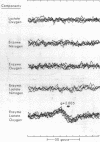
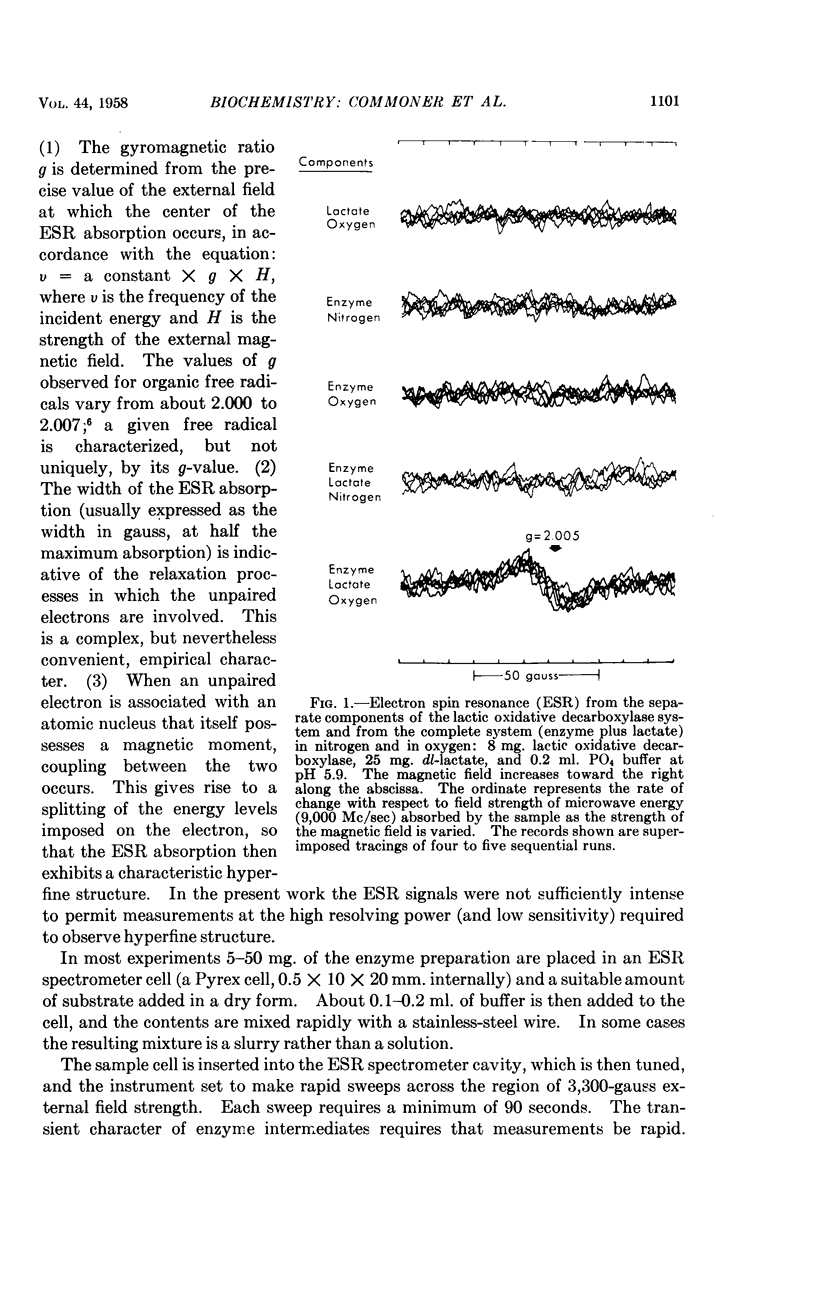
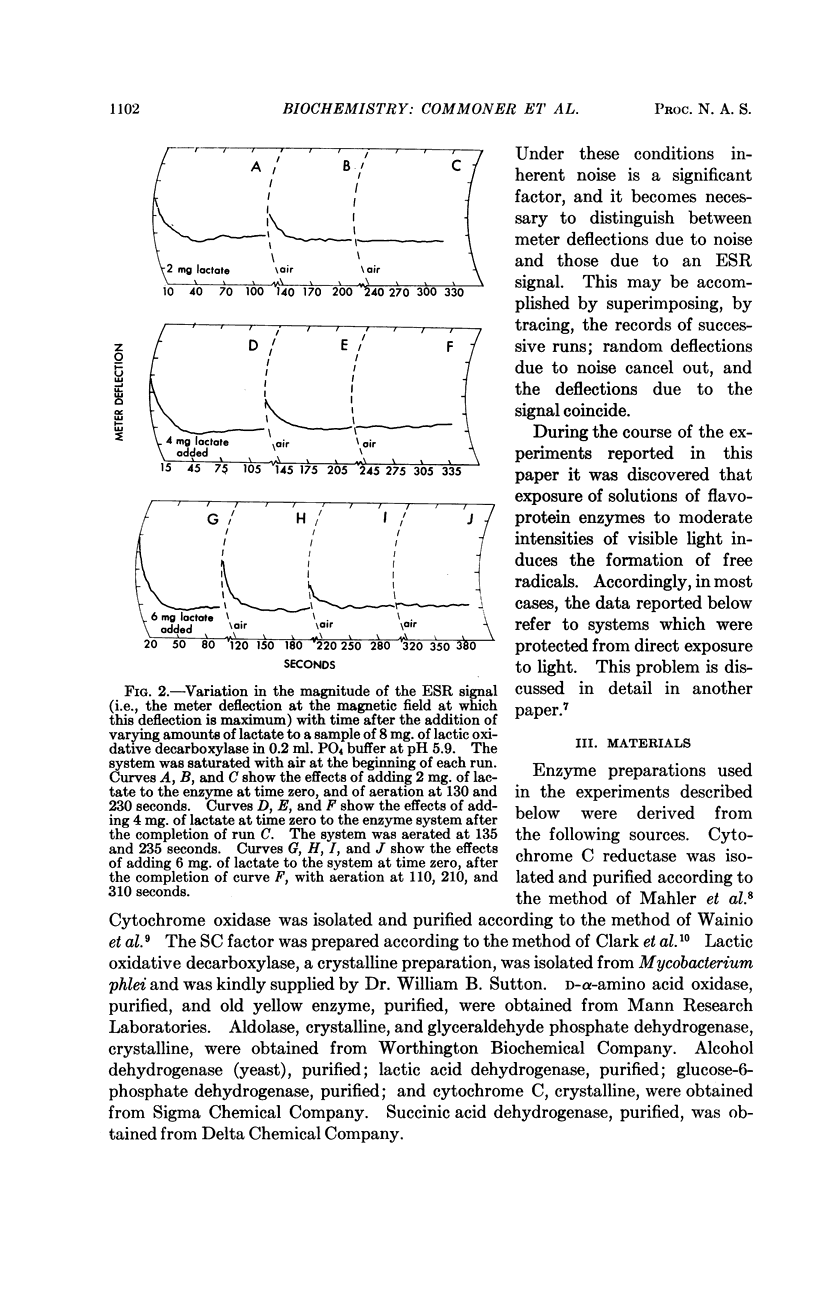
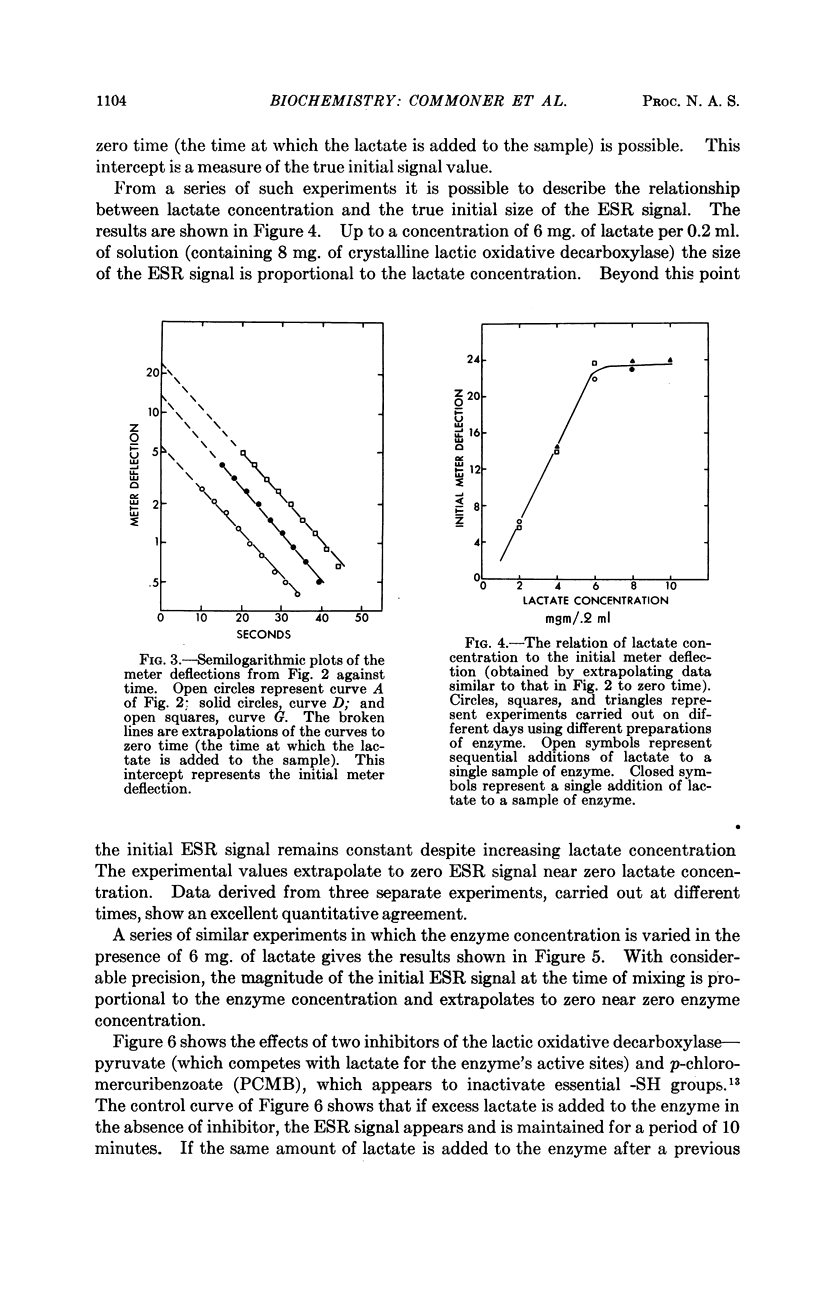
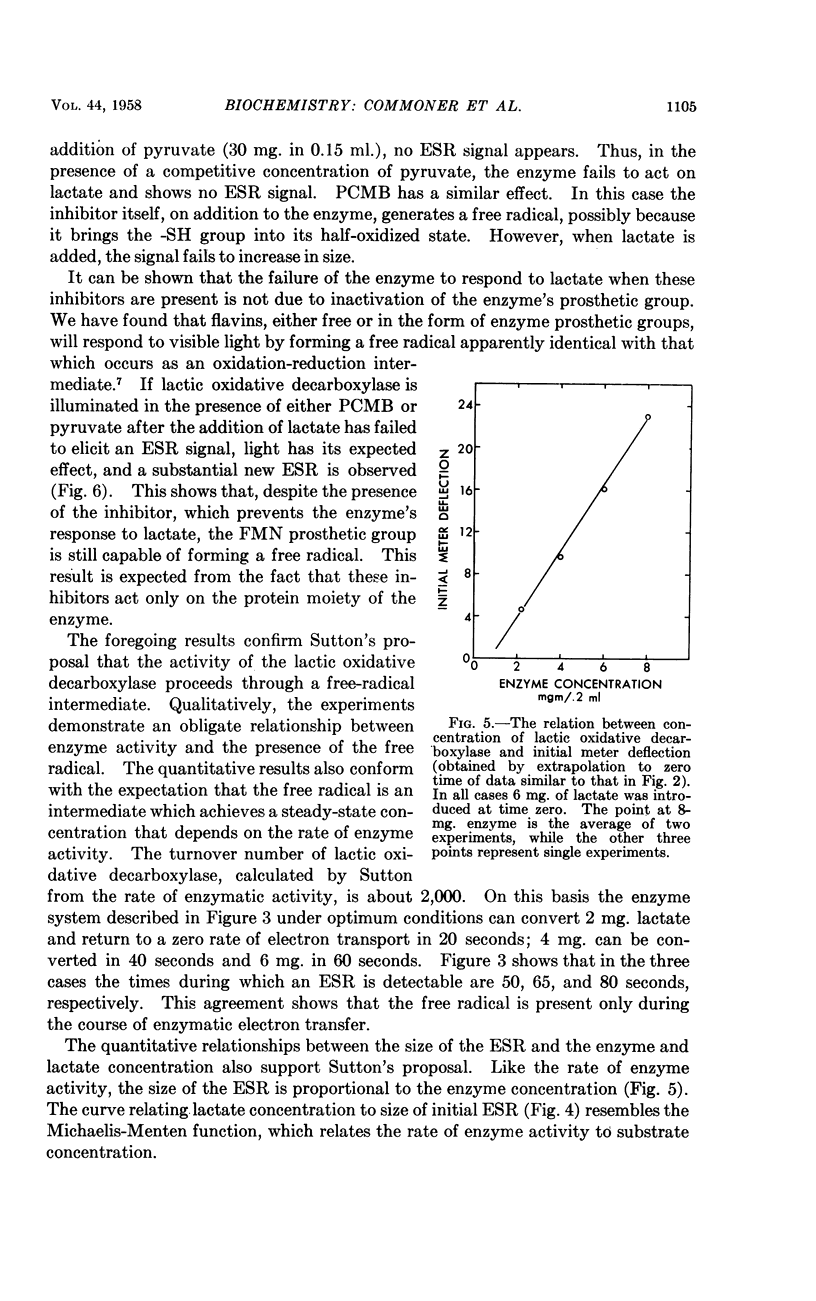
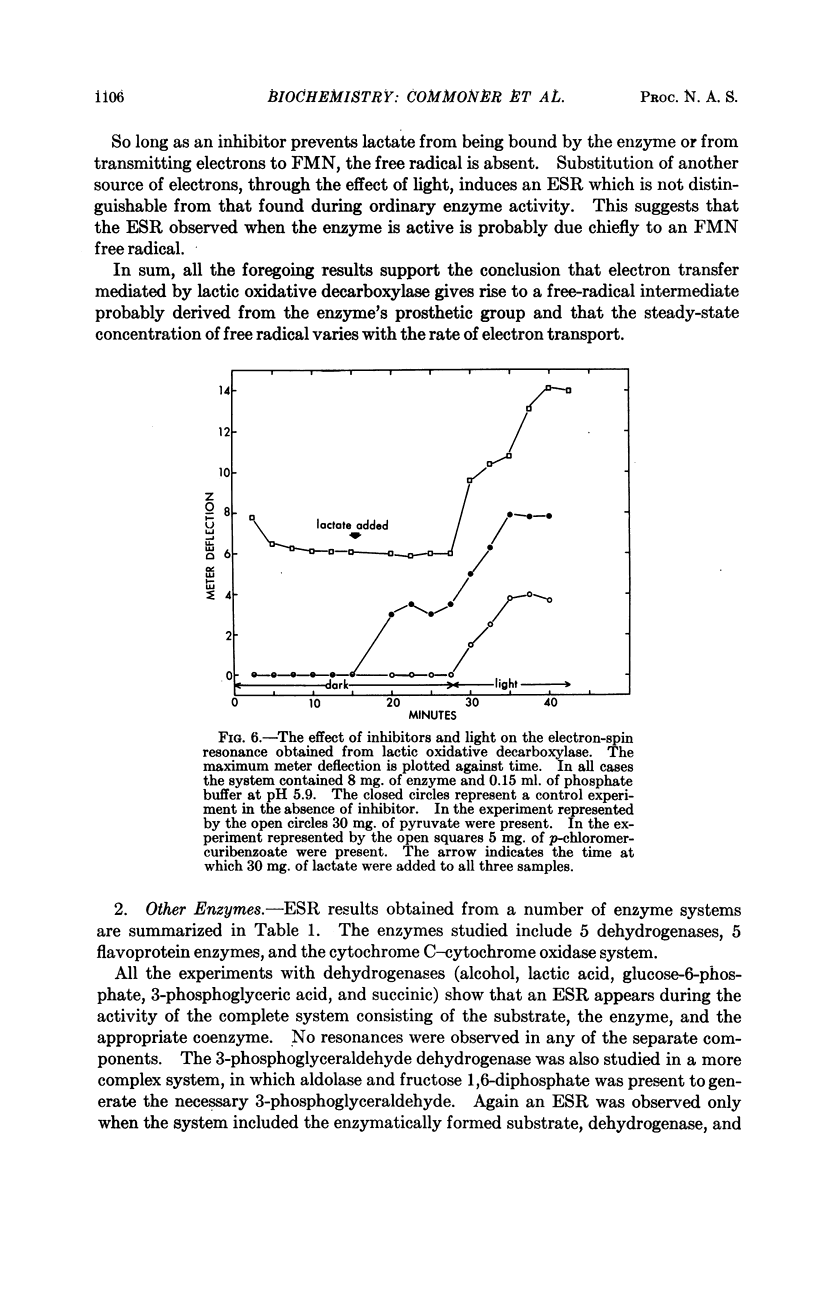
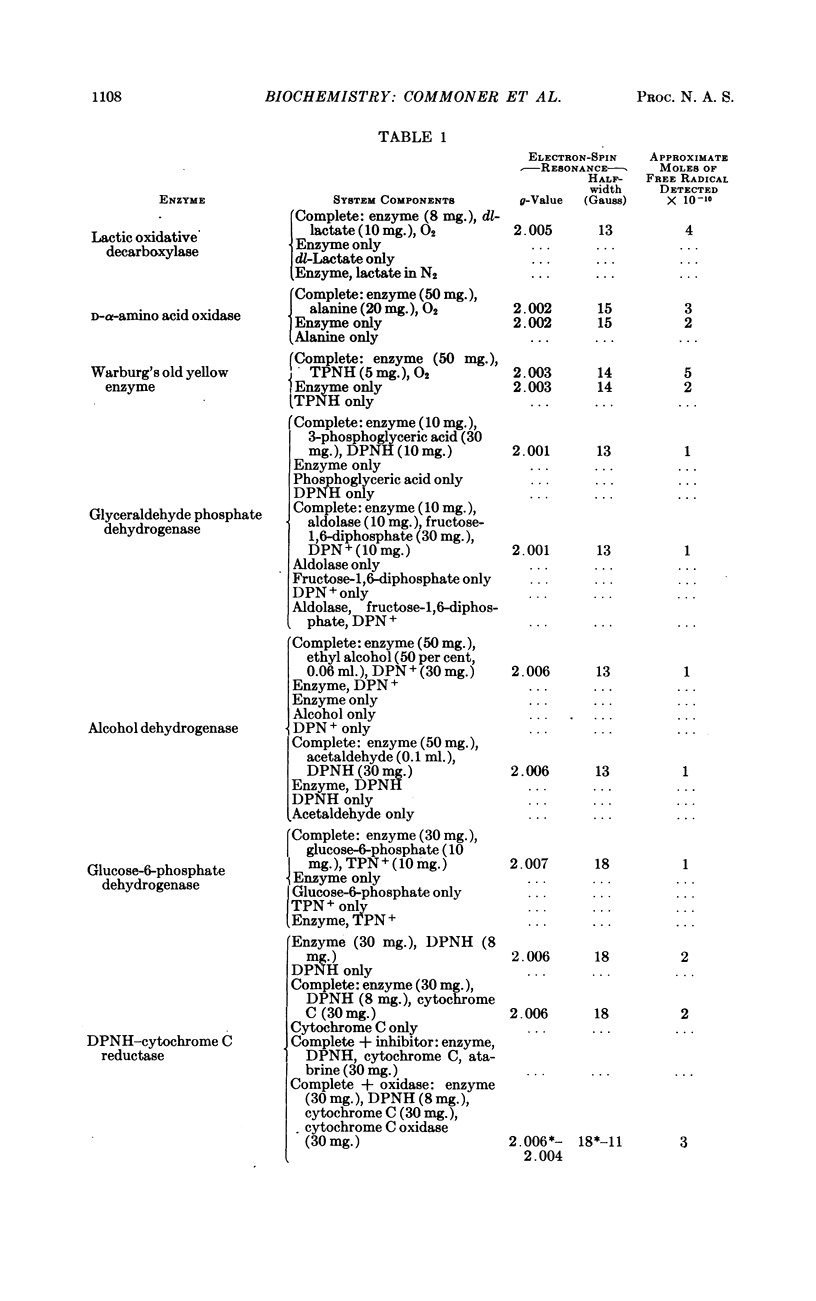
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEINERT H. Evidence for an intermediate in the oxidation-reduction of flavoproteins. J Biol Chem. 1957 Mar;225(1):465–478. [PubMed] [Google Scholar]
- CLARK H. W., NEUFELD H. A., WIDMER C., STOTZ E. Purification of a factor linking succinic dehydrogenase with cytochrome c. J Biol Chem. 1954 Oct;210(2):851–859. [PubMed] [Google Scholar]
- COMMONER B., HEISE J. J., LIPPINCOTT B. B., NORBERG R. E., PASSONNEAU J. V., TOWNSEND J. Biological activity of free radicals. Science. 1957 Jul 12;126(3263):57–63. doi: 10.1126/science.126.3263.57. [DOI] [PubMed] [Google Scholar]
- COMMONER B., HEISE J. J., LIPPINCOTT B. B., NORBERG R. E., PASSONNEAU J. V., TOWNSEND J. Biological activity of free radicals. Science. 1957 Jul 12;126(3263):57–63. doi: 10.1126/science.126.3263.57. [DOI] [PubMed] [Google Scholar]
- COMMONER B., TOWNSEND J., PAKE G. E. Free radicals in biological materials. Nature. 1954 Oct 9;174(4432):689–691. doi: 10.1038/174689a0. [DOI] [PubMed] [Google Scholar]
- EHRENBERG A., LUDWIG G. D. Free radical formation in reaction between old yellow enzyme and reduced triphosphopyridine nucleotide. Science. 1958 May 16;127(3307):1177–1178. doi: 10.1126/science.127.3307.1177. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., SARKAR N. K., VERNON L. P. Studies on diphosphopyridine nucleotide-cytochrome c reductase. II. Purification and properties. J Biol Chem. 1952 Dec;199(2):585–597. [PubMed] [Google Scholar]
- SUTTON W. B. Mechanism of action and crystallization of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1957 May;226(1):395–405. [PubMed] [Google Scholar]
- SUTTON W. B. Sulfhydryl and prosthetic groups of lactic oxidative decarboxylase from Mycobacterium phlei. J Biol Chem. 1955 Oct;216(2):749–761. [PubMed] [Google Scholar]