Abstract
The paper continues the series of publications from the International Nuclear Workers Study cohort (INWORKS) that comprises 308,297 workers from France, the United Kingdom and the United States, providing 8.2 million person-years of observation from a combined follow-up period (at earliest 1944 to at latest 2005). These workers' external radiation exposures were primarily to photons, resulting in an estimated average career absorbed dose to the colon of 17.4 milligray. The association between cumulative ionizing radiation dose and cancer mortality was evaluated in general relative risk models that describe modification of the excess relative risk (ERR) per gray (Gy) by time since exposure and age at exposure. Methods analogous to a nested-case control study using conditional logistic regression of sampled risks sets were used. Outcomes included: all solid cancers, lung cancer, leukemias excluding chronic lymphocytic, acute myeloid leukemia, chronic myeloid leukemia, multiple myeloma, Hodgkin lymphoma, and non-Hodgkin lymphoma.
Significant risk heterogeneity was evident in chronic myeloid leukemia with time since exposure, where we observed increased ERR per Gy estimates shortly after exposure (2-10 year) and again later (20-30 years). We observed delayed effects for acute myeloid leukemia although estimates were not statistically significant. Solid cancer excess risk was restricted to exposure at age 35+ years and also diminished for exposure 30 years prior to attained age. Persistent or late effects suggest additional follow-up may inform on lifetime risks. However, cautious interpretation of results is needed due to analytical limitations and a lack of confirmatory results from other studies.
Keywords: cancer, epidemiology, longitudinal studies, dose-response, mortality studies
Introduction
Exposure to ionizing radiation, a known human carcinogen, is unavoidable.1 Ubiquitous natural and man-made environmental sources account for about one-half of the average annual per capita effective dose in developed countries. The remaining dose stems primarily from radiation used in diagnostic and therapeutic medicine.2 Moreover, the trend in population dose is increasing as a consequence of a proliferation in radiologic and nuclear medicine procedures in recent years. In the United States of America (USA), the annual per capita effective dose has risen two-fold in the last 25 years from a concurrent 10-fold increase in diagnostic and interventional radiologic examinations and a 2.5-fold increase in nuclear medicine procedures.3
In addition to environmental and medical exposures, millions of workers worldwide are exposed in the course of their employment in many occupational settings, such as healthcare, research, military, general industry and commercial nuclear power.2 Protection standards adopted to mitigate health risks are based on our current understanding of the relation between ionizing radiation and cancer, which relies heavily on studies of acutely exposed populations, such as the Japanese atomic bomb survivors or radiation therapy patients who received highly fractionated doses.4 Yet exposure conditions among these populations differ greatly from that of nuclear workers, whose radiation dose accrues from protracted low dose and low dose rate exposures. Clearly, direct information from occupational studies promises better estimates of nuclear worker cancer risks; however, occupational studies have lacked sufficient precision to project population-based risks.5
Recent studies have pooled information from several occupational sources to increase study size. The International Agency for Research on Cancer (IARC) has led efforts to conduct pooled studies of nuclear workers from several countries.6-8 The most recent IARC study, the International Nuclear Workers Study (INWORKS), involves a consortium from France, the United Kingdom (UK) and the USA who are examining mortality patterns in about 300,000 nuclear workers.8 Initial examinations of the INWORKS cohort have yielded relatively precise estimates of the linear excess relative risk (ERR) of all solid cancers (ERR per Gy =0.47; 90% CI: 0.18, 0.79) and leukemia, excluding chronic lymphocytic leukemia (ERR per Gy =2.96; 90% CI: 1.17, 5.21).9, 10
An important consideration in assessing radiation-related risk is how the risk is modified by temporal factors. The Life Span Study of atomic bomb survivors (LSS) provides a number of general observations on temporal patterns following acute exposure.11 First, exposure-related cancer risk has persisted over the follow-up period (1950-2003). Second, the risk is not immediately apparent following exposure but is observed after a latent period, which appears longer for solid cancers. Lastly, the ERR per unit dose for some cancers can significantly vary by temporal factors, such as time since exposure (TSE) or age at exposure (AE). The degree to which these observations hold true for protracted radiation exposure is poorly understood. To that end, the current study examined the effects of age at exposure and time since exposure on the cancer risk from protracted low-dose ionizing radiation exposure in the INWORKS cohort of nuclear workers.
Methods and Materials
Study cohort
The INWORKS cohort is described elsewhere.8 Briefly, the cohort comprised 308,297 nuclear workers of both genders who were employed for one or more years in at least one of 13 study facilities/companies and were individually monitored for occupational external exposure to ionizing radiation. The cohort was assembled using data obtained from: the Commissariat à l'Energie Atomique Civil, AREVA Nuclear Cycle, and Electricité de France in France 12; the National Registry for Radiation Workers in the UK 13; and the Hanford Site, the Savannah River Site, the Oak Ridge National Laboratory, the Idaho National Laboratory, and the Portsmouth Naval Shipyard in the USA.14 Vital status was ascertained between years 1946-2001, 1968-2004, and 1944-2005 for the UK, France, and USA subcohorts, respectively. Case status was defined by the underlying cause of death determined from death certificates and generally coded according to the 6th to 10th revision of the International Classification of Diseases (ICD) in effect at the time of death. The observation period began the later of: the date first monitored for radiation exposure; the start date of the applicable death registry; or one year after date first hired. The end of observation was on the earliest of the death date, date lost to follow-up, or the end of follow-up.
This research was approved by the Institutional Review Board (IRB) of the National Institute for Occupational Safety and Health (NIOSH). As required by the French Data Protection Authority, workers in France were given the opportunity to refuse participation; however, none refused to participate. UK workers can also refuse participation in the NRRW and associated studies; however, less than 1% refused to participate. Based on its review, the NIOSH IRB waived requirements for informed consent of USA participants.
Outcomes of interest
Analyses were restricted to outcomes from the previous INWORKS studies (except for CLL) with 100 or more observed deaths and includes: all solid cancers, leukemia excluding chronic lymphocytic leukemia (CLL), acute myeloid leukemia (AML), chronic myeloid leukemia (CML), Hodgkin lymphoma (HL), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).9, 10 CLL was excluded because it lacked evidence of radiogenicity in the previous analysis. In addition, we examined lung cancer as the leading cause of cancer death (Table S1). It is acknowledged that all solid cancers and non-CLL leukemia comprise heterogeneous groups of diseases with varying etiologies; however, both outcomes are frequently assessed because of their importance in radiation protection and risk assessment.
Exposure
Details on the exposure assessment methods are presented elsewhere.15, 16 Briefly, dose reconstruction methods were an extension of methods used in the previous IARC pooled study, whereby individual records of personal monitoring data were used to estimate annual doses over the course of employment of each worker. The majority of occupational radiation exposure was attributed to penetrating gamma and x-ray radiations of energies between 0.1 and 3.0 mega-electron volt (MeV); however, some measurements of neutron exposures were also recorded. For photons, measurements were adjusted for exposure geometry, body attenuation, dosimeter response, dosimetry practices, and facility-specific exposure conditions to estimate the absorbed dose to the lung, active red bone marrow (RBM), and colon. RBM dose was used for all lymphohematopoietic outcomes. Colon dose, a common surrogate for “deep dose”, was used in analyses of all solid cancers combined. Neutron exposure was estimated as a time-dependent categorical variable of whether a worker had a positive recorded neutron dose, and if so, whether their neutron dose ever exceeded ten percent of their total external penetrating radiation dose. As in previous examinations, doses from internally deposited radionuclides were not quantified for this analysis. 9, 10
Statistical Methods
All analyses were conducted using SAS software.17 General relative risk models were fit under a nested case-control design using methods analogous to conditional logistic regression with age as the time scale as described by Langholz and Richardson.18 This approach allows for exact control of attained age as a confounder and time-dependent exposures are precisely calculated at the failure time of the case. Cumulative dose was calculated by summing annual organ doses from age at first exposure to attained age of the associated case (i.e., age at death of the case) minus any applied exposure lag period. For each death in each outcome, 200 controls were drawn from cohort risk sets by incidence density sampling.19 Sampling was necessary due to computational restrictions; however, sampling a sufficient number of controls has been shown to give unbiased, efficient results.20 Controls were matched to cases on: attained age, sex, and country in all models; birth year (10-year intervals), socioeconomic status (5 categories based on job titles or employment category: professional and technical, skilled non-manual, skilled manual, unskilled, and uncertain), neutron exposure, (3 categories) and employment duration (10-year intervals) for all solid cancers and lung cancer; and calendar period (5-year intervals) for all lymphohematopoietic cancers.9, 10 Point estimates were expressed as the excess relative risk (ERR) per cumulative absorbed tissue dose in gray (Gy) for consistency with previous publications. In some cases, negative ERR per Gy estimates were below the boundary for relative risk (i.e., ERR < -1.0) because of linear extrapolation to a dose of one Gy. We present these artefactual results for consistency with previous results. Linear models allow estimates to be easily scaled to values typical to workplaces and within the boundary of relative risk.
All modeling was conducted using the NLMIXED procedure. Models used a linear rate function, based on findings from previous studies and an examination of alternative (nonlinear) rate functions during model development. Modeling was conducted in six basic steps. First, we examined cumulative dose-mortality associations under a fixed exposure lag specified a priori: 2 year lag for leukemias and 10 year lag for all other cancers. The lag discounted exposures that occurred during the period immediately prior to attained age and serves as the ‘latent’ period. Second, we compared a range of lags (2-40 years in one year increments) using a grid search to find the lag that maximized the likelihood function (i.e., fitted lag). Third, we fit a piecewise constant model (i.e., time windows), with exposure accrued in windows of 2<10 (for leukemias and MM), 10<20, 20<30 and 30+ years prior to attained age to examine TSE effects. Fourth, we fit a cubic B-spline model to describe variation in ERR per Gy with TSE.21 The B-spline function used two evenly spaced knots and models were restricted to decrease to zero at 60 years prior to attained age. Fifth, time-windows and B-splines were also used to assess variation in the ERR per Gy with AE. These models used age windows of <35, 35<50, and 50+ and a 2-year lag for hematopoietic cancers (leukemias and MM) and 10-years for all others. Thus, the estimate in the 35<50 year window corresponds to the dose accrued between age 35 and 50 years. Finally, joint analysis was conducted using AE windows (<35, 35<50, and 50+) cross classified with TSE windows (2<10, 10<20, and 20+). Given the large number of parameters to be estimated, joint analyses were restricted to all solid cancers and non-CLL leukemia.
For consistency with previous studies, model estimates included 90% confidence intervals (CI). The CIs for all models except B-splines were likelihood-based. Likelihood-based CIs were computationally expensive for B-spline models; therefore, 90% Wald-based CIs were estimated. Likelihood-based confidence bounds that did not converge or were on the boundary of parameter space were annotated as ‘not calculable’ (NC). Model comparisons were made by likelihood ratio tests. Based on reviewer comments, we also examined effect modification by categories of attained age (<60, 60<80, and 80+ years) in fixed lag models as a means to validate proportional hazards. Other post hoc analyses combining exposure windows in AE analyses were conducted based on inspection of risk patterns in a priori models.
Results
The cohort was mostly male (87%). A large proportion was still alive (77%) at study end. Less than 2% of persons were lost to follow-up. Among 66,632 decedents, there were 19,748 deaths identified having cancer as the underlying cause. The average age at first exposure was 31 years. About 83% of the study population had a positive recorded dose (Table 1). The radiation dose distribution was right-skewed, with mean and median cumulative colon doses through the end of follow-up for each worker of 17.4 mGy and 2.3 mGy, respectively. We found no evidence of significant interaction between exposure and attained age for any outcome (Table S2).
Table 1. Characteristics of the INWORKS cohort.
| Characteristic | France | United Kingdom | United States | INWORKS |
|---|---|---|---|---|
| Workers | 59,003 | 147,866 | 101,428 | 308,297 |
| With cumulative dose >0 | 42,206 | 130,373 | 84,587 | 257,166 |
| Sex (%) | ||||
| Male | 51,567 (87.4) | 134,812 (91.2) | 81,883 (80.7) | 268,262 (87.0) |
| Female | 7,436 (12.6) | 13,054 (8.8) | 19,545 (19.3) | 40,035 (13.0) |
| Year of Birth | ||||
| Average | 1947 | 1944 | 1934 | 1941 |
| Range | 1894-1975 | 1877-1983 | 1873-1973 | 1873-1983 |
| Follow-up | ||||
| Calendar period | 1968-2004 | 1946-2001 | 1944-2005 | 1944-2005 |
| Average duration (years) | 25 | 23 | 33 | 27 |
| Total person-years | 1,469,500 | 3,410,483 | 3,341,049 | 8,221,032 |
| Vital status (%) | ||||
| Alive | 52,565 (89.1) | 118,775 (80.3) | 65,573 (64.7) | 236,913 (76.9) |
| Deceased | 6,310 (10.7) | 25,307 (17.1) | 35,015 (34.5) | 66,632 (21.6) |
| Emigrated or Lost to follow-up | 128 (0.22) | 3,784 (2.6) | 840 (0.83) | 4,752 (1.5) |
| Exposure | ||||
| Year of first exposure1 | ||||
| Average | 1977 | 1975 | 1966 | 1972 |
| Range | 1950-2004 | 1946-1999 | 1932-2005 | 1932-2005 |
| Duration (years)2 | ||||
| Average | 12 | 10 | 12 | 11 |
| Range | 1-41 | 1-51 | 1-56 | 1-56 |
| Age (years) at first exposurea | ||||
| Average | 30 | 30 | 32 | 31 |
| 5%-95% Range | 21-45 | 18-52 | 19-51 | 18-51 |
| Mean Dose (50th, 95th percentile) | ||||
| RBM mGy | 11.6 (1.3, 58.9) | 18.2 (2.6, 83.3) | 15.2 (1.9, 80.5) | 15.9 (2.1, 78.0) |
| Colon mGy | 12.7 (1.5, 63.8) | 19.9 (2.9, 94.3) | 16.7 (2.1, 88.2) | 17.4 (2.3, 85.1) |
| Lung mGy | 12.6 (1.4, 64.1) | 19.8 (2.9, 94.1) | 16.6 (2.0, 87.7) | 17.4 (2.3, 85.0) |
First exposure is the first positive photon dose record.
Duration in years between first and last positive photon dose record before the end of follow-up.
Time Since Exposure (TSE)
In comparing similar models, our estimates differed slightly from those in the previous studies. These differences were likely from the different statistical approaches used. Significant positive dose-response associations were evident in fixed lag models for all solid cancers (ERR per Gy =0.42; 90% CI: 0.13, 0.73), non-CLL leukemia (ERR per Gy =2.80; 90% CI: 0.96, 5.10), and CML (ERR per Gy =11.17; 90% CI: 4.44, 21.26). Compared to fixed exposure lags, fitted lags decreased by 7-8 years for all solid cancers, lung cancer, and MM, but increased for leukemias (+3 to 17 years) and lymphomas (+8 to 17 years). However, none of these differences were statistically significant. These changes had little effect on the ERR per Gy for all solid cancers, lung cancer, CML, and MM. However, marked increases in the ERR per Gy were observed for non-CLL leukemia (67%), AML (183%), HL (221%), and NHL (210%) in fitted lag models compared to fixed lags. The most improved fit (p-value =0.09) was observed for non-CLL leukemia, where the ERR per Gy increased nearly two-fold using a 19 year lag (ERR per Gy =4.68; 90% CI: 1.26, 9.37) (Table 2).
Table 2. Linear ERR per Gy for fixed and fitted exposure lag periods 1,2.
| Outcome | deaths | Fixed lag3 | Fitted lag4 | Heterogeneity (LRT p-value, 1 df) | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| lag (years) | ERR per Gy (90% CI) | lag (years) | ERR per Gy (90% CI) | |||
| All solid cancers | 17957 | 10 | 0.42 (0.13, 0.73) | 3 | 0.41 (0.07, 0.79) | 0.420 |
| MN trachea, bronchus and lung | 5802 | 10 | 0.46 (-0.03, 1.02) | 3 | 0.44 (-0.14, 1.13) | 0.717 |
| Leukemia excl. CLL | 531 | 2 | 2.80 (0.96, 5.1) | 19 | 4.68 (1.26, 9.37) | 0.091 |
| Acute myeloid leukemia | 254 | 2 | 1.09 (-1.12, 4.21) | 19 | 3.09 (-1.02, 9.67) | 0.341 |
| Chronic myeloid leukemia | 100 | 2 | 11.17 (4.66, 21.26) | 5 | 12.45 (3.7, 28.14) | 0.356 |
| Multiple myeloma | 293 | 10 | 1.21 (-0.59, 3.76) | 2 | 1.24 (-0.84, 4.46) | 0.626 |
| Hodgkin lymphoma | 104 | 10 | 3.15 (-2.05, 12.07) | 18 | 10.1 (-1.81, 36.77) | 0.156 |
| Non Hodgkin lymphoma | 710 | 10 | 0.73 (-0.53, 2.32) | 27 | 2.26 (-0.54, 6.08) | 0.161 |
Matching variables were: attained age, sex, and country in all models; birth year (10-year intervals), socioeconomic status (in 5 categories), neutron exposure, (3 categories) and employment (10-year intervals) for lung cancer and all solid cancers combined; and calendar period (5-year intervals) for all lymphohematopoietic cancers.
Extrapolating estimates to 1Gy resulted in artefactual values (i.e., ERR per Gy <-1) for some outcomes. These estimates are shown for completeness only.
Lag period selected a priori. Profile-likelihood confidence intervals calculated with 1 df.
Lag period solved to maximize likelihood. Profile-likelihood confidence intervals calculated with 2 df.
Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; df, degrees of freedom; ERR, excess relative risk; LRT, likelihood ratio test; MN, malignant neoplasm.
Results from time series windows of TSE are shown in Table 3 and Table S3. TSE effects were more evident in CML (p-value=0.02), HL (p-value =0.11), and non-CLL leukemia (p-value =0.17) compared to other outcomes, although only CML effects were statistically significant. The pattern in CML suggested two peak periods prior to attained age; one for exposures within 2-10 years (ERR per Gy =23.12; 90% CI: 5.57, 50.25) and another for exposures within 20<30 years (ERR per Gy =59.94; 90% CI: 29.18, 103.83). Sandwiched between these periods, the ERR per Gy was strongly attenuated (ERR per Gy =-28.84; 90% CI: NC, -5.30). Excess HL risk was observed only in the 20<30 year TSE window (ERR per Gy =29.16, 90% CI: NC, 75.55). Peak excess AML risk was associated with exposures in the 20<30 year window (ERR per Gy =5.62; 90% CI: -0.46, 14.02). The pattern in non-CLL excess risk reflected the combination of CML and AML patterns. Findings for all solid cancers and lung cancer suggested persistent excess risk over the first two periods followed by an absence of excess risk at 30+ years TSE. There were also indications of persistent excess risk of MM and NHL over TSE. The excess risk was greatest in the 20<30 year window for MM (ERR per Gy =3.97; 90% CI: NC, 11.89) and in the 30+ year window for NHL (ERR per Gy =1.86, 90% CI: -0.98, 5.31).
Table 3. Linear ERR per Gy by time since exposure (TSE) for (a) non-hematopoietic and (b) hematopoietic outcomes.1.
| (a) 10-year lag imposed | ||||
|---|---|---|---|---|
|
| ||||
| Outcome | ERR per Gy (90% CI)2 by TSE (years) | Heterogeneity (LRT p-value, 2 df)3 | ||
|
| ||||
| 10<20 | 20<30 | 30+ | ||
| All solid cancers | 0.74 (-0.01, 1.54) | 0.57 (-0.11, 1.29) | 0.03 (-0.54, 0.65) | 0.411 |
| MN trachea, bronchus and lung | 0.71 (-0.56, 2.14) | 0.84 (-0.23, 2.11) | -0.23 (-1.24, 0.94) | 0.516 |
| Hodgkin lymphoma | -7.79 (NC, 4.24) | 29.16 (NC, 75.55) | -1.18 (NC, 21.27) | 0.107 |
| Non Hodgkin lymphoma | -2.58 (-5.36, 1.39) | 1.51 (-1.93, 5.63) | 1.86 (-0.98, 5.31) | 0.285 |
| (b) 2-year lag imposed | |||||
|---|---|---|---|---|---|
|
| |||||
| Outcome | ERR per Gy (90% CI)2 by TSE (years) | Heterogeneity (LRT p-value, 3 df)3 | |||
|
| |||||
| 2<10 | 10<20 | 20<30 | 30+ | ||
| Leukemia excl. CLL | -0.68 (NC, 6.1) | -1.71 (NC, 4.5) | 9.43 (3.37, 16.71) | 1.39 (-2.51, 6.7) | 0.171 |
| Acute myeloid leukemia | -0.14 (-4.63, 11.04) | -6.31 (NC, 1.03) | 5.62 (-0.46, 14.02) | 2.53 (-2.3, 9.99) | 0.256 |
| Chronic myeloid leukemia | 23.12 (5.57, 50.25) | -28.84 (NC, -5.30) | 59.94 (29.18, 103.83) | -13.5 (NC, 13.74) | 0.021 |
| Multiple myeloma | 2.35 (NC, 19.90) | 2.46 (NC, 13.02) | 3.97 (NC, 11.89) | -2.79 (-6.16, 1.94) | 0.450 |
Extrapolating estimates to 1Gy resulted in artefactual values (i.e., ERR per Gy <-1) for some outcomes. These estimates are shown for completeness only.
Profile-likelihood confidence intervals calculated with 1 df.
Compared to a model where all parameters are equal, which is equivalent to the baseline lag model.
Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; df, degrees of freedom; ERR, excess relative risk; LRT, likelihood ratio test; MN, malignant neoplasm; NC, not calculable.
The findings from TSE B-spline models were in reasonable agreement with those in time series windows for all outcomes except HL (which did not converge) and CML (Figure 1 and Figure S1). The wide variation in the CML piecewise model was far less evident in the B-spline model (Figure 1); however, the shape of the curve followed the general pattern suggested by time series windows.
Figure 1.
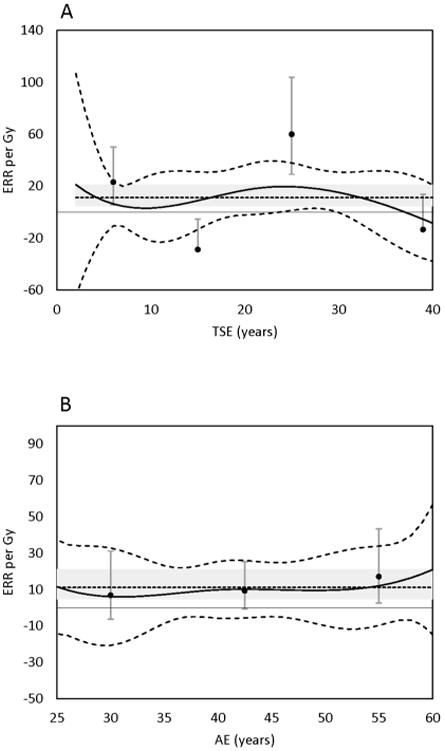
Excess relative risk (ERR) of chronic myeloid leukemia by: Panel A, time since exposure (TSE), Panel B, age at exposure (AE). Solid line, B-Spline models with 90% CI indicated by dashed line. Midpoints of piecewise windows, filled circles with 90% CI indicated by error bars. Small dash line, fixed lag estimate with 90% CI in grey field.
Age at Exposure (AE)
Results from piecewise constant models are shown in Table 4 and Table S4. Statistically significant modification by AE was not observed in any outcome tested. Evidence of heterogeneity by AE was strongest for all solid cancers (p-value =0.086) and lung cancer (p-value =0.093), where excess risk first appeared in the 35<50 year AE window and the ERR per Gy appeared consistent thereafter. Combining the last two age groups provided an ERR per Gy estimate for all solid cancers of 0.66 (90% CI: 0.31, 1.03) for AE of 35 years or older (p-value = 0.03). Similar patterns were observed for lung cancer (ERR per Gy =0.85; 90% CI: 0.25, 1.55; p-value = 0.03) and to a lesser extent MM (ERR per Gy =2.23; 90% CI: -0.21, 5.56; p-value = 0.27). For AML, the ERR per Gy was greatest for exposure ages <35 years (ERR per Gy =6.36; 90% CI: -0.35, 15.93), attenuated in the middle window, and then positive again in the 50 year or older age window (ERR per Gy =2.77; 90% CI: -1.76, 10.07). The NHL excess risk appeared to decrease with increasing AE; the greatest ERR per Gy was observed for exposure ages 35 years or less (ERR per Gy =3.03; 90% CI: -1.38, 8.47), although the estimate for exposure ages between 35-50 years was also positive (ERR per Gy =1.42; 90% CI: -1.17, 4.50). Combining these two age groups resulted in an ERR per Gy of 1.93 (90% CI: 0.04, 4.21; p-value = 0.13) for exposures at ages <50 years.
Table 4.
Linear ERR per Gy by age at exposure (AE).1
| Outcome | Fixed Lag (years) | ERR per Gy (90% CI)2 by AE (years) | Heterogeneity (LRT p-value, 2 df)3 | ||
|---|---|---|---|---|---|
|
| |||||
| <35 | 35<50 | 50+ | |||
| All solid cancers | 10 | -0.64 (-1.4, 0.19) | 0.75 (0.23, 1.32) | 0.51 (-0.15, 1.23) | 0.086 |
| MN trachea, bronchus and lung | 10 | -1.44 (-2.70, 0.05) | 0.97 (0.06, 2.00) | 0.68 (-0.46, 2.00) | 0.093 |
| Leukemia excl. CLL | 2 | 5.29 (-0.19, 12.45) | -0.29 (-3.17, 3.30) | 6.16 (1.89, 11.9) | 0.213 |
| Acute myeloid leukemia | 2 | 6.36 (-0.35, 15.93) | -2.36 (-5.68, 2.35) | 2.77 (-1.76, 10.07) | 0.292 |
| Chronic myeloid leukemia | 2 | 6.84 (-6.30, 31.15) | 9.33 (-0.58, 25.54) | 17.1 (2.61, 43.48) | 0.788 |
| Multiple myeloma | 2 | -3.30 (-8.71, 4.57) | 1.52 (-2.15, 6.28) | 3.22 (-2.03, 10.73) | 0.472 |
| Hodgkin lymphoma | 10 | NC | NC | NC | NC |
| Non Hodgkin lymphoma | 10 | 3.03 (-1.38, 8.47) | 1.42 (-1.17, 4.50) | -1.59 (NC, 1.74) | 0.299 |
Extrapolating estimates to 1Gy resulted in artefactual values (i.e., ERR per Gy <-1) for some outcomes. These estimates are shown for completeness only.
Profile-likelihood confidence intervals calculated with 1 df.
Compared to a model where all parameters are equal, which is equivalent to the baseline lag model.
Abbreviations: CI, confidence interval; CLL, chronic lymphocytic leukemia; df, degrees of freedom; ERR, excess relative risk; LRT, likelihood ratio test; MN, malignant neoplasm; NC, not-calculable.
As in TSE analyses, the results from AE B-spline models were in reasonable agreement with the findings from time series windows, except for HL, which again did not converge (Figure 1 and Figure S2).
Joint Analyses of AE and TSE Effects
The results of the joint analyses were largely consistent with separate TSE and AE analyses (Table 5 and Table S5). The ERR per Gy for solid cancers combined was largest for doses accrued at ages 35 to <50 years and 2 to <10 years prior to attained age; however, the most precise estimate (ERR per Gy =0.75; 90% CI: 0.03, 1.42) was obtained for exposures accrued 20 or more years prior to attained age and exposure ages 35 to <50 years. The combined temporal effect on the radiation risk of non-CLL leukemia was pronounced (p-value =0.06) relative to that for solid cancers. The excess risk of non-CLL leukemia was largest for the cross-classification of the youngest exposure age group and fewest years prior to attained age. Also for non-CLL leukemia, significantly positive ERR per Gy estimates were observed in adjacent cells representing AE ages 35 years or older and TSE of 20 or more years. Of particular note is the pattern in which the excess risk diminished with increasing time-since-exposure for exposures received below age 35 years, while the opposite occurred for exposures received above age 50 years.
Table 5.
Linear ERR per Gy and 90% confidence intervals (CI) by age at exposure (AE) and time since exposure (TSE).1,2
| Outcome | AE (years) | TSE (years) | Heterogeneity (LRT p-value, 8 df)3 | ||
|---|---|---|---|---|---|
|
| |||||
| 2<10 | 10<20 | 20+ | |||
| All solid cancers | <35 | -0.88 (NC, 6.53) | 0.54 (-2.27, 4.14) | -0.77 (-1.56, 0.11) | 0.682 |
| 35<50 | 1.25 (-1.26, 4.23) | 0.74 (-0.60, 2.20) | 0.75 (0.03, 1.42) | ||
| 50+ | 0.01 (-1.44, 1.61) | 0.74 (-0.30, 1.91) | 0.27 (-0.75, 1.44) | ||
|
| |||||
| Leukemia excl. CLL | <35 | 16.56 (NC, 52.6) | 8.11 (NC, 35.86) | 2.86 (-3.42, 11.44) | 0.064 |
| 35<50 | 0.93 (NC, 11.19) | -13.09 (NC, -2.0) | 5.11 (0.07, 11.37) | ||
| 50+ | -6.66 (-10.69, 3.16) | 6.65 (-2.96, 20.72) | 8.75 (1.04, 20.66) | ||
Extrapolating estimates to 1Gy resulted in artefactual values (i.e., ERR per Gy <-1) for some outcomes. These estimates are shown for completeness only.
Profile-likelihood confidence intervals calculated with 1 df.
Compared to a model where all parameters are equal.
Abbreviations: AE, age at exposure; CI, confidence interval; CLL, chronic lymphocytic leukemia; df, degrees of freedom; ERR, excess relative risk; LRT, likelihood ratio test; NC, not calculable; TSE, time since exposure.
Discussion
Solid Cancers
Risk patterns for all solid cancers combined were largely similar to that of lung cancer, although lung cancer estimates appeared slightly less precise given fewer cases. Estimates for all solid cancers from fixed and fitted exposure lag models were compatible with the previous study 10 and the estimate for LSS men of working ages (ERR per Gy =0.37; 90% CI: 0.17, 0.60).12 As in the LSS, the all solid cancers excess risk persisted several decades after exposure. The evidence of lasting effects from ionizing radiation exposure underscores the importance of continued follow-up to gain full understanding of lifetime risks among nuclear workers.
The excess risk of all solid cancers and lung cancer appeared restricted to exposure ages 35 years or greater. Evidence from other studies have also suggested associations between radiation dose and lung cancer that are stronger at older exposure ages.7, 22-24 However, methods have varied among studies and differences in age-risk patterns between studies are apparent. These inconsistencies may result from underpowered analyses given low baseline cancer risk at young attained ages. Another possible explanation is temporal variation in confounding.24 There was no evidence of decreasing ERR by increasing AE, which is contrary to the 29% reduction per 10-year increase of AE recently found in the LSS.11 However, LSS AE effects were largely influenced by relatively high risks among persons exposed before working ages; therefore, these findings may poorly translate to nuclear workers.
Hematopoietic cancers
We report a positive association between non-CLL leukemia and radiation exposure (under a 2-year lag) that is compatible with an estimate for LSS males of working ages (ERR at 1 Sv = 2.63; 90% CI 1.50, 4.27). 12 Our leukemia risk estimates are similar to previous INWORKS estimates using Poisson regression, with significant risk also observed for non-CLL leukemia (ERR per Gy = 2.96; 90% CI 1.17, 5.21) and CML (ERR per Gy = 10.45; 90% CI 4.48, 19.65) in that study.9 Extending the lag period to 19 years under the fitted model markedly increased the magnitude of the non-CLL leukemia ERR per Gy estimate, suggesting late onset of leukemia risk. This late onset was most evident in AML, which was also best-fit to a lag period of 19 years. A similar pattern was suggested by Leuraud et al. (2015), who reported improved model fit and increased excess risk for non-CLL leukemia and AML using the alternative lag of 10 years.9 These findings were consistent with piecewise and B-spline models that showed peak non-CLL leukemia, AML and CML risks from exposures 20-30 years prior to attained age. Richardson et al. (2009) reported similar TSE patterns in the LSS, which can be described as rapidly increasing non-CLL leukemia excess risk within 10 years of exposure, followed by a long period absent of excess risk, and then a slight uptick in excess risk occurring 45 years after exposure that is observed in all-leukemias and AML, but not in CML.25 However, these effects were greatest at early exposure ages and disappeared in survivors who were aged 30 or more years at time of the bombings. Late onset leukemia has not been reported in previous worker studies, although these studies may lack sufficient follow-up to observe late effects.
Significant heterogeneity was observed for CML risk by TSE in the piecewise model. In contrast to AML, increased excess CML risk appeared shortly after exposure (2-10 year) and again much later (20-30 years). The first peak is consistent with previous studies of nuclear workers that report early onset of leukemia excess risk.26-28 The onset of the second peak is consistent AML, again suggesting delayed effects. However, CML piecewise model estimates appear exaggerated compared to those from the B-spline Model (Table 3). Both opposing piecewise estimates, which together indicate the largest change in excess risk with TSE (between 10 and 30 years), lie outside of the B-spline model confidence interval (Figure 1). Overly large estimates may indicate model instability from sparse data, which can inflate estimates downward when the ERR estimate is below zero and upward when it is above zero.29 This may explain the differences observed between piecewise and B-spline models for CML, where the latter shows a smoothed effect. Cautious interpretation of CML findings is recommended given the inconsistencies between these models, especially in the absence of confirmatory results from other studies.
AML excess risk was greatest in the earliest and latest exposure age groups, suggesting a U-shaped AE effect. In the recent study of cancer incidence in the LSS, Hsu et al. (2013) found a non-monotone AML dependence on AE, whereby the ERR per Gy was lowest among survivors exposed around age 30 years compared to younger or older ages, and if exposed as an adult, the ERR per Gy increased with increasing attained age.30 Long latency in radiation-induced myelodysplastic syndromes (MDS) has also been found in the LSS.31, 32 MDS is a hematologic disorder that is associated with an increased risk of developing AML. However, we note that information on MDS radiogenicity is sparse and findings are possibly affected by MDS diagnosis and disease classification that have varied over time; therefore, patterns in risk MDS risk remain unclear.
Direct evidence of an association between ionizing radiation exposure and MM is limited.7, 11, 14, 33-35 Ichimaru et al. (1982) reported increased MM incidence in the LSS followed through 1976 that was restricted to AE between 20 and 59 years; however, the excess was not apparent for bone marrow doses less than 0.5 Gy or TSE less than 20 years.33 Significant excess MM mortality risk was first reported after extending follow-up through 1985 (ERR per Gy to bone marrow = 2.29; 90% CI: 0.67, 5.31).34 Subsequent LSS updates; however, failed to report significant excesses and point estimates were diminished.11, 30, 35, 36 Among overlapping occupational studies, both the 15-Country Study (ERR per Sv =6.5; 90% CI: NC, 20.6; n=83) and the recent study of USA nuclear workers (ERR per Gy of 3.9 (95% CI: 0.60, 9.5; n=188) reported positive ERR per Gy estimates for MM.7, 14 Our estimates from fitted and fixed lags were also positive but less so, and appeared reasonably compatible with most recent LSS estimates.11,30 Our analyses of temporal effects on MM risk, though imprecise, suggested risk persisted with TSE and was primarily restricted to exposure ages 35 years or more. These findings appear consistent with the previous case control-study of workers from four U.S. nuclear facilities, including three facilities currently studied. Wing et al., (2000) reported dose response associations for MM and ionizing radiation that increased in magnitude with exposure age.37
Lymphatic cancers
Previous epidemiology has provided only limited evidence of ionizing radiation exposure as a risk factor for NHL and no evidence of causing HL.38, 39 Our study also provides little evidence supporting a dose-response for lymphatic cancers. Overall, our ERR per Gy estimates under fixed and fitted lags were positive but highly imprecise for both cancers. Poor estimate precision should be expected for HL given so few cases, but there was a relatively large number of NHL cases available.
Our NHL estimate using a 10-year lag was compatible with, but slightly less than an estimate for LSS males who were aged 15-64 years at the time of the bombing (ERR per Gy =1.12; 90% CI: 0.26, 2.51).40 However, the preferred lag in our study was nearly 30 years prior to attained age, resulting in an increase in the ERR per Gy of about three-fold compared to the fixed lag estimate. Excess NHL risk was also observed for dose accrued at age 50 years or less; the estimate of the ERR per Gy in this age range gained statistical significance. The long latent period observed for NHL mortality in our study was consistent with the LSS data, which showed that most of the excess risk in A-bomb survivors occurred 35 years or more after irradiation.40 This extended latency may explain the lack of evidence of association in previous studies with comparably less observation time.
Study Limitations
As in all observational studies, our study has a number of noteworthy limitations. The primary limitation is low statistical power to detect significant effect modification by age and time since exposure. Of all the effects tested, only CML by TSE was statistically significant. Furthermore, estimates from complex models necessary to examine temporal effects may be particularly vulnerable to sparse data. Although the INWORKS cohort is large, with long follow-up and many observed cancers, the low average doses and concomitant low excess risk preclude making conclusive statements about temporal or age effect modification, for most outcomes.
Other limitations were discussed in detail in the preceding reports;9, 10 therefore, they are only briefly reintroduced in this report. First, information on exposures to neutrons and incorporated radionuclides was insufficient to quantify dose. However, excluding workers flagged for potential neutron exposures (13%) and internal contamination (17%) had only modest effects on point estimates in previous analyses.9, 10 Second, we lacked information on important risk factors, such as exposures to other occupational carcinogens (e.g., benzene and asbestos) and lifestyle factors (e.g., smoking habits, alcohol consumption, and diet) that may distort risk estimates. Yet previous estimates of the ERR for solid cancer with and without lung cancer did not appreciably differ; suggesting that strong confounding by smoking or asbestos exposure is unlikely.10 We observed significant temporal effects for CML, which has sparse evidence of an association with benzene exposure 41 or other exogenous risk factors aside from ionizing radiation exposure. Thus, although residual confounding cannot be ruled out, unmeasured risk factors are unlikely to fully explain our findings. Third, for some cancers, mortality may be a poor substitute for cancer incidence, and many non-occupational factors can strongly influence cancer survival. Fourth, our study involved several statistical tests; therefore, the potential for spurious results is increased. Nevertheless, similar results across multiple methods were observed for most outcomes, which is evidence against chance findings. Fifth, errors in ascertainment and exposure measurement are also unavoidable sources of information bias given that records quality, disease classification, and dosimetry methods have changed over the observation period. Lastly, we are mindful that we report modest associations that are most vulnerable to bias or that may have simply resulted from chance alone.
Although the TSE and AE cubic regression B-spline plots were restricted to best show the informative range, some plots give warnings of large instability in tail regions, as depicted by rapidly increasing confidence intervals. Estimates in tail areas of splines are prone to instability; 42 therefore, we caution against over interpretation of estimates in these areas.
Conclusion
This study provides direct information on the effect of temporal modifiers of cancer mortality risk from occupational ionizing radiation exposure. Given its size, length of follow-up, and quality of exposure information, the INWORKS cohort is well-suited to study the relation between occupational exposure to ionizing radiation and cancer. Yet although considerable statistical power has been realized by INWORKS, estimates of temporal effects on radiation risk are still largely imprecise. Statistically significant temporal effect modification was observed only for CML by TSE, which points to generally modest temporal effects on the relatively weak association between low-dose ionizing radiation exposure and cancer.
In conclusion, we found temporal risk patterns that appeared to vary by cancer type; emphasizing the need for more tumor-specific analyses. We also found that the excess risk of certain cancers persisted over the entire observation period spanning several decades, which suggests additional follow-up is needed to fully describe lifetime risks. Finally, this study is among the first reporting late onset of exposure-related leukemia mortality in nuclear workers. These findings add to our understanding of cancer risk from protracted low dose rate exposure to ionizing radiation. Nevertheless, there are a number of study limitations that encourage cautious interpretation.
Novelty and Impact.
We examine cancer mortality risk from occupational radiation exposure in the International Nuclear Workers Study (INWORKS) cohort, comprising over 300,000 nuclear workers contributing over eight million person-years at risk. The current work assesses effects of age at exposure and time since exposure on linear excess relative risk estimates. This study is first to report late onset leukemia from protracted occupational radiation exposure. These findings add to our understanding of cancer risk from ionizing radiation.
Acknowledgments
The work was funded, in part, by the National Institute for Occupational Safety and Health, the United States (U.S.) Department of Energy through an agreement with the U.S. Department of Health and Human Services and through a grant received by the University of North Carolina from the National Institute for Occupational Safety and Health (R03.OH-010056) and by the Ministry of Health, Labour and Welfare of Japan (GA No 2012-02-21-01). The construction of the French cohort was realized by the Institut de Radioprotection et de Sûreté Nucléaire (IRSN), with partial funding from AREVA Nuclear Cycle (AREVA NC) and Electricité de France (EDF). The IRSN thanks all persons from the French Atomic Energy Commission, AREVA, and Electricité de France who cooperated in the elaboration of the French cohort. The construction of the UK cohort was undertaken by Public Health England (PHE) who operates the UK's National Registry for Radiation Workers (NRRW). The PHE thank all of the organizations and individuals participating in the NRRW for their cooperation, and the NRRW Steering Group for their continued support.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- 1.International Agency for Research on Cancer, IARC. A review of human carcinogens Part D: Radiation / IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. 100D. Lyon: IARC Press; 2012. [Google Scholar]
- 2.United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR. Sources and effects of Ionizing Radiation, UNSCEAR 2008 Report, Volume I, Annex B: Exposures of the public and workers from various sources of radiation. New York: United Nations; 2010. [Google Scholar]
- 3.Mettler FA, Jr, Bhargavan M, Faulkner K, Gilley DB, Gray JE, Ibbott GS, Lipoti JA, Mahesh M, McCrohan JL, Stabin MG, Thomadsen BR, Yoshizumi TT. Radiologic and nuclear medicine studies in the United States and worldwide: frequency, radiation dose, and comparison with other radiation sources--1950-2007. Radiology. 2009;253:520–31. doi: 10.1148/radiol.2532082010. [DOI] [PubMed] [Google Scholar]
- 4.United Nations Scientific Committee on the Effects of Atomic Radiation, UNSCEAR. Effects of Ionizing Radiation, UNSCEAR 2006 Report, Volume 1, Annex A: Epidemiological studies of radiation and cancer. New York: United Nations; 2009. [Google Scholar]
- 5.National Research Council, Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, NRC. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII – Phase 2. Washington, DC: National Academys Press; 2005. [PubMed] [Google Scholar]
- 6.Cardis E, Gilbert ES, Carpenter L, Howe G, Kato I, Armstrong BK, Beral V, Cowper G, Douglas A, Fix J, Fry SA, Kaldor J, Lave C, Salmon L, Smith PG, Voelz GL, Wiggs LD. Effects of low doses and low dose rates of external ionizing-radiation: cancer mortality among nuclear industry workers in 3 countries. Radiat Res. 1995;142:117–32. [PubMed] [Google Scholar]
- 7.Cardis E, Vrijheid M, Blettner M, Gilbert E, Hakama M, Hill C, Howe G, Kaldor J, Muirhead CR, Schubauer-Berigan M, Yoshimura T, Bermann F, Cowper G, Fix J, Hacker C, Heinmiller B, Marshall M, Thierry-Chef I, Utterback D, Ahn YO, Amoros E, Ashmore P, Auvinen A, Bae JM, Bernar J, Biau A, Combalot E, Deboodt P, Sacristan AD, Eklof M, Engels H, Engholm G, Gulis G, Habib RR, Holan K, Hyvonen H, Kerekes A, Kurtinaitis J, Malker H, Martuzzi M, Mastauskas A, Monnet A, Moser M, Pearce MS, Richardson DB, Rodriguez-Artalejo F, Rogel A, Tardy H, Telle-Lamberton M, Turai I, Usel M, Veress K. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: Estimates of radiation-related cancer risks. Radiat Res. 2007;167:396–416. doi: 10.1667/RR0553.1. [DOI] [PubMed] [Google Scholar]
- 8.Hamra GB, Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan JA, Haylock R, Laurier D, Leuraud K, Moissonnier M, Schubauer-Berigan M, Thierry-Chef I, Kesminiene A. Cohort Profile: The International Nuclear Workers Study (INWORKS) Int J Epidemiol. 2015 doi: 10.1093/ije/dyv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leuraud K, Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan JA, Hamra GB, Haylock R, Laurier D, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. The Lancet Haematology. 2015;2:e276–e81. doi: 10.1016/S2352-3026(15)00094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson DB, Cardis E, Daniels RD, Gillies M, O'Hagan JA, Hamra GB, Haylock R, Laurier D, Leuraud K, Moissonnier M, Schubauer-Berigan MK, Thierry-Chef I, Kesminiene A. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS) Bmj. 2015;351:h5359. doi: 10.1136/bmj.h5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, report 14, 1950-2003: An overview of cancer and noncancer diseases. Radiat Res. 2012;177:229–43. doi: 10.1667/rr2629.1. [DOI] [PubMed] [Google Scholar]
- 12.Metz-Flamant C, Laurent O, Samson E, Caer-Lorho S, Acker A, Hubert D, Richardson DB, Laurier D. Mortality associated with chronic external radiation exposure in the French combined cohort of nuclear workers. Occup Environ Med. 2013;70:630–8. doi: 10.1136/oemed-2012-101149. [DOI] [PubMed] [Google Scholar]
- 13.Muirhead CR, O'Hagan JA, Haylock RG, Phillipson MA, Will cock T, Berridge GL, Zhang W. Mortality and cancer incidence following occupational radiation exposure: third analysis of the National Registry for Radiation Workers. Br J Cancer. 2009;100:206–12. doi: 10.1038/sj.bjc.6604825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubauer-Berigan MK, Daniels RD, Bertke SJ, Tseng CY, Richardson DB. Cancer Mortality through 2005 among a Pooled Cohort of U.S. Nuclear Workers Exposed to External Ionizing Radiation Radiat Res. 2015;183:620–31. doi: 10.1667/RR13988.1. [DOI] [PubMed] [Google Scholar]
- 15.Thierry-Chef I, Marshall M, Fix JJ, Bermann F, Gilbert ES, Hacker C, Heinmiller B, Murray W, Pearce MS, Utterback D, Bernar K, Deboodt P, Eklof M, Griciene B, Holan K, Hyvonen H, Kerekes A, Lee MC, Moser M, Pernicka F, Cardis E. The 15-Country collaborative study of cancer risk among radiation workers in the nuclear industry: study of errors in dosimetry. Radiat Res. 2007;167:380–95. doi: 10.1667/RR0552.1. [DOI] [PubMed] [Google Scholar]
- 16.Thierry-Chef I, Richardson DB, Daniels RD, Gillies M, Hamra GB, Haylock R, Kesminiene A, Laurier D, Leuraud K, Moissonnier M, O'Hagan J, Schubauer-Berigan MK, Cardis E, Consortium I. Dose Estimation for a Study of Nuclear Workers in France, the United Kingdom and the United States of America: Methods for the International Nuclear Workers Study (INWORKS) Radiat Res. 2015;183:632–42. doi: 10.1667/RR14006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SAS and all other SAS Institute Inc product or service names are registered trademarks or trademarks of SAS Institute Inc. Cary, NC, USA: SAS Institute Inc; SAS Software, Version 9.2, Copyright (2002-2003) [Google Scholar]
- 18.Langholz B, Richardson DB. Fitting general relative risk models for survival time and matched case- control analysis. Am J Epidemiol. 2010;171:377–83. doi: 10.1093/aje/kwp403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beaumont JJ, Steenland K, Minton A, Meyer S. A computer program for incidence density sampling of controls in case-control studies nested within occupational cohort studies. Am J Epidemiol. 1989;129:212–9. doi: 10.1093/oxfordjournals.aje.a115111. [DOI] [PubMed] [Google Scholar]
- 20.Bertke S, Hein M, Schubauer-Berigan M, Deddens J. A simulation study of relative efficiency and bias in the nested case-control study design. Epidemiol Method. 2013;2:85–93. doi: 10.1515/em-2013-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hauptmann M, Wellmann J, Lubin JH, Rosenberg PS, Kreienbrock L. Analysis of exposure-time-response relationships using a spline weight function. Biometrics. 2000;56:1105–8. doi: 10.1111/j.0006-341x.2000.01105.x. [DOI] [PubMed] [Google Scholar]
- 22.Wing S, Richardson DB. Age at exposure to ionising radiation and cancer mortality among Hanford workers: follow up through 1994. Occup Environ Med. 2005;62:465–72. doi: 10.1136/oem.2005.019760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ritz B, Morgenstern H, Moncau J. Age at exposure modifies the effects of low-level ionizing radiation on cancer mortality in an occupational cohort. Epidemiology. 1999;10:135–40. [PubMed] [Google Scholar]
- 24.Richardson DB, Ashmore JP. Investigating time patterns of variation in radiation cancer associations. Occup Environ Med. 2005;62:551–8. doi: 10.1136/oem.2004.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson D, Sugiyama H, Nishi N, Sakata R, Shimizu Y, Grant EJ, Soda M, Hsu WL, Suyama A, Kodama K, Kasagi F. Ionizing radiation and leukemia mortality among Japanese Atomic Bomb Survivors, 1950-2000. Radiat Res. 2009;172:368–82. doi: 10.1667/RR1801.1. [DOI] [PubMed] [Google Scholar]
- 26.Shilnikova NS, Preston DL, Ron E, Gilbert ES, Vassilenko EK, Romanov SA, Kuznetsova IS, Sokolnikov ME, Okatenko PV, Kreslov VV, Koshurnikova NA. Cancer mortality risk among workers at the Mayak nuclear complex. Radiat Res. 2003;159:787–98. doi: 10.1667/0033-7587(2003)159[0787:cmrawa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Richardson DB, Wing S. Leukemia mortality among workers at the Savannah River Site. Am J Epidemiol. 2007;166:1015–22. doi: 10.1093/aje/kwm176. [DOI] [PubMed] [Google Scholar]
- 28.Daniels RD, Bertke S, Waters KM, Schubauer-Berigan MK. Risk of leukaemia mortality from exposure to ionising radiation in US nuclear workers: a pooled case-control study. Occup Environ Med. 2013;70:41–8. doi: 10.1136/oemed-2012-100906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S, Mansournia MA, Altman DG. Sparse data bias: a problem hiding in plain sight. Bmj. 2016;352:i1981. doi: 10.1136/bmj.i1981. [DOI] [PubMed] [Google Scholar]
- 30.Hsu WL, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, Iwanaga M, Miyazaki Y, Cullings HM, Suyama A, Ozasa K, Shore RE, Mabuchi K. The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950-2001. Radiat Res. 2013;179:361–82. doi: 10.1667/RR2892.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwanaga M, Hsu WL, Soda M, Takasaki Y, Tawara M, Joh T, Amenomori T, Yamamura M, Yoshida Y, Koba T, Miyazaki Y, Matsuo T, Preston DL, Suyama A, Kodama K, Tomonaga M. Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors. J Clin Oncol. 2011;29:428–34. doi: 10.1200/JCO.2010.31.3080. [DOI] [PubMed] [Google Scholar]
- 32.Tsushima H, Iwanaga M, Miyazaki Y. Late effect of atomic bomb radiation on myeloid disorders: leukemia and myelodysplastic syndromes. Int J Hematol. 2012;95:232–8. doi: 10.1007/s12185-012-1002-4. [DOI] [PubMed] [Google Scholar]
- 33.Ichimaru M, Ishimaru T, Mikami M, Matsunaga M. Multiple myeloma among atomic bomb survivors in Hiroshima and Nagasaki, 1950-76: Relationship to radiation dose absorbed by marrow. J Natl Cancer Inst. 1982;69:323–8. [PubMed] [Google Scholar]
- 34.Shimizu Y, Kato H, Schull WJ, Preston DL, Fujita S, Pierce DA. Studies of the mortality of A-bomb survivors. 9. Mortality, 1950-1985: Part 1. Comparison of risk coefficients for site-specific cancer mortality based on the DS86 and T65DR shielded kerma and organ doses Radiat Res. 1989;118:502–24. [PubMed] [Google Scholar]
- 35.Preston DL, Kusumi S, Tomonaga M, Izumi S, Ron E, Kuramoto A, Kamada N, Dohy H, Matsuo T, Matsui T, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950-1987 Radiat Res. 1994;137:S68–97. [PubMed] [Google Scholar]
- 36.Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, Part I. Cancer: 1950-1990 Radiat Res. 1996;146:1–27. [PubMed] [Google Scholar]
- 37.Wing S, Richardson D, Wolf S, Mihlan G, Crawford-Brown D, Wood J. A case control study of multiple myeloma at four nuclear facilities. Annals of epidemiology. 2000;10:144–53. doi: 10.1016/s1047-2797(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 38.Boice JD., Jr Radiation and non-Hodgkin's lymphoma. Cancer Res. 1992;52:5489s–91s. [PubMed] [Google Scholar]
- 39.Kim CJ, Freedman DM, Curtis RE, Berrington de Gonzalez A, Morton LM. Risk of non-Hodgkin lymphoma after radiotherapy for solid cancers. Leuk Lymphoma. 2013;54:1691–7. doi: 10.3109/10428194.2012.753543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson D, Sugiyama H, Wing S, Sakata R, Grant E, Shimizu Y, Nishi N, Geyer S, Soda M, Suyama A, Kasagi F, Kodama K. Positive associations between ionizing radiation and lymphoma mortality among men. Am J Epidemiol. 2009;169:969–76. doi: 10.1093/aje/kwp018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Agency for Research on Cancer. IARC A review of human carcinogens Part F: Chemical agents and related occupations / IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, Volume 100F. Lyon: IARC Press; 2012. [PMC free article] [PubMed] [Google Scholar]
- 42.Hastie T, Tibshirani R. Monographs on Statistics and Applied Probability. 1st. Vol. 43. New York: Chapman and Hall/CRC; 1990. Generalized additive models; p. 335p. [Google Scholar]


