Abstract
Seagrasses play an essential ecological role within coastal habitats and their worldwide population decline has been linked to different types of anthropogenic forces. We investigated, for the first time, the combined effects of future ocean warming and acidification on fundamental biological processes of Zostera noltii, including shoot density, leaf coloration, photophysiology (electron transport rate, ETR; maximum PSII quantum yield, Fv/Fm) and photosynthetic pigments. Shoot density was severely affected under warming conditions, with a concomitant increase in the frequency of brownish colored leaves (seagrass die-off). Warming was responsible for a significant decrease in ETR and Fv/Fm (particularly under control pH conditions), while promoting the highest ETR variability (among experimental treatments). Warming also elicited a significant increase in pheophytin and carotenoid levels, alongside an increase in carotenoid/chlorophyll ratio and De-Epoxidation State (DES). Acidification significantly affected photosynthetic pigments content (antheraxanthin, β-carotene, violaxanthin and zeaxanthin), with a significant decrease being recorded under the warming scenario. No significant interaction between ocean acidification and warming was observed. Our findings suggest that future ocean warming will be a foremost determinant stressor influencing Z. noltii survival and physiological performance. Additionally, acidification conditions to occur in the future will be unable to counteract deleterious effects posed by ocean warming.
Atmospheric carbon dioxide (CO2) levels have stepwise risen from preindustrial levels of 280 μatm to ~390 μatm (present day), being expected to increase up to 420–940 μatm by 21001. As carbon dioxide concentration increases in Earth’s atmosphere, CO2 exchange between atmosphere and sea surface layer will vary, with ocean CO2 uptake increasing and seawater chemistry changing, accordingly2. According to the Intergovernmental Panel on Climate Change (IPCC), average ocean pH is expected to decrease between 0.13–0.42 units by the end of the 21st century, depending upon the different representative concentration pathways (RCP) IPCC scenarios1. As a consequence, changes in seawater chemistry and subsequent shift in the relative proportion of dissolved inorganic carbon species (DIC), will ultimately lead to a continuous process known as ocean acidification, with predictable cascading effects on marine ecosystems2. Concomitantly, and as a result of enhanced greenhouse effect, sea surface temperature (SST) has risen. Even though global SST trends will differ at a regional, seasonal and interannual scale, it is expectable (IPCC’s 8.5 RCP scenario) that mean SST will increase between 2.6 °C−4.8 °C until 21003.
Future changes in ocean’s physical and chemical properties are expected to pose severe impairments to marine biota, by directly affecting organisms’ ability to regulate acid-base equilibrium, as well as their growth, survival, metabolism, physiology and reproduction2,4. Within marine coastal environments, seagrasses play an essential ecological key role as ecosystem builders providing food, shelter and crucial nursery habitats for a wide range of species5. Additionally, they act as “ecological service providers” by promoting sea bottom stabilization and nutrient cycling, acting as buffer/trophic transfer zones to sensitive/neighboring habitats (i.e. coral reefs) and as “environmental status beacons” of coastal ecosystems5,6. Their worldwide distribution and key ecological importance within coastal environments is such that seagrasses may represent, to more or less extent, the marine “counter part” of tropical rainforests, in terms of carbon cycle/storage potential as well as biodiversity promoters7,8. As a result of seagrass meadows exposure to a variety of both biotic and abiotic environmental stressors, these ecosystems are increasingly experiencing a global decline9,10. Most of these pressures result from anthropogenic forcing, such as dredging activities (hydrodynamic shifts), runoffs from urban and agriculture areas (e.g. household chemicals, metals, fertilizers/pesticides, animal waste), commercial/recreational human activities (e.g. fishing, boat groundings) and increasing climate change derived impacts10,11.
Within a climate change perspective, increasing temperature (and heat wave events) can cause detrimental changes upon seagrass growth, survival and distribution12,13. Additionally, temperature may also influence seagrass photosynthesis, nutrient uptake and act as a sexual reproduction trigger10,14,15. In fact, as temperature rises, seagrass photosynthesis increases up until a point where an optimal value is reached, followed by a subsequent rapid decline16. Concomitantly, an increase in photosynthesis is accompanied by a faster growth and consequent higher respiration rate, which in turn leads to a net primary productivity compromise status and a negative carbon balance14. Therefore, it is of particular concern to understand how ecologically important species (i.e. seagrasses) will survive, adapt or even geographically relocate, when faced upon an increasing warming habitat. Ocean acidification conditions have also proven to promote deleterious effects in marine organisms2. Opposingly, photosyntetic organisms (e.g. marine angiosperms) are expected to benefit from these changes, once aqueous CO2 is a crucial photosynthesis resource (primary carbon source) and as oceans become more acidic so will DIC species in seawater tend to increase. Although nearly 90% of total DIC in seawater is available as bicarbonate ions17, seagrasses tend to use this ionic carbon source, for photosynthesis, rather inefficiently, although further studies are needed as to confirm this assumption18,19,20. Nevertheless, it is important to highlight that environmental stressors can act synergistically and subsequent effects will be dictated as a result of species ability to cope with combined stress increase or an augmented antagonistic output towards stress tolerance21,22. Thus, understanding the combined effects between increasing temperature and rising CO2 levels is of utmost importance and should be carefully addressed in order to empirically predict potential cascading effects over the marine environment and associated biota.
The dwarf eelgrass Zostera noltii (Hornemann 1832) has a wide geographical distribution, covering several Atlantic coastal areas, from Norway to Mauritania, including the Mediterranean and adjacent seas23. Although not critically endangered as other seagrass species, there is an increasing concern towards the conservation of Z. noltii and its ecological status, mainly as a result of habitat degradation and decreasing trends at a population level24. As a foundation species of many marine coastal habitats, ecological and socio-economic importance, it is imperative to understand if Z. noltii will be able to cope with the scenarios predicted for the oceans of tomorrow.
The aim of our study is to understand, for the first time, the combined effects of ocean warming and acidification upon shoot density, leaf coloration, photophysiology (electron transport rate: ETR and maximum PSII quantum yield: Fv/Fm) and photosynthetic pigments of a pioneer temperate seagrass species (Z. noltii). As seagrass response towards the combined effects of ocean warming and acidification is still poorly understood, this study will be a major contribution as to improve our knowledge on potential cascading effects at a species and subsequently population and community levels.
Results
Shoot density and leaf coloration
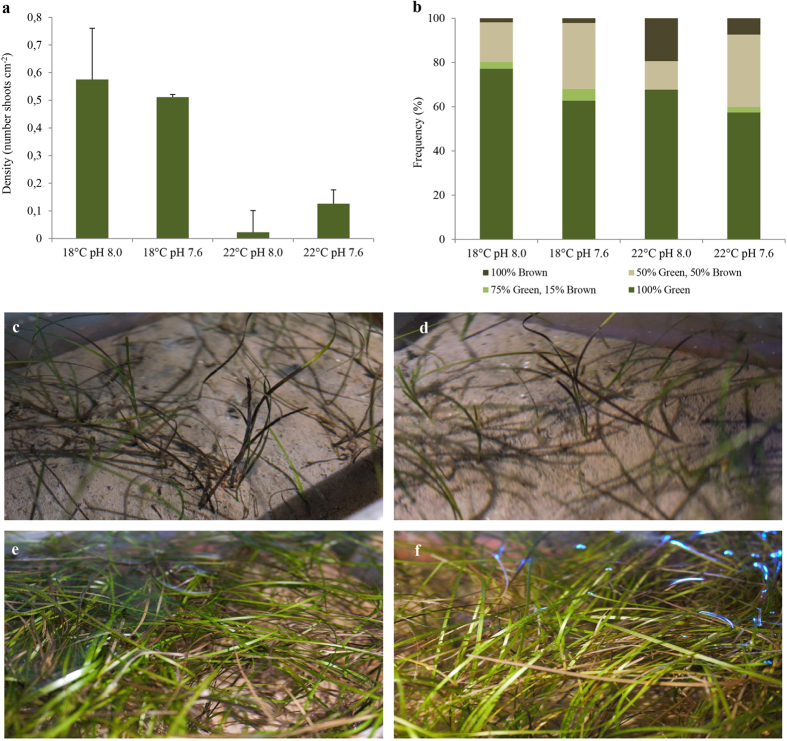
At the beginning of the experimental treatments (T0), Z. noltii shoot density was 0.69 ± 0.16 shoots cm−2 (n = 16). Thirty days (T30) after exposure to the four scenarios investigated, Z. noltii shoot density was significantly reduced under warming conditions (Fig. 1; Supplementary Table S2). Shoot density varied from 0.58 shoots cm−2 under control conditions (18 °C, pH 8.0; Fig. 1a,e) to 0.02 shoots cm−2 under the warming and normocapnic (control pH) scenario (22 °C, pH 8.0; Fig. 1a,c). It is worth noting that hypercapnia induced a slight increase, although non-significant, in seagrass density under the warming scenario (Supplementary Table S2).
Figure 1. Impact of ocean warming (+4 °C) and acidification (ΔpH = 0.4) on Zostera noltii.
(a) Shoot density (number of shoots cm−2) and (b) frequency of leaf colors per shoot (%). Exemplificative images of shoot density (T30), under the different experimental conditions [(c) 22 °C pH 8.0; (d) 22 °C pH 7.6; (e) 18 °C pH 8.0 and (f) 18 °C pH 7.6] are given. Values in panel (a) represent mean ± SD (i.e., n = 4 independent replicate systems, per experimental treatment).
Regarding leaf coloration, seagrass kept under control conditions revealed a higher frequency of shoots with green leaves (77%; Fig. 1b), while seagrass exposed to the warming and normocapnic scenario displayed higher frequency of shoots with brown leaves (19%; Fig. 1b).
Photobiology and pigments
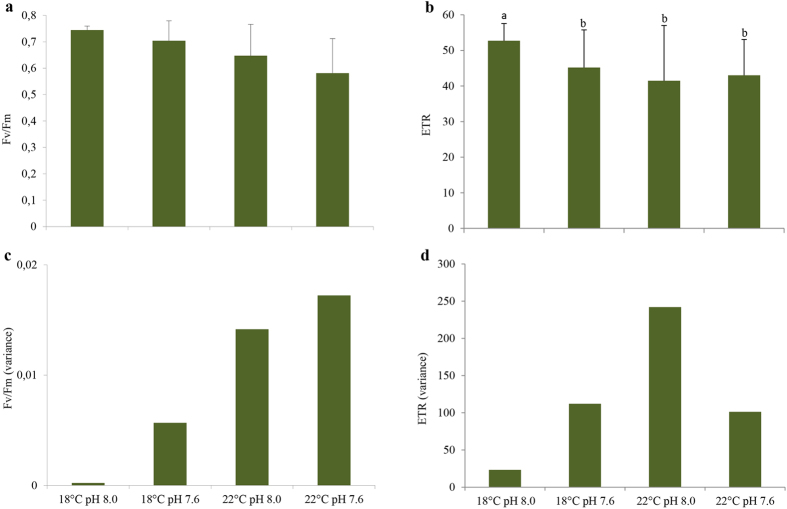
Only temperature significantly affected the photophysiological parameters (ETR and Fv/Fm) of seagrass (two-way MANOVA: F(2,151) = 25.96; p < 0.001; Pillai’s trace = 0.256) with no significant effects of pH or interaction between the explaining variables (two-way MANOVA: F(2,151) = 2.76; p = 0.066; Pillai’s trace = 0.035; F(2,151) = 1.60; p = 0.205; Pillai’s trace = 0.021, respectively). More specifically, warming caused a significant decrease in both Fv/Fm and ETR levels, although it was not statistically significant in the case of ETR measured in seagrass exposed to acidification (Tukey post-hoc test: p < 0.013; Fig. 2a,b, respectively; Supplementary Table S2). It is worth noting that warming and hypercapnic treatment revealed the greatest Fv/Fm variability (Fig. 2c), whereas the warming and normocapnic treatment showed the greatest ETR variability (Fig. 2d), among experimental treatments. A highly positive correlation between ETR variability and the frequency of shoots with 100% brown leaves was observed (r = 0.97; p < 0.05; Table 1).
Figure 2. Impact of ocean warming (+4 °C) and acidification (ΔpH = 0.4) on Zostera noltii.
(a) Maximum PSII quantum yield (Fv/Fm); (b) electron transport rate (ETR); (c) Fv/Fm variance and (d) ETR variance among treatments. Values represent mean ± SD [(n = 40 per experimental treatment (i.e., n = 10 per replicate system (n = 4), per experimental treatment)]. Different letters represent statistical differences between temperature and pH treatments, following a significant interaction (p < 0.013).
Table 1. Pearson’s correlation coefficients between density, frequency of leaf colors, ETR, Fv/Fm and pigments profile, with respective ratios and indices in the seagrass (Zostera noltii) under ocean warming and acidification.
| Dens | CF 100% Green | CF100% Brown | CF 75–15% | CF 50–50% | ETR | ETR Var | Fv/Fm | Fv/FmVar | Chl a | Chl b | Total Chl | Pheo a | Pheo b | Auro | Anthe | β car | Lut | Viol | Zea | Total Car | Carot/Chl | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dens | 1.00 | |||||||||||||||||||||
| CF - 100% G | 0.49 | 1.00 | ||||||||||||||||||||
| CF- 100% B | −0.88 | −0.11 | 1.00 | |||||||||||||||||||
| CF - 75% G, 15%B | 0.09 | −0.13 | −0.89 | 1.00 | ||||||||||||||||||
| CF - 50% G, 50%B | 0.14 | −0.75 | −0.56 | 0.66 | 1.00 | |||||||||||||||||
| ETR | 0.91 | 0.80 | −0.63 | 0.48 | −0.28 | 1.00 | ||||||||||||||||
| ETR Variance | −0.88 | −0.30 | 0.97 | −0.75 | −0.39 | −0.70 | 1.00 | |||||||||||||||
| Fv/Fm | 0.84 | 0.81 | −0.48 | 0.41 | −0.40 | 0.98 | −0.54 | 1.00 | ||||||||||||||
| Fv/Fm Var | −0.94 | −0.75 | 0.68 | −0.55 | 0.21 | −0.99 | 0.74 | −0.97 | 1.00 | |||||||||||||
| Chl a | 0.68 | 0.85 | −0.52 | 0.14 | −0.34 | 0.82 | −0.71 | 0.72 | −0.80 | 1.00 | ||||||||||||
| Chl b | 0.51 | 0.98 | −0.21 | −0.12 | −0.67 | 0.78 | −0.42 | 0.76 | −0.74 | 0.93 | 1.00 | |||||||||||
| Total Chl | 0.65 | 0.86 | −0.49 | −0.11 | −0.37 | −0.81 | −0.68 | 0.71 | −0.78 | 0.99 | 0.94 | 1.00 | ||||||||||
| Pheo a | −0.96 | −0.38 | 0.96 | −0.80 | −0.31 | −0.81 | 0.98 | −0.68 | 0.85 | −0.70 | −0.45 | −0.68 | 1.00 | |||||||||
| Pheo b | −0.90 | −0.37 | 0.96 | −0.73 | −0.33 | −0.75 | 0.99 | −0.59 | 0.78 | −0.74 | −0.47 | −0.72 | 0.99 | 1.00 | ||||||||
| Auro | −0.92 | −0.50 | 0.91 | −0.67 | −0.19 | −0.83 | 0.98 | −0.69 | 0.85 | −0.82 | −0.59 | −0.80 | 0.98 | 0.99 | 1.00 | |||||||
| Anthe | −0.84 | −0.05 | 0.99 | −0.89 | −0.61 | −0.57 | 0.96 | −0.41 | 0.62 | −0.48 | −0.15 | −0.45 | 0.94 | 0.94 | 0.89 | 1.00 | ||||||
| β car | −0.80 | 0.09 | 0.97 | −0.96 | −0.70 | −0.49 | 0.88 | −0.36 | 0.55 | −0.30 | −0.03 | −0.26 | 0.88 | 0.86 | 0.78 | 0.98 | 1.00 | |||||
| Lut | −0.75 | 0.15 | 0.96 | −0.93 | −0.75 | −0.42 | 0.88 | −0.27 | 0.48 | −0.28 | −0.06 | −0.25 | 0.86 | 0.85 | 0.77 | 0.98 | 0.99 | 1.00 | ||||
| Viol | 0.32 | 0.97 | −0.00 | −0.32 | −0.79 | −0.66 | −0.23 | 0.65 | −0.60 | 0.85 | 0.98 | 0.86 | −0.26 | −0.28 | −0.42 | 0.05 | 0.23 | 0.26 | 1.00 | |||
| Zea | −0.64 | 0.32 | 0.90 | −0.93 | −0.85 | −0.27 | 0.78 | −0.13 | 0.34 | −0.11 | 0.23 | −0.08 | 0.75 | 0.75 | 0.65 | 0.93 | 0.97 | 0.98 | 0.43 | 1.00 | ||
| Total Car | −0.80 | −0.03 | 0.99 | −0.86 | −0.63 | −0.53 | 0.96 | −0.36 | 0.58 | −0.48 | −0.14 | −0.45 | 0.92 | 0.94 | −0.80 | 0.99 | 0.97 | −0.25 | 0.06 | 0.93 | 1.00 | |
| Carot / Chl | −0.82 | −0.51 | 0.85 | −0.53 | −0.16 | −0.75 | 0.96 | −0.59 | 0.77 | −0.87 | −0.63 | −0.86 | 0.92 | 0.97 | 0.88 | 0.83 | 0.70 | 0.98 | −0.48 | 0.58 | −0.86 | 1.00 |
| DES | −0.40 | −0.72 | 0.73 | −0.41 | 0.10 | −0.87 | 0.87 | −0.75 | 0.87 | −0.96 | −0.81 | −0.95 | 0.87 | 0.90 | 0.95 | 0.70 | 0.55 | 0.53 | −0.68 | 0.38 | −0.95 | 0.96 |
Values in bold represent statistical significance at the 5% level of significance. Abbreviations: Dens – density; CF – leaf color frequency (G-Green; B- Brown); ETR – electron transport rate; ETR Var: electron transport rate variance; FV/Fm: maximum PSII quantum yield; FV/Fm Var: maximum PSII quantum yield variance; Chl a: chlorophyll a; Chl b: chlorophyll b; Total Chl: total chlorophyll; Pheo a: pheophytin a; Pheo b: pheophytin b; Auro - Auroxanthin; Anthe: Antheraxanthin; β car: β-carotene; Lut: luteín; Viol: violaxanthin; Zea: zeaxanthin; Total Car: total carotenoid; Carot/Chl: carotenoid/chlorophyll ratio; DES: De-epoxidation state.
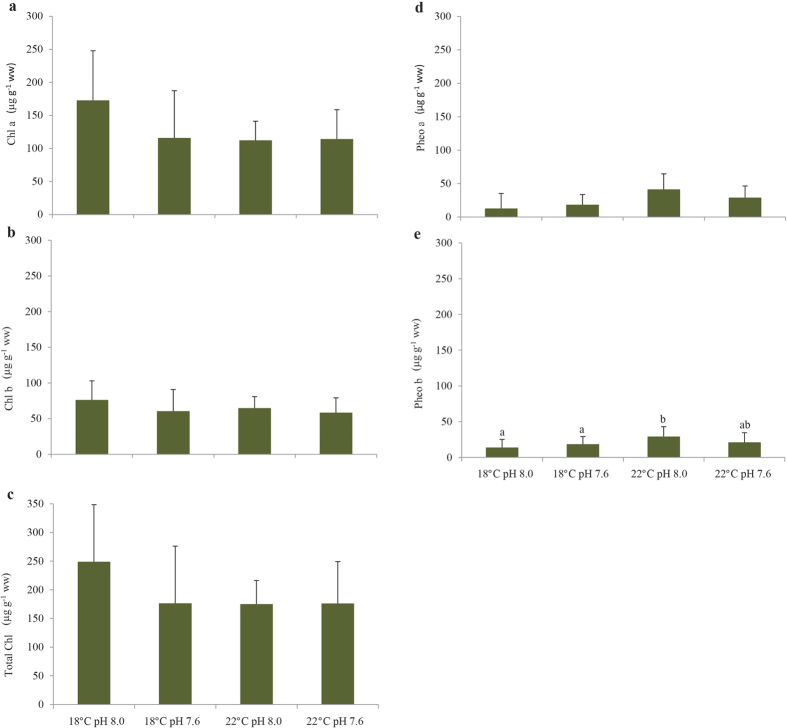
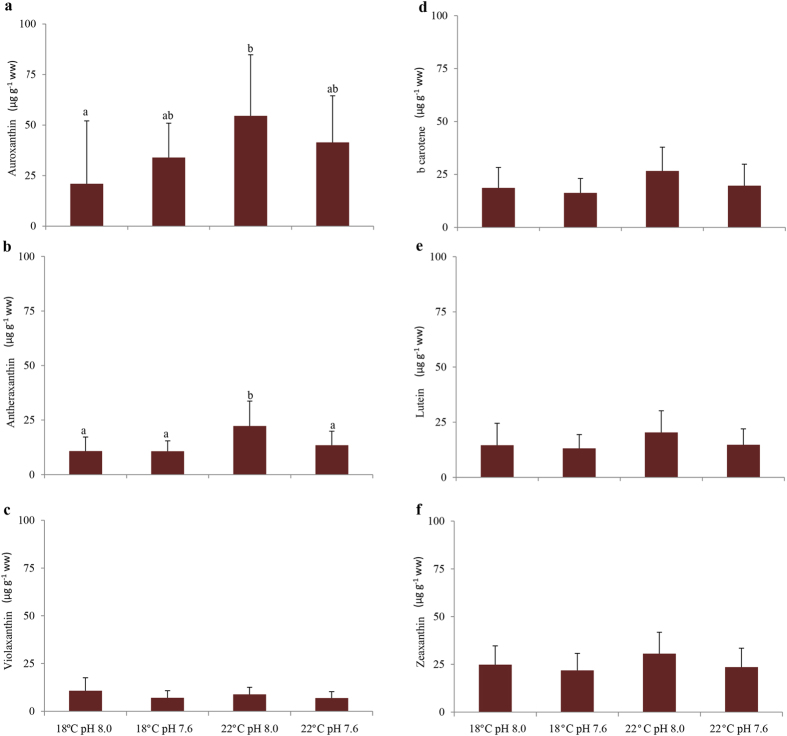
Temperature and pH significantly affected photosynthetic pigment content (two-way MANOVA: F(14, 65) = 2.44; p < 0.008; Pillai’s trace = 0.344; and F(14, 65) = 2.01; p = 0.030; Pillai’s trace = 0.302, respectively) with a significant interaction between the two explaining variables (two-way MANOVA: F(14, 65) = 1.99; p = 0.032; Pillai’s trace = 0.300). More specifically, chlorophyll a content was significantly lower under warming, whereas chlorophyll b content was influenced by both environmental factors (Fig. 3a,b; Supplementary Table S2). As for total chlorophyll content, it was not affected by either temperature or pH (Fig. 3c; Supplementary Table S2). It is worth noting that a highly positive correlation between chlorophyll b and the frequency of shoots with 100% green leaves was observed (r = 0.98; p < 0.05; Table 1). Pheophytin a and b showed an increasing trend with warming, under both normocapnic and hypercapnic conditions (Fig. 3d,e; Supplementary Table S2). Pheophytin a levels were highly negatively correlated with seagrass density (r = −0.96; p < 0.05; Table 1) and positively correlated with: i) frequency of shoots with 100% brown leaves (r = 0.96; p < 0.05; Table 1) and ii) ETR variability (r = 0.98; p < 0.05; Table 1). Identically, a significant positive correlation was recorded between these variables and pheophytin b, with the exception of seagrass density (p > 0.05; Table 1). Auroxanthin and antheraxanthin contents increased under warming and normocapnic condition (Fig. 4a,b), although it was only statistically significant for the latter accessory pigment (Supplementary Table S2). As for β-carotene, its content was significantly affected by both temperature and pH (Supplementary Table S2) with warming causing a significant increase in β-carotene, whereas acidification lead to a significant decrease in the content of this pigment (Fig. 4d). Regarding violaxanthin, only pH significantly affected the content of this pigment, similarly to what was observed regarding zeaxanthin content (Supplementary Table S2). In both cases, seagrasses exposed to hypercapnia showed a significant decrease in their pigments (Fig. 4c,f). Lutein did not significantly vary with both temperature and pH (Fig. 4e; Supplementary Table S2). Similar to pheophytin a and b, auroxanthin and antheraxanthin concentrations were positively correlated with ETR variability (r = 0.98 and 0.96, respectively; p < 0.05; Table 1). Moreover, while auroxanthin levels were positively correlated with pheophytin a and b (r = 0.98 and 0.99, respectively; p < 0.05; Table 1), antheraxanthin concentrations were also significantly correlated with the frequency of shoots with 100% brown leaves (r = 0.99; p < 0.05; Table 1). β-carotene levels were positively correlated with: i) antheraxanthin (r = 0,98; p < 0.05; Table 1), ii) lutein (r = 0,99; p < 0.05; Table 1), zeaxanthin (r = 0.97; p < 0.05; Table 1) and the frequency of shoots with 100% brown leaves (r = 0.97; p < 0.05; Table 1).
Figure 3. Impact of ocean warming (+4 °C) and acidification (ΔpH = 0.4) on Zostera noltii.
(a) Chlorophyll a; (b) chlorophyll b ; (c) total chlorophyll; (d) pheophytin a and (e) pheophytin b contents (μg g−1 ww). Values represent mean ± SD [(n = 40 per experimental treatment (i.e., n = 10 per replicate system (n = 4), per experimental treatment)]. Different letters represent statistical differences between temperature and pH treatments, following a significant interaction (p < 0.013). Abbreviations: Chl a: chlorophyll a; Chl b: chlorophyll b; Total Chl: total chlorophyll; Pheo a: pheophytin a; Pheo b: pheophytin b; μg: microgram; g: gram; ww: wet weight.
Figure 4. Impact of ocean warming (+4 °C) and acidification (ΔpH = 0.4) on Zostera noltii.
(a) Auroxanthin; (b) antheraxanthin; (c) violaxanthin; (d) β-carotene; (e) luteín and (f) zeaxanthin contents (μg g−1 ww). Values represent mean ± SD [(n = 40 per experimental treatment (i.e., n = 10 per replicate system (n = 4), per experimental treatment)]. Different letters represent statistical differences between temperature and pH treatments, following a significant interaction (p < 0.013). Abbreviations: μg: microgram; g: gram; ww: wet weight.
Total carotenoid concentrations were significantly higher in seagrasses exposed to warming and normocapnia, in comparison to Z. noltii exposed to remaining scenarios (Fig. 5a; Supplementary Table S2). Additionally, total carotenoid concentration was positively correlated with: i) frequency of shoots with 100% brown leaves (r = 0.99; p < 0.05; Table 1); ii) ETR variability (r = 0.96; p < 0.05; Table 1); iii) antheraxanthin (r = 0.99; p < 0.05; Table 1) and iv) β-carotene (r = 0.97; p < 0.05; Table 1). While seagrasses exposed to warming scenario presented higher carotenoids/chlorophylls ratios, differences were only statistically significant when compared with those recorded for specimens under control conditions (Fig. 5b; Supplementary Table S2). Additionally, carotenoids/chlorophylls ratio was positively correlated with: i) ETR variability (r = 0.96; p < 0.05; Table 1); ii) pheophytin b (r = 0.97; p < 0.05; Table 1) and iii) lutein (r = 0.98; p < 0.05; Table 1). De-Epoxidation State (DES) showed significantly lower values in Z. noltii exposed to control conditions in comparison to warming scenarios (both under normocapnic and hypercapnic conditions, Fig. 5c; Supplementary Table S2). Moreover, DES was negatively correlated with: i) chlorophyll a (r = −0.96; p < 0.05; Table 1); ii) total chlorophylls (r = −0.95; p < 0.05; Table 1) and iii) total carotenoids (r = −0.95; p < 0.05; Table 1).
Figure 5. Impact of ocean warming (+4 °C) and acidification (ΔpH = 0.4) warming on Zostera noltii.
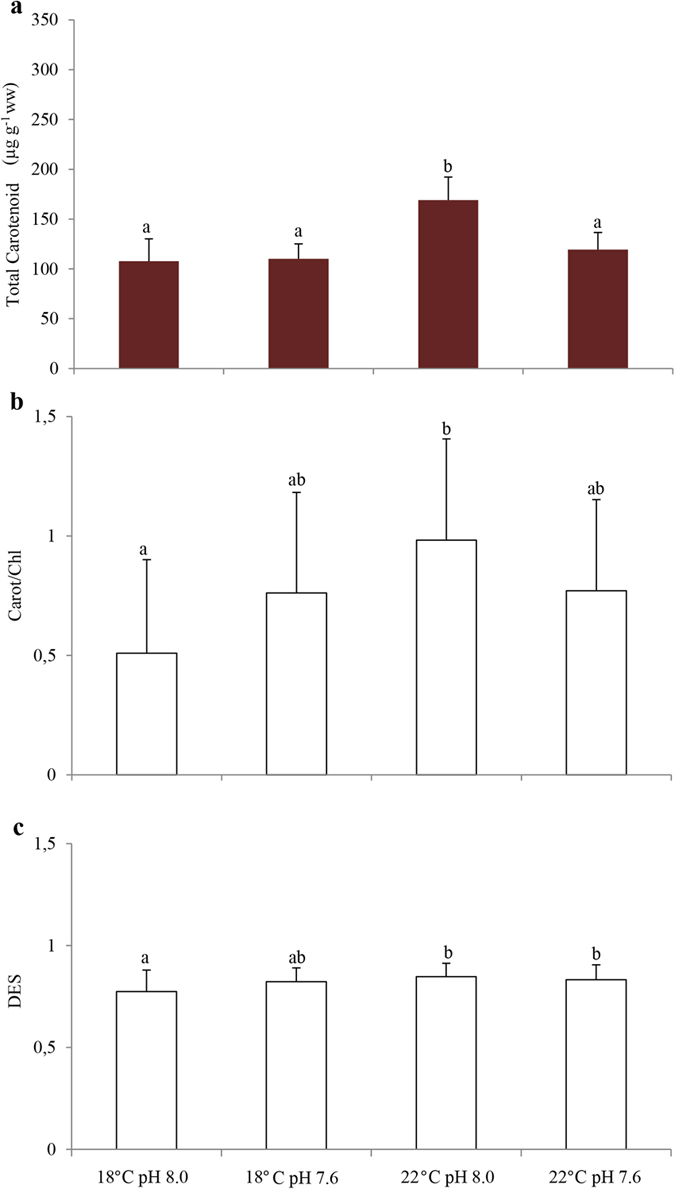
(a) Total carotenoid content (μg g−1 ww); (b) carotene/chlorophyll ratio and (c) De-Epoxidation State (DES). Values represent mean ± SD [(n = 40 per experimental treatment (i.e., n = 10 per replicate system (n = 4), per experimental treatment)]. Different letters represent statistical differences between temperature and pH treatments, following a significant interaction (p < 0.013). Abbreviations: Carot/Chl: carotenoid/chlorophyll ratio; DES: De-epoxidation state; μg: microgram; g: gram; ww: wet weight.
Discussion
Seagrass ability to cope with increasing temperature is dependent upon thermal tolerance of each individual species and environmental conditions25. As temperature rises and thermal tolerance upper limits are exceeded, cellular and organism’s death will potentially occur26,27. In our study, increasing temperature (+4 °C above control conditions) was the most determinant stressor in Z. noltii survival and physiology, severely affecting shoot density, leaf coloration and profile of photosynthetic pigments. When comparing lower shoot density values within warming/hypercapnic scenario versus control conditions, a plausible explanation could derive from limited recovery capacity of seagrass to counterbalance increasing temperature13,28,29, enhanced by slow CO2 diffusion capacity into seagrass leaves30,31.
Increasing temperature has been recognized to promote deleterious effects to health and survival of seagrass species. Indeed, Z. marina shoot survival and production was found to be adversely affected by increasing temperature32. In another study, increasing temperature was found to enhance Posidonia oceanica shoot mortality events, even exceeding recruitment rates13. Likewise, a swift shoot mortality and leaf loss were observed for Z. muelleri exposed to an increase temperature (32 °C), merely 5 °C above this species optimal growth value28. Moreover, a mass mortality episode (>90%) was reported for Amphibolis antartica, after the occurrence of a heat wave event15. Although Z. noltii inhabits the intertidal zone and therefore, to a more or less extent, can be physiologically adapted to a fluctuating environment33, our findings clearly show that increasing temperature, can pose a major threat to this species ability to survive. Consequently, we argue that, under a continuous and prolonged exposure to ocean warming conditions, Z. noltii will be closer or even above its physiological performance thresholds.
It is generally accepted that ocean acidification will benefit photosynthesis and growth rates of primary producers. As aqueous carbon dioxide will tend to increase, it is expected that a physiological competiveness enhancement may occur in all those species that rely upon and are capable to promptly seize CO210,19,34. Most marine seagrasses are considered to be under-saturated with respect to dissolved inorganic carbon10,31. The majority of seagrasses have been described as photosynthetic C3 plants, although some exceptions are in order, including Z. noltii C4 capability35,36 or facultative photosynthetic pathways under low CO2 levels10. Consequently, an increase in dissolved CO2 concentration could bring a beneficial output in terms of seagrass growth and photosynthetic machinery performance, regardless of their capacity to use CO2, HCO3− or other inorganic carbon sources for photosynthesis related processes10,18. Although not completely in accordance with this theory, our results do not contradict these previous considerations. In fact, pH was not a determinant factor, but when taking into consideration some analyzed endpoints, Z. noltii was, to a certain extent, beneficiated. Our findings suggest that Z. noltii has the ability to partially overcome negative effects of ocean warming through the use of CO2, HCO3− or both, as inorganic carbon sources to obtain energy for metabolic processes and consequently survive. Moreover, we argue that the present findings also indicate to a carbon-limitation status (under present day conditions), with increasing dissolved carbon availability unlocking arrested photochemical potential of plants37.
Seagrass stress level was also assessed by measuring photosystem II (PSII) photochemical efficiency through chlorophyll fluorescence emitted from plant leaves38. Maximum photochemical efficiency (Fv/Fm ratio) provides a measurement of photosynthetic stress, i.e. gives an insight if PSII disturbance is caused by plant stress. Additionally, higher Fv/Fm ratios (0.79–0.84) are considered optimal for the majority of plant species, indicating healthier plants39. Zostera noltii exposed to control conditions exhibited nearly optimal Fv/Fm ratios, which can indicate a healthy photosynthetic condition. On the contrary, lowest values for Fv/Fm ratio were observed in seagrasses exposed under warming conditions, thus clearly demonstrating a stress related condition as a result of increased temperature. Likewise, similar findings were previously reported regarding tropical seagrass species40.
In our study, increased CO2 levels did not significantly affected photochemical efficiency of Z. noltii. Indeed, several studies point out towards an enhancement of photobiological fitness of C3 plants (under increasing CO2 scenarios), in opposition to C4 plants10,37,41. Indeed, while C3 species appear to respond positively to increasing atmospheric CO2 levels, enhancing its light harvesting mechanisms and its photosynthetic efficiency, C4 plants appear to suffer from stress induced by rising CO2. In C3 plants, CO2 fertilization induces Rubisco full carboxylation capacity and photosynthetic enhancement, mostly due to an enhancement of ETR and lower quantities of dissipated energy. On the contrary, C4 plants are usually working at full potential, presenting little or even any photosynthetic enhancement under CO2 fertilization. Although carbon-harvesting mechanisms and incorporation seem to be improved by higher dissolved CO2 availability, the light-harvesting component of the photosynthetic apparatus show some signs of stress. Again, this confirms the above mentioned hypothesis of a typical response of a C4 species37,42,43. Nonetheless, our results also contradict, to a certain degree, previous studies where high ETR values and photosynthesis downregulation were reported (under CO2 enriched conditions), indicating an inorganic carbon photosynthesis limitation of Z. noltii, at present day conditions44. Thus, it seems that Z. noltii might retain both C3–C4 photosynthetic capacity, but further experimental work needs to be performed in order to sustain this hypothesis. In our study, Z. noltii also showed a decrease in ETR capacity under warming conditions. Thus, warming seems to directly impact the electron transport chain (ETC), probably by affecting the PSII donor side45.
Levels of photosynthetic related pigments (e.g. chlorophylls and carotenoids) can provide an insight on plant growth, photosynthetic and physiological status46. Under heat stress, PSII donor side is highly affected due to its lower thermostability47, thus reducing its ability to supply the ETC with electronic energy generated during light harvesting. This inevitably generates high amounts of excessive energy within the stroma that needs to be dissipated as to prevent PSII permanent damage47. In our study this seemed to be achieved through two pathways: i) energy quenching by auroxanthin and ii) xanthophyll cycle activity. Zostera noltii individuals exposed to warming showed a significant increase in auroxanthin content, a violaxanthin analogue48. Auroxanthin is a C5,8 epoxy carotenoid that has previously been shown to enhance aggregation-associated quenching in isolated LHC IIb49. The effectiveness of auroxanthin has been suggested to derive from the fact that its S1 energy level is higher than the one of violaxanthin48. This effect is explained by its structure, where the end groups of auroxanthin lie in the plane of the conjugated carbon double bond chain, as in zeaxanthin, whereas they are expected to be out-of-plane in violaxanthin. In fact, the C5,8 epoxide would hold the end group rigidly in-plane, explaining why it is even more effective than zeaxanthin and thus be an efficient energy quencher, under stress conditions49,50. The activation of energy dissipation pathway is typical from heat stress plants damaged at the PSII donor side51. The coupled activation of the xanthophyll cycle (increased DES) along with a higher auroxanthin synthesis suggests that warming-exposed individuals had higher needs to dissipate excessive energy than seagrass under control temperature treatments. It is worth noting that the increase in antioxidant pigments (e.g. β-carotene) in seagrass exposed to warming and acidification conditions, reinforces the need to counteract the effects of excessive redox potential accumulated inside its chloroplasts and sub consequent generation of reactive oxygen species (ROS). Another clear evidence of cellular stress was the production of chlorophyll degradation products (pheophytin a and pheophytin b), generated by the loss of the chlorophyll molecule, under stress conditions. This fact was clearly perceived in leaf coloration. Here, not only there was an elimination of chlorophyll molecules, but a concomitant increase in carotenoid content; this could point towards a stress situation in opposition to natural senescence where only chlorophyll is reabsorbed52. Once again this was not recorded for specimens in treatments with higher dissolved CO2 availability, yet again indicating an ameliorating effect, reduced stress levels and in this case by supplying the required carbon to dissipate the excessive energy within the cells37.
As biological catalyst substances, enzymes play an important role in chlorophyll biosynthesis. Once temperature directly affects enzyme activity, chlorophyll synthesis is expected to be affected53. Such trend was not observed in our study with temperature influence not being recorded in total chlorophyll content. However, our findings conflict with previous studies, that recorded a rise in chlorophyll concentration within Ruppia drepanensis leaves, with increasing temperature conditions54. These contradictory results could eventually be explained by species specific strategies by means of distinct acclimation capabilities54.
To the best of our knowledge, no previous studies have reported the combined effects of ocean warming and acidification on Z. noltii survival, photophysiology and photosynthetic output. Our results show that Z. noltii exhibited down-regulated acclimation potential to future projected warming conditions, with possible severe consequences at individual and subsequently ecosystem level15. Although this species inhabits unstable and fluctuating marine coastal environments, it seems that predictable climate change scenarios will pose major constraints to Z. noltii ability to cope with additional stressors, particularly ocean warming. Due to its key ecological role within coastal environments, seagrass long-term acclimation capacity and/or adaptation response to anthropogenic-derived stressors should be further investigated, in order to elucidate and predict species resilience towards predicted climate change scenarios10,21,44. Moreover, since seagrasses are important ecosystem builders and biological richness “enhancers” within coastal habitats, future research should address how marine biota (e.g. epiphytes, macroalgae and bacterial communities) that co-exist and biologically interact with marine angiosperms can be affected and influence seagrasses adaptive plasticity to climate change55,56.
Methods
Seagrass collection and acclimation
Dwarf seagrass Zostera noltii was collected with its natural sediment using a stainless steel core (300 × 200 × 100 mm, AISI 316), at a pristine area (38°29′ 18.42″N; 8°53′ 15.12″W, Caldeira de Tróia, Portugal)57. Following collection, each sample (seagrass and natural sediment; n = 16) was covered with seawater soaked towels (air dissection avoidance) within thermal insulated cases and immediately transported to the aquatic facilities of Laboratório Marítimo da Guia (FCUL, Portugal). Upon arrival, samples were laboratory acclimated, in order to minimize any changes in the natural sediment structure (collected with Z. noltii). Laboratory acclimation lasted 30 days, under seawater conditions mimicking those at collection site: salinity = 35 ± 1 (V2 refractometer, TMC Iberia, Portugal); water temperature = 18 ± 1 °C (TFX 430 Thermometer, WTW GmbH, Germany) and pH = 8.0 ± 0.1 (SG8 – SevenGo pro™ pH/Ion meter, Mettler-Toledo International Inc., Switzerland). After initial laboratory acclimation, seagrass was exposed to the following experimental conditions: i) control scenario (18 °C, pH 8.0); ii) hypercapnic scenario (18 °C, pH 7.6); iii) warming scenario (22 °C, pH 8.0) and iv) combined warming and hypercapnic scenario (22 °C, pH 7.6), following IPCC’s RCP scenario 8.5. Normocapnia [i.e. experimental conditions: i) and iii)] and hypercapnia [i.e. experimental conditions: ii) and iv)] conditions were defined as experimental settings where pH = 8.0 and 7.6, respectively. Experimental exposure lasted an additional 30 days and consisted in sixteen 10-L tank setups (4 independent replicate tanks per treatment; Supplementary Figure S1).
Flow-through aquatic systems were set (as described in Fig. 3d schematics)58, in order to maintain total alkalinity, dissolved inorganic carbon speciation due to bacterial activity and acidification of treatments. Experimental tanks were set in a completely randomized outline58. Natural seawater (NSW) was pumped from the sea into a seawater storage tank (5 m3 capacity). Subsequently, NSW was 0.35 μm filtered (Harmsco, Florida, USA) and UV-irradiated (Vecton 600, TMC Iberia, Portugal), before being supplied to mixing (n = 16) and experimental (n = 16) tanks. Overhead tank illumination was provided through artificial overhead lightning apparatus (Aquabeam 1500 Ultima NP, TMC Iberia, Portugal), with a correlated color temperature of 20.000 K, total luminous flux of 1965 lumens and a 120° beam angle. Photosynthetically active radiation (PAR) was measured using a portable fluorometer (FluorPen FP 100, Photo Systems Instruments, Czech Republic) and maintained at 180 ± 10 μmol photons m−2 s−1 (mid-light intensity for Zostera spp.)59. Experimental tanks were kept under a photoperiod of 14 h/10 h (light/dark cycle), according to prevailing natural light conditions (collection site). Ammonia and nitrite levels were daily checked using colorimetric tests (Salifert Profi Test, Holland), while individual pH values were adjusted automatically (Profilux 3.1, GHL, Germany). Monitoring of pH was performed every 2 seconds interval, with pH values being lowered through the injection of certified CO2 gas (Air Liquide, Portugal) or upregulated through aeration using CO2 filtered atmospheric air (soda lime, Sigma-Aldrich). Seawater temperature was controlled through seawater chilling systems (Frimar, Fernando Ribeiro Lda, Portugal) and submerged heaters (150 W, Eheim GmbH & Co. KG, Germany). Additionally, handheld equipment was used in order to perform a daily monitoring of seawater temperature (TFX 430 thermometer, WTW GmbH, Germany) and salinity (V2 refractometer, TMC Iberia, Portugal). Seawater carbonate system speciation was calculated weekly from total alkalinity (spectrophometrically at 595 nm) and pH measurements60. Seawater temperature and pH of different experimental setups is summarized in Supplementary Table S1. Quantification of pH was determined using a pH meter (826 pH mobile, Metrohm, Germany) connected to a glass electrode (Schott IoLine, SI analytics,±0.001), calibrated with TRIS-HCl (TRIS) and 2-aminopyridine-HCl (AMP) (Mare, Belgium) seawater buffers61. Recording of pH values was performed under temperature controlled conditions using a water bath (±0.1 °C, Lauda, Germany). Bicarbonate and pCO2 values (Supplementary Table S1) were calculated using the CO2SYS software (Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, USA), with dissociation constants62, as refitted63.
Shoot density and leaf color intensity
Zostera noltii shoot density was determined at the beginning of the experiment (T0) and 30 days (T30) after exposure to experimental conditions. Shoot density was recorded as the number of shoots per area (shoots.cm−2). Seagrass leaves color was assessed through direct observation (T30) and scored using the following criteria: i) 100% green; ii) 75% green and 25% brown; iii) 50% green and 50% brown and iv) 100% brown.
Photobiology
Pulse amplitude modulation (PAM) fluorometry was used to monitor photosynthetic activity [40 leaves per treatment, 30 days (T30) after seagrass exposure to experimental conditions], by measuring non-intrusively variable chlorophyll fluorescence64. The PAM fluorometer comprised a computer-operated PAM-control unit (Junior-Pam, Heinz Walz GmbH, Germany) and a water-EDF-Universal emitter-detector unit (Gademann Instruments GmbH, Germany). Actinic and saturating light was provided by a blue LED-lamp (450 nm peak, 20 nm half-band width), supplied to the experimental biological sample by a fiber optic (fo) bundle (1.5 mm-diameter). The fo bundle was perpendicularly positioned to seagrass leaf surface. Measurements were taken 30 days after the beginning of experimental exposure. Seagrass replicates were dark-adapted for 30 min, after which one saturation pulse (0.8 s) was applied to determine the minimum- or dark-level fluorescence (Fo), a parameter expected to correlate with chlorophyll a (chl a) content65 and the maximum fluorescence (Fm). Photosystem II (PSII) quantum yield was determined using Fo and Fv64 :
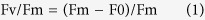 |
The Fv/Fm ratio indicates the proportion of PSII reaction centers capable of converting captured light into photosynthetic energy (PSII quantum yield). This ratio is a convenient measure of the maximum potential quantum yield of PSII, and therefore is inversely proportional to photochemical stress.
Seagrasses were light-adapted for 30 minutes as to calculate the electron transport rate, a light-adapted parameter that is directly related to PS(II) by the equation:
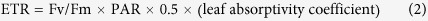 |
where 0.5 compensates for irradiance being split between two photosystems. The absorption coefficient used was 0.84.
Gauss peak spectra pigment analysis
Zostera noltii leaves (n = 40 per treatment) were collected after 30 days of exposure to experimental conditions (T30) and immediately frozen. For pigment extraction, leaves were freeze-dried (48 h dark), after which were grinded (pure acetone, using a glass rod). To ensure complete disaggregation of leaf material, samples with acetone were subjected to a cold ultra-sound bath (2 min)66. Extraction occurred at −20 °C (24 h, dark) to prevent pigment degradation. After extraction, samples were centrifuged (4,000 rpm, 15 min at 4 °C). The Gauss-Peak Spectra method67 was employed for pigment analysis. This method has been widely applied for higher plants40,47 and proved to analyze pigment profile efficiently even when compared with HPLC analysis67. This method has the advantage of being less expensive and faster than HPLC analysis, providing a statistical analysis of the results while comparted to the peak shapes of standard materials. All analyses in which results did not match statistical standards were discarded, with extraction and subsequent analysis being repeated. Samples were scanned in a dual beam spectrophotometer (350 nm to 750 nm, 0.5 nm steps). Absorbance spectrum was introduced in the Gauss Peak Spectra (GPS) fitting library, thus allowing the identification and quantification of pigments (i.e. chlorophyll a, chlorophyll b, antheraxanthin, β-carotene, auroxanthin, lutein, violoxanthin and zeaxanthin), using SigmaPlot Software (Systat Software, Inc., USA). In order to better evaluate light harvesting and photo-protection mechanisms, De-Epoxidation State (DES) was calculated as:
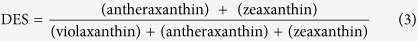 |
The xanthophyll cycle consists of light-dependent conversions of three xanthophylls in a cyclic reaction. Succinctly, it involves a deepoxidation sequence from the diepoxide violaxanthin, via the monoepoxide antheraxanthin to the epoxide-free form zeaxanthin, facilitating the de-excitation of accumulated chlorophyll singlet excited states68. This allows PS(II) to dissipate excessive energy in the form of heat. De-Epoxidation State evaluates the activity of this cyclic reaction, with higher values corresponding to higher conversions of the epoxides to zeaxanthin.
Statistical analyses
In order to detect significant statistical differences in shoot density, a two-way ANOVA (temperature and pH as explaining factors) was performed. In addition, two separate two-way multivariate analysis of variance (MANOVA), using temperature and pH as explaining factors, were performed to identify significant effects of temperature and pH on: i) photophysiological parameters (ETR and Fv/Fm), and ii) pigment levels. As MANOVA revealed significant differences, two-way ANOVA followed by Tukey post-hoc tests were conducted (whenever the interaction between temperature and pH was significant) in order to better scrutinize the effect of explaining variables on each measured endpoint. Dunn-Sidak procedure was used to adjust the associated significance level of the family-wise type-I error. A total of 4 comparisons were applied (2 temperature levels combined with 2 pH values), resulting in a corrected significance level of 0.013. Prior to all analyses of variance, data were checked for normality and homoscedasticity (Kolmogorov-Smirnov and Levene’s tests, respectively).
A Pearson correlation analysis was also applied in order to investigate potential relationships between biological (shoot density, frequency of shoots’ coloration), photophysiological (ETR and Fv/Fm) and biochemical (pigments) variables. Unless stated otherwise, all statistical analyses were performed using a significance level of 0.05, using Statistica 10.0 software (StatSoft Inc., USA).
Additional Information
How to cite this article: Repolho, T. et al. Seagrass ecophysiological performance under ocean warming and acidification. Sci. Rep. 7, 41443; doi: 10.1038/srep41443 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
The Portuguese Foundation for Science and Technology (FCT) supported this study through the strategic project UID/MAR/04292/2013 granted to MARE, project Mutualchange (PTDC/MAREST/5880/2014), postdoctoral (Tiago Repolho: SFRH/BPD/94523/2013 and Tiago Grilo: SFRH/BPD/98590/2013) and doctoral grants (José Ricardo Paula: SFRH/BD/111153/2015; Ana Lopes: SFRH/BD/97070/2013; Bernardo Duarte: SFRH/BD/75951/2011 and Gisela Dionísio: SFRH/BD/73205/2010) and programa investigador FCT 2013 to Rui Rosa.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.R. and R.R. conceived and designed the experiment. T.R., A.R.L., T.F.G. and R.R. performed the experiment. G.D. and B.D. performed the photobiology and the gauss peak spectra pigment analysis, respectively. T.R., B.D., J.R.P., I.C.R. and R.R. analyzed and interpreted the data. T.R., R.C., I.C. and R.R. wrote the paper. All authors reviewed the manuscript. All authors read and approved the final manuscript.
References
- Pörtner H.-O. et al. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Field C. B. et al.) 411–484 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2014). [Google Scholar]
- Fabry V. J., Seibel B. A., Feely R. A. & Orr J. C. Impacts of ocean acidification on marine fauna and ecosystem processes. ICES J. Mar. Sci. 65, 414–432 (2008). [Google Scholar]
- Collins M. et al. In Climate Change 2013: The Physical Science Basis. Contribution of Working GroupI to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (eds Stocker T. F. et al.) 1029–1136 (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 2013). [Google Scholar]
- Rosa R. et al. Early-life exposure to climate change impairs tropical shark survival. Proc. R. Soc. B Biol. Sci. 281, 20141738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth P. J. The Biology of Mangroves and Seagrasses. (Oxford University Press, UK, 2007). [Google Scholar]
- Marbà N. et al. Diversity of European seagrass indicators: patterns within and across regions. Hydrobiologia 704, 265–278 (2012). [Google Scholar]
- Duarte C. M., Middelburg J. J. & Caraco N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences Discuss. 1, 659–679 (2004). [Google Scholar]
- Fourqurean J. W. et al. Seagrass ecosystems as a globally significant carbon stock. Nat. Geosci. 5, 505–509 (2012). [Google Scholar]
- Waycott M. et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 106, 12377–12381 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M., Bowes G., Ross C. & Zhang X.-H. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Glob. Chang. Biol. 19, 103–132 (2013). [DOI] [PubMed] [Google Scholar]
- Short F. T. et al. Extinction risk assessment of the world’s seagrass species. Biol. Conserv. 144, 1961–1971 (2011). [Google Scholar]
- Diaz-Almela E., Marbà N. & Duarte C. M. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 13, 224–235 (2007). [Google Scholar]
- Marbà N. & Duarte C. M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 16, 2366–2375 (2010). [Google Scholar]
- Lee K.-S., Park S. R. & Kim Y. K. Effects of irradiance, temperature, and nutrients on growth dynamics of seagrasses: A review. J. Exp. Mar. Bio. Ecol. 350, 144–175 (2007). [Google Scholar]
- Thomson J. A. et al. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Glob. Chang. Biol. 21, 1463–1474 (2015). [DOI] [PubMed] [Google Scholar]
- Dhir B. Status of aquatic macrophytes in changing climate: A perspective. J. Environ. Sci. Technol. 8, 139–148 (2015). [Google Scholar]
- Zeebe R. E. & Wolf-Gladrow D. A. CO2 in seawater: equilibrium, kinetics, isotopes. (Elsevier, 2001). [Google Scholar]
- Beer S., Bjork M., Hellblom F. & Axelsson L. Inorganic carbon utilization in marine angiosperms (seagrasses). Funct. Plant Biol. 29, 349–354 (2002). [DOI] [PubMed] [Google Scholar]
- Palacios S. L. & Zimmerman R. C. Response of eelgrass Zostera marina to CO2 enrichment: possible impacts of climate change and potential for remediation of coastal habitats. Mar. Ecol. Prog. Ser. 344, 1–13 (2007). [Google Scholar]
- Invers O., Zimmerman R. C., Alberte R. S., Pérez M. & Romero J. Inorganic carbon sources for seagrass photosynthesis: an experimental evaluation of bicarbonate use in species inhabiting temperate waters. J. Exp. Mar. Bio. Ecol. 265, 203–217 (2001). [Google Scholar]
- Ralph P. J. Photosynthetic response of Halophila ovalis (R. Br.) Hook. f. to combined environmental stress. Aquat. Bot. 65, 83–96 (1999). [Google Scholar]
- Sunda W. G. & Cai W.-J. Eutrophication induced CO2-acidification of subsurface coastal waters: interactive effects of temperature, salinity, and atmospheric PCO2. Environ. Sci. Technol. 46, 10651–10659 (2012). [DOI] [PubMed] [Google Scholar]
- Larkum A. W. D., Orth R. J. & Duarte C. M. Seagrasses: Biology, Ecology and Conservation. (Springer, The Netherlands, 2006). [Google Scholar]
- Valle M. et al. Projecting future distribution of the seagrass Zostera noltii under global warming and sea level rise. Biol. Conserv. 170, 74–85 (2014). [Google Scholar]
- Short F. T. & Neckles H. A. The effects of global climate change on seagrasses. Aquat. Bot. 63, 169–196 (1999). [Google Scholar]
- Kaldy J. E. & Shafer D. J. Effects of salinity on survival of the exotic seagrass Zostera japonica subjected to extreme high temperature stress. Bot. Mar. 56, 75–82 (2013). [Google Scholar]
- Kaldy J. E. Effect of temperature and nutrient manipulations on eelgrass Zostera marina L. from the Pacific Northwest, USA. J. Exp. Mar. Bio. Ecol. 453, 108–115 (2014). [Google Scholar]
- York P. H. et al. Physiological and morphological responses of the temperate seagrass Zostera muelleri to multiple stressors: investigating the interactive effects of light and temperature. PLoS One 8, e76377 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa S. I., Arnaud-Haond S., Pearson G. A. & Serrão E. A. Temperature tolerance and survival of intertidal populations of the seagrass Zostera noltii (Hornemann) in Southern Europe (Ria Formosa, Portugal). Hydrobiologia 619, 195–201 (2008). [Google Scholar]
- Papenbrock J. Highlights in Seagrasses’ Phylogeny, Physiology, and Metabolism: What Makes Them Special? ISRN Bot. 2012, 1–15 (2012). [Google Scholar]
- Mackey K. R. M., Morris J. J., Morel F. M. M. & Kranz S. A. Response of photosynthesis to ocean acidification. Oceanography 28, 74–91 (2015). [Google Scholar]
- Bintz J. C., Nixon S. W., Buckley B. A. & Granger S. L. Impacts of temperature and nutrients on coastal lagoon plant communities. Estuaries 26, 765–776 (2003). [Google Scholar]
- Peralta G., Brun F. G., Hernández I., Vergara J. J. & Pérez-Lloréns J. L. Morphometric variations as acclimation mechanisms in Zostera noltii beds. Estuar. Coast. Shelf Sci. 64, 347–356 (2005). [Google Scholar]
- Zimmerman R. C., Kohrs D. G., Steller D. L. & Alberte R. S. Impacts of CO2 enrichment on productivity and light requirements of eelgrass. Plant Physiol. 115, 599–607 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez C., Xavier Niell F. & Algarra P. Photosynthetic adaptation of Zostera noltii Hornem. Aquat. Bot. 29, 217–226 (1987). [Google Scholar]
- Mercado J. M., Niell F. X., Silva J. & Santos R. Use of light and inorganic carbon acquisition by two morphotypes of Zostera noltii Hornem. J. Exp. Mar. Bio. Ecol. 297, 71–84 (2003). [Google Scholar]
- Duarte B. et al. Light-dark O2 dynamics in submerged leaves of C3 and C4 halophytes under increased dissolved CO2: clues for saltmarsh response to climate change. AoB Plants 6, plu067 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph P. J. & Burchett M. D. Photosynthetic responses of the seagrass Halophila ovalis (R. Br.) Hook. f. to high irradiance stress, using chlorophyll a fluorescence. Aquat. Bot. 51, 55–66 (1995). [Google Scholar]
- Maxwell K. & Johnson G. N. Chlorophyll fluorescence-a practical guide. J. Exp. Bot. 51, 659–668 (2000). [DOI] [PubMed] [Google Scholar]
- Campbell S. J., McKenzie L. J. & Kerville S. P. Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J. Exp. Mar. Bio. Ecol. 330, 455–468 (2006). [Google Scholar]
- Pedersen O., Colmer T. D., Borum J., Zavala-Perez A. & Kendrick G. A. Heat stress of two tropical seagrass species during low tides - impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytol. 210, 1207–1218 (2016). [DOI] [PubMed] [Google Scholar]
- Duarte B., Santos D., Silva H., Marques J. C. & Caçador I. Photochemical and biophysical feedbacks of C3 and C4 Mediterranean halophytes to atmospheric CO2 enrichment confirmed by their stable isotope signatures. Plant Physiol. Biochem. 80, 10–22 (2014). [DOI] [PubMed] [Google Scholar]
- Geissler N., Hussin S., El-Far M. M. M. & Koyro H.-W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 118, 67–77 (2015). [Google Scholar]
- Alexandre A., Silva J., Buapet P., Björk M. & Santos R. Effects of CO2 enrichment on photosynthesis, growth, and nitrogen metabolism of the seagrass Zostera noltii. Ecol. Evol. 2, 2625–2635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte B., Marques J. C. & Caçador I. Ecophysiological responses of native and invasive Spartina species to extreme temperature events in Mediterranean marshes. Biol. Invasions 18, 2189–2205 (2016). [Google Scholar]
- Liu J., Zhang P., Guo D., Niu S. & Zhang X. Annual change in photosynthetic pigment contents of Zostera marina L. in Swan Lake. African Journal of Biotechnology 10, 18194–18199 (2011). [Google Scholar]
- Duarte B., Goessling J. W., Marques J. C. & Caçador I. Ecophysiological constraints of Aster tripolium under extreme thermal events impacts: Merging biophysical, biochemical and genetic insights. Plant Physiol. Biochem. 97, 217–228 (2015). [DOI] [PubMed] [Google Scholar]
- Wentworth M., Ruban A. V. & Horton P. Chlorophyll fluorescence quenching in isolated light harvesting complexes induced by zeaxanthin. FEBS Lett. 471, 71–74 (2000). [DOI] [PubMed] [Google Scholar]
- Ruban A. V., Phillip D., Young A. J. & Horton P. Excited-state energy level does not determine the differential effect of violaxanthin and zeaxanthin on chlorophyll fluorescence quenching in the isolated light-harvesting complex of photosystem II. Photochem. Photobiol. 68, 829–834 (1998). [Google Scholar]
- Horton P., Ruban A. V. & Young A. J. In Advances in Photosynthesis and Respiration (eds Frank H. A., Young A. J., Britton G. & Cogdell R. J.) 271–291 (Kluwer Academic Publishers, the Netherlands, 1999). doi: 10.1007/0-306-48209-6. [DOI] [Google Scholar]
- Duarte B., Santos D., Marques J. C. & Caçador I. Impact of heat and cold events on the energetic metabolism of the C3 halophyte Halimione portulacoides. Estuar. Coast. Shelf Sci. 167, 166–177 (2015). [Google Scholar]
- Duarte B., Couto T., Marques J. C. & Caçador I. Scirpus maritimus leaf pigment profile and photochemistry during senescence: implications on carbon sequestration. Plant Physiol. Biochem. 57, 238–244 (2012). [DOI] [PubMed] [Google Scholar]
- Niu S., Zhang P., Liu J., Guo D. & Zhang X. The effect of temperature on the survival, growth, photosynthesis, and respiration of young seedlings of eelgrass Zostera marina L. Aquaculture 350–353, 98–108 (2012). [Google Scholar]
- Santamaría L. & Vierssen W. Photosynthetic temperature responses of fresh- and brackish-water macrophytes: a review. Aquat. Bot. 58, 135–150 (1997). [Google Scholar]
- Semesi S., Beer S. & Björk M. Seagrass photosynthesis controls rates of calcification and photosynthesis of calcareous macroalgae in a tropical seagrass meadow. Mar. Ecol. Prog. Ser. 382, 41–47 (2009). [Google Scholar]
- Hendriks I. E. et al. Photosynthetic activity buffers ocean acidification in seagrass meadows. Biogeosciences 11, 333–346 (2014). [Google Scholar]
- Caeiro S. et al. Ecological risk assessment of sediment management areas: application to Sado Estuary, Portugal. Ecotoxicology 18, 1165–75 (2009). [DOI] [PubMed] [Google Scholar]
- Cornwall C. E. & Hurd C. L. Experimental design in ocean acidification research: problems and solutions. ICES J. Mar. Sci. 72, fsv118 (2015). [Google Scholar]
- Silva J., Barrote I., Costa M. M., Albano S. & Santos R. Physiological responses of Zostera marina and Cymodocea nodosa to light-limitation stress. PLoS One 8, e81058 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarazin G., Michard G. & Prevot F. A rapid and accurate spectroscopic method for alkalinity measurements in sea water samples. Water Res. 33, 290–294 (1999). [Google Scholar]
- Dickson A. G., Sabine C. L. & Christian J. Guide to best practices for ocean CO2 measurements. (North Pacific Marine Science Organization, 2007). [Google Scholar]
- Mehrbach C., Culberson C. H., Hawley J. E. & Pytkowicz R. M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907 (1973). [Google Scholar]
- Dickson A. G. & Millero F. J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A. Oceanogr. Res. Pap. 34, 1733–1743 (1987). [Google Scholar]
- Schreiber U., Schliwa U. & Bilger W. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62 (1986). [DOI] [PubMed] [Google Scholar]
- Serôdio J., Marques da Silva J. & Catarino F. Use of In vivo chlorophyll a fluorescence to quantify short-term variations in the productive biomass of intertidal microphytobenthos. Mar. Ecol. Prog. Ser. 218, 45–61 (2001). [Google Scholar]
- Duarte B., Santos D., Marques J. C. & Caçador I. Ecophysiological constraints of two invasive plant species under a saline gradient: Halophytes versus glycophytes. Estuar. Coast. Shelf Sci. 167, 154–165 (2015). [Google Scholar]
- Küpper H., Seibert S. & Parameswaran A. Fast, sensitive, and inexpensive alternative to analytical pigment HPLC: quantification of chlorophylls and carotenoids in crude extracts by fitting with Gauss peak spectra. Anal. Chem. 79, 7611–7627 (2007). [DOI] [PubMed] [Google Scholar]
- Adams W. & Demmig-Adams B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 186, 390–398 (1992). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.