Abstract
The chemical contamination of food is among the main public health issues in developing countries. With a view to find new natural bioactive products against fungi responsible for chemical contamination of staple food such as maize, the antifungal activity tests of scopoletin extracted from different components of the cassava root produced in Benin were carried out. The dosage of scopoletin from parts of the root (first skin, second skin, whole root, and flesh) was done by High Performance Liquid Chromatography. The scopoletin extract was used to assess the activity of 12 strains (11 strains of maize and a reference strain). The presence of scopoletin was revealed in all components of the cassava root. Scopoletin extracted from the first skin cassava root was the most active both as inhibition of sporulation (52.29 to 87.91%) and the mycelial growth (36.51–80.41%). Scopoletin extract from the cassava root skins showed significant inhibitory activity on the tested strains with fungicide concentration (MFC) between 0.0125 mg/mL and 0.1 mg/mL. The antifungal scopoletin extracted from the cassava root skins may be well beneficial for the fungal control of the storage of maize.
1. Introduction
Cereals are fundamental staple food in developing countries especially for rural populations of low revenues [1]. Among cereals, maize is considered as the main agricultural sector and therefore has an important political support in West Africa [2]. Maize is one of the most important cereal grain crops worldwide, together with rice and wheat, and it is an important forage crop [3]. Its added value is more than two billion euros, mainly helps rural people, and provides more than 10 million permanent jobs [4]. In Benin, maize is grown nationwide and thus ranking first among cereals such as sorghum and millet [5]. The increase in maize production draws the attention of farmers to its storage and conservation. The problems faced by maize producers during postharvest have long been overlooked [6]. Adégbola et al. [7] reported that inadequate storage facilities are often the cause of these postharvest losses. In effect, every year, producers record postharvest losses ranging from 20 to 50% after six (06) months of storage [8]. It was observed that women recorded postharvest losses up to 75% when they failed to apply any phytosanitary treatment [9]. Besides, Borbély et al. [10] have examined mycotoxin levels in cereal samples and mixed feed samples collected in eastern Hungary and detected aflatoxin B1 levels above the EU limit in 4.8% of the samples. Recently, Dobolyi et al. [11] identified aflatoxin producing A. flavus from maize kernel collected in various parts of Hungary. Furthermore, Tóth et al. [3] have examined the occurrence of potential mycotoxigenic fungi on Hungarian maize kernels between 2010 and 2012. Chemical contamination caused by toxigenic fungi of staple foods such as maize is a critical issue for public health. Maize kernels are often infected with fungi that synthesize mycotoxins, which pose major threats to human and animal health [12, 13]. Mycotoxins are secondary metabolites of filamentous fungi, which are harmful to animals and humans, and are able to induce various disease symptoms [14–16]. Although direct evidence that fumonisins cause serious human and animal health problems is lacking, human esophageal cancer is reportedly associated with the consumption of maize-based products contaminated with fumonisins [17]. In warmer regions, Fusarium ear rot is more prevalent; in this disease, kernels are infected by Fusarium verticillioides or other species of the Gibberella fujikuroi complex, including F. proliferatum and F. subglutinans [18]. Over the last decade, the most commonly isolated pathogen worldwide has been F. verticillioides Leslie and Summerell [19]; the fungus has been identified in Spain [18]; Brazil [20]; Canada [21]; Nepal [22]; and Nigeria [23]. Chemical control remains the primary measure to reduce the incidence of postharvest contamination [24]. Similarly, the restriction imposed by the food industry and regulatory agencies on the use of some synthetic food additives has led to renewed interest in the search for alternatives, such as natural antimicrobial compounds, especially those of vegetable origin [25]. Coumaric compound, belonging to the groups of phytoalexins, is the active ingredient present in several plants' scopoletin [26] and was therefore the subject of several studies. But, for the best of our knowledge, very few investigations have been conducted on the use of scopoletin extracted from different parts of cassava root to control the growth of maize mold strains in their storage. Herein, we aim to assess the antifungal activity of scopoletin extracted from cassava root parts on mold strains isolated from maize in storage.
2. Materials and Methods
2.1. Plant Material
The plant material consists of cassava roots of variety BEN 86052 harvested from the fields of the National Institute of Agricultural Research of Benin situated at Abomey-Calavi Township. The cassava root samples were dried in the sun on grid at room temperature to obtain cassava chips.
2.2. Microbiological Materials
Microbiological materials comprise 12 strains including 11 isolated from the maize in storage and a reference strain (A. parasiticus CMBB20) (Table 1). The reference strain was provided by the Hygiene Section of Water and Food of the Benin National Public Health Laboratory. The identification of fungal species was based on macroscopic observation (mycelium morphology, growth rate, color, and texture of thallus) and microscopic examination of a fungal colony [27–29].
Table 1.
Isolated strains from the maize in storage.
| Number of strains | Species | Origin |
|---|---|---|
| (1) | Penicillium sp. | Storage maize |
| (2) | Aspergillus niger | |
| (3) | Penicillium griseofulvum | |
| (4) | Aspergillus ochraceus | |
| (5) | Aspergillus flavus | |
| (6) | Fusarium graminearum | |
| (7) | Aspergillus parasiticus | |
| (8) | Fusarium oxysporum | |
| (9) | Fusarium sp. | |
| (10) | Fusarium verticillioides | |
| (11) | Penicillium roqueforti | |
|
| ||
| (12) | A. parasiticus (CMBB20) | Reference |
Be et al. [29].
2.3. Extraction and Dosage of Scopoletin of Cassava Roots
Scopoletin was extracted from each part of cassava roots. It includes the first skin (P1); the second skin (P2); the flesh (P3); and the whole root (P4) (Figure 1). These different parts were dried in the sun on grid at room temperature for six days to obtain cassava chips (Figure 2). After drying, the skin, the flesh, and the whole root samples were ground with an electric mill. Scopoletin was extracted with absolute ethanol as described by Buschmann et al. [30]. For each part of the cassava root, 10 mL of absolute ethanol was added to 2 g and 4 g of powder, respectively. After 3 days of maceration, the resulting mixture was then filtered and concentrated to a final volume of 3 mL. Scopoletin was then assayed on a high performance liquid chromatograph in the analytical conditions described by the method developed by Ba et al. [31].
Figure 1.
Different parts of cassava root.
Figure 2.

Drying parts of cassava root in the sun on grid at room temperature (28°C ± 2).
2.4. Qualitative Assessment of Scopoletin in Cassava Root
The presence of scopoletin in the various components of the cassava root was assessed by thin layer chromatography as described by the French Pharmacopoeia [32]. The extracts obtained above were deposited on a grafted silica gel plate RP 18. The mixture Toluene-Ethyl Acetate (15; 85) was used as eluent (mobile phase). After elution the plate was observed at 356 nm under UV. Pure scopoletin (Sigma®, S2500-50MG) was used as standard and the spots of the various extracts were compared to that of the reference scopoletin.
2.5. Assessment of the In Vitro Antifungal Activity of Scopoletin on Fungal Strains Isolated
The antifungal activity of scopoletin extracted from cassava roots was investigated in vitro and determining the Minimum Inhibitory Concentration (MIC) of the extracts was aimed. These tests were carried out on a solid medium and consisted primarily of the realization of antifungal screening and the determination of Minimum Fungistatic Concentration (MFC) and Minimum Fungicidal or Lethal Concentrations (MLC).
Antifungal Screening. The antifungal screening was carried out following the method described by Dohou et al. [33]. This method permits to identify and select efficient plant extracts against fungi. Scopoletin activity was evaluated on eleven (11) strains isolated from maize in storage and the reference strain A. parasiticus (CMBB20). Two stages of fungal growth (mycelial growth and sporulation) were observed. Powders of different parts of the cassava root dissolved in absolute ethanol were tested at concentrations of 0.2 mg/mL and 0.4 mg/mL. The extracts thus obtained were mixed with hot Potato Dextrose Agar (PDA) (50°C) and 10 mL of each mixture (extract-PDA) was poured into Petri dishes. After the polymerization of agar, a fungal inoculum volume equivalent to 100 spores was deposited in the middle of each Petri dish. The fluconazole (0.1 mg/mL) and the PDA culture medium (without extract) were used as positive and negative control, respectively. The tests were performed in triplicate and the plates were incubated at 28°C ± 2. After 5 days of incubation, the mycelium diameter was measured each on test plates and on control plates. With the aid of a Malassez cell (Germany; P 0.200 mm; S 0.0025 mm2), the number of spores was determined microscopically (microscope XSZ-107BN). The percentages of inhibition of mycelial growth and the sporulation of the extracts were calculated relative to a negative control (plate without extract) using the formula below:
| (1) |
See [34].
PI is % of inhibition, A is average diameter of the mycelium or the average number of spores on control medium, and B is average diameter of the mycelium or the average number of spores in the presence of the tested extract.
When the percentage of inhibition is between 75 and 100%, the extract is very active and the fungal strain is considered very sensitive. When this percentage is between 50 and 75%, the extract is regarded as active and the fungal strain sensitive. Between 25 and 50%, the extract is moderately active and the strain is called limit; the extract is either little or nonactive when the percent inhibition is between 0 and 25%; in this case the strain is considered insensitive or resistant [35].
2.6. Determination of Minimum Inhibitory Concentrations
Minimum Inhibitory Concentrations (MIC) and Minimum Fungicidal or Fungistatic Concentrations (MFC) were determined using microdilution method with Sabouraud liquid broth [36]. Each fungal inoculum was obtained from a suspension in physiological water of spores from a culture of seven days on PDA. Fungal suspensions were distributed in 96-well plates at 100 μL per well. The increasing concentrations of pure scopoletin and extracts of scopoletin (scopoletin from the first skin, second skin, and the mixture of the two skins) were obtained by serial dilution (1/2 to 1/64) of a stock solution 0.4 mg/mL. Thus, the corresponding concentrations 0.2 mg/mL; 0.1 mg/mL; 0.05 mg/mL; 0.025 mg/mL; 0.0125 mg/mL; and 0.00625 mg/mL were tested by their addition to the culture broth. A fungal inoculum volume corresponding to opacity of 5 to 105 spores was determined by counting the spores in a Malassez cell (Germany; P 0.200 mm; S 0.0025 mm2) from control wells without extract (negative control). The microplates were covered with wax paper and incubated at 28°C ± 2 for 72 h. Two other controls were also performed, that is, the positive control (broth plus suspension) and ethanol control (ethanol plus suspension). The MIC corresponds to the lowest concentration at which no fungal growth was observed with the naked eye. To the experimental concentrations where no growth or sprouting was observed, Fungicidal or Fungistatic activity was tested (Boniface et al. [37]). The Minimum Fungicidal Concentration is defined as the minimum concentration that kills 99.99% of the initial inoculum. The MFC was determined by taking 100 μL of suspension in the wells without growth on nutrient agar PDA. PDA plates were incubated at 28 ± 2°C for 5 days. The ratios MFC/MIC were determined and were used to determine the type of effect produced by extracts on the fungi tested. Thus, the effect of the extract is considered as Fungistatic if the ratio MFC/MIC is higher than 1 and is considered as Fungicidal if this ratio is less than or equal to 1 [38].
2.7. Statistical Analysis
The data matrix with treatments, strains, doses, diameter, and the number of spores in the columns was subjected to ANOVA, fixed model in order to assess the effect of treatments, and doses on the growth of strains. It was carried out after checking the normality of logged data and the equality of variances. The structure test of averages SNK (Student-Newman-Keuls) was performed to identify the best treatment and the most effective dose. A treatment or dose is considered as effective when the mean diameter measured is the smallest. But there must be a significant difference between the terms of the factor considered. These tests were followed by the comparison test two by two of Bonferroni.
3. Results and Discussion
3.1. Presence of Scopoletin in Cassava Roots
The qualitative assessment of scopoletin in the cassava roots as described above in the methodology is presented in Figure 3. The extracts of various cassava root parts, namely, 1st skin (P1), 2nd skin (P2), flesh (P3), and whole root (P4), migrated at the same retention factor (RF) as reference scopoletin (S). Thus, we concluded that scopoletin was present in each part of the cassava root. These results are similar to those of Gnonlonfin et al. [39] who showed the presence of scopoletin in cassava roots produced in Benin. Furthermore, our results corroborated with those obtained by Tanaka et al. [40] and Buschmann et al. [30], who demonstrated the accumulation of scopoletin in the tuberous cassava roots during postharvest deterioration time.
Figure 3.
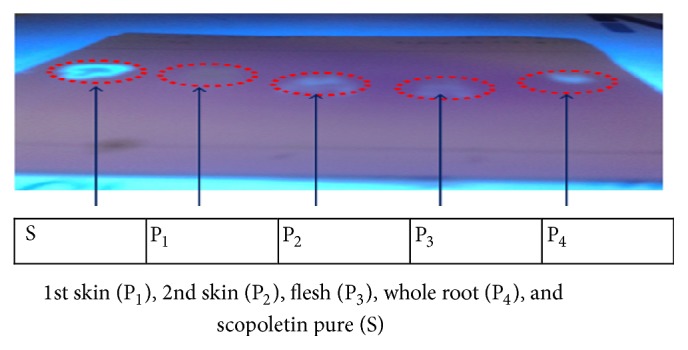
Qualitative assessment of scopoletin in the cassava roots.
3.2. Quantification of Scopoletin by HPLC
As previously described by Gnonlonfin et al. [41], high concentration (containing up to 242.5 mg/kg of dry matter) of scopoletin was found in cassava chips from BEN 86052. This justifies the use of cassava roots of this variety in the present study. The assay of scopoletin in the flour obtained from cassava chips with pure scopoletin showed a recovery rate of the method which was 98% in average with a linearity of R2 = 0,999 in the concentration interval between 200 ng/mL and 2000 ng/mL.
3.3. Antifungal Activity of Scopoletin on the Isolated Strains
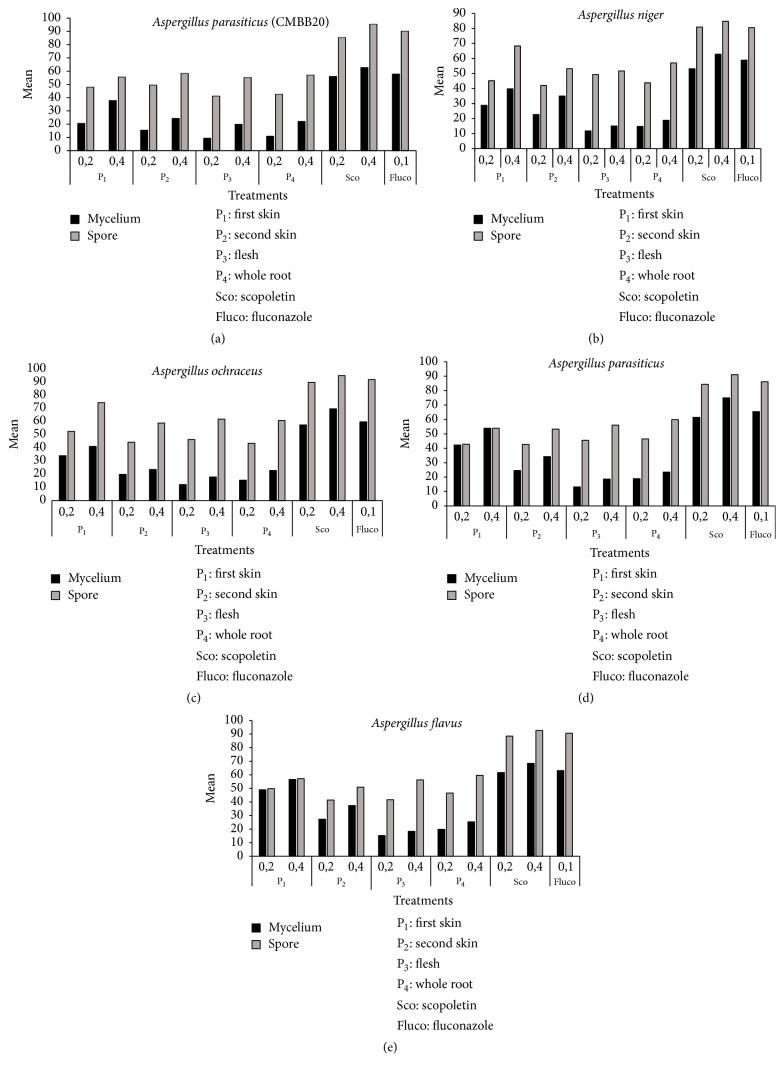
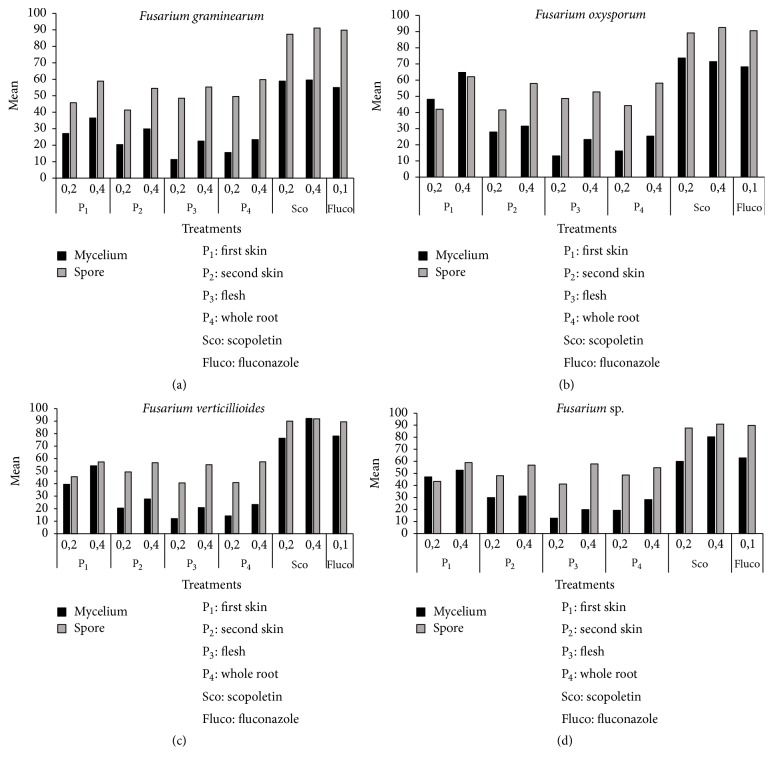
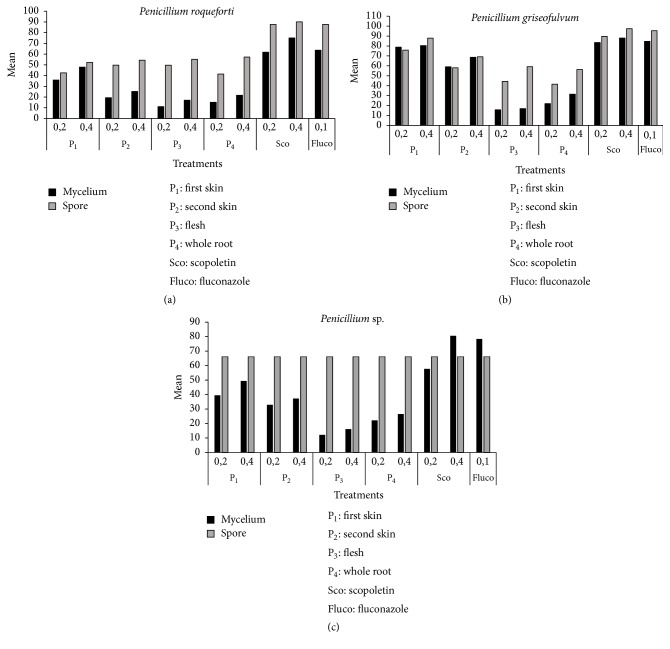
The percentages of inhibition of spores and mycelium growth of different fungi isolated from maize in storage are presented in Figures 4, 5, and 6. After five days of incubation at 28°C ± 2, we observed in all series of experiments compared to the controls a gradual decrease in the number of spores and mycelial diameter as the dose of the factor considered increases (Figures 4, 5, and 6). This was observed for all the series of tests. The analysis of variance performed on the diameter of the mycelial growth and on the number of spores was found highly significant (p < 0.001). The six treatments showed variable activity on fungal strains tested. Overall, only scopoletin extracts at the concentration of 0.4 mg/mL demonstrated the best percentages of inhibition for all strains tested. From the analysis of the results, it appears that the dose of 0.2 mg/mL was too small to significantly reduce mycelial growth. Pure scopoletin at the concentration of 0.4 mg/mL was the most active with the percentages of inhibition (PI) ranging from 59.50 to 92.14% for all strains. The use of fluconazole at a concentration of 0.1 mg/mL has shown PI ranging from 55.03 to 84.58%. These values obtained were lower than those of pure scopoletin. Scopoletin extracts from the cassava root also showed antifungal activity on the tested strains since they revealed inhibition rates ranging from 36.51 to 80.41% if they were from the first skin and between 24.33 and 68.56% if they were from the second skin and ranging from 18.86 to 28.18% if they were from the whole root. In fact, the diameters of the tested strains induced by their action were significantly lower than that showed under the action of pure scopoletin and fluconazole. Moreover, scopoletin extracted from the flesh of the cassava root showed, as in all the other cases, inhibition of mycelial growth compared to the negative control. However, these percentages of inhibition were lower than those of scopoletin extracted from the other components and this is regardless of the tested strain. These results confirm those of Gnonlonfin et al. [39] who proved the inhibitory effect of scopoletin on A. flavus. Furthermore, the inhibition of sporulation by the extracts varies according to the strains tested. The inhibitory effect of the extracts at the same concentration was more marked on sporulation than on the mycelial growth. The different treatments showed a percentage of inhibition of sporulation higher than 40% (Figures 4, 5, and 6). These inhibition percentages vary between 41.27% and 97.35%. Treatment with pure scopoletin was the most active on sporulation of fungal strains with inhibition percentages ranging from 90.11% to 97.35%. Scopoletin extracted from the first skin of cassava root inhibited sporulation of the strains tested, but these inhibitions' percentages were lower than those of pure scopoletin. As for treatment with scopoletin derived from the second skin of the cassava root, we also observed an inhibition of sporulation whatever the strain, but the recorded content was low when compared with the pure scopoletin and that extracted from the first skin (Figures 4, 5, and 6). For all strains, treatment with scopoletin extracted from the flesh revealed percentages of inhibition (51.70% to 59.16%) lower than those found for the treatment with scopoletin extracted from the skins and the whole root. From the results obtained, it appears that scopoletin was present in all parts of the cassava root. Furthermore, treatment with scopoletin extracted from the whole root revealed, as in other cases, an inhibition of sporulation, but the percentages found were lower than those of scopoletin extracted from the skins of cassava root. However, further studies are undergoing in our group to elucidate this preliminary observation. P. griseofulvum was more sensitive both to the inhibition of sporulation and to that of the mycelial growth. On the other hand, strains 2 and 6 were the least sensitive to the inhibition of mycelial growth and strains A. parasiticus, F. oxysporum, and P. roqueforti to the inhibition of sporulation. To the best of our knowledge this is the first time that the biological activity of scopoletin extracted from the cassava root components was evaluated on strains isolated from maize in storage. These results are similar to those obtain by Gómez-Vásquez et al. [42]. They have proved that scopoletin has Fungistatic and Fungicidal properties.
Figure 4.
Inhibition percentages of mycelial growth and sporulation by Aspergillus.
Figure 5.
Inhibition percentages of mycelial growth and sporulation by Fusarium.
Figure 6.
Inhibition percentages of mycelial growth and sporulation by Penicillium.
3.4. Determination of Minimum Inhibitory Concentrations of Scopoletin
The results of the determination of the MIC of scopoletin extracted from skins of cassava roots (first skin, second skin) are summarized in Table 2. From these results, it appears that the extract of scopoletin from the skins of cassava roots showed no inhibitory activity on the isolated fungi from maize in storage at the concentration of 0.00625 mg/mL. On the other hand, the pure scopoletin showed at this concentration antifungal activity. Above this concentration, the antifungal activity was observed on the 11 species of fungi isolated from maize and as well as the reference strain. The Minimum Fungicidal Concentration observed was between 0.0125 mg/mL and 0.1 mg/mL (Table 2). The analysis of the results shows that the isolated fungal strains of maize in storage and tested reference strain had varying sensitivities to the activity of the scopoletin extract. This could be justified by the fact that 9 out of 12 tested strains showed MFC between 0.0125 mg/mL and 0.1 mg/mL. A. parasiticus; P. sp.; A. niger; A. ochraceus; F. verticillioides; F. sp.; P. griseofulvum; and A. parasiticus (CMBB20) were the most sensitive strains. On the other hand, the following species: A. flavus; F. oxysporum; F. graminearum; and P. roqueforti were the least susceptible strains to the raw extract of scopoletin having revealed MFC/MIC ratios greater than or equal to 2. Thus, the fungal species A. parasiticus (CMBB20) and P. griseofulvum were the most susceptible because the concentrations of 0.0125 mg/mL and 0.025 mg/mL were enough to stop their growth. Moreover, the various MFC observed indicate that the extract of pure scopoletin demonstrated, at 0.00625 mg/mL, interesting antifungal activity on the tested fungal strains. Apart from the pure scopoletin, that obtained from the first skin of the cassava root has also shown an interesting antifungal activity. But the MFC observed were higher than that of pure scopoletin. This observation is probably due to the purity of the standard. In addition, the same antifungal activity was observed for scopoletin extracts from the second skin of the cassava root and the mixture of the first and second skins. This inhibitory effect could be explained by the fact that there was no difference between scopoletin extract from the first skin and the second skin. Overall, scopoletin, being a coumarin, shows an inhibitory effect on enzymes and the interactions with DNA [43, 44]. The antifungal activity of scopoletin from cassava root skins corroborates the studies of Rodriguez et al. [45] who showed that the antifungal activity is among different biological activities of scopoletin. As for Buschmann et al. [30], the accumulation of scopoletin in the chips is a defense response against invading microorganisms. Furthermore, the concentration of 0.1 mg/mL was not enough to stop the growth of A. flavus. This result is consistent with previous research conducted in Benin by Gnonlonfin et al. [46] and Gnonlonfin et al. [47]. These authors showed the absence of aflatoxin despite the presence of A. flavus in the samples of cassava chips. Similar results were also observed in Tanzania by Muzanila et al. [48], in Ghana by Wareing et al. [49], and in Nigeria by Jimoh and Kolapo [50]. The antifungal activity of the extract of the scopoletin from cassava root skins observed during this study will not only permit to value the waste from this plant but also to ensure the fungal control of maize in storage.
Table 2.
Minimum inhibitory concentrations of scopoletin extracted from skins of cassava roots (CMI and CMF).
| Skins of cassava roots | Concentration (mg/mL) | P. sp. | A. niger | P. griseofulvum | A. ochraceus | A. flavus | F. graminearum | A. parasiticus | F. oxysporum | F. sp. | F. verticillioides | P. roqueforti | A. parasiticus (CMBB20) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st skin (P1) | CMI | 0.05 | 0 | 0.025 | 0.05 | 0.025 | 0.025 | 0.025 | 0.05 | 0.025 | 0.1 | 0.03 | 0.0125 |
| CMF | 0.05 | 0 | 0.025 | 0.05 | 0.2 | 0.2 | 0.025 | 0.2 | 0.025 | 0.1 | 0.2 | 0.0125 | |
| CMF/CMI | 1 | 1 | 1 | 1 | 8 | 8 | 1 | 4 | 1 | 2 | 8 | 1 | |
|
| |||||||||||||
| 2nd skin (P2) | CMI | 0.1 | 0.1 | 0.025 | 0.1 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.2 | 0.03 | 0.025 |
| CMF | 0.1 | 0.1 | 0.025 | 0.1 | 0.1 | 0.2 | 0.05 | 0.2 | 0.05 | 0.2 | 0.2 | 0.025 | |
| CMF/CMI | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 4 | 1 | 1 | 8 | 1 | |
|
| |||||||||||||
| Mix of 1st skin and 2nd skin | CMI | 0.1 | 0.1 | 0.025 | 0.1 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.2 | 0.03 | 0.025 |
| CMF | 0.1 | 0.1 | 0.025 | 0.1 | 0.1 | 0.2 | 0.05 | 0.2 | 0.05 | 0.2 | 0.2 | 0.025 | |
| CMF/CMI | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 4 | 1 | 1 | 8 | 1 | |
|
| |||||||||||||
| Pure scopoletin | CMI | 0.025 | 0 | 0.0125 | 0.05 | 0.025 | 0.025 | 0.013 | 0.01 | 0.013 | 0.1 | 0.03 | 0.00625 |
| CMF | 0.025 | 0 | 0.0125 | 0.05 | 0.05 | 0.1 | 0.013 | 0.05 | 0.013 | 0.1 | 0.1 | 0.00625 | |
| CMF/CMI | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 4 | 1 | 1 | 4 | 1 | |
P: Penicillium; F: Fusarium; and A: Aspergillus.
4. Conclusion
The study on controlling the growth of fungi in storage of maize by using scopoletin extracted from cassava roots produced in Benin revealed the presence of the latter in the various components of the cassava root. As a matter of fact, the various extracts showed antifungal activity on growth of the strains tested in vitro. The reference scopoletin at a concentration of 0.4 mg/mL was the most active. Among the extracts, only scopoletin from the first skin showed significant antifungal activity both for the inhibition of sporulation (52.29 and 87.91%) and for inhibition of mycelial growth (36.51–80.41%). The crude extracts from the manioc root skins showed, in vitro, a significant antifungal activity against the isolated maize fungi. This high bioactive power observed is attributed to the presence of scopoletin in the skins of the cassava root. Thus, the use of cassava root skins has a practical benefit for the fungal control of maize in storage.
Acknowledgments
The authors wish to thank the West Africa Agricultural Productivity Project (WAAPP) of Benin for funding the realization of this study and the University Agency of Francophonie for its scientific contribution.
Competing Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Guèye M. T., Seck D., Wathelet J.-P., Lognay G. Lutte contre les ravageurs des stocks de céréales et de légumineuses au Sénégal et en Afrique occidentale: synthèse bibliographique. Biotechnologie, Agronomie, Société et Environnement. 2011;15:183–194. [Google Scholar]
- 2.Guèye T. M., Seck D., Wathelet J. P., Lognay G. Typologie des systèmes de stockage et de conservation du maïs dans l'est et le sud du Sénégal. Biotechnologie, Agronomie, Société et Environnement. 2012;16(1):49–58. [Google Scholar]
- 3.Tóth B., Kótai É., Varga M., et al. Climate change and mycotoxin contamination in Central Europe: an overview of recent findings. Review on Agriculture and Rural Development. 2013;2(1):461–466. [Google Scholar]
- 4.Baris P., Demay S. Les Cultures Vivrières Pluviales en Afrique de l'Ouest et du Centre. Éléments d'Analyse et Propositions pour l'Action. Paris, France: AFD, CIRAD, FIDA; 2009. Analyse des pratiques des projets de développement des cultures vivrières en Afrique de l'Ouest et du Centre; p. p. 19. [Google Scholar]
- 5.Ba R., Monteiro N. M. F., Houngue U., Donou Hounsode M. T., Gbaguidi F., Baba-Moussa L. Perception des producteurs et impact des facteurs socio-économiques sur la connaissance des mycotoxines du maïs en stockage au Bénin. International Journal of Biological and Chemical Sciences. 2016;10(1):155–166. [Google Scholar]
- 6.Ba R., Monteiro N. M. F., Koudjega H., et al. Synthèse Bibliographique sur l'utilisation de la Scopolétine pour la Réduction des Aflatoxines du Maïs en Stock au Bénin. Annales des Sciences Agronomiques. 2015;19(2):201–211. [Google Scholar]
- 7.Adégbola P. Y., Arouna A., Ahoyo N. R. A. Acceptabilité des structures améliorées de stockage du maïs au Sud-Bénin. Bulletin de la Recherche Agronomique du Bénin. :76. Numéro spécial 2 Aspects économiques du stockage et de la conservation du maïs au Sud-Bénin, 2011. [Google Scholar]
- 8.Gansou G., Yabi M., Kiki E. Etude des Modes de “Vente à Terme” et de “Vente Précoce” des Récoltes du Maïs par les Producteurs. Cotonou, Bénin: PADSA/DANIDA; 2000. [Google Scholar]
- 9.PADSA (Programme d'Appui au Développement du Secteur Agricole) Rapport Final. Cotonou, Bénin: PADSA/DANIDA; 2000. Evaluation à mi-parcours du PADSA. [Google Scholar]
- 10.Borbély M., Sipos P., Pelles F., Győri Z. Mycotoxin contamination in cereals. Journal of Agroalimentary Processes and Technologies. 2010;16:96–98. [Google Scholar]
- 11.Dobolyi C., Sebők F., Varga J., et al. Occurrence of aflatoxin producing Aspergillus flavus isolates in maize kernel in Hungary. Acta Alimentaria. 2013;42(3):451–459. doi: 10.1556/aalim.42.2013.3.18. [DOI] [Google Scholar]
- 12.Samapundo S., Devlieghere F., De Meulenaer B., Lamboni Y., Osei-Nimoh D., Debevere J. M. Interaction of water activity and bicarbonate salts in the inhibition of growth and mycotoxin production by Fusarium and Aspergillus species of importance to corn. International Journal of Food Microbiology. 2007;116(2):266–274. doi: 10.1016/j.ijfoodmicro.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Qiu J., Xu J., Dong F., Yin X., Shi J. Isolation and characterization of Fusarium verticillioides from maize in eastern China. European Journal of Plant Pathology. 2015;142(4):791–800. doi: 10.1007/s10658-015-0652-5. [DOI] [Google Scholar]
- 14.Richard J. L., Bhatnagar D., Peterson S., Sandor G. Assessment of aflatoxin and cyclopiazonic acid production by Aspergillus flavus isolates from Hungary. Mycopathologia. 1992;120(3):183–188. doi: 10.1007/bf00436397. [DOI] [Google Scholar]
- 15.Varga J., Frisvad J. C., Samson R. A. A reappraisal of fungi producing aflatoxins. World Mycotoxin Journal. 2009;2(3):263–277. doi: 10.3920/WMJ2008.1094. [DOI] [Google Scholar]
- 16.Baranyi N., Kocsubé S., Kiss N., et al. Identification of potential mycotoxin producing fungi on agricultural products in Hungary and Serbia. Acta Biologica Szegediensis. 2014;58(2):167–170. [Google Scholar]
- 17.Gong H.-Z., Ji R., Li Y.-X., et al. Occurrence of fumonisin B1 in corn from the main corn-producing areas of China. Mycopathologia. 2009;167(1):31–36. doi: 10.1007/s11046-008-9146-8. [DOI] [PubMed] [Google Scholar]
- 18.Aguín O., Cao A., Pintos C., Santiago R., Mansilla P., Butrón A. Occurrence of Fusarium species in maize kernels grown in northwestern Spain. Plant Pathology. 2014;63(4):946–951. doi: 10.1111/ppa.12151. [DOI] [Google Scholar]
- 19.Leslie J. F., Summerell B. A. The Fusarium Laboratory Manual. Ames, Iowa, USA: Blackwell Professional; 2006. [Google Scholar]
- 20.Pamphile J. A., Azevedo J. L. Molecular characterization of endophytic strains of Fusarium verticillioides (= Fusarium moniliforme) from maize (Zea mays. L) World Journal of Microbiology and Biotechnology. 2002;18(5):391–396. doi: 10.1023/a:1015507008786. [DOI] [Google Scholar]
- 21.Schaafsma A. W., Limay-Rios V., Tamburic-Illincic L. Mycotoxins and Fusarium species associated with maize ear rot in Ontario, Canada. Cereal Research Communications. 2008;36(6):525–527. doi: 10.1556/crc.36.2008.suppl.b.43. [DOI] [Google Scholar]
- 22.Desjardins A. E., Manandhar G., Plattner R. D., Maragos C. M., Shrestha K., McCormick S. P. Occurrence of Fusarium species and mycotoxins in nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. Journal of Agricultural and Food Chemistry. 2000;48(4):1377–1383. doi: 10.1021/jf991022b. [DOI] [PubMed] [Google Scholar]
- 23.Bankole S. A., Mabekoje O. O. Occurrence of aflatoxins and fumonisins in preharvest maize from south-western Nigeria. Food Additives and Contaminants. 2004;21(3):251–255. doi: 10.1080/02652030310001639558. [DOI] [PubMed] [Google Scholar]
- 24.Adjou E., Aoumanou M. Efficacité des extraits de plantes dans la lutte contre les moisissures toxinogènes isolées de l’arachide en post-récolte au Bénin. Journal of Applied Biosciences. 2013;70:5555–5566. doi: 10.4314/jab.v70i1.98755. [DOI] [Google Scholar]
- 25.Hammer K. A., Carson C. F., Riley T. V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. Journal of Applied Microbiology. 2003;95(4):853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 26.Silva W. P. K., Deraniyagala S. A., Wijesundera R. L. C., Karunanayake E. H., Priyanka U. M. S. Isolation of scopoletin from leaves of Hevea brasiliensis and the effect of scopoletin on pathogens of H. brasiliensis. Mycopathologia. 2002;153(4):199–202. doi: 10.1023/a:1014910132595. [DOI] [PubMed] [Google Scholar]
- 27.Pitt J. I., Hocking A. D. Fungi and Food Spoilage. Springer US; 1997. [DOI] [Google Scholar]
- 28.Samson R. A., Pitt J. I. Integration of Modern Taxonomic Methods for Penicillium and Aspergillus Classification. CRC Press; 2000. [Google Scholar]
- 29.Ba R., Yehouenou B., Houngue U., et al. Performance of maize storage technologies in Benin: fungal ecology and mycotoxin contamination. British Microbiology Research Journal. 2016;15(1):1–10. doi: 10.9734/bmrj/2016/26297. [DOI] [Google Scholar]
- 30.Buschmann H., Rodriguez M. X., Tohme J., Beeching J. R. Accumulation of hydroxycoumarins during post-harvest deterioration of tuberous roots of Cassava (Manihot esculenta Crantz) Annals of Botany. 2000;86(6):1153–1160. doi: 10.1006/anbo.2000.1285. [DOI] [Google Scholar]
- 31.Ba R., Gbaguidi F., Houngbeme G. A., et al. Influence of the drying on the scopoletin induction of cassava chips produced in Benin. International Journal of Applied Biology and Pharmaceutical Technology. 2016;7(2):235–242. [Google Scholar]
- 32.ANSM. Pharmacopée Française. Les Prescriptions Générales et les Monographies Générales de la Pharmacopée Européenne ainsi que le Préambule de la Pharmacopée Française s'Appliquent. Aurône Mâle Pour Préparations Homéopathiques. ANSM; 2008. [Google Scholar]
- 33.Dohou N., Yamni K., Gmira N., Idrissi Hassani L. M. Etude de polyphenols des feuilles d'une endemique ibero marocaine, Thymelaea lythroides. Acta Botanica Malacitana. 2004;29:233–239. [Google Scholar]
- 34.Kumar R., Kamra D. N., Agarwal N., Chaudhary L. C. In vitro methanogenesis and fermentation of feeds containing oil seed cakes with rumen liquor of buffalo. Asian-Australasian Journal of Animal Sciences. 2007;20(8):1196–1200. doi: 10.5713/ajas.2007.1196. [DOI] [Google Scholar]
- 35.Reyes C. R., Quiroz V. R. I., Jiménez E. M., Navarro-ocaňa A., Cassani H. J. Antifungal activity of selected plant secondary metabolites against Coriolus versicolor. Journal of Tropical Forest Products. 1997;3(1):110–113. [Google Scholar]
- 36.NCCLS. NCCLS Document. M44-A. Wayne, Pa, USA: National Committee for Clinical Laboratory Standards; 2004. Reference method for antifungal disk diffusion susceptibility testing of yeasts; approved guideline. [Google Scholar]
- 37.Boniface Y., Ahoussi E., Sessou P., Alitonou G. A., Toukourou F., Sohounhloue C. K. D. Chemical composition and antimicrobial activities of essential oils (EO) extracted from leaves of Lippia rugosa A. Chev against foods pathogenic and adulterated microorganisms. African Journal of Microbiology Research. 2012;6(26):5496–5505. doi: 10.5897/ajmr12.698. [DOI] [Google Scholar]
- 38.Karou S. D., Tchacondo T., Ouattara L., et al. Antimicrobial, antiplasmodial, haemolytic and antioxidant activities of crude extracts from three selected Togolese medicinal plants. Asian Pacific Journal of Tropical Medicine. 2011;4(10):808–813. doi: 10.1016/S1995-7645(11)60199-5. [DOI] [PubMed] [Google Scholar]
- 39.Gnonlonfin G. J. B., Gbénou J., Gbaguidi F., Amadou L. Etude du mécanisme d'autodéfense du manioc contre l'infection par les moisissures et la production de mycotoxines. Rapport technique de recherche. Protocole. 2008;(117/APRA 2007 Code PV)
- 40.Tanaka H., Itakura S., Enoki A. Hydroxyl radical generation by an extracellular low-molecular-weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor. Journal of Biotechnology. 1999;75(1):57–70. doi: 10.1016/s0168-1656(99)00138-8. [DOI] [PubMed] [Google Scholar]
- 41.Gnonlonfin G. J. B., Katerere D. R., Adjovi Y., Brimer L., Shephard G. S., Sanni A. Determination of aflatoxin in processed dried cassava root: validation of a new analytical method for cassava flour. Journal of AOAC International. 2010;93(6):1882–1887. [PubMed] [Google Scholar]
- 42.Gómez-Vásquez R., Day R., Buschmann H., Randles S., Beeching J. R., Cooper R. M. Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged cassava (Manihot esculenta) suspension cells and leaves. Annals of Botany. 2004;94(1):87–97. doi: 10.1093/aob/mch107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zobel A. M. Thomas-Barabare. Phytochemistry of Fruit and Vegetables. Vol. 41. Oxford, UK: Claredon Press; 1997. Coumarins in fruit and vegetables; pp. 173–203. (Proceedings of the Phytochemical Society of Europe). [Google Scholar]
- 44.Ojala T., Vuorela P., Kiviranta J., Vuorela H., Hiltunen R. A bioassay using Artemia salina for detecting phototoxicity of plant coumarins. Planta Medica. 1999;65(8):715–718. doi: 10.1055/s-1999-14049. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez M. X., Buschmann H., Iglesis C., Beeching J. R. Production of antimicrobial compounds in cassava (Manihot esculenta Crantz) roots during post-harvest physiological deterioration. In: Carvalho L. J. C. B., Thro A. M., Vilarinho A. D., editors. Proceedings of the 4th International Scientific Meeting Cassava Biotechnology Network. Cassava Biotechnology; 2000; Brasilia, Brazil. Embrapa; pp. 320–328. [Google Scholar]
- 46.Gnonlonfin G. J. B., Adjovi C. S. Y., Katerere D. R., Shephard G. S., Sanni A., Brimer L. Mycoflora and absence of aflatoxin contamination of commercialized cassava chips in Benin, West Africa. Food Control. 2012;23(2):333–337. doi: 10.1016/j.foodcont.2011.07.026. [DOI] [Google Scholar]
- 47.Gnonlonfin B. G., Gbaguidi F., Gbenou J. D., Sanni A., Brimer L. Changes in scopoletin concentration in cassava chips from four varieties during storage. Journal of the Science of Food and Agriculture. 2011;91(13):2344–2347. doi: 10.1002/jsfa.4465. [DOI] [PubMed] [Google Scholar]
- 48.Muzanila Y. C., Brennan J. G., King R. D. Residual cyanogens, chemical composition and aflatoxins in cassava flour from Tanzanian villages. Food Chemistry. 2000;70(1):45–49. doi: 10.1016/S0308-8146(00)00062-5. [DOI] [Google Scholar]
- 49.Wareing P. W., Westby A., Gibbs J. A., Allotey L. T., Halm M. Consumer preferences and fungal and mycotoxin contamination of dried cassava products from Ghana. International Journal of Food Science and Technology. 2001;36(1):1–10. doi: 10.1046/j.1365-2621.2001.00419.x. [DOI] [Google Scholar]
- 50.Jimoh K. O., Kolapo A. L. Mycoflora and aflatoxin production in market samples of some selected Nigerian foodstuffs. Research Journal of Microbiology. 2008;3(3):169–174. doi: 10.3923/jm.2008.169.174. [DOI] [Google Scholar]