Abstract
Background. Currently, mutations in rpoB, KatG, and rrs genes and inhA promoter were considered to be involved in conferring resistance to rifampicin, isoniazid, and streptomycin in Mycobacterium tuberculosis (MTB). Objective. The aims of this study were to detect the prevalence of first-line tuberculosis (TB) drug resistance among a group of previously treated and newly detected TB patients, to determine the association between prevalence of multidrug resistance (MDR) and demographic information (age and sex), to explain genes correlated with MDR Mycobacterium tuberculosis, and to characterize MTB via 16S ribosomal RNA (16S rRNA) analysis. Methods. A hundred MTB isolates from Sudanese pulmonary TB patients were included in the study. The proportional method of drug susceptibility test was carried out on Löwenstein-Jensen media. Multiplex PCR of rpoB and KatG genes and inhA promoter was conducted; then rrs genes were amplified by conventional PCR and were sequenced. The sequences of the PCR product were compared with known rrs gene sequences in the GenBank database by multiple sequence alignment tools. Result. The prevalence of MDR was 14.7% among old cases and 5.3% among newly diagnosed cases. Conclusion. Mutations in rrs could be considered as a diagnostic marker.
1. Introduction
Monitoring of tuberculosis (TB) caused by drug-resistant Mycobacterium tuberculosis (MTB) has become one of the major problems throughout the world [1]. However, the detection of drug-resistant phenotypes of MTB takes at least 3–6 weeks by direct and indirect methods, respectively. Thus, treatment was prescribed empirically. Patients who fail to respond to drugs remain infectious [2]. They may be a source of transmission of infections [3].
Sudan is surrounded by high burden countries [4] and it harbors a high TB incidence among the East Mediterranean countries. The prevalence of TB infection in Sudan is probably variable in different regions [5]. The country has been severely affected by war, famine, and flood in recent decades and has a large population of internally displaced persons, which is considered risk factor of spreading TB. Cases among men exceeded those found in women by a ratio of 2 : 1 [6].
Multidrug-resistant TB (MDR-TB) is defined as MTB that is resistant to first-line anti-TB drugs such as rifampicin (RIF) and isoniazid (INH) [7]. RIF is a broad spectrum antimicrobial agent, which remains the most effective drug against M. tuberculosis. Resistance of rifampicin occurs via mutation in rpoB gene that encodes the β-subunit of RNA polymerase [8]. INH has the most powerful bactericidal activity against TB and has good tolerance and low price [7]. It is a prodrug, requiring activation through oxidation by a mycobacterial catalase-peroxidase enzyme encoded by katG gene [9]. Activated isoniazid interferes with the biosynthesis of essential mycolic acids through inhibition of nicotinamide adenine dinucleotide hydrogen (NADH) dependent enoyl-acyl carrier protein reductase, which is encoded by inhA promoter. Alterations in katG gene and inhA promoter were strongly associated with isoniazid resistance [10, 11]. Streptomycin (SM) was the foremost antibiotic successfully used against TB. Resistance to SM emerged as a result of monotherapy administration [12]. SM is an aminocyclitol glycoside that acts against actively growing bacilli by inhibiting the initiation of translation in protein synthesis at the level of the 30S subunit of the ribosome, specifically at the ribosomal protein S12 and the 16S rRNA coded by rpsL and rrs genes, respectively [13, 14]. Consequently, mutations in rpsL and rrs are the major mechanisms of resistance [10]. The aims of the current study were to detect the prevalence of monoresistance and multidrug-resistant Mycobacterium tuberculosis (MDR), to determine the association between prevalence of MDR and demographic information (age and sex), to explain the molecular characterization of M. tuberculosis through 16S rRNA, and to illustrate the frequency of rrs mutations among streptomycin-resistant M. tuberculosis isolates.
2. Materials and Methods
This study was approved by the Ethics Committee of the Africa City of Technology and the University of Khartoum, Sudan.
2.1. Mycobacterium tuberculosis Growth Properties
A hundred sputum samples were collected from pulmonary TB patients as a cross-sectional descriptive study conducted at the National Reference Laboratory-Tuberculosis (NRL-TB), Khartoum, Sudan. Samples were processed under biosafety cabinet level II via adding twice the volume of 4% NaOH to sputum in 50 mL centrifuge tube for decontamination and homogenization. Samples were neutralized by buffer, followed by centrifugation at 3000 RCF (Relative Centrifuge Force) for 20 min. Inoculation was adjusted by pipetting 3–5 drops of deposit in three Löwenstein-Jensen media (two containing glycerol and one containing pyruvate) which are egg based media. Then, cultured media were incubated at 37°C in slant position. Subsequently, weekly observation was made to check the growth of bacteria [15–17].
2.2. Drugs Susceptibility Test
Drugs susceptibility test was done through the conventional proportional method on Löwenstein-Jensen (LJ) media containing drugs according to CDC standard procedures [16, 18]. The grown colonies were picked up from the media and emulsified in a thick wall glass tube, containing DW + glass beads, by shaking the tubes well; furthermore, turbidity was adjusted with McFarland standard (number 0.5). The diluted suspension 10−4 was cultured on LJ drug-free media as a control and drug containing 0.2 μg/mL isoniazid (INH), 40.0 μg/mL rifampicin (RIF), 4.0 μg/mL streptomycin (SM), and 2.0 μg/mL ethambutol (EMB). The cultured media were incubated at 37°C and were observed after 4 weeks. If there are no colonies or if the ratio between the number of colonies in the media containing drug and the number of colonies in drug-free media is less than 1%, it will be considered sensitive, while if the ratio between the number of colonies in media including drug and the number of colonies in drug-free media is more than 1%, it will be interpreted as resistant to all four drugs. Species identification of Mycobacterium was carried out via testing the ability to grow on p-nitrobenzoic acid (PNB). All isolates were tested twice in media to confirm the accuracy of the results [19, 20].
2.3. Guanidine Chloride DNA Extraction Method
All scraped colonies were washed with phosphate buffer saline (PBS), followed by the addition of 2 mL lysis buffer, 5 μL proteinase K, 1 mL guanidine chloride, and 300 μL ammonium acetate. Suspensions were incubated overnight at 37°C; on the next day, 2 mL of chilled chloroform was added. After centrifugation, the clear upper layer was collected in a new tube and cold absolute ethanol was added to enhance precipitation of DNA. The pellet was washed with 70% ethanol and then decanted by 70% ethanol and allowed to dry. The pellet was then resuspended with nuclease-free water and qualified using NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, USA). The DNA samples were stored at −20°C until used for conventional PCR [21, 22].
2.4. GenoLyse Extraction Method
DNA extraction was performed as recommended by the manufacturer (Hain Lifescience). Firstly, collected bacterial colonies were heated for 20 minutes at 100°C in a water bath and then suspended in 100 μL of kit lysis buffer, followed by incubation at 95°C for 5 min; finally, 100 μL of neutralization buffer was added. The mixture was spun at full speed in a tabletop centrifuge with an aerosol tight rotor and then stored at −20°C and then used for multiplex PCR in Line Probe Assay [23].
2.5. Conventional PCR
Fifteen genomic DNA were used as templates for PCR amplification of complete rrs gene (16S rRNA). The two primers used were forward primer, namely, 27F (5′-AGAGTTTGATCCTGGCTCAG-3′), and reverse primer, namely, 1495R (5′-CTACGGCTACCTTGTTACGA-3′). The 25 μL reaction mixture contained 1 μL DNA, 1x reaction buffer (10x) with 3 mM MgCl2, 2.5 U i-Taq™ DNA polymerase (5 U/μL), 2.5 mM dNTPs, 1 μL of 10 pmol of each primer, and 1x of gel loading buffer, followed by completing the volume to 25 μL by DW. PCR amplifying procedure was as follows: 5 min at 94°C, 30 cycles of 1 min at 94°C, 1 min at 58°C, 2 min at 72°C, and then 10 min at 72°C, which was performed on a Bio-Rad (DNA engine/Dyad Peltier) automatic thermal cycler. Duplicate PCR of every sample were carried out for more confirmation. The products of amplification were checked through running on 0.6% agarose gel electrophoresis [24, 25].
2.6. Multiplex PCR
Hybridization and detection were performed using the hybridization kits. Seventy-five isolated DNA were used by taking 5 μL of each DNA and mixing it with 10 μL Amplification Mix A and 35 μL Amplification Mix B containing biotinylated primers. The PCR amplifying procedure was as follows: 15 min at 95°C, 10 cycles of 30 seconds at 95°C, 20 cycles of 40 seconds at 50°C, and 8 min at 70°C, which was performed on a Hain Lifescience thermal cycler. The amplification product was visualized through reverse hybridization probes complementary to amplified nucleic acids on membrane strips. [26].
2.7. Sequencing of 16S rRNA
Isolates were packaged according to the International Air Transport Association guidelines and shipped with authorized permission to Macrogen Company (Seoul, South Korea). Purification and standard forward sequencing of 16S rRNA were done by ABI Genetic Analyser (Applied Biosystems).
2.8. Statistical and Bioinformatic Analysis
The result was analysed statistically using IBM SPSS Statistics version 21 (Statistical Package for the Social Sciences) which is a software package for statistical analysis; the chi-square test was used to check the statistical significance [27]. The chromatogram sequences were visualized through Finch TV program version 1.4.0 [28]. The nucleotides sequences of the rrs gene were searched for sequences similarity using nucleotide BLAST [29]. Highly similar sequences have accession numbers HM007576, KF796661, JX303293, and GU142936 and sequences of the reference M. tuberculosis H37Rv strain [X55588.1] were retrieved from NCBI (https://www.ncbi.nlm.nih.gov/) and subjected to multiple sequence alignment using BioEdit software version 7.2.5 [30]. Newick format was withdrawn from ClustalW (http://www.ebi.ac.uk/Tools/msa/clustalw2) [31] to create a phylogenetic tree in Phylogeny.fr software [32].
2.9. Nucleotide Sequence Accession Numbers
The nucleotide sequences of the rrs genes containing novel mutations were deposited in the GenBank database (National Center for Biotechnology Information; https://www.ncbi.nlm.nih.gov/) under the following accession numbers: KU372152, KU372153, KU372154, KU372155, KU372156, and KU372157.
3. Results
Among 100 sputum samples, twenty of them revealed no growth, two were contaminated, one was lysed, and two had grown in PNB indicating Mycobacterium other than tuberculosis (MOTT). The growth rate on LJ media is as follows: 39% (29/75) showed +3 (200–500 colonies), 33% (25/75) showed +2 (100–200 colonies), 26.7% (20/75) showed +1 (10–99 colonies), and 1.3% (1/75) showed 4 colonies. Most of the MDR growth appeared as +1 and probably had grown at 2–4 weeks (P value 0.426), showing a statistically insignificant association between growth rate and MDR. The prevalence of MDR was 20% (15/75) and 14.7% (11/15) among previously treated and old cases and 5.3% (4/15) among newly diagnosed cases (P value 0.003), which means drug resistance was associated with previous treatment. 73.3% of MDR cases were males, while 26.7% were females (P value 0.536). Sixty percent (9/15) of MDR cases were found in the age group ≤ 30 years (P value 0.652) (Table 1). Among seventy-five Mycobacterium tuberculosis isolates, 56% (42/75) were streptomycin-resistant, 29% (22/75) were rifampicin-resistant, 20% (15/75) were ethambutol-resistant, and 28% (21/75) were isoniazid-resistant. 20% (15/75) were resistant to RIF and INH. 16% (12/75) were resistant to all four drugs. The run progression of the final separation of PCR products was visualized under UV transilluminator documentation machine. Most of them fluoresced sharply, but two of them fluoresced faintly and were excluded. The most common mutation detected by LPA in the rpoB gene was S531L (80%, 12/15), followed by H526D; in KatG gene, the most common mutation was S315T (67%, 10/15) and in InhA promoter it was C15T (33.3%, 7/21).
Table 1.
Percentage of MDR in different age groups.
| Age group | MDR % | Account (number) |
|---|---|---|
| ≤30 | 60% | 9 |
| 31–40 | 13.3% | 2 |
| 41–50 | 13.3% | 2 |
| 51–60 | 13.3% | 2 |
| >60 | 0% | 0 |
Sixty-two percent (8/13 isolates) of streptomycin-resistant isolates revealed mutations in rrs gene which were identified in seven groups: four isolates (31%) have G → A transition at nucleoside position 892; three isolates (23%) have C → A transversion at nucleoside position 222; two isolates (15%) have A → G transition at nucleoside position 904; one isolate (8%) has G → T transversion at nucleoside position 855; one isolate (8%) has A → G transition at nucleoside position 906; one isolate (8%) has T → A transversion at nucleoside position 1238; and two isolates have G → A/G → C at nucleoside position 1400, which are more explained in Table 2.
Table 2.
Explaining the position and frequency of polymorphism with resistance pattern of isolates.
| Mutation position | Polymorphism | Frequency (%) | Resistance pattern | |||
|---|---|---|---|---|---|---|
| RIF | INH | STM | EMB | |||
| 222 | C → A | 23% | R | R | R | S |
| S | S | R | S | |||
| R | S | R | S | |||
|
| ||||||
| 885 | G → T | 8% | S | S | R | S |
|
| ||||||
| 892 | G → A | 31% | S | R | R | S |
| S | S | R | S | |||
| R | R | R | S | |||
| R | R | R | R | |||
|
| ||||||
| 904 | A → G | 15% | S | R | R | S |
| R | R | R | R | |||
|
| ||||||
| 906 | A → G | 8% | S | R | R | S |
| T → A | 8% | R | S | R | S | |
|
| ||||||
| 1400 | G → A | 8% | R | R | R | R |
| G → C | 8% | S | S | R | S | |
R: resistance; S: sensitive; RIF: rifampicin; INH: isoniazid; STM: streptomycin; EMB: ethambutol.
3.1. Phylogenetic Tree
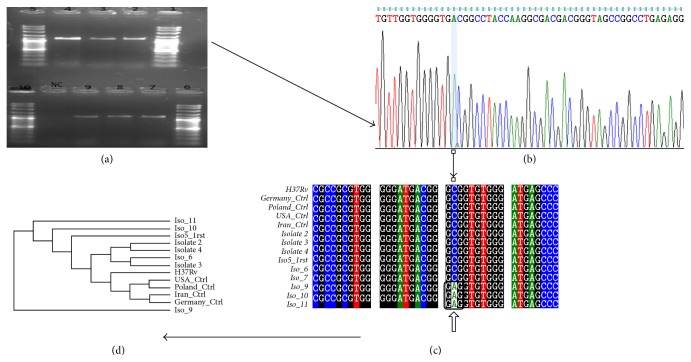
A phylogenetic tree represents the relationships among a set of Mycobacterium tuberculosis. The tree is classified into two branches. All isolates have a common ancestor except for isolate-9 which was outgroup. Isolate-2 and isolate-4 were sister groups as shown in Figure 1.
Figure 1.
(a) Gel electrophoresis of PCR product. Lanes 1 and 5: DNA marker 1000+. Lanes 2, 3, and 4: amplified DNA products, 1500 bp. (b) Chromatogram sequence of 16S rRNA visualized via Finch TV software. (c) Alignment determining novel mutation at position 222, transversion of C to A in isolates 9, 10, and 11 via BioEdit software. (d) Phylogenetic tree using Phylogeny.fr software.
4. Discussions
The study found novel transversion mutation of C → A at position 222. To our knowledge, the present study is the first study that demonstrated 16S rRNA analysis among multidrug-resistant M. tuberculosis isolates from Sudan, and thus the prevalence of MDR was 20% (15/75) and 14.7% (11/15) among old cases and 5.3% (4/15) among newly diagnosed cases, which was lower than France's study with a prevalence of 23/323 (7.1%) in newly diagnosed patients and 33/105 (31.4%) in re-treated patients [33]; this difference could be due to the geographical variation. In the present study, 73.3% of MDR cases were males, while 26.7% were females, which agreed with Raizada et al.'s study where MDR-TB was predominant in males (72%, 230/320) [34] contrary to Melzer et al.'s study where 56.7% in males and 43.3% in females [35] may be due to the large numbers of males when compared with females included in this study. Most MDR cases were found in the age group ≤ 30 years (60%), corresponding with Melzer et al.'s study [35]. The genotype MTBDRplus test identified most frequent mutations involved in resistance to RIF and INH as follows: in rpoB gene, S531L was 54% (12/22); this is similar to the findings of Barnard et al. [36]. The final result of mutation frequency in rrs gene is 62% which is approximately similar to Asho's study conducted in Pakistan that detected 35/50 (70%) strains [37]. Also, additional studies in China, Japan, and Latvia have reported the highest frequencies, 85.7%, 77.8%, and 85%, respectively [38–40], whereas, in North India, no mutation had been detected in streptomycin-resistant isolates [41]. Regarding mutation in the 912 region, 23% of the isolates have been revealed. A → G transition at position 904 corresponds to studies in Germany [42]. On the other hand, a study in Barcelona did not detect mutation in the rrs912 region [43]. The mutation at loop 530 of the rrs coding region had not been identified, which agreed with New York's study [44] and conflicted with Poland's study [45]. Novel mutation could be used as a diagnostic marker that represents a tool for rapid monitoring of streptomycin resistance and could be of value to the clinician.
Small sample size was one of the drawbacks that limited our study; therefore, a large sample size in further studies could be useful for the determination of other biomarkers that assist the diagnosis of streptomycin-resistant MTB.
5. Conclusion
Analysis of 16S rRNA sequences is considered the golden standard method for the identification and assessment of phylogenetic relationships among bacterial isolates.
Acknowledgments
The authors are grateful to Africa City of Technology, National Public Health Laboratory, Molecular Tuberculosis Laboratory, and all members of the National Reference Laboratory of Tuberculosis (NRL-TB), Sudan, Khartoum.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.World Health Organization. Global Tuberculosis Report 2015. 20th. chapter 4. Geneva, Switzerland: WHO/HTM/TB/2015.22; 2015. [Google Scholar]
- 2.Mitchison D. A., Nunn A. J. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. American Review of Respiratory Disease. 1986;133(3):423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 3.Kritski A. L., Ozorio Marques M. J., Rabahi M. F., et al. Transmission of tuberculosis to close contacts of patients with multidrug-resistant tuberculosis. American Journal of Respiratory and Critical Care Medicine. 1996;153(1):331–335. doi: 10.1164/ajrccm.153.1.8542139. [DOI] [PubMed] [Google Scholar]
- 4.WHO. Global Tuberculosis Control 2014. Geneva, Switzerland: WHO; 2014. http://www.who.int/tb/publications/global_report/ [Google Scholar]
- 5.World Health Organization/IUATLD. Report. 3. Geneva, Switzerland: World Health Organization/IUATLD; 2003. Global project on anti-tuberculosis drug resistance surveillance. [Google Scholar]
- 6.Rhines A. S. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis. 2013;93(1):104–107. doi: 10.1016/j.tube.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Caminero J. A., editor. Guidelines for Clinical and Operational Management of Drug-Resistant Tuberculosis. Paris, France: International Union Against Tuberculosis and Lung Disease; 2013. [Google Scholar]
- 8.Somoskovi A., Parsons L. M., Salfinger M. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respiratory Research. 2001;2(3):164–168. doi: 10.1186/rr54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Heym B., Allen B., Young D., Cole S. The catalase—peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature. 1992;358(6387):591–593. doi: 10.1038/358591a0. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie S. H. Evolution of drug resistance in Mycobacterium tuberculosis: clinical and molecular perspective. Antimicrobial Agents and Chemotherapy. 2002;46(2):267–274. doi: 10.1128/aac.46.2.267-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heym B., Zhang Y., Poulet S., Young D., Cole S. T. Characterization of the katG gene encoding a catalase-peroxidase required for the isoniazid susceptibility of Mycobacterium tuberculosis. Journal of Bacteriology. 1993;175(13):4255–4259. doi: 10.1128/jb.175.13.4255-4259.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crofton J., Mitchison D. A. Streptomycin resistance in pulmonary tuberculosis. British Medical Journal. 1948;2:1009–1015. doi: 10.1136/bmj.2.4588.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moazed D., Noller H. F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327(6121):389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 14.Finken M., Kirschner P., Meier A., Wrede A., Böttger E. C. Molecular basis of streptomycin resistance in Mycobacterium tuberculosis: alterations of the ribosomal protein S12 gene and point mutations within a functional 16S ribosomal RNA pseudoknot. Molecular Microbiology. 1993;9(6):1239–1246. doi: 10.1111/j.1365-2958.1993.tb01253.x. [DOI] [PubMed] [Google Scholar]
- 15.WHO. Laboratory Services in Tuberculosis Control, Part II and III: Microscopy and Culture. WHO; 1998. [Google Scholar]
- 16.Kent P. T., Kubica G. P. Public Health Mycobacteriology, Guides For Level III Laboratory. US Department of Health and Human Services, Public Health Service, CDC; 1985. [Google Scholar]
- 17.Kim S.-J., Bai G.-H., Hwang H.-D. Efficiency of the direct and concentration methods in demonstrating tubercle bacilli in the sputum specimens. Tuberculosis and Respiratory Diseases. 1989;36(4):354–361. [Google Scholar]
- 18.Vincent V., Gutiérrez M. C. Manual of Clinical Microbiology. Washington, DC, USA: American Society for Microbiology; 2007. Mycobacterium: laboratory characteristics of slowly growing mycobacteria; pp. 573–588. [Google Scholar]
- 19.Collins C., Grange J., Yates M. Organization and Practice in Tuberculosis Bacteriology. London, UK: Butterworth; 1985. [Google Scholar]
- 20.Canetti G., Fox W., Khomenko A., et al. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bulletin of the World Health Organization. 1969;41(1):21–43. [PMC free article] [PubMed] [Google Scholar]
- 21. DNA Extraction Guanidine Method, Modified from Black well Lab, Cambridge, UK.
- 22.Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Research. 1988;16(3):p. 1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hain Life Science, Hardwiesenstraße, Nehren, Germany, http://www.hain-lifescience.de.
- 24.Liu W., Bao Q., Jirimutu, et al. Isolation and identification of lactic acid bacteria from Tarag in Eastern Inner Mongolia of China by 16S rRNA sequences and DGGE analysis. Microbiological Research. 2012;167(2):110–115. doi: 10.1016/j.micres.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 25. iNtRON BIOTECHNOLOGY, Maxime PCR PreMix Kit (i-Taq) for 20μl rxn, Cat. NO. 25025, http://www.intronbio.com/
- 26.Genotype MTBDRplus™, version 1.0 [product insert] Nehren, Germany: Hain Lifescience, GmbH. Hain lifescience website, http://www.hainlifescience.com/pdf/304xx_pbl.pdf.
- 27. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp, Released 2012, http://www.ibm.com/analytics/us/en/technology/spss/
- 28. http://www.geospiza.com/Products/finchtv.shtml.
- 29. https://blast.ncbi.nlm.nih.gov/Blast.cgi.
- 30.Hall T. A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- 31.McWilliam H., Li W., Uludag M., et al. Analysis Tool Web Services from the EMBL-EBI. Nucleic acids research. 2013;41:W597–W600. doi: 10.1093/nar/gkt376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dereeper A., Guignon V., Blanc G., et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research. 2008;36:W465–W469. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyu J., Kim M.-N., Song J. W., et al. GenoType® MTBDRplus assay detection of drug-resistant tuberculosis in routine practice in Korea. The International Journal of Tuberculosis and Lung Disease. 2013;17(1):120–124. doi: 10.5588/ijtld.12.0197. [DOI] [PubMed] [Google Scholar]
- 34.Raizada N., Sachdeva K. S., Chauhan D. S., et al. A Multi-site validation in India of the line probe assay for the rapid diagnosis of multi-drug resistant tuberculosis directly from sputum specimens. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0088626.e88626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melzer M., Gupta N., Petersen I., Cook S., Hall B. Previous treatment in predicting drug-resistant tuberculosis in an area bordering East London, UK. International Journal of Infectious Diseases. 2010;14(8):e717–e722. doi: 10.1016/j.ijid.2010.02.2247. [DOI] [PubMed] [Google Scholar]
- 36.Barnard M., Albert H., Coetzee G., O'Brien R., Bosman M. E. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. American Journal of Respiratory and Critical Care Medicine. 2008;177(7):787–792. doi: 10.1164/rccm.200709-1436OC. [DOI] [PubMed] [Google Scholar]
- 37.Ali A., Hasan R., Jabeen K., Jabeen N., Qadeer E., Hasan Z. Characterization of mutations conferring extensive drug resistance to Mycobacterium tuberculosis isolates in Pakistan. Antimicrobial Agents and Chemotherapy. 2011;55(12):5654–5659. doi: 10.1128/aac.05101-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X.-Q., Lu Y., Zhang J.-X., et al. Detection of streptomycin resistance in Mycobacterium tuberculosis clinical isolates using four molecular methods in China. Acta Genetica Sinica. 2006;33(7):655–663. doi: 10.1016/s0379-4172(06)60096-6. [DOI] [PubMed] [Google Scholar]
- 39.Katsukawa C., Tamaru A., Miyata Y., Abe C., Makino M., Suzuki Y. Characterization of the rpsL and rrs genes of streptomycin-resistant clinical isolates of Mycobacterium tuberculosis in Japan. Journal of Applied Microbiology. 1997;83(5):634–640. doi: 10.1046/j.1365-2672.1997.00279.x. [DOI] [PubMed] [Google Scholar]
- 40.Tracevska T., Jansone I., Nodieva A., Margab O., Skendersc G., Baumanisa V. Characterization of rpsl, rrs and embB mutation associated Streptomycin and Euthambutol resistance in Mycobacterium Tuberculosis. Microbiological Research. 2007;155:830–834. doi: 10.1016/j.resmic.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqi N., Shamim M., Hussain S., et al. Molecular characterization of multidrug-resistant isolates of Mycobacterium Tuberculosis from patients in North India. Antimicrobial Agents and Chemotherapy. 2002;46(2):443–450. doi: 10.1128/aac.46.2.443-450.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier A., Kirschner P., Bange F.-C., Vogel U., Bottger E. C. Genetic alterations in streptomycin-resistant Mycobacterium tuberculosis: mapping of mutations conferring resistance. Antimicrobial Agents and Chemotherapy. 1994;38(2):228–233. doi: 10.1128/aac.38.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudó G., Rey E., Borrell S., et al. Characterization of mutations in streptomycin-resistant Mycobacterium tuberculosis clinical isolates in the area of Barcelona. Journal of Antimicrobial Chemotherapy. 2010;65(11):2341–2346. doi: 10.1093/jac/dkq322.dkq322 [DOI] [PubMed] [Google Scholar]
- 44.Cooksey R. C., Morlock G. P., Mcqueen A., Glickman S. E., Crawford J. T. Characterization of streptomycin resistance mechanisms among Mycobacterium tuberculosis isolates from patients in New York City. Antimicrobial Agents and Chemotherapy. 1996;40(5):1186–1188. doi: 10.1128/aac.40.5.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jagielski T., Ignatowska H., Bakuła Z., et al. Screening for streptomycin resistance-conferring mutations in Mycobacterium tuberculosis clinical isolates from Poland. PLoS ONE. 2014;9(6) doi: 10.1371/journal.pone.0100078.e100078 [DOI] [PMC free article] [PubMed] [Google Scholar]