Abstract
Purpose
Although adjuvant radiation to the tumor bed has been reported to improve the clinic outcomes of esthesioneuroblastoma (ENB) patients, the role of elective neck irradiation (ENI) in clinically node negative (N0) patients remains controversial. Here, we evaluated the effects of ENI on neck nodal relapse risk in ENB patients treated with radiotherapy as a component of multi-modality treatment.
Methods and Materials
Seventy-one N0 ENB patients irradiated at XXXXXXXXX between 1970 and 2013 were identified. ENI was performed on 22 of these patients (31%). Survival analysis was performed with focus on comparative outcomes of those patients who did and did not receive ENI.
Results
The median follow up time for our cohort is 80.8 months (range 6 – 350 month). Among N0 patients, 13 (18.3%) developed neck nodal relapses, with a median time to progression of 62.5 months. None of these 13 patients received prophylactic neck irradiation. ENI was associated with significantly improved regional nodal control at 5-year (regional control rate of 100% for ENI vs 82%, p < 0.001), but not overall survival or disease-free survival. Eleven patients without ENI developed isolated neck recurrences. All had further treatment for their neck disease, including neck dissection (n=10), radiation (n=10), or chemotherapy (n=5). Six of these 11 patients (54.5%) demonstrated no evidence of further recurrence with a median follow up of 55.5 month.
Conclusion
ENI significantly reduces the risk of cervical nodal recurrence in ENB patients with clinically N0 neck but this did not translate to a survival benefit. Multimodality treatment for isolated neck recurrence provides a reasonable salvage rate. The greatest benefit for ENI appeared to be among younger patients who presented with Kadish C disease. Further studies are needed to confirm these findings.
INTRODUCTION
Esthesioneuroblastoma (ENB) is a rare neuroectodermal malignancy that arises from the olfactory epithelium lining the roof of the nasal cavity.1 The rarity of this malignancy has hampered the formulation of a standard treatment regimen for ENB.2, 3 Surgical resection followed by local field radiation therapy is the main approach for local management of the tumor, with additional chemotherapy considered for advanced stage diseases. This multi-modality approach has resulted in improved clinical outcomes according to multiple retrospective series.4–6
There is a general consensus among practitioners that adjuvant radiation therapy provides a local tumor control benefit for ENB patients.7–9 However, the role of prophylactic neck irradiation to prevent regional nodal recurrence is less clear. In particular, for patients with clinically node negative disease, it is controversial whether elective neck irradiation (ENI) should be performed. The nodal recurrence rate of ENB approaches 30%,10 yet studies examining the addition prophylactic radiation to the neck have yielded conflicting results.11–13 While investigators from The University of Florida and other institutions have found ENI reduces the rate of neck failures,12 others failed to observe similar benefits, especially in patients who are also receiving systemic chemotherapy.13–15 These studies were limited by small patient numbers (in the 20 to 30 range) and none have examined the effect of salvage therapies in those who developed neck recurrences when upfront ENI was omitted.
Here, we present a large series of ENB patients treated with radiation therapy with or without neck irradiation. Our aim is to assess the role of ENI in providing regional control and whether omission of ENI is possible if successful salvage therapies are available.
METHODS AND MATERIALS
Patient data
As a part of an Institutional Review Board-approved study, a total of 79 patients with histologically confirmed diagnosis of ENB that were treated at the Department of Radiation Oncology at XXXXXXXXXXX from 1970 to 2013 were retrospectively identified. Eight patients with clinically node positive disease by examination or imaging were excluded from further analyses. The medical records of all these patients were then reviewed for clinical and pathologic characteristics, as well as chemotherapy, surgery and radiation therapy details.
Clinicopathological confirmation
All pathological specimens were evaluated by head and neck pathologists at the time of patient’s presentation to XXXXXXXX, with diagnosis confirmed by microscopic findings and immunohistochemical staining for synaptophysin, chromogranin, S100, among other markers. Disease staging was determined from medical record or imaging studies using both the American Joint Committee on Cancer (AJCC) stage and the modified Kadish system.
Statistical analysis
Chi-square or Fisher’s exact tests were used to compare all categorical variables and t-test was used to compare continuous variables in the study when appropriate. The Kaplan-Meier method was used to analyze overall survival (OS), progression free survival (DFS), locoregional control (LRC), and distant metastasis (DM). The log-rank test was used to compare survival curves between different groups. Time to event was defined as completing date of radiation treatment to the event of interest. Disease free survival was defined as the number of patients alive and without evidence of disease. Locoregional failure was defined as relapse within the primary site or neck lymph node. The impact of covariates on clinical outcomes was determined by multivariate Cox proportional hazard regression analysis. The proportional hazard assumption was tested graphically. All tests were 2-sided, and a p value of <0.05 was considered significant. All statistical analysis was performed using the IBM SPSS V22.0 software package.
RESULTS
Patient characteristics and treatment
Table 1 outlines the basic demographic and clinical data for the 71 N0 patients. Using the modified Kadish staging system, 4 (5.6%) patients had stage A, 15 (21.1%) had stage B, and 51 (71.8%) had stage C diseases. One (1.4%) patient had stage D disease due to distant metastasis without evidence of cervical lymph node involvement. A total of 22 (31%) patients received ENI, while 49 (69%) did not.
Table 1.
Clinical Characteristics of N0 ENB Patients (n=71)
| Characteristic | Value or Number of Patients (%) | ||||
|---|---|---|---|---|---|
| All (n=71) |
With ENI (n=22) |
Without ENI (n=49) |
P Value | ||
| Age | |||||
| Median | 50.4 | 45.5 | 51.0 | ||
| Range | 12.9–77.4 | 15.3–76.0 | 12.9–77.4 | ||
| Sex | 0.292 | ||||
| Male | 44 (62.0) | 16 (72.7) | 28 (57.1) | ||
| Female | 27 (38.0) | 6 (27.3) | 21 (42.9) | ||
| Race | 0.323 | ||||
| White | 58 (81.7) | 16 (72.7) | 42 (85.7) | ||
| African American | 3 (4.2) | 2 (9.1) | 1 (2.0) | ||
| Asian | 1 (1.4) | 0 (0) | 1 (2.0) | ||
| Hispanic | 9 (12.7) | 4 (18.2) | 5 (10.2) | ||
| Kadish Stage | 0.087 | ||||
| A/B | 19 (26.8) | 9 (40.9) | 10 (20.4) | ||
| C | 52 (73.2) | 13 (59.1) | 39 (79.6) | ||
| T Category | 0.552 | ||||
| T1 | 4 (5.6) | 1 (4.5) | 3 (6.1) | ||
| T2 | 5 (7.0) | 0 (0.0) | 5 (10.2) | ||
| T3 | 14 (19.7) | 5 (22.7) | 9 (18.4) | ||
| T4 | 58 (81.7) | 16 (72.7) | 32 (65.3) | ||
| Chemotherapy | 13 (18.3) | 6 (27.3) | 7 (14.3) | 0.203 | |
| Surgery | 65 (91.5) | 21 (95.5) | 44 (89.8) | >0.999 | |
Abbreviations: ENI, elective neck irradiation; DNI, definitive neck irradiation
Surgical resection of the primary tumor was performed in 65 patients (91.5%), and all 65 patients received post-operative radiation. The remaining 6 non-surgical patients were treated with definitive radiation (n=3) or chemoradiation (n=3). Eight patients who underwent surgery were treated with induction chemotherapy (all preoperatively), and 5 were treated with concurrent chemoradiation postoperatively. None of the patients with stage A or B received systemic therapies. No elective neck dissection was performed at the time of resection of primary tumor.
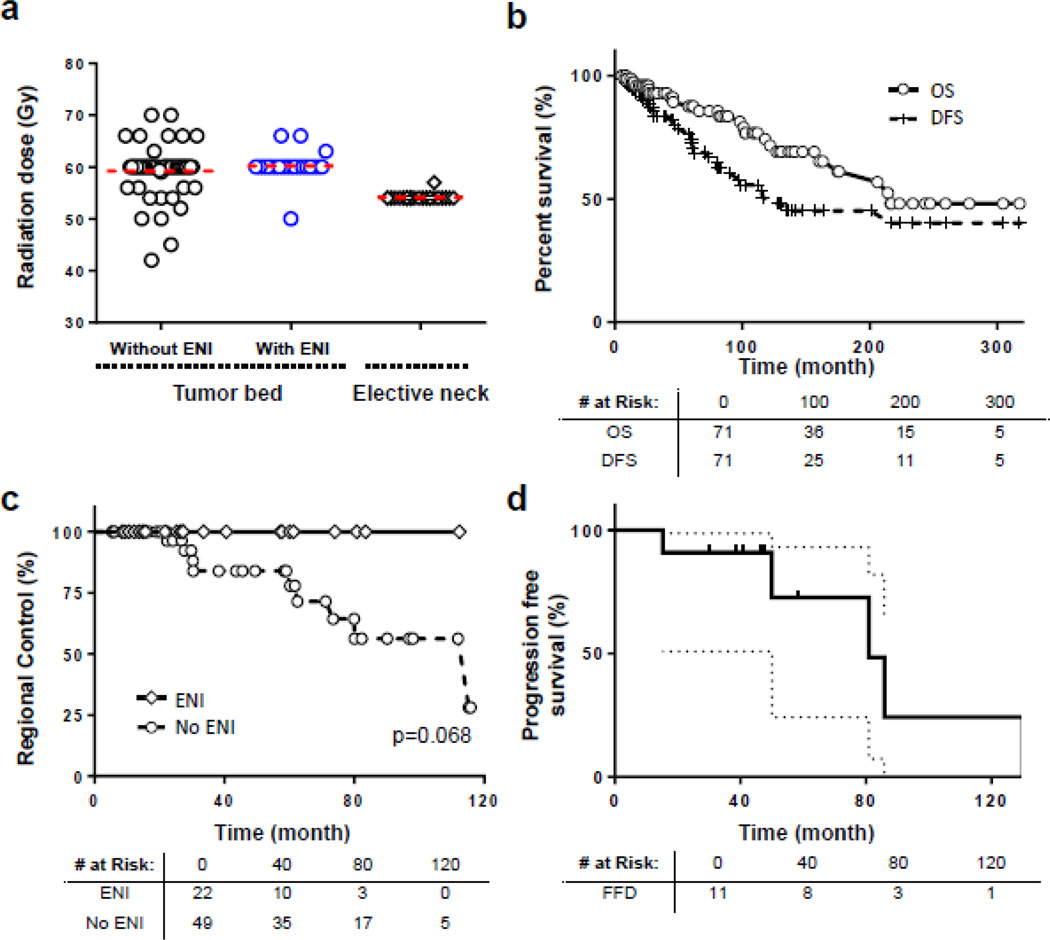
Among the 65 patients treated with adjuvant radiotherapy, 44 received radiation to the tumor bed alone (68%) and 21 patients (32%) received ENI. ENI typically included the retropharyngeal (RP), level IB and II nodes. Of these 21 ENI patients, 15 patients received bilateral ENI (71.4%), while 6 patients with lateralized lesions received ipsilateral ENI (28.5%). The mean radiation doses to the tumor bed and elective neck are shown in Figure 1a. There was no statistically significant differences among patients who received elective neck irradiation versus those who did not (Table 1) in terms of gender, race, tumor T stage, chemotherapy or surgery status. Of the 6 patients who received definitive radiation or chemoradiation, 1 patient received ENI (17%) and the rest received radiation to the primary tumor alone.
Figure 1.
Clinical outcomes
The median follow up time was 82.3 months (range 6–350 months) for all living patients. The median OS and DFS for the entire cohort were 216.0 and 128.1 months, respectively. The 5- and 10-years survival OS rates were 87.3% and 73.1%, and the 5- and 10-year DFS rate were 68.6% and 50.4%, respectively (Figure 1b). The 5- and 10-year locoregional control (LRC) rates for the entire cohort were 82.8% and 67.8%. The 5- and 10-years LRC rates were 100% and 100% for patients who received ENI compared to 77.9% and 62.6%, respectively for those who did not receive ENI. Among those with isolated regional neck recurrence in the absence of local failure, ENI resulted in an improved 5-year regional control rate of 100% versus 82.1% for patients who did not receive ENI (Figure 1c). Among the 7 non-ENI patients who received chemotherapy, 2 developed isolated neck recurrences (28.6%), which is similar to the nodal failure rate for rest of the non-ENI cohort.
On univariate analysis, older age was associated with a worse OS and DFS, and a trend towards worse OS was observed for patient receiving ENI (Table 2). On multivariate analysis, older age was the only statistically significant risk factor for both OS and DFS. After stratifying our patient cohort by median age, we found that ENI was associated with worse OS for patients older than 50 years (Table 3). The patient characteristics for patients older than 50 years are listed in Supplemental Table 1.
Table 2.
Analysis of Patient- and Treatment-Related Factors Affecting Outcomes
| Covariate | OS | DFS | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Univariate | ||||
| ENI | 0.065 | 0.311 | ||
| No | 1.00 | 1.00 | ||
| Yes | 2.98 (0.917–9.49) | 1.64 (0.63–4.27) | ||
| Age* | 1.10 (1.04–1.16) | <0.001 | 1.07 (1.03–1.12) | <0.001 |
| Chemotherapy | 0.726 | 0.334 | ||
| No | 1.00 | 1.00 | ||
| Yes | 0.89 (0.48–1.66) | 0.79 (0.48–1.23) | ||
| Kadish Stage* | 1.03 (0.37–2.84) | 0.954 | 1.34 (0.55–3.25) | 0.523 |
| Multivariate | ||||
| ENI | 0.343 | 0.945 | ||
| No | 1.00 | 1.00 | ||
| Yes | 1.29 (0.47–6.16) | 1.04 (0.35–3.13) | ||
| Age* | 1.09 (1.03–1.15) | 0.002 | 1.08 (1.03–1.12) | 0.001 |
| Chemotherapy | 0.597 | 0.837 | ||
| No | 1.00 | 1.00 | ||
| Yes | 1.15 (0.31–6.42) | 1.14 (0.34–3.86) | ||
| Kadish Stage* | 1.33 (0.49–3.61) | 0.583 | 1.31 (0.53–3.23) | 0.562 |
analyzed as continuous covariate with increment of 1
Abbreviations: ENI, elective neck irradiation; HR, hazard ratio; CI, confidence interval;
Table 3.
Multivariate Analysis of Patient- and Treatment-Related Factors Affecting Outcomes for patients ≥ 50
| Covariate | Overall Survival | Disease-Free Survival | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| ENI | 0.018 | 0.317 | ||
| No | 1.00 | 1.00 | ||
| Yes | 3.48 (1.37–8.59) | 1.90 (0.54–6.69) | ||
| Chemotherapy | 0.298 | 0.562 | ||
| No | 1.00 | 1.00 | ||
| Yes | 0.38 (0.06–2.38) | 0.56 (0.16–2.76) | ||
| Kadish Stage | 1.34 (0.52–3.49) | 0.548 | 1.12 (0.46–2.70) | 0.804 |
Patterns of failure
A total of 21 patients (29.6%) developed biopsy-proven recurrent disease. Of these, 3 patients (14.3%) had local failures, 13 patients (61.9%) had regional cervical lymph node failures, and 7 patients (25.9%) had DM. The median time to disease recurrence was 60.4 months (range 22.3 – 134.9 months). Twelve of these 13 patients had Kadish C disease. Among the 13 patients who developed regional failure, 11 had isolated nodal failures and 2 had both regional recurrences with DM. Two patients developed recurrences on both sides of the neck. Among patients with Kadish A/B disease, 1 patient developed neck recurrence who did not receive ENI, and no patient had neck failure with ENI. This corresponds to a neck failure rate of 5% among stage A/B patients. A detailed description of regional failure rate stratified by Kadish stages are shown in Supplemental Table S2.
Anatomically, level II lymph nodes were the most common site of neck failure, with 11 patients (84.6%) developing recurrences in this location. Level IB and RP nodal recurrences were observed in 3 patients (23.1%). Twelve of the 13 patients (92.3%) with regional failures had stage C disease. All 13 patients with regional failures did not receive ENI, which accounted for 26.4% (13 of 49) of patients in that treatment group. There were no nodal failures in the ENI group.
Salvage therapy
For patients who developed recurrent disease, salvage therapies included a combination of surgery, radiation and chemotherapy. For patients who developed distant metastasis, all patients received chemotherapy as part of their salvage treatments. Of the 11 patients who developed isolated regional nodal failure, 1 received a neck dissection only, 1 received neck radiation only, 5 received surgery and radiotherapy, and 4 received surgery and chemoradiation. With a median follow up time of 55.5 months, 6 of the 11 patients were free of disease. Details of the 5 patients that failed after salvage treatment are listed in Table 4. One patient developed DM, 2 developed recurrences within the paranasal sinuses or masticator space, and 2 developed additional nodal disease, albeit in sites (retropharyngeal and contralateral neck) not managed with surgery. This corresponds to a median time to progression of 80.9 months and a 5-year disease control rate of 72.7% (Figure 1d).
Table 4.
Detailed information for patients with neck recurrence
| Patients | Initial recurrence site |
Salvage treatments |
Salvage RT target | Failure location |
|---|---|---|---|---|
| 1 | Left level II, RP | ND, RT | BL neck | R maxillary sinus |
| 2 | Left level II, RP | ND, RT | BL neck | Lung, vertebral bodies |
| 3 | Left level II | ND | - | R RP node |
| 4 | Left level IB, BL level II | ND, RT | BL neck | R Infratemporal fossa |
| 5 | Left level IB | ND, CRT | L neck | R Level II node |
| 6 | R level II, RP | ND, RT | R neck | NED |
| 7 | L level II, III | ND, CRT | L neck | NED |
| 8 | L level II | ND, CRT | L neck | NED |
| 9 | L Level II | RT | L neck | NED |
| 10 | L level II | ND, RT | BL neck | NED |
| 11 | R level IB | ND, CRT | R neck | NED |
| 12 | R level II, dura | ND, craniotomy, chemotherapy | R neck, dura | Multiple dural locations |
| 13 | R level IB, dura, C1 spine | ND, craniotomy, RT, SRS, | R neck, spine, dura | NED |
Abbreviations: BL, bilateral; RP, retropharyngeal; ND, neck dissection; RT, radiation; CRT, chemoradiation, SRS, stereotactic radiosurgery, NED, no evidence of disease
DISCUSSION
Radiation is an important component of multimodality treatment to improve local control in patients with ENB; however, the role of ENI in patients with a clinically negative neck is controversial. Our current report demonstrates that for those whose neck was not irradiated, over 25% will develop recurrent disease in the neck. Further, our study showed that while ENI is effective, as none of the patients who received ENI developed neck recurrence, an impact on survival was not seen. In addition, subsequent recurrence in the neck after salvage therapies is uncommon, and has a fairly long latency, as the median time to neck failure was over 5 years.
It has been reported that in the absence of any therapy directed to the neck, patients with clinically negative necks can experience an overall nodal failure rate as high as 33%.13 This appears similar to our observed nodal failure rate of 27% in N0 patients that did not receive ENI. The limited number of reported cases on use of ENI to prevent nodal recurrence further complicates the picture.12–14, 16 A recently study published by Noh and co-workers, based on 4 patients treated with ENI or neck dissection, showed a 75% rate of cervical failure if no systemic therapies were given.13 Conversely, earlier studies by University of Florida showed that in 11 patients treated with ENI, none experienced nodal recurrence, whereas 4 of 9 patients who had ENI omitted developed neck failure.12 Other smaller retrospective studies have also reported conflicting findings after ENI, ranging from no locoregional benefit to significantly improved regional control in clinically N0 patients.14–16 Given the small number patients of each study and the significant amount of heterogeneity in patient and treatment characteristics, it is difficult to draw a definitive conclusion from the existing literature on the role of ENI in ENB.
Our data suggests a benefit of ENI in providing regional nodal control in clinically N0 patients. In the 22 patients who received ENI, the 5-year regional control rate was 100% compared to 82% in patients who did not receive ENI. One study has suggested that systemic therapy may provide adequate nodal control in lieu of ENI.13 We observed that ENI appears to improve regional control independent of systemic therapy use.
In terms of patient selection criteria, some have suggested that ENI is warranted in Kadish stage C or D patients and can be omitted in those with earlier stage of disease or receiving systemic therapy.10, 12, 17, 18 We observed that only 7.7% of the non-ENI patients who developed neck recurrence had stage A/B disease. This corresponds to a neck failure rate of 10% among stage A/B patients compared to 31% of Kadish C patients who did not have ENI. Studies by University of Michigan and the University of Florida also demonstrated that Kadish C patients without elective neck treatment had nodal recurrence rate of 20% and 44%, respectively.11, 12 Our study demonstrated a similar regional failure rate that are consistent with these studies but with a much larger patient population. Similar to the University of Florida experience, we also demonstrated that majority of the nodal failures occurred in Kadish C patients (92.3% in our series versus 100% for University of Florida). In addition, we had similar proportion of Kadish C patients (73.2 % versus 68.2% for University of Florida) and none of these patients developed neck recurrence after ENI.12 However, despite the fact that nearly one-third of patients with Kadish C disease may recur in the neck if unirradiated, the relatively long latency period, and the apparent efficacy of salvage therapy makes one question that while ENI is effective in controlling neck disease, whether it has an ultimate benefit. Among those patients who did not get ENI, the disease control in the neck was over 80% after 5-years, and the distant recurrence rate was 6%. In the 11 patients that underwent salvage treatment for an isolated neck recurrence, the DFS rate was 72.7% at 5-years. This includes 2 patients who only had neck dissection or ipsilateral radiation as part of their salvage treatment for the neck (Table 4). Our results are consistent with other studies, albeit small and retrospective in nature, that have suggested an overall salvage rate after regional failure approaching 50–60%.13, 19
Our series adds a significant number of patients to the existing literature regarding the role of ENI in clinically node-negative ENB patients, and support a potential role for ENI to reduce regional recurrence rate in these patients. Although the finding presented here comes from a single institutional retrospective series, and is subjected to inherent weaknesses such as small absolute patient number and potential selection bias, it represents the largest homogeneous cohort to date for this particular rare disease. With a median follow up time of 82.3 months, patients with ENB can experience disease recurrence even 10-years after completion of their initial treatment.4, 20–22 As such, while we observed a trend towards improved neck control with ENI, this difference may become significant with longer follow-up. Unfortunately, overcoming the aforementioned limitations is difficult given the rarity and natural history of ENB. In addition, even with CT and PET imaging, in the absence of neck surgery, the rate of occult nodal metastases in clinically N0 patients is unknown. For patients over the age of 50, ENI may be associated with a worse OS. One possible explanation is that in older patients, the long-term benefit of improved neck control may be offset by the potential toxicities associated with ENI. Treatment related toxicity was not analyzed in this study and thus its influence on non-cancer related deaths is unknown. Also, patients receiving ENI may have more adverse risk factors that were not adjusted for in our study among those 50 years or older, including Hyam’s histological grade or chromosomal re-arrangements that are associated with increased aggressiveness and worse prognosis,23 In addition, radiation delivery techniques have drastically changed in early-mid 2000s when IMRT was widely implemented in the use of head and neck radiotherapy. Thus, how the adaptation of IMRT compared to conventional 3D radiation on clinical outcomes would be of interest to explore in ENB patients.
CONCLUSION
In summary, our study provides a large cohort of patients with ENB treated at a single institution and confirms that ENB has a high cervical nodal recurrence rate even in the setting of clinically negative neck. ENI can result in improved regional control but this did not translate into a survival benefit, possibly due to the long latency of nodal metastasis and the availability of effective salvage options. The greatest benefit for ENI was in younger patients who presented with Kadish C disease. Larger patient numbers and longer follow up are needed to confirm these findings.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interests
Reference
- 1.Iezzoni JC, Mills SE. “Undifferentiated” small round cell tumors of the sinonasal tract: differential diagnosis update. Am J Pathol. 2005;124:S110–S121. doi: 10.1309/59RBT2RK6LQE4YHB. [DOI] [PubMed] [Google Scholar]
- 2.Morita A, Ebersold MJ, Olsen KD, et al. Esthesioneuroblastoma: Prognosis and management. Neurosurgery. 1993;32:706–715. doi: 10.1227/00006123-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Olsen KD, DeSanto LW. Olfactory neuroblastoma. Biological and clinical behavior. Arch Otolaryngol. 1983;109:797–802. doi: 10.1001/archotol.1983.00800260019005. [DOI] [PubMed] [Google Scholar]
- 4.Dulguerov P, Allal AS, Calcaterra TC. Esthesioneuroblastoma: a meta-analysis and review. Lancet Oncol. 2001;2:683–690. doi: 10.1016/S1470-2045(01)00558-7. [DOI] [PubMed] [Google Scholar]
- 5.Foote RL, Morita A, Ebersold MJ, et al. Esthesioneuroblastoma: the role of adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 1993;27:835–842. doi: 10.1016/0360-3016(93)90457-7. [DOI] [PubMed] [Google Scholar]
- 6.Platek ME, Mezianu M, Mashtare TL, et al. Improved survival following surgery and radiation therapy for olfactory neuroblastoma: analysis of the SEER database. Radiat Oncol. 2001;6:41. doi: 10.1186/1748-717X-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz EM, Johnigan RH, 3rd, Pero C, et al. Olfactory neuroblastoma: The 22-year experience at one comprehensive cancer center. Head Neck. 2005;27:138–149. doi: 10.1002/hed.20127. [DOI] [PubMed] [Google Scholar]
- 8.Chao KS, Kaplan C, Simpson JR, et al. Esthesioneuroblastoma: The impact of treatment modality. Head Neck. 2001;23:749–757. doi: 10.1002/hed.1107. [DOI] [PubMed] [Google Scholar]
- 9.Ow TJ, Hanna EY, Roberts DB, et al. Optimization of long-term outcomes for patients with esthesioneuroblastoma. Head Neck. 2014;36:524–530. doi: 10.1002/hed.23327. [DOI] [PubMed] [Google Scholar]
- 10.Beitler JJ, Fass DE, Brenner HA, et al. Esthesioneuroblastoma: Is there a role for elective neck treatment? Head Neck. 1991;13:321–326. doi: 10.1002/hed.2880130409. [DOI] [PubMed] [Google Scholar]
- 11.Demiroz C, Gutfeld O, Aboziada M, et al. Esthesioneuroblastoma: Is there a need for elective neck treatment? Int J Radiat Oncol Biol Phys. 2011;81:e255–e261. doi: 10.1016/j.ijrobp.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Monroe AT, Hinerman RW, Amdur RJ, et al. Radiation therapy for esthesioneuroblastoma: Rationale for elective neck irradiation. Head Neck. 2003;25:529–534. doi: 10.1002/hed.10247. [DOI] [PubMed] [Google Scholar]
- 13.Noh OK, Lee S, Yoon SM, et al. Radiotherapy for Esthesioneuroblastoma: Is Elective Nodal Irradiation Warranted in the Multimodality Treatment Approach? Int J Radia Oncol Biol Phys. 2011;79:443–449. doi: 10.1016/j.ijrobp.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 14.Resto VA, Chan AW, Deschler DG, et al. Extent of surgery in the management of locally advanced sinonasal malignancies. Head Neck. 2008;30:222–229. doi: 10.1002/hed.20681. [DOI] [PubMed] [Google Scholar]
- 15.Zafereo ME, Fakhri S, Prayson R, et al. Esthesioenuroblastoma: 25-year experience at a single institution. Otolaryngol Head Neck Surg. 2008;138:452–458. doi: 10.1016/j.otohns.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 16.Koka VN, Julieron M, Bourhis J, et al. Esthesioneuroblastoma. J Laryngol Otol. 1998;112:628–633. doi: 10.1017/s0022215100141295. [DOI] [PubMed] [Google Scholar]
- 17.Davis RE, Weissler MC. Esthesioneuroblastoma and neck metastasis. Head Neck. 1992;14:477–482. doi: 10.1002/hed.2880140610. [DOI] [PubMed] [Google Scholar]
- 18.Rinaldo A, Ferlito A, Shaha AR, et al. Esthesioneuroblastoma and cervical lymph node metastases: Clinical and therapeutic implications. Acta Otolaryngol. 2002;122:215–221. doi: 10.1080/00016480252814261. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Cho HJ, Kim KS, et al. Results of salvage therapy after failure of initial treatment for advanced olfactory neuroblastoma. J Craniomaxillofac Surg. 2008;36:47–52. doi: 10.1016/j.jcms.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Levine PA, Gallagher R, Cantrell RW. Esthesioneuroblastoma: Reflection of a 21-year experience. Laryngoscope. 1999;109:1539–1543. doi: 10.1097/00005537-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Theilgaard SA, Buchwald C, Ingeholm P, et al. Esthesioneuroblastoma: A Danish demographic study of 40 patients registered between 1978 and 2000. Acta Otolaryngol. 2003;123:433–439. doi: 10.1080/00016480310001295. [DOI] [PubMed] [Google Scholar]
- 22.Loy AH, Reibel JF, Read PW, et al. Esthesioneuroblastoma: Continued follow-up of a single institution’s experience. Arch Otolaryngol Head Neck Surg. 2006;132:134–138. doi: 10.1001/archotol.132.2.134. [DOI] [PubMed] [Google Scholar]
- 23.Bockmuhl U, You X, Pacyna-Gengelback M, et al. CGH Pattern of Esthesioneuroblastoma and their Metastases. Brain Pathology. 2004;14:158–163. doi: 10.1111/j.1750-3639.2004.tb00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.