Abstract
Background
Therapy with evidence-based heart failure (HF) medications has been shown to be associated with lower risk of 30-day all-cause readmission in patients with HF and reduced ejection fraction (HFrEF).
Methods
We examined the association of aldosterone antagonist use with 30-day all-cause readmission in this population. Of the 2443 Medicare beneficiaries with HF and left ventricular EF ≤35% discharged home from 106 Alabama hospitals during 1998–2001, 2060 were eligible for spironolactone therapy (serum creatinine ≤2.5 for men and ≤2 mg/dl for women, and serum potassium <5 mEq/L). After excluding 186 patients already receiving spironolactone on admission, the inception cohort consisted of 1874 patients eligible for a new discharge prescription for spironolactone, of which 329 received one. Using propensity scores for initiation of spironolactone therapy, we assembled a matched cohort of 324 pairs of patients receiving and not receiving spironolactone balanced on 34 baseline characteristics (mean age 72 years, 42% women, 33% African American).
Results
30-day all-cause readmission occurred in 17% and 19% of matched patients receiving and not receiving spironolactone, respectively (hazard ratio {HR}, 0.92; 95% confidence interval {CI}, 0 64 1.32; p=0.650). Spironolactone had no association with 30-day all-cause mortality (HR, 0.84; 95% CI, 0.38–1.88; p=0.678) or HF readmission (HR, 0.74; 95% CI, 0.41 1.31; p=0.301). These associations remained unchanged during 12 months of post-discharge follow-up.
Conclusion
A discharge prescription for spironolactone had no association with 30-day all-cause readmission among older, hospitalized Medicare beneficiaries with HFrEF eligible for spironolactone therapy.
Keywords: spironolactone, 30-day all-cause readmission, Medicare beneficiaries, heart failure
1. Introduction
HF is a major public health problem and is the leading cause of 30-day all-cause readmission, an outcome which has been identified by the Affordable Care Act as a potentially preventable reason for high Medicare cost and a target for reduction [1–3]. Transitions of care-type interventions have not been successful in consistently lowering the rate of 30-day all-cause readmission [4]. Therapy with a number of evidence-based HF drugs has been shown to be associated with a lower 30-day all-cause readmission in eligible HF patients with reduced left ventricular ejection fraction (EF) [5–7]. However, little is known about the role of aldosterone antagonists on 30-day all-cause readmission in this population. We examined the association of discharge prescription of spironolactone with 30-day all-cause readmission and other outcomes in hospitalized patients with HF and reduced EF (HFrEF).
2. Materials and Methods
2.1 Source of data
We used data from the Alabama HF Project, which has been previously described [8–10]. Briefly, charts of 9649 fee-for-service Medicare beneficiaries hospitalized for acute HF from 106 Alabama hospitals between July 1, 1998 and October 31, 2001 were abstracted. All patients had a principal discharge diagnosis of HF based on International Classification of Diseases 9 coding. The 9649 hospitalizations occurred in 8555 unique Medicare beneficiaries, of which 8049 were discharged alive.
2.2 History of HF and Spironolactone Use
We excluded 27 patients receiving potassium-sparing diuretics either on admission or discharge other than aldosterone antagonists. We then created a cohort of 2060 patients with HF and EF ≤35%, serum creatinine ≤2 5 mg/dL for men and ≤2 mg/dL for women, and serum potassium <5 mEq/L, who were eligible for therapy with aldosterone antagonists based on the 2013 American College of Cardiology/American Heart Association HF guidelines criteria [11]. To minimize bias due to prevalent drug use, we excluded 186 patients who were receiving spironolactone on admission [12]. Of the final cohort of 1874 patients eligible for a new prescription for aldosterone antagonist, 329 received a discharge prescription. None of the patients were receiving eplerenone as the drug was not approved for use in HF during the study period.
2.3 Assembly of a balanced cohort
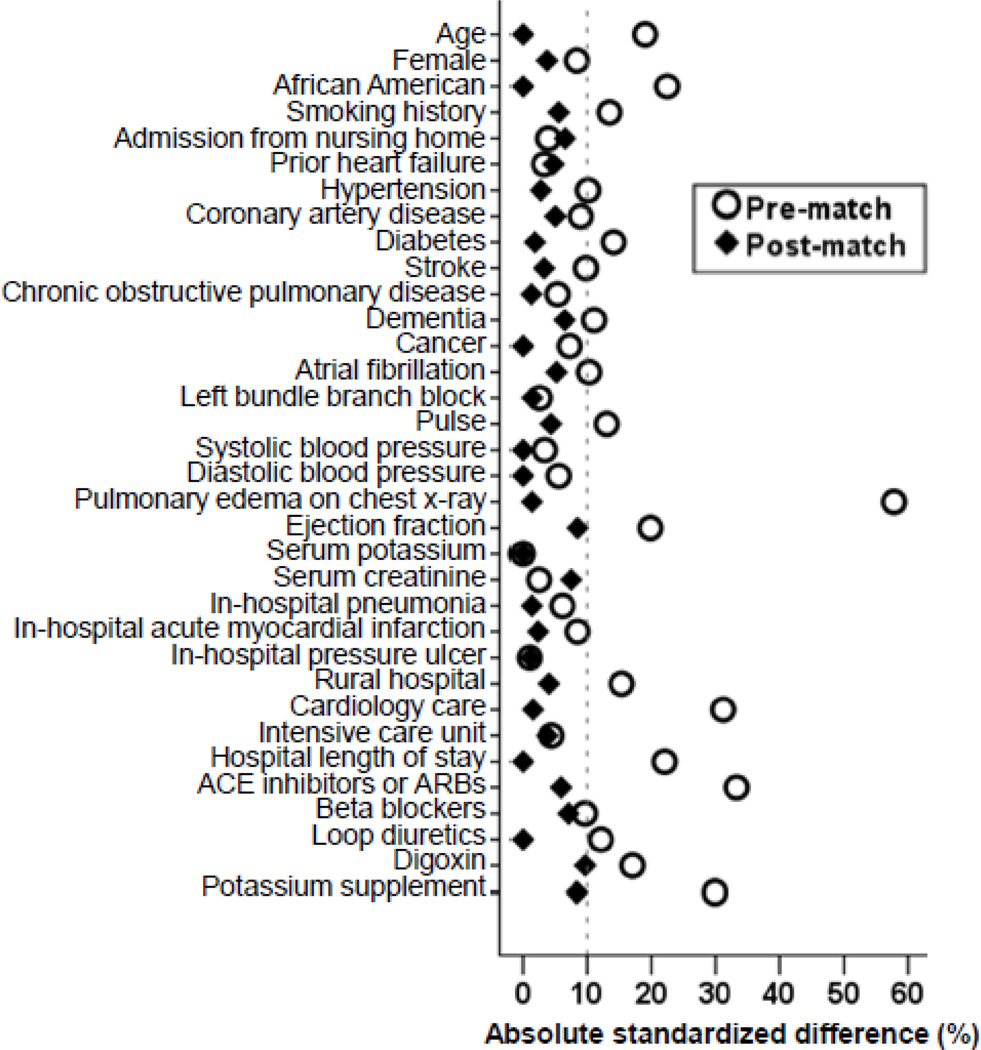
Because patients receiving and not receiving a therapy in the real world often have differences in baseline characteristics that may be of prognostic consequences, we used propensity scores to assemble a matched balanced cohort [13–17]. We used a multivariable logistic regression model to estimate propensity scores for the receipt of a discharge prescription for spironolactone for each of the 1874 patients using 34 baseline characteristics displayed in Figure 1 [10, 18–20]]. We then matched 324 patients receiving spironolactone with 324 patients who did not receive this drug but had similar propensity to receive it [21]. The resultant matched cohort of 648 patients was balanced on all 34 baseline characteristics. Between-group balance in baseline characteristics before and after matching were assessed by absolute standardized differences and the results were presented as a Love plot [22]. An absolute standardized difference of 0% would indicate no residual bias and a difference of <10% indicates inconsequential bias.
Fig. 1.
Love plot displaying absolute standardized differences for 34 baseline characteristics of hospitalized patients with heart failure and reduced ejection fraction receiving and not receiving spironolactone, before and after propensity score matching (ACE=angiotensin-converting enzyme; ARB=angiotensin receptor blockers)
2.4 Study outcomes
The primary outcome of the current analysis was 30-day all-cause readmission. Secondary outcomes included 30-day all-cause mortality, 30-day HF readmissions, and a combined end-point of all-cause mortality or all-cause readmission at 30 days. We also examined these outcomes at 12 months. Data on outcomes and time to events were obtained from the Centers for Medicare and Medicaid Services Medicare data [8].
2.5 Statistical analysis
Number (%) and mean (standard deviation) for categorical and continuous variables, respectively, for baseline characteristics were compared using Pearson’s Chi-square and Wilcoxon rank-sum tests. Cox regression models were used to examine associations of spironolactone use and outcomes among matched patients. Kaplan-Meier survival analysis was used to generate plots for 30-day all-cause readmissions by spironolactone use among patients. We also examined the association of spironolactone use with the primary outcome among the 1874 pre-match patients using Cox regression models, separately adjusting for propensity scores and the 34 variables used to estimate propensity scores. All statistical tests were two-tailed with a p-value <0.05 considered significant. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp. was used for data analyses.
3. Results
3.1 Baseline characteristics
After matching, patients (n=648) had a mean age of 72 years, 42% percent were women and 33% were African American. Before matching, patients receiving spironolactone were generally younger, and more likely to be African American. They were also more likely to have a lower ejection fraction and be discharged on angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs) (Table 1). These and other imbalances were balanced in the matched cohort (Table 1 and Figure 1).
Table 1.
Baseline patient characteristics of hospitalized patients with heart failure and reduced ejection fraction, prescription of spironolactone, before and after propensity score matching by discharge
| n (%) or mean (±SD) | Before propensity score matching Spironolactone on discharge |
After propensity score matching Spironolactone on discharge |
||||
|---|---|---|---|---|---|---|
| No (n=1545) | Yes (n=329) | P value | No (n=324) | Yes (n=324) | P value | |
| Age (years) | 74 (±10) | 72 (±11) | <0.001 | 72.3 (±11) | 71.9 (±11) | 0.644 |
| Female | 698 (45%) | 135 (41%) | 0.170 | 140 (43%) | 134 (41%) | 0.633 |
| African American | 361 (23%) | 110 (33%) | <0.001 | 106 (33%) | 106 (33%) | 1.000 |
| Smoking history | 231 (15%) | 66 (20%) | 0.021 | 57 (18%) | 64 (20%) | 0.480 |
| Admission from nursing home | 58 (4%) | 10 (3%) | 0.529 | 14 (4%) | 10 (3%) | 0.405 |
| Past medical history | ||||||
| Prior heart failure | 1131 (73%) | 236 (72%) | 0.585 | 226 (70%) | 233 (72%) | 0.545 |
| Hypertension | 1046 (68%) | 238 (72%) | 0.100 | 239 (74%) | 235 (73%) | 0.723 |
| Coronary artery disease | 997 (65%) | 198 (60%) | 0.136 | 188 (58%) | 196 (61%) | 0.522 |
| Diabetes mellitus | 620 (40%) | 155 (47%) | 0.020 | 154 (48%) | 151 (47%) | 0.813 |
| Stroke | 302 (20%) | 52 (16%) | 0.115 | 56 (17%) | 52 (16%) | 0.673 |
| Chronic obstructive pulmonary disease | 556 (36%) | 110 (33%) | 0.380 | 108 (33%) | 110 (34%) | 0.868 |
| Dementia | 122 (8%) | 17 (5%) | 0.086 | 22 (7%) | 17 (5%) | 0.409 |
| Cancer | 41 (3%) | 13 (4%) | 0.201 | 12 (4%) | 12 (4%) | 1.000 |
| Clinical findings | ||||||
| Pulse (beats per minute) | 93 (±23) | 96 (±23) | 0.045 | 97 (±23) | 96 (±23) | 0.766 |
| Systolic blood pressure (mm Hg) | 144 (±30) | 145 (±29) | 0.690 | 146 (±28.5) | 146 (±29.3) | 0.925 |
| Diastolic blood pressure (mm Hg) | 81 (±18) | 82 (±18) | 0.303 | 82.1 (±18) | 82.3 (±18) | 0.931 |
| Pulmonary edema by chest x-ray | 1103 (71%) | 241 (73%) | 0.496 | 240 (74%) | 238 (74%) | 0.858 |
| Atrial fibrillation by electrocardiogram | 421 (27%) | 75 (23%) | 0.096 | 68 (21%) | 75 (23%) | 0.507 |
| Left bundle branch block | 374 (24%) | 76 (23%) | 0.670 | 77 (24%) | 75 (23%) | 0.853 |
| Ejection fraction | 25.4 (±7.0) | 24.0 (±7.2) | 0.001 | 24.1 (±6.8) | 24.7 (±7.3) | 0.288 |
| Tests and procedures | ||||||
| Serum potassium (mEq/L) | 4.19 (±0.6) | 4.18 (±0.6) | 0.923 | 4.2 (±0.55) | 4.2 (±0.64) | 0.874 |
| Serum creatinine (mEq/L) | 1.30 (±0.4) | 1.29 (±0.4) | 0.488 | 1.26 (±0.4) | 1.29 (±0.4) | 0.311 |
| In-hospital events | ||||||
| Pneumonia | 381 (25%) | 90 (27%) | 0.306 | 85 (26%) | 87 (27%) | 0.859 |
| Acute myocardial infarction | 85 (6%) | 25 (8%) | 0.142 | 25 (8%) | 23 (7%) | 0.764 |
| Pressure ulcer | 117 (8%) | 24 (7%) | 0.862 | 23 (7%) | 24 (7%) | 0.880 |
| Hospital and care characteristics | ||||||
| Rural hospital | 384 (25%) | 61 (19%) | 0.015 | 55 (17%) | 60 (19%) | 0.607 |
| Cardiology care | 1035 (67%) | 265 (81%) | <0.001 | 262 (81%) | 260 (80%) | 0.843 |
| Intensive care unit | 74 (5%) | 19 (6%) | 0.455 | 22 (7%) | 19 (6%) | 0.628 |
| Length of stay (days) | 6.6 (±4) | 7.6 (±5) | <0.001 | 7.5 (±5.1) | 7.5 (±4.8) | 0.994 |
| Discharge medications | ||||||
| ACE inhibitors and angiotensin receptor blockers |
1106 (71%) | 280 (85%) | <0.001 | 269 (83%) | 276 (85%) | 0.452 |
| Beta blockers | 511 (33%) | 124 (38%) | 0.108 | 111 (34%) | 122 (38%) | 0.368 |
| Loop diuretics | 1362 (88%) | 302 (92%) | 0.058 | 297 (92%) | 297 (92%) | 1.000 |
| Digoxin | 916 (59%) | 222 (68%) | 0.006 | 202 (62%) | 217 (67%) | 0.218 |
| Potassium supplements | 795 (52%) | 121 (37%) | <0.001 | 108 (33%) | 121 (37%) | 0.285 |
ACE = angiotensin converting enzyme
3.2 Spironolactone use and outcomes
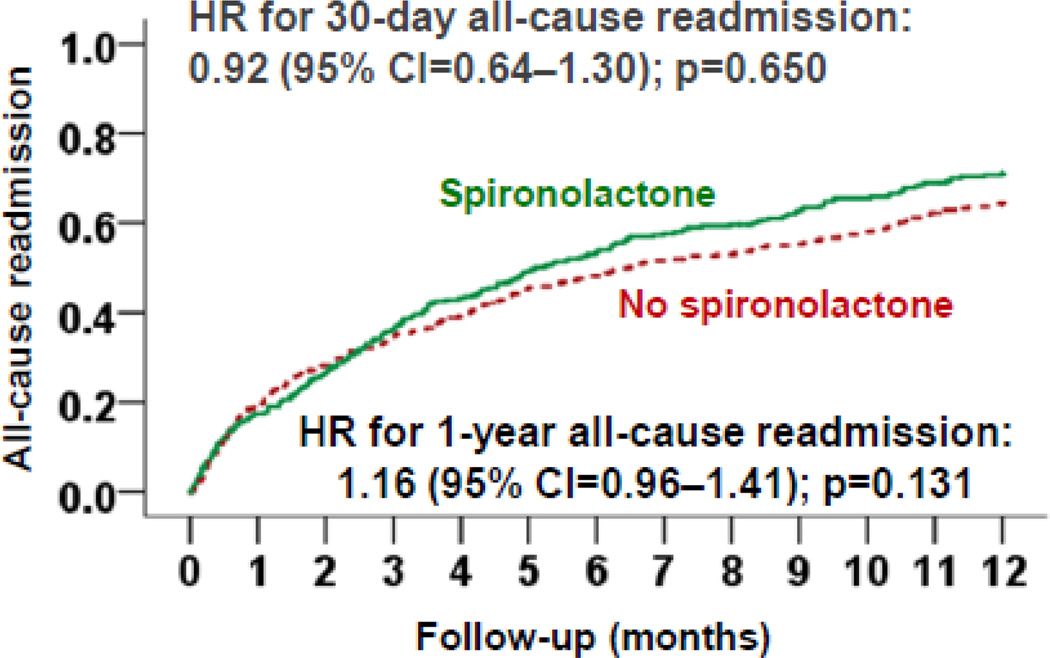
Among matched patients, 30-day all-cause readmission occurred in 17% and 19% of those receiving and receiving a discharge prescription for spironolactone, respectively (hazard ratio {HR}, 0.92; 95% confidence interval {CI}, 0.64–1.30; p=0.650; Table 2 and Figure 2). Among pre-match patients, propensity score-adjusted and multivariable-adjusted HRs associated with spironolactone use for 30-day all-cause readmission were 0.98 (95% CI, 0.73–1.32; p=0.913) and 0.94 (95% CI, 0.70–1.27; p=0.699), respectively.
Table 2.
Associations of discharge prescription for spironolactone with outcomes at 30 days post-discharge in a propensity score matched cohort of hospitalized patients for heart failure and reduced ejection fraction
| (Events %) Spironolactone on discharge |
HR (95% CI); p-value | ||
|---|---|---|---|
| No (n=324) | Yes (n=324) | ||
| All-cause readmission | 18.8% | 17.3% | 0.92 (0.64–1.30); 0.650 |
| Heart failure readmission | 8.3% | 6.2% | 0.74 (0.41–1.31); 0.737 |
| All-cause mortality | 4.0% | 3.4% | 0.84 (0.38–1.88); 0.844 |
| All-cause mortality or readmission | 21.9% | 19.8% | 0.90 (0.64–1.27); 0.902 |
HR = hazard ratio; CI = confidence interval
Fig. 2.
Kaplan-Meier plots displaying association between pre-discharge initiation of spironolactone and all-cause readmission in a propensity-matched cohort of hospitalized Medicare beneficiaries with heart failure and reduced ejection fraction (CI=confidence interval)
Spironolactone use had no association with 30-day HF readmission, 30-day all-cause mortality, or the combined end point of 30-day all-cause readmission or 30-day all-cause mortality (Table 2). Similarly, spironolactone had no association with any outcomes at 12 months (Table 3 and Figure 2).
Table 3.
Associations of discharge prescription for spironolactone with outcomes at 12 months post-discharge in a propensity score matched cohort of hospitalized patients for heart failure and reduced ejection fraction
| (Events %) Spironolactone on discharge |
HR (95% CI); p-value | ||
|---|---|---|---|
| No (n=324) | Yes (n=324) | ||
| All-cause readmission | 61% | 68% | 1.16 (0.96–1.41); 0.131 |
| Heart failure readmission | 31% | 29% | 0.92 (0.70–1.22); 0.575 |
| All-cause mortality | 26.2% | 29.0% | 1.11 (0.83–1.49); 0.483 |
| All-cause mortality or readmission | 68.8% | 74.7% | 1.13 (0.94–1.36); 0.181 |
HR = hazard ratio; CI = confidence interval
4. Discussion
Findings from our current study demonstrate that a discharge prescription of spironolactone had no association with all-cause readmission, HF readmission, or all-cause mortality during 30 days following hospital discharge among older patients with HFrEF who were eligible for spironolactone therapy. Furthermore, spironolactone use had no association with these outcomes at 12 months. These findings are consistent with data suggesting a lack of clinical effectiveness of spironolactone in real world eligible patients despite robust evidence of efficacy of aldosterone antagonists from previous major randomized controlled trials [23–28]. Taken together, these findings suggest potential efficacy-effectiveness dissociation for outcomes associated with the use of these drugs. To the best of our knowledge, the current study extends this efficacy-effectiveness dissociation to 30-day all-cause readmission, an important contemporary outcome measure identified for Medicare cost reduction under the Affordable Care Act [29].
The efficacy-effectiveness dissociation of aldosterone antagonists for long-term clinical outcomes is intriguing and has been attributed to a lack of adherence and stringent follow-up and patient selection bias including disease severity and comorbidity burden [23, 24]. These reasons may also in part explain the efficacy-effectiveness dissociation of spironolactone for short-term outcomes including 30-day all-cause readmission. However, the impact of lack of adherence is unlikely to be a major factor during short-term follow-up [30, 31]. The impact of lack of stringent laboratory follow-up, on the other hand, may be more substantial during short-term follow-up. Hyperkalemia and worsening kidney function are the two most common adverse effects of aldosterone antagonists and the guidelines recommend closer laboratory monitoring of these values [32]. In the Randomized Aldactone Evaluation Study (RALES) trial, worsening kidney function was more common in the spironolactone group, which mostly occurred during the first 30 days, and the kidney function plateaued thereafter [33]. The incidence of hyperkalemia was significantly higher in the spironolactone group compared to placebo, and the risk was higher among those with worsening kidney function [33]. Impaired kidney function is associated with poor outcomes in HF and spironolactone therapy may further worsen outcome in these patients [34, 35]. Although patients in our study had normal kidney function, a lack of stringent monitoring may have cancelled out a potential clinical benefit during the first 30 days. Imbalances in baseline disease severity and comorbidity burden between the treatment groups may have played a lesser role in explaining the lack of association of spironolactone with short-term outcomes in our study as all key measured baseline characteristics were balanced after propensity score matching. Further, even before matching, there were no significant differences in the prevalence of comorbidities except diabetes and in hospital events.
To the best of our knowledge this is the first study to examine the association of spironolactone use with 30-day all-cause readmission in real world patients with HFrEF who were eligible for therapy with this drug. Findings from the current study suggest that spironolactone may not be beneficial for the purpose of lowering 30-day all-cause readmission among patients who are otherwise eligible for this drug. Prior studies of associations of HF pharmacotherapy and 30-day all-cause readmission have demonstrated that while ACE inhibitors or ARBs and digoxin are effective in lowering this outcome, beta-blockers were not, suggesting all evidence-based HF drugs may not be beneficial for 30-day all-cause readmission [5, 7, 36]. Future studies need to examine the underlying reasons for this effectiveness-efficacy dissociation and if a more careful patient selection and a more stringent follow-up may result in better short-term outcomes in patients receiving aldosterone antagonists.
Our study has several limitations. Despite our use of rigorous methodology with propensity score-matched inception cohort design, potential bias due to unmeasured confounders and residual bias from measured confounders is possible. A formal sensitivity analysis may estimate if the observed association could be explained away by a potential unmeasured confounder [37]. However, we did not perform sensitivity analysis due to the null association with our primary outcome. We did not have data on post-discharge adherence, and regression dilution due to crossover of therapy during follow-up may in part explain the null association observed in our study [38]. However, adherence of spironolactone is as high as that of ACE inhibitors, [30, 31] the use of which has been shown to be associated with lower 30-day all-cause readmission [7]. Lack of data on dose of spironolactone, and follow-up data on serum creatinine and potassium are other limitations. Finally, findings of the current study are based on Medicare fee-for-service patients from a single state from 1999–2001 and may limit generalizability to more contemporary HF patients.
In conclusion, a discharge prescription for spironolactone had no association with 30-day all-cause readmission among older, hospitalized Medicare beneficiaries with heart failure and reduced ejection fraction who were eligible for spironolactone therapy. Taken together with lack of association with other 30-day or longer-term outcomes, these findings add to the growing body of literature that demonstrate a potential efficacy-effectiveness dissociation of spironolactone therapy. This highlights the need for further studies in a carefully selected real-world patient population with a stringent follow-up protocol comparable to those used in randomized controlled trials.
Acknowledgments
Funding/Support: Dr. Ali Ahmed was in part supported by the National Institutes of Health through grants (R01-HL085561, R01-HL085561-S and R01-HL097047) from the National Heart, Lung, and Blood Institute.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology [39].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. New England Journal of Medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Rau J. Hospitals Face Pressure to Avert Readmissions. [Accessed July 10, 2016];The New York Times. 2012 Nov 16; http://www.nytimes.com/2012/11/27/health/hospitals-face-pressure-from-medicare-to-avert-readmissions.html.
- 3.Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4–25. doi: 10.1002/ehf2.12005. [DOI] [PubMed] [Google Scholar]
- 4.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed A, Bourge RC, Fonarow GC, et al. Digoxin use and lower 30-day all-cause readmission for Medicare beneficiaries hospitalized for heart failure. Am J Med. 2014;127:61–70. doi: 10.1016/j.amjmed.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourge RC, Fleg JL, Fonarow GC, et al. Digoxin reduces 30-day all-cause hospital admission in older patients with chronic systolic heart failure. Am J Med. 2013;126:701–708. doi: 10.1016/j.amjmed.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanam K, Bhatia V, Bajaj NS, et al. Renin-Angiotensin System Inhibition and Lower 30-day All-cause Readmission in Medicare Beneficiaries with Heart Failure. Am J Med. 2016 doi: 10.1016/j.amjmed.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feller MA, Mujib M, Zhang Y, et al. Baseline characteristics, quality of care, and outcomes of younger and older Medicare beneficiaries hospitalized with heart failure: findings from the Alabama Heart Failure Project. Int J Cardiol. 2012;162:39–44. doi: 10.1016/j.ijcard.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed A, Fonarow GC, Zhang Y, et al. Renin-angiotensin inhibition in systolic heart failure and chronic kidney disease. Am J Med. 2012;125:399–410. doi: 10.1016/j.amjmed.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed A, Rich MW, Zile M, et al. Renin-angiotensin inhibition in diastolic heart failure and chronic kidney disease. Am J Med. 2013;126:150–161. doi: 10.1016/j.amjmed.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Danaei G, Tavakkoli M, Hernan MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol. 175:250–262. doi: 10.1093/aje/kwr301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenbaum PRRD. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 14.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 15.Ahmed MI, Ekundayo OJ, Mujib M, et al. Mild hyperkalemia and outcomes in chronic heart failure: a propensity matched study. Int J Cardiol. 2010;144:383–388. doi: 10.1016/j.ijcard.2009.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie C, Ekundayo OJ, Muchimba M, et al. Effects of diabetes mellitus in patients with heart failure and chronic kidney disease: a propensity-matched study of multimorbidity in chronic heart failure. Int J Cardiol. 2009;134:330–335. doi: 10.1016/j.ijcard.2008.12.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ekundayo OJ, Adamopoulos C, Ahmed MI, et al. Oral potassium supplement use and outcomes in chronic heart failure: a propensity-matched study. Int J Cardiol. 2010;141:167–174. doi: 10.1016/j.ijcard.2008.11.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adamopoulos C, Meyer P, Desai RV, et al. Absence of obesity paradox in patients with chronic heart failure and diabetes mellitus: a propensity-matched study. Eur J Heart Fail. 2011;13:200–206. doi: 10.1093/eurjhf/hfq159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel K, Fonarow GC, Kitzman DW, et al. Angiotensin receptor blockers and outcomes in real-world older patients with heart failure and preserved ejection fraction: a propensity-matched inception cohort clinical effectiveness study. Eur J Heart Fail. 2012;14:1179–1188. doi: 10.1093/eurjhf/hfs101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed MI, White M, Ekundayo OJ, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30:2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahle C, Adamopoulos C, Ekundayo OJ, Mujib M, Aronow WS, Ahmed A. A propensity-matched study of outcomes of chronic heart failure (HF) in younger and older adults. Arch Gerontol Geriatr. 2009;49:165–171. doi: 10.1016/j.archger.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernandez AF, Mi X, Hammill BG, et al. Associations between aldosterone antagonist therapy and risks of mortality and readmission among patients with heart failure and reduced ejection fraction. JAMA. 2012;308:2097–2107. doi: 10.1001/jama.2012.14795. [DOI] [PubMed] [Google Scholar]
- 24.Lund LH, Svennblad B, Melhus H, Hallberg P, Dahlstrom U, Edner M. Association of spironolactone use with all-cause mortality in heart failure: a propensity scored cohort study. Circ Heart Fail. 2013;6:174–183. doi: 10.1161/CIRCHEARTFAILURE.112.000115. [DOI] [PubMed] [Google Scholar]
- 25.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 26.Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 28.Cowie MR, Anker SD, Cleland JGF, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. ESC Heart Fail. 2014;1:110–145. doi: 10.1002/ehf2.12021. [DOI] [PubMed] [Google Scholar]
- 29.Konstam MA. Heart Failure in the Lifetime of Musca Domestica (The Common Housefly) JACC: Heart Failure. 2013;1:178–180. doi: 10.1016/j.jchf.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Butler J, Arbogast PG, Daugherty J, Jain MK, Ray WA, Griffin MR. Outpatient utilization of angiotensin-converting enzyme inhibitors among heart failure patients after hospital discharge. J Am Coll Cardiol. 2004;43:2036–2043. doi: 10.1016/j.jacc.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 31.Curtis LH, Mi X, Qualls LG, et al. Transitional adherence and persistence in the use of aldosterone antagonist therapy in patients with heart failure. Am Heart J. 2013;165:979–986. doi: 10.1016/j.ahj.2013.03.007. e1. [DOI] [PubMed] [Google Scholar]
- 32.Svensson M, Gustafsson F, Galatius S, Hildebrandt PR, Atar D. How prevalent is hyperkalemia and renal dysfunction during treatment with spironolactone in patients with congestive heart failure? J Card Fail. 2004;10:297–303. doi: 10.1016/j.cardfail.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 33.Vardeny O, Wu DH, Desai A, et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients With severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study) J Am Coll Cardiol. 2012;60:2082–2089. doi: 10.1016/j.jacc.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 34.Inampudi C, Parvataneni S, Morgan CJ, et al. Spironolactone use and higher hospital readmission for Medicare beneficiaries with heart failure, left ventricular ejection fraction <45%, and estimated glomerular filtration rate <45 ml/min/1.73 m(2.) Am J Cardiol. 2014;114:79–82. doi: 10.1016/j.amjcard.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schefold JC, Lainscak M, Hodoscek LM, Blöchlinger S, Doehner W, S vH. Single baseline serum creatinine measurements predict mortality in critically ill patients hospitalized for acute heart failure. ESC Heart Fail. ESC Heart Fail. 2015;2:122–128. doi: 10.1002/ehf2.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatia V, Bajaj NS, Sanam K, et al. Beta-blocker Use and 30-day All-cause Readmission in Medicare Beneficiaries with Systolic Heart Failure. Am J Med. 2015;128:715–721. doi: 10.1016/j.amjmed.2014.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenbaum PR. Sensitivity to hidden bias. In: Rosenbaum PR, editor. Observational Studies. Vol. 1. NewYork: Springer-Verlag; 2002. pp. 105–170. [Google Scholar]
- 38.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 39.Shewan LG, Coats AJ. Ethics in the authorship and publishing of scientific articles. Int J Cardiol. 2010;144:1–2. [Google Scholar]