin fibre density in and around the paraventricular nucleus (data not shown), the kisspeptin projection to the perinuclear zone might also co‐ordinate bursts across the population of oxytocin neurones to facilitate pulsatile oxytocin release for episodic contraction of the uterus during parturition.
By contrast to a recent report of a direct NKB projection from the arcuate nucleus to the supraoptic nucleus that excites vasopressin neurones but not oxytocin neurones (Pineda et al. 2016), there was little NKB expression in the perinuclear zone–supraoptic nucleus and almost no co‐expression with kisspeptin, despite clear double‐labelling in arcuate nucleus cell bodies. Hence, it appears unlikely that the kisspeptin projection to the perinuclear zone arises from the arcuate nucleus kisspeptin neurones that co‐express NKB (Burke et al. 2006; Navarro et al. 2009). Retrograde tracer injected into the perinuclear zone–supraoptic nucleus did not label kisspeptin cell bodies in the arcuate nucleus, confirming that the kisspeptin fibres did not arise from the arcuate nucleus. Retrograde label was differentially distributed within the RP3V; while there was almost no label evident in kisspeptin neurones in the AVPV, there was labelling of periventricular nucleus kisspeptin neurones in late‐pregnant rats. The modest retrograde labelling of periventricular nucleus kisspeptin neurones is probably an underestimate because the tracer was injected unilaterally, and so the projections to the contralateral side would not be labelled, nor would projections to the paraventricular nucleus. Hence, it is probable that a substantial proportion of periventricular nucleus kisspeptin neurones interact with the oxytocin system in late pregnancy. While the total number of retrogradely labelled neurones was similar in non‐pregnant rats and late‐pregnant rats, a larger proportion of these neurones expressed kisspeptin in late‐pregnant rats. The simplest explanation for this observation is that periventricular nucleus neurones that project to the perinuclear zone–supraoptic nucleus increase their expression of kisspeptin in late pregnancy.
Kisspeptin expression in the arcuate nucleus and RP3V in late pregnancy
Similarly to previous work in non‐pregnant rats, we found a substantial number of kisspeptin‐positive cell bodies in the arcuate nucleus but very few in the RP3V (Desroziers et al. 2010; Overgaard et al. 2013). Consistent with increased kisspeptin expression in perinuclear zone–supraoptic nucleus fibres arising from the periventricular nucleus, there were more kisspeptin‐positive cell bodies in the RP3V of pregnant rats than non‐pregnant rats. Indeed, in rats treated with the axonal transport inhibitor, colchicine, kisspeptin neurones are clearly discernible in the RP3V of non‐pregnant rats (Overgaard et al. 2013), suggesting that these neurones are always capable of synthesizing kisspeptin but do not do so in measurable quantities under basal conditions. By contrast to the RP3V, there were less kisspeptin‐positive cell bodies in the arcuate nucleus of late‐pregnant rats than in non‐pregnant rats. The cause of these changes in kisspeptin expression levels is unclear but increased oestrogen levels during pregnancy might be responsible because oestrogen administration increases Kiss1 mRNA expression in the RP3V but decreases Kiss1 mRNA expression in the arcuate nucleus (Smith et al. 2005).
Central kisspeptin excitation of oxytocin neurones in late‐pregnant rats
i.c.v. kisspeptin consistently increased the firing rate of oxytocin neurones in late‐pregnant rats but did not affect the firing rate of oxytocin neurones in non‐pregnant rats, early‐pregnant rats or late‐pregnant rats. While i.c.v. kisspeptin could excite oxytocin neurones via afferent inputs from other brain areas, it appears more likely that kisspeptin acts locally because kisspeptin fibre density increased in the vicinity of the supraoptic nucleus during pregnancy. The emergence of the periventricular nucleus kisspeptin input to the oxytocin system occurs independent of circulating kisspeptin actions because i.v. kisspeptin caused a transient excitation of oxytocin neurones that was similar in non‐pregnant rats and in rats across all stages of pregnancy. Kisspeptin does not cross the blood–brain barrier but excites oxytocin neurones via activation of vagal afferents (Scott & Brown, 2011); this regulatory mechanism persists throughout pregnancy, which might allow placental kisspeptin to impact oxytocin neurone activity.
The simplest explanation for the emergence of central kisspeptin excitation of oxytocin neurones in late pregnancy is upregulation of Kiss1R expression. Consistent with a recent in situ hybridization study that reported low supraoptic nucleus Kiss1R mRNA expression (Higo et al. 2016), little Kiss1R mRNA was present in the perinuclear zone–supraoptic nucleus of non‐pregnant rats, based on the number of PCR cycles at which fluorescence rose above background. Unexpectedly, there was no difference in Kiss1R mRNA expression levels in the perinuclear zone–supraoptic nucleus between non‐pregnant, early‐pregnant, mid‐pregnant and late‐pregnant rats, despite the clear emergence of kisspeptin excitation of oxytocin neurones over pregnancy. Kiss1R mRNA expression might remain low while translation of mRNA into Kiss1R expression is nevertheless increased; this possibility will require further investigation once reliable antibodies become available.
Alternatively, Kiss1R intracellular signalling pathways might be differentially regulated in late‐pregnancy. Kiss1R is coupled to Gq/11 (Bianco & Kaiser, 2013) and excites GnRH neurones and proopiomelanocortin neurones via multiple mechanisms (Liu et al. 2008; Fu & van den Pol, 2010). Hence, stronger coupling of Gq/11 activation to activation of non‐specific cation channels or inhibition of potassium channels might underpin the emergence of kisspeptin excitation in late pregnancy.
Finally, kisspeptin might act via other receptors. Kisspeptin is an RF‐amide (Ebling & Luckman, 2008) and has affinity for other RF‐amide receptors, including neuropeptide FF 1 and 2 receptors (NPFF1/2Rs) (Lyubimov et al. 2010; Oishi et al. 2011). NPFF1/2Rs are expressed in various brain regions, including the paraventricular nucleus and regions that send excitatory projections to the supraoptic nucleus, such as the SFO and nucleus tractus solitarius (Liu et al. 2001). However, i.c.v. administration of NPFF1R agonists increases plasma oxytocin concentrations in male rats (Kaewwongse et al. 2011), suggesting that NPFF1R is unlikely to mediate the effects of kisspeptin on oxytocin neurones evident in our experiments because this would be expected to excite oxytocin neurones in non‐pregnant rats, unless there are sex differences in NPFF1R expression. Further studies will be required to determine whether NPFF1/2R expression in the perinuclear zone–supraoptic nucleus mediates kisspeptin effects on oxytocin neurones.
Effects of kisspeptin on vasopressin neurones
The firing rate of vasopressin neurones was unaffected by i.c.v. kisspeptin in non‐pregnant and in pregnant rats. i.c.v. kisspeptin has been shown to increase plasma vasopressin concentrations in male rats (Han et al. 2010; Ten et al. 2010), and to increase mEPSC frequency in vasopressin neurones in brain slices from male rats (Yokoyama et al. 2014). While this might represent a sex difference in the regulation of vasopressin neurones by kisspeptin, it is possible that paraventricular nucleus vasopressin neurones respond differently than supraoptic nucleus oxytocin neurones to i.c.v. kisspeptin, perhaps via activation of different afferent inputs; while paraventricular nucleus and supraoptic nucleus oxytocin neurones share many inputs, there is some functional independence (Hatton, 1990).
In non‐pregnant rats, ∼25% of vasopressin neurones display a short burst of firing after i.v. kisspeptin administration (Scott & Brown, 2011); here, we found that this transient excitation of a subset of vasopressin neurones is maintained throughout pregnancy. The mechanisms that underpin these bursts are unknown but, as we have previously proposed (Scott & Brown, 2011), they might arise from a more prominent fast afterdepolarization in this subset of vasopressin neurones (Teruyama & Armstrong, 2007).
Concluding remarks
We have characterized a periventricular nucleus projection to the perinuclear zone–supraoptic nucleus that increases kisspeptin expression in late pregnancy to excite oxytocin neurones. The functional significance of this pregnancy‐specific input has yet to be determined but it appears likely that it will enhance the excitability of oxytocin neurones to promote episodic contraction of the uterus during delivery. Hence, Kiss1R antagonists might provide a therapeutic target for a new generation of drugs to reduce the risk of pre‐term delivery for at‐risk pregnancies.
Additional information
Competing interests
The authors declare no competing financial interests.
Author contributions
C.H.B., V.S. and R.E.C. conceived the work. C.H.B., A.J.S., V.S. and R.E.C. designed the work. V.S., A.J.S. and G.T.B. acquired, analysed and interpreted the data. All authors were involved in drafting the work or revising it critically for important intellectual content. All authors approved the final version of the manuscript, agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved and all persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This work was supported by the Health Research Council of New Zealand (C.H.B.) and a University of Otago Postgraduate Scholarship (A.J.S.).
Translational perspective.
Oxytocin is critical for normal parturition and the prevention of early activation of oxytocin neurones is important to reduce the risk of pre‐term delivery. This paper shows that a new excitatory projection from kisspeptin neurones to the oxytocin system emerges only in late pregnancy. Hence, antagonism of kisspeptin actions might constitute a novel therapeutic target for the management of pregnancies at risk of pre‐term delivery.
Linked articles This article is highlighted by a Perspective by Armstrong. To read this Perspective, visit http://dx.doi.org/10.1113/JP273364.
This is an Editor's Choice article from the 1 February 2017 issue.
References
- Antonijevic IA, Douglas AJ, Dye S, Bicknell RJ, Leng G & Russell JA (1995). Oxytocin antagonists delay the initiation of parturition and prolong its active phase in rats. J Endocrinol 145, 97–103. [DOI] [PubMed] [Google Scholar]
- Armstrong WE & Stern JE (1997). Electrophysiological and morphological characteristics of neurons in perinuclear zone of supraoptic nucleus. J Neurophysiol 78, 2427–2437. [DOI] [PubMed] [Google Scholar]
- Augustine RA, Bouwer GT, Seymour AJ, Grattan DR & Brown CH (2016). Reproductive regulation of gene expression in the hypothalamic supraoptic and paraventricular nuclei. J Neuroendocrinol 28, DOI: 10.1111/jne.12350. [DOI] [PubMed] [Google Scholar]
- Bianco SD & Kaiser UB (2013). Molecular biology of the kisspeptin receptor: signaling, function, and mutations. Adv Exp Med Biol 784, 133–158. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Di S & Tasker JG (2003). Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol 15, 803–810. [DOI] [PubMed] [Google Scholar]
- Boudaba C, Schrader LA & Tasker JG (1997). Physiological evidence for local excitatory synaptic circuits in the rat hypothalamus. J Neurophysiol 77, 3396–3400. [DOI] [PubMed] [Google Scholar]
- Boudaba C & Tasker JG (2006). Intranuclear coupling of hypothalamic magnocellular nuclei by glutamate synaptic circuits. Am J Physiol Regul Integr Comp Physiol 291, R102–R111. [DOI] [PubMed] [Google Scholar]
- Brown CH, Bains JS, Ludwig M & Stern JE (2013). Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. J Neuroendocrinol 25, 678–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CH, Han SY, Moaddab M, Scott V & Schwenke DO (2014). Peptidergic control of oxytocin and vasopressin neurons and its role in reproductive and hypertension‐associated plasticity In Neurophysiology of Neuroendocrine Neurons, eds Armstrong WE. & Tasker JG, pp. 65–86. Wiley‐Blackwell, UK. [Google Scholar]
- Brown CH, Munro G, Murphy NP, Leng G & Russell JA (1996). Activation of oxytocin neurones by systemic cholecystokinin is unchanged by morphine dependence or withdrawal excitation in the rat. J Physiol 496, 787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MC, Letts PA, Krajewski SJ & Rance NE (2006). Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: Morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498, 712–726. [DOI] [PubMed] [Google Scholar]
- Clarkson J, d'Anglemont de Tassigny X, Colledge WH, Caraty A & Herbison AE (2009). Distribution of kisspeptin neurones in the adult female mouse brain. J Neuroendocrinol 21, 673–682. [DOI] [PubMed] [Google Scholar]
- Desroziers E, Mikkelsen J, Simonneaux V, Keller M, Tillet Y, Caraty A & Franceschini I (2010). Mapping of kisspeptin fibres in the brain of the pro‐oestrous rat. J Neuroendocrinol 22, 1101–1112. [DOI] [PubMed] [Google Scholar]
- Ebling FJ & Luckman SM (2008). RFAmide‐related peptide: another sexy peptide? Endocrinology 149, 899–901. [DOI] [PubMed] [Google Scholar]
- Fu LY & van den Pol AN (2010). Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci 30, 10205–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA & Herbison AE (2005). Activation of gonadotropin‐releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci 25, 11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Yan M, An XF, He M & Yu JY (2010). Central administration of kisspeptin‐10 inhibits natriuresis and diuresis induced by blood volume expansion in anesthetized male rats. Acta Pharmacol Sin 31, 145–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI (1990). Emerging concepts of structure–function dynamics in adult brain: the hypothalamo‐neurohypophysial system. Prog Neurobiol 34, 437–504. [DOI] [PubMed] [Google Scholar]
- Herbison AE (2008). Estrogen positive feedback to gonadotropin‐releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev 57, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo S, Honda S, Iijima N & Ozawa H (2016). Mapping of kisspeptin receptor mRNA in the whole rat brain and its co‐localisation with oxytocin in the paraventricular nucleus. J Neuroendocrinol 28, DOI: 10.1111/jne.12356. [DOI] [PubMed] [Google Scholar]
- Kaewwongse M, Takayanagi Y & Onaka T (2011). Effects of RFamide‐related peptide (RFRP)‐1 and RFRP‐3 on oxytocin release and anxiety‐related behaviour in rats. J Neuroendocrinol 23, 20–27. [DOI] [PubMed] [Google Scholar]
- Kim J, Semaan SJ, Clifton DK, Steiner RA, Dhamija S & Kauffman AS (2011). Regulation of Kiss1 expression by sex steroids in the amygdala of the rat and mouse. Endocrinology 152, 2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, Schutz G & Herbison AE (2013). Dependence of fertility on kisspeptin‐Gpr54 signaling at the GnRH neuron. Nat Commun 4, 2492. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez‐Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G & Parmentier M (2001). The metastasis suppressor gene KiSS‐1 encodes kisspeptins, the natural ligands of the orphan G protein‐coupled receptor GPR54. J Biol Chem 276, 34631–34636. [DOI] [PubMed] [Google Scholar]
- Liu Q, Guan XM, Martin WJ, McDonald TP, Clements MK, Jiang Q, Zeng Z, Jacobson M, Williams DL Jr, Yu H, Bomford D, Figueroa D, Mallee J, Wang R, Evans J, Gould R & Austin CP (2001). Identification and characterization of novel mammalian neuropeptide FF‐like peptides that attenuate morphine‐induced antinociception. J Biol Chem 276, 36961–36969. [DOI] [PubMed] [Google Scholar]
- Liu X, Lee K & Herbison AE (2008). Kisspeptin excites gonadotropin‐releasing hormone neurons through a phospholipase C/calcium‐dependent pathway regulating multiple ion channels. Endocrinology 149, 4605–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyubimov Y, Engstrom M, Wurster S, Savola JM, Korpi ER & Panula P (2010). Human kisspeptins activate neuropeptide FF2 receptor. Neuroscience 170, 117–122. [DOI] [PubMed] [Google Scholar]
- Mayer C & Boehm U (2011). Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nat Neurosci 14, 704–710. [DOI] [PubMed] [Google Scholar]
- Meddle SL, Leng G, Selvarajah JR, Bicknell RJ & Russell JA (2000). Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience 101, 1013–1021. [DOI] [PubMed] [Google Scholar]
- Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CG, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL & Harrison DC (2001). AXOR12, a novel human G protein‐coupled receptor, activated by the peptide KiSS‐1. J Biol Chem 276, 28969–28975. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK & Steiner RA (2009). Regulation of gonadotropin‐releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29, 11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O & Fujino M (2001). Metastasis suppressor gene KiSS‐1 encodes peptide ligand of a G‐protein‐coupled receptor. Nature 411, 613–617. [DOI] [PubMed] [Google Scholar]
- Oishi S, Misu R, Tomita K, Setsuda S, Masuda R, Ohno H, Naniwa Y, Ieda N, Inoue N, Ohkura S, Uenoyama Y, Tsukamura H, Maeda K, Hirasawa A, Tsujimoto G & Fujii N (2011). Activation of neuropeptide FF receptors by kisspeptin receptor ligands. ACS Med Chem Lett 2, 53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard A, Tena‐Sempere M, Franceschini I, Desroziers E, Simonneaux V & Mikkelsen JD (2013). Comparative analysis of kisspeptin‐immunoreactivity reveals genuine differences in the hypothalamic Kiss1 systems between rats and mice. Peptides 45, 85–90. [DOI] [PubMed] [Google Scholar]
- Patisaul HB, Fortino AE & Polston EK (2008). Sex differences in serotonergic but not gamma‐aminobutyric acidergic (GABA) projections to the rat ventromedial nucleus of the hypothalamus. Endocrinology 149, 397–408. [DOI] [PubMed] [Google Scholar]
- Pineda R, Sabatier N, Ludwig M, Millar RP & Leng G (2016). A direct neurokinin B projection from the arcuate nucleus regulates magnocellular vasopressin cells of the supraoptic nucleus. J Neuroendocrinol 28, DOI: 10.1111/jne.12342. [DOI] [PubMed] [Google Scholar]
- Polston EK & Simerly RB (2003). Sex‐specific patterns of galanin, cholecystokinin, and substance P expression in neurons of the principal bed nucleus of the stria terminalis are differentially reflected within three efferent preoptic pathways in the juvenile rat. J Comp Neurol 465, 551–559. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M & Leng G (2004). Phasic spike patterning in rat supraoptic neurones in vivo and in vitro . J Physiol 558, 161–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott V & Brown CH (2011). Kisspeptin activation of supraoptic nucleus neurons in vivo . Endocrinology 152, 3862–3870. [DOI] [PubMed] [Google Scholar]
- Scott V & Brown CH (2013). Beyond the GnRH axis: kisspeptin regulation of the oxytocin system in pregnancy and lactation. Adv Exp Med Biol 784, 201–218. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK & Steiner RA (2005). Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146, 3686–3692. [DOI] [PubMed] [Google Scholar]
- Ten SC, Gu SY, Niu YF, An XF, Yan M & He M (2010). Central administration of kisspeptin‐10 inhibits water and sodium excretion of anesthetized male rats and the involvement of arginine vasopressin. Endocr Res 35, 128–136. [DOI] [PubMed] [Google Scholar]
- Teruyama R & Armstrong WE (2007). Calcium‐dependent fast depolarizing afterpotentials in vasopressin neurons in the rat supraoptic nucleus. J Neurophysiol 98, 2612–2621. [DOI] [PubMed] [Google Scholar]
- Weiss ML & Hatton GI (1990). Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res Bull 24, 231. [DOI] [PubMed] [Google Scholar]
- Wyatt AK, Zavodna M, Viljoen JL, Stanton JA, Gemmell NJ & Jasoni CL (2013). Changes in methylation patterns of kiss1 and kiss1r gene promoters across puberty. Genet Epigenet 5, 51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Kaga S, Mochiduki A, Tsubomizu J, Adachi S, Sakai T, Inoue K & Adachi AA (2012). Immunocytochemical localization of kisspeptin neurons in the rat forebrain with special reference to sexual dimorphism and interaction with GnRH neurons. Endocr J 59, 161–171. [DOI] [PubMed] [Google Scholar]
- Yokoyama T, Minami K, Terawaki K, Miyano K, Ogata J, Maruyama T, Takeuchi M, Uezono Y & Ueta Y (2014). Kisspeptin‐10 potentiates miniature excitatory postsynaptic currents in the rat supraoptic nucleus. Brain Res 1583, 45–54. [DOI] [PubMed] [Google Scholar]


