Abstract
Many coral reefs have phase shifted from coral to macroalgal dominance. Ocean acidification (OA) due to elevated CO2 is hypothesised to advantage macroalgae over corals, contributing to these shifts, but the mechanisms affecting coral-macroalgal interactions under OA are unknown. Here, we show that (i) three common macroalgae are more damaging to a common coral when they compete under CO2 concentrations predicted to occur in 2050 and 2100 than under present-day conditions, (ii) that two macroalgae damage corals via allelopathy, and (iii) that one macroalga is allelopathic under conditions of elevated CO2, but not at ambient levels. Lipid-soluble, surface extracts from the macroalga Canistrocarpus (=Dictyota) cervicornis were significantly more damaging to the coral Acropora intermedia growing in the field if these extracts were from thalli grown under elevated vs ambient concentrations of CO2. Extracts from the macroalgae Chlorodesmis fastigiata and Amansia glomerata were not more potent when grown under elevated CO2. Our results demonstrate increasing OA advantages seaweeds over corals, that algal allelopathy can mediate coral-algal interactions, and that OA may enhance the allelopathy of some macroalgae. Other mechanisms also affect coral-macroalgal interactions under OA, and OA further suppresses the resilience of coral reefs suffering blooms of macroalgae.
Coral reefs are one of the most diverse and complex ecosystems on the planet and provide livelihoods, food, and important ecosystem services for hundreds of millions of people1. However, a large proportion of reefs worldwide are severely degraded, and many reefs are on a trajectory of decline2,3. A symptom of decline is a reduced cover of reef-building corals and an increased abundance of upright macroalgae4,5. Understanding the drivers of macroalgal increases and the effects of coral-macroalgal interactions on reef degradation and resilience is of critical importance for the conservation of reef ecosystems6,7,8.
The drivers of coral loss and macroalgal increase are varied and include herbivore loss through overfishing or disease, decreased water quality associated with nutrient enrichment and sedimentation, coral mortality caused by predators, hurricanes and cyclones, bleaching and diseases, algal colonization associated with coral morality, and a failure of juvenile corals and fishes to recruit once reefs become algal dominated2,9,10,11,12. Problems arising from human-induced ocean acidification, that occurs as atmospheric CO2 is absorbed by the ocean and lowers pH13, have also been suggested as potential drivers of elevated algal populations on reefs14,15,16. Elevated seawater CO2 concentrations may enhance fleshy algal growth rates15,17,18, reduce coral growth and calcification rates19, and strengthen space competition between macroalgae and corals, with outcomes that favour macroalgae over corals14,15,20. Habitats with naturally elevated CO2 concentrations show increased fleshy macroalgal abundance21 and decreased coral cover compared to control sites16, suggesting shifts in competitive interactions between taxa under conditions of ocean acidification. Manipulative experiments on the role of elevated CO2 in coral-macroalgal competition are currently limited to a single pair of species15 and it is unclear whether this coral-algal pairing is representative of such interactions. Moreover, the underlying mechanism for altered competitive ability has not been identified.
Some algal competitive mechanisms have been recognised. Macroalgae may directly overgrow coral tissue, reduce light levels required for photosynthesis, abrade tissue, or produce chemical compounds that directly6,22,23,24or indirectly stress or kill corals25,26. Macroalgae may also harm corals by modifying coral microbial community composition27,28. However, the effect of ocean acidification on the potency of algal allelopathy to corals is uninvestigated.
In terrestrial systems, elevation of CO2 tends to increase the production of plant secondary metabolites, but responses are variable29,30. The limited investigations in marine systems suggest that elevated CO2 decreases phenolics in seagrasses31 and brown macroalgae32 or has little effect on terpenes in calcareous algae33. Elevated CO2 could increase the production and release of macroalgal primary metabolites (dissolved organic carbon -DOC), and release of DOC has been associated with a detrimental increase in microbial activity and hypoxic conditions on coral surfaces25,34, however, water flow may limit hypoxic conditions at coral-algal interfaces35,36. At present, effects of ocean acidification on chemically-mediated coral-macroalgal interactions are unknown.
Because most macroalgal secondary metabolites are carbon-based37,38,39, we evaluate the hypothesis that ocean acidification might intensify the potency of allelopathic interactions, strengthen the competitive abilities of macroalgae over corals, contribute to coral mortality, and possibly exacerbate coral-to-algal phase shifts. We use a combination of outdoor tank and in-situ field experiments to quantify the effects of elevated CO2 on the strength of allelopathic interactions between macroalgae and a common coral on the Great Barrier Reef (GBR), Australia. We assess the allelopathic potency, rather than the concentrations, of extracts from the surfaces of macroalgae because many allelopathic compounds are unknown, may act synergistically or additively and thus their combined effects cannot be quantified by evaluating the concentrations of a few known compounds. To examine the generality of macroalgal effects, we used three species of macroalgae (seaweeds) common in GBR reefs: Chlorodesmis fastigiata, Canistrocarpus cervicornis (synonym Dictyota cervicornis) and Amansia glomerata and tested the impact of each on the common coral Acropora intermedia.
First, we conducted an experiment to assess competitive outcomes between the coral A. intermedia and each macroalga when in direct contact and exposed to either present day levels of CO2 or to levels projected to occur by the mid and end of the century under the RCP 8.5 CO2 emission scenarios (ambient = 380 CO2 ppm; mid-century = 540 CO2 ppm; end-of-century = 936 CO2 ppm, see Methods). Once the ability of macroalgae to damage coral tissue under elevated CO2 levels was established, we conducted a second experiment to assess the effect of algal allelochemicals alone by embedding natural concentrations of lipid-soluble extracts from algal surfaces into experimental gels and applying these, and appropriate controls, to A. intermedia growing in the field. For this experiment, the three species of macroalgae were incubated (in isolation) under either high CO2 concentrations (936 CO2 ppm) or ambient CO2 concentrations (380 CO2 ppm) and their lipid-soluble, surface chemicals extracted at the end of the 14 day incubation period. Although macroalgae may induce allelopathy when competing with corals40 we incubated the algae in isolation to evaluate the effects of elevated CO2 alone. Surface extracts were then imbedded in gel pads that were placed in contact with un-manipulated corals in situ on the reef. We used un-manipulated corals to avoid confounding altered potency of the extracts with changed susceptibility of the corals exposed to differing levels of CO2. The chlorophyll fluorescence of the coral’s symbiotic dinoflagellates, which indicates the overall photosynthetic efficiency of corals, was assessed as an indicator of stress41 caused by macroalgal allelopathy22,25,38.
Results
Effects of CO2 and coral-macroalgal competition on coral tissue loss (partial morality)
The coral-macroalgal competition and CO2 treatments significantly affected coral tissue loss. Macroalgal contact with corals increased the rate of coral tissue loss in all CO2 conditions (Fig. 1, Table 1), but the rate of tissue death per day increased significantly under elevated concentrations of CO2 (Figs 1 and 2, Table 1). Coral tissue death also varied by algal species, with C. cervicornis being strongly and significantly more damaging than A. glomerata. Corals exposed to any macroalga experienced significantly higher partial mortality (Table 1), and experienced it more rapidly, than control corals or corals exposed to the algal mimic (Fig. 2). This suggests a chemical or biological mechanism rather than simple impacts due to shading or abrasion. However, corals isolated from all macroalgae and corals in contact with plastic macroalgal mimics (mimicking the physical presence of algae) exposed to medium or high CO2 levels also experienced some tissue loss, indicating that OA alone led to some coral damage (Figs 1 and 2).
Figure 1. Mean rate of tissue loss (percent per day) in Acropora intermedia corals exposed to varying levels of ocean acidification and coral-macroalgal competition treatments.
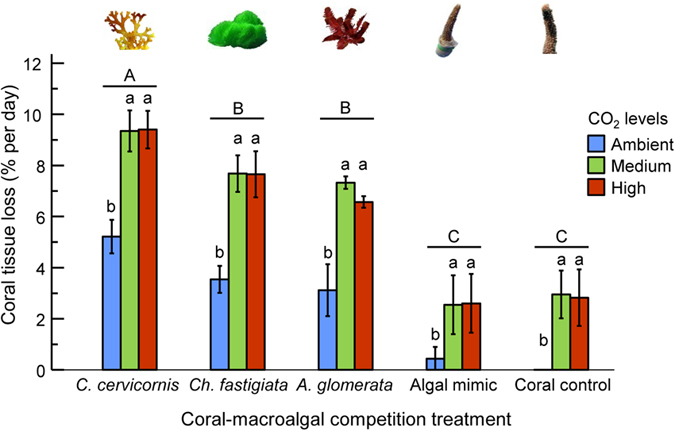
The rate was calculated by estimating the amount of coral tissue loss per day and values were then averaged by the number of days until complete loss of coral tissue. N = 12 coral branches (±SEM). Corals were exposed to contact with the macroalgae Canistrocarpus (=Dictyota) cervicornis, Chlorodesmis fastigiata, Amansia glomerata or a plastic mimic under three levels of CO2: ambient (380 ppm); medium (540 ppm) or high (936 ppm). Controls were corals without macroalgal contact. Data were analysed with a two-way ANOVA. Lowercase (a,b) and uppercase (A,B,C) letters indicate significant differences among CO2 and competition treatments, respectively, via Tukey tests. Complete ANOVA and Tukey test results are in Table 1.
Table 1. Two-way ANOVA to test for the effects of CO2 and coral-macroalgal competition on the rate of coral tissue loss per day (Fig. 1).
| Source of variation | df | MS | F | p-value | Conclusion (Tukey Test) |
|---|---|---|---|---|---|
| CO2 | 2 | 234.6 | 35.2 | <0.001 | High = Med > Amb |
| Algal competition (AC) | 4 | 267.9 | 40.2 | <0.001 | Can > Chlo = Ama > Mimic = Control |
| CO2*AC | 8 | 3.5 | 0.5 | 0.840 | |
| Error | 164 | 6.7 |
CO2 levels included: ambient (Amb), medium (Med), and high. Coral–macroalgal competition levels included: coral with the algae Canistrocarpus (=Dictyota) cervicornis (Can), Chlorodesmis fastigiata (Chlo), and Amansia glomerata (Ama), coral without algae (coral control = Control) and coral with an inert algal mimic (Mimic). df: degrees of freedom; MS: mean squares; F: test statistic.
Figure 2. Percentage of coral tissue loss through time during a coral-algal competition and ocean acidification experiment.
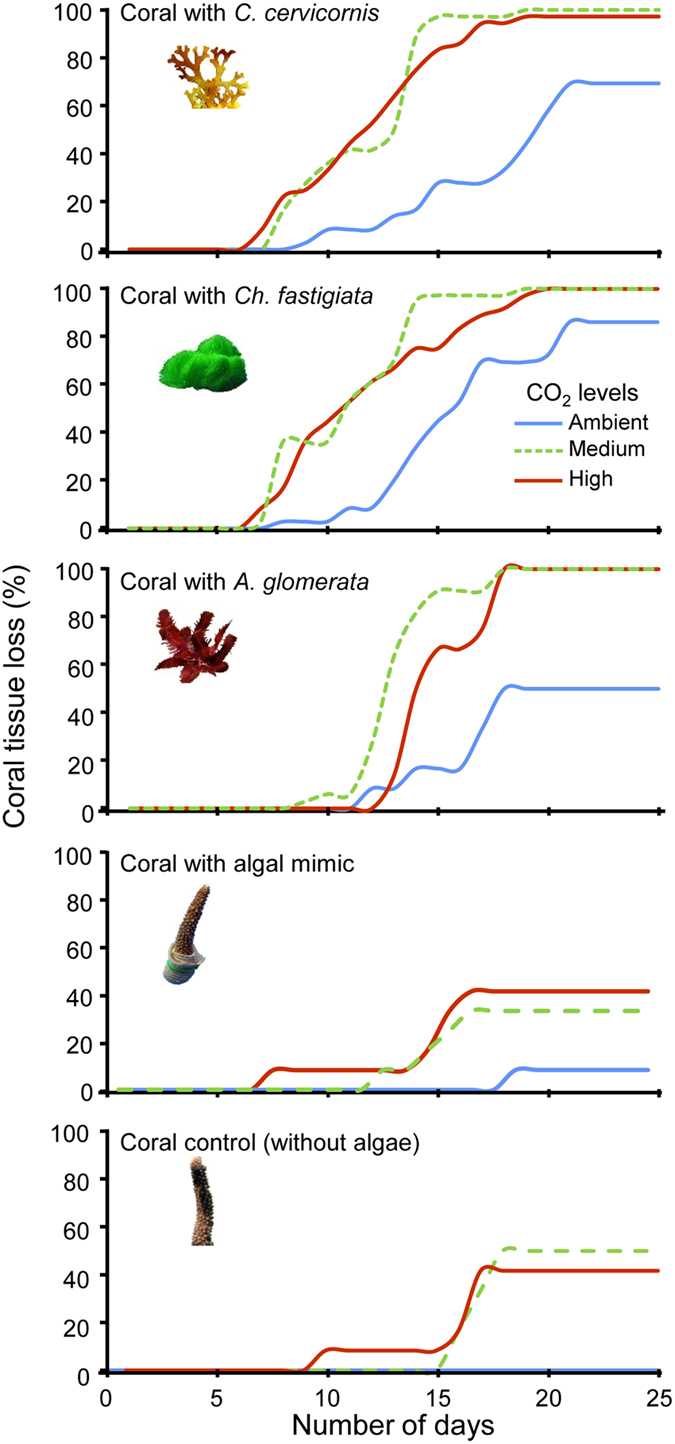
Acropora intermedia corals were exposed to contact with Canistrocarpus (=Dictyota) cervicornis, Chlorodesmis fastigiata, Amansia glomerata or a plastic mimic under three levels of CO2: ambient (380 ppm); medium (540 ppm) or high (936 ppm). Controls were corals without macroalgal contact. Coral tissue loss was estimated daily by counting the number of coral branches that exhibited any tissue loss and data are presented as frequency of dead colonies over the experimental period (25 days).
Effects of CO2 on the potency of macroalgal allelochemicals
Elevated CO2 enhanced macroalgal allelopathy against A. intermedia but only for the alga C. cervicornis (Fig. 3, Table 2). Allelochemicals from C. cervicornis incubated under high CO2 conditions suppressed effective quantum yield (EQY) significantly more than extracts from thalli grown in ambient CO2 (p = 0.037, Table 2). Thus, for C. cervicornis, elevated CO2 concentrations enhanced the negative effects of allelochemicals on coral tissue. In contrast, surface extracts from Ch. fastigiata and A. glomerata were not significantly more potent when grown under elevated CO2 concentrations (Fig. 3, Table 2). The allelopathic effect on corals varied among macroalgal species, with surface extracts from Ch. fastigiata [grown under both ambient and high CO2 (Dunnett test, p = 0.007 and 0.022, respectively)] and C. cervicornis (only under high CO2) significantly reducing effective quantum yield relative to control treatments (Phytagel controls, i.e. gel pads without allelochemicals) (Fig. 3). Extracts from A. glomerata did not cause significant reduction in effective quantum yield at any CO2 level.
Figure 3. Mean effective quantum yield (±SEM, n = 15) of symbiotic dinoflagellates in in-situ corals exposed to contact with lipid-soluble surface extracts from the macroalgae Canistrocarpus (=Dictyota) cervicornis, Chlorodesmis fastigiata and Amansia glomerata grown under elevated CO2 (936 CO2 ppm) and ambient conditions (380 ppm).
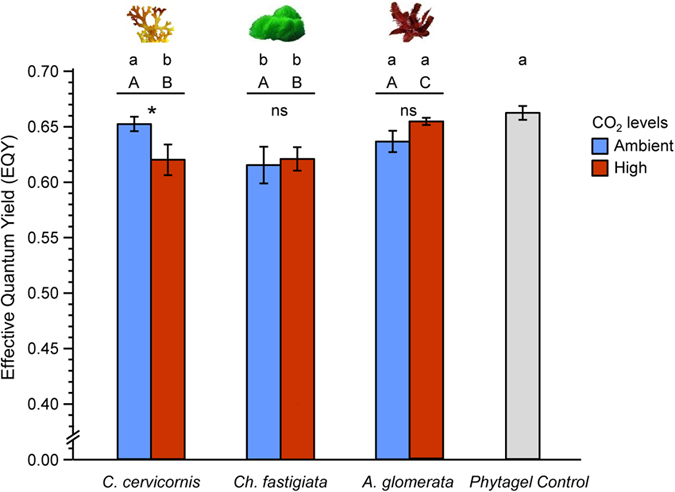
Control corals were in contact with gel pads treated with carrier solvent but without inclusion of algal extract (i.e. Phytagel controls). Extract from C. cervicornis grown under enriched CO2 was significantly more suppressive of photosynthetic efficiency (EQY) compared to extracts from C. cervicornis grown under ambient CO2. Elevated CO2 did not affect the potency of extracts from the other two algal species. Data were analysed with a two-way ANOVA: Asterisks and uppercase (A,B,C) letters indicate significant differences among CO2 and macroalgal treatments, respectively, via posthoc Tukey tests (*p < 0.05, ns: not significant). Complete ANOVA and Tukey test results are in Table 2. Lowercase letters (a,b) indicate significant differences in EQY of the coral compared to Phytagel controls via Dunnett test. Note y-axis break.
Table 2. Two-way ANOVA to test for the effects of extracts from macroalgae [Canistrocarpus (=Dictyota) cervicornis (Can), Chlorodesmis fastigiata (Chlo), and Amansia glomerata (Ama)] grown under two CO2 levels [ambient (Amb) and high] on effective quantum yield (EQY) of Acropora intermedia corals (as a measure of the potency of allelopathic extracts; Fig. 3).
| Source of variation | df | MS | F | p-value | Conclusion (Tukey Test) |
|---|---|---|---|---|---|
| CO2 | 1 | <0.001 | 0.111 | Can: 0.037 | Can: Ambient > High |
| Chlo: 0.775 | Chlo: ns | ||||
| Ama: 0.074 | Ama: ns | ||||
| Algae | 2 | 0.006 | 3.511 | Ambient: 0.078 | Ambient: ns |
| High: 0.025 | High: Ama > Can = Chlo | ||||
| CO2*Algae | 2 | 0.005 | 3.073 | 0.052 | |
| Error | 164 | 6.7 |
Discussion
Corals have declined and seaweeds have increased in abundance on reefs world-wide1,2,3,7,9,10. The relative roles of seaweeds damaging corals directly versus simply colonizing coral skeletons following their death from other causes is unclear and variable6, but numerous experimental manipulations indicate that an increase in macroalgae can directly suppress coral growth, survivorship, and recruitment12,42. As corals decline and macroalgae increase, coral-macroalgal contact increases in frequency43, making it critical to understand the mechanisms determining the outcome of coral-macroalgal competition6,25,26,34,36,38.
All three macroalgae we investigated induced considerable coral tissue loss and two were allelopathic to Acropora intermedia under some conditions, but C. cervicornis became allelopathic only under conditions of OA (Fig. 3, Table 2). This increase in allelopathic potency was modest (about a 5% greater suppression of PSII), but occurred after only 24 h of exposure, using only surface extractions, and would be expected to increase in effect over longer durations. A strengthening of macroalgal competition against reef-building corals under enhanced OA was documented previously for a single macroalgal species (Lobophora cf. papenfussii), but the mechanism was unknown and it was unclear if the cause was enhanced algal allelopathy, altered role of DOC, enhanced coral susceptibility, or a combination of processes15. Here we document for the first time that enhanced allelopathy with increasing OA can be a mechanism by which some macroalgae cause higher levels of coral damage under elevated concentrations of CO2. Investigations have documented macroalgal allelopathy against corals in about 75% of the 40 + coral-macroalgal combinations assayed to date22,24,38,44,45, and elevated effects of macroalgae on corals have been seen for two of the four contrasts tested under conditions of OA [i.e. C. cervicornis (Fig. 3), and L.cf. papenfussii15). Thus, macroalgal allelopathy against corals is common; if enhanced allelopathic potency under OA occurs in Canistrocarpus (=Dictyota), and likely in Lobophora15, which are widespread and abundant globally3,46,47,48,49,50, corals will experience greater stress from macroalgal competition as OA accelerates.
Surface extracts from the brown macroalga C. cervicornis grown under CO2 enriched conditions in flow through tanks caused greater damage to in-situ corals than extracts from C. cervicornis grown under ambient CO2 in equivalent flow through tanks. Because the extract experiment was conducted using in situ corals that had not been exposed to elevated CO2, CO2 effects on the coral can be ruled out. The stronger allelopathy noted in Fig. 3 was due to C. cervicornis becoming more allelopathic rather than the coral becoming more susceptible. The magnitude of the stress caused by the algal extracts in our experimental coral (quantified as a decline in fluorescence levels, EQY, Fig. 3) is smaller than that observed in corals in other coral-algal competition experiments22,25,38. It is likely that different coral species used in these studies have different susceptibilities to algal contact [(e.g. A. prolifera was more resistant to algal allelopathy than A. millepora using same exposure times in pilot studies conducted by the authors GDP, MEH (unpubl. data)], or that the use of different methodologies and exposure times to algal interactions causes variable damage to the corals (e.g. exposure times > 24 hrs would have caused higher coral damage). Importantly, however, coral fluorescence levels were significantly lower when exposed to extracts from C. cervicornis grown under high CO2 levels, compared to extracts from algae grown under ambient CO2, indicating a reduction in the photosynthetic efficiency of the coral symbionts due to lipid-soluble surface extracts alone.
Increased atmospheric CO2 generally enhances production of secondary metabolites in terrestrial plants29,51. Effects of elevated CO2 on secondary metabolites in marine plants appear variable. For example, concentrations of polyphenolics decline while concentrations of dimethylsulfoniopropionate (DMSP) appear to increase with elevated CO231,32,52. In these examples, both phenolics and DMSP are more polar (i.e. more water soluble) secondary compounds that can mediate trophic interactions. In our experiment, we extracted lipid-soluble constituents from the surface of macroalgae because surface associated lipids have been identified as the active components in contact competitive interactions with reef corals, and have been shown to cause bleaching, suppression of photosynthesis, and sometimes mortality of corals22,38,44. The allelopathic compounds identified earlier38,44 are hydro-phobic terpenes associated with macroalgal surfaces. Production of some terpenes increases under elevated CO2 in some terrestrial plants due to higher availability of carbon for plant metabolism53,54. Elevation of CO2 might have enhanced production of allelopathic lipids in C. cervicornis, but not in the other two macroalgae we investigated (in line with33) (Fig. 3). Because Ch. fastigiata and C. cervicornis both make bioactive terpenes37,38,39, the response appears to vary by species and not simply by the class of compound involved. Enhanced concentrations of allelopathic compounds could have also occurred due to reduced macroalgal growth (as suggested for terrestrial plants53), or changes in other processes not considered in this study. However, reduced macroalgal growth is unlikely as parallel experiments showed the photosynthetic efficiency of our experimental Canistrocarpus did not change with CO2 (One-Way ANOVA, n = 12, F = 0.69, p = 0.55) (see Supplementary Fig. S1), and our own previous work on a closely related Dictyotaceae macroalga (Lobophora)15 and that of others on Dictyota has demonstrated increased growth under OA conditions17,18. The mechanisms by which elevated CO2 modifies the potency of Canistrocarpus allelopathy, or the chemical compounds involved in these interactions require additional investigation.
In our outdoor mesocosm experiment, the rates of tissue loss for corals in contact with A. glomerata and Ch. fastigiata also increased under OA conditions, but surface extracts from these species grown under elevated CO2 did not show enhanced allelopathic activity on corals in situ. Thus, other competitive mechanisms or some processes affecting coral physiology may be driving interactions among these species. Corals exposed to OA but free of macroalgae experienced some tissue loss, suggesting that elevated CO2 alone is a physiological stressor19, and this stress may have made the corals in our experimental tanks more susceptible to algal effects (including allelopathic effects) and space competition than the in situ corals we used for testing allelopathic potency (Fig. 3)55,56. In addition to producing allelopathic lipids, macroalgae may also stress corals indirectly by releasing dissolved organic carbon (DOC), which may alter microbial community composition and the balance between beneficial versus pathogenic microbes on coral surfaces and lead to coral death25,34,47. Little is known about the effects of elevated CO2 on DOC production by macroalgae, but it is possible that macroalgae under conditions of OA may uptake excess CO2 and increase release of DOC, as suggested for planktonic microalgae57. Increased release of DOC could have contributed to coral tissue loss in our experimental tank experiment (Figs 1 and 2), but could not explain the allelopathy seen in our field assays using gel-strips (Fig. 3) because these contained the non-polar, not the polar, extracts from macroalgal surfaces. A combination of enhanced coral sensitivity to macroalgal allelochemicals, shifts in microbial communities, and DOC release may have acted in concert to compromise coral health in the presence of A. glomerata and Ch. fastigiata (and possibly C. cervicornis) under elevated CO2. Our results show that although all experimental macroalgae caused damage to the corals when exposed to elevated CO2 levels, there is variability in the mechanisms by which algae stress and kill corals, suggesting that the relative importance of each mechanism varies under OA. Allelopathy in C. cervicornis, and possibly an altered role of DOC in A. glomerata and Ch. fastigiata, in addition to direct impacts on coral holobiont physiology and coral microbiomes may play important roles in driving coral-macroalgal interactions as concentrations of CO2 increase in future oceans.
Our study has implications for understanding the impacts of OA on the dynamics of coral-macroalgal interactions, which may cascade to affect coral-algal phase shifts. First, we demonstrate that common macroalgae are allelopathic to corals and that this allelopathy is strengthened for one of the three macroalgae we investigated under elevated CO2. This suggests that the competitive advantage of some macroalgae over corals may increase and further accelerate phase shifts as OA continues. This may be exacerbated by enhanced macroalgal growth and cover under projected near-future CO215,16,17,18,20,58. Secondly, our data demonstrate variance in competitive mechanisms and intensity between coral and macroalgae under elevated CO2 levels. For example, the brown alga C. cervicornis and the green alga Ch. fastigiata both damage corals faster (Fig. 2) than the red alga A. glomerata. C. cervicornis and Ch. fastigiata both produce potent allelochemicals22,38,43. A. glomerata is not known to produce strong, bioactive compounds that damage corals (although it may be allelopathic against bacteria59), and it affects corals more slowly. This variability implies that coral reefs dominated by Dictyotaceae algae like C. cervicornis and Lobophora papenfussii15, may be more susceptible to damage than reefs dominated by A. glomerata as CO2 concentrations increase. The macroalga Ch. fastigiata is common but rarely abundant on reefs43 and may be a lesser concern under future OA scenarios.
Our results may be particularly important given the current dominance of Dictyota (now changed to Canistrocarpus for some species) and Lobophora on many Caribbean3,23,48 and a number of Indo-Pacific reefs49. The dominance of these Dictyotaceae seaweeds has been attributed mainly to reductions of grazing by reef herbivores and to additional space made available by coral mortality from cyclones, disease, thermal bleaching, and crown-of-thorn-starfish49,60,61. Dictyotaceae seaweeds, however, may also actively damage corals via a variety of direct (e.g. overgrowth and allelopathy22,24) and indirect (e.g. DOC release and alteration of coral microbial activity and coral microbiome26,28) mechanisms (or combination of both, e.g. ref. 26), contributing to coral decline and macroalgal dominance. The increased potency of allelopathic interactions with C. cervicornis (and potentially Lobophora papenfussii15) due to OA conditions, suggests that the 75% increase in CO2 concentrations that have occurred since preindustrial times13 may already be advantaging these macroalgae over corals. Dictyotaceae species such as Dictyota21, Padina62 and Spatoglossum63 are known to proliferate in naturally acidified reefs, although whether these taxa also enhance the potency of allelopathic interactions against corals under elevated CO2 needs to be investigated. Further, enhancement of algal allelochemicals due to CO2 enrichment may not only alter competitive interactions, but also trophic interactions by reducing macroalgal palatability and consequently macroalgal removal by herbivores (via reduction in grazing), although this hypothesis needs testing (e.g. ref. 33) in Dictyotaceae algae. Reduction in herbivory pressure due to overfishing9 as well as enhanced coral mortality due to increased sea surface temperature19,64, declining water quality47, and reductions of coral recruitment due to algal competition and dominance12,65 will further contribute to algal proliferation, ultimately eroding reef resilience. Numerous seaweeds may be competitively advantaged over corals as OA increases. This will occur not only from corals being weakened by OA, but also by the allelopathic potency of some seaweeds being enhanced by OA or by other mechanisms not tested in this study (e.g. altered DOC production/consumption). This adds to the complex list of interacting stressors that tropical reefs face now, as well as in the future.
Methods
Effects of elevated CO2 on coral-macroalgal competition
To evaluate the effects of elevated CO2 on coral-macroalgal competition and how this varies among species pairings, we conducted a factorial outdoor experiment at the Heron Island Research Station (HIRS), GBR (23°26′30″S–151°54′46″E). We used the branching coral Acropora intermedia and three fleshy macroalgae, the green alga Chlorodesmis fastigiata, the brown alga Canistrocarpus (=Dictyota) cervicornis, and the red alga Amansia glomerata. These seaweeds were common and frequently in contact with corals on the reef66. Corals and macroalgae were collected by hand at 3–6 m depth from Harry’s-Bommie, Heron Island, GBR (23°27′32″S, 151°55′45″E) and acclimatised separately in shaded outdoor tanks using a flow-through system with ambient conditions for two weeks. Light levels in the acclimatisation tanks were similar to those of the experimental containers (see below). A. intermedia was the assay coral because it is abundant in the study area (average cover of 22% in the colleting site, but can be locally dominant, Del Monaco, unpubl. data). C. cervicornis and A. glomerata are also abundant in Heron reefs, the latter particularly so at the base of coral branches in coral dominated areas. Transect surveys show C. cervicornis, A. glomerata cover in average 4% and 5% respectively in shallow reefs of Heron Island (Del Monaco, unpubl. data). Ch. fastigiata is common but less abundant (1% cover), and similar to C. cervicornis, it produces bioactive terpenoids that harm corals38.
Our factorial experiment consisted of a coral-algal competition treatment with five levels and an ocean acidification treatment with three levels of CO2/pH manipulation. The coral-algal competition treatment included (1) coral- Ch. fastigiata interaction pair (a live coral branch with the alga Ch. fastigiata attached to the base with a plastic cable tie); (2) coral- C. cervicornis interaction pair (as for the previous treatment level), (3) coral- A. glomerata interaction pair, (4) coral control (coral alone, no alga present); and (5) a coral branch in contact with an inert algal mimic (monofilament fabric) attached to the base of the coral with a plastic cable tie; this assessed the effect of an alga’s physical, but not chemical or biological, presence on the coral (as per15)(see also Supplementary Fig. S2 for diagram of the experimental design). Due to logistic constrains, we could only use one type of algal mimic morphology for the three algal species. Coral branches were 8–10 cm long and the algal biomass was 3–7 g (wet weight).
Corals and macroalgae were exposed to three CO2 levels; ambient (380 CO2 ppm, pH = 8.16 ± 0.02), medium (540 CO2 ppm; pH = 7.86 ± 0.03), and high (936 CO2 ppm; pH = 7.70 ± 0.02) CO2, simulating current levels and those predicted for the years 2050 and for 2100, respectively, under the RCP 8.5 model of the Intergovernmental Panel for Climate Change (IPCC)13. CO2 concentrations were achieved using established methods15,67. In brief, pH was regulated by computer operated solenoid valves (Aquatronica-AEB Technologies, Cavriago, Italy) that controlled the amount of analytical grade CO2 bubbled into 200 l mixing sumps. pH sensors (Mettler-Toledo, InPro4501VP) logged measurements every 30 minutes, and calibration was linearly performed using three NIST-certified pH buffers (Mettler Toledo, Switzerland). Ambient and CO2 treated seawater from the mixing sumps continuously fed the experimental tanks (20 l plastic containers, see below) at a flow rate of 1.79 ± 0.08 (SEM) l/min using unfiltered seawater from the reef flat. Total alkalinity was estimated from each CO2 treatment by an open-cell potentiometric titrator (Model T50 Mettler Toledo) using triplicate water samples taken from the experimental containers every 4 hours during a 24 h-period. We also collected water samples from the sumps and did not find significant differences in carbonate chemistry between sumps and experimental tanks (Table 3, t-student, p = 0.95). Total alkalinity and pH values determined in the samples were utilised to constrain the carbonate chemistry of the experimental system68,69 (Table 3).
Table 3. Seawater carbonate chemistry parameters estimated in experimental tanks and mixing sumps for each CO2 level in both experiments.
| CO2 level | Temp (°C) | pH NBS/NIST | pCO2 (ppm) | DIC (μmol/KgSW) | TA (μmol/Kg SW) | ΩArag |
|---|---|---|---|---|---|---|
| High | ||||||
| Tank | 25.58 | 7.70 | 936 | 2109.95 | 2245.28 | 1.52 |
| (0.58) | (0.02) | (13.86) | (1.65) | (0.04) | ||
| Sump | 25.58 | 7.70 | 936 | 2108.72 | 2243.99 | 1.51 |
| (0.59) | (0.03) | (11.87) | (2.71) | (0.01) | ||
| Medium | ||||||
| Tank | 25.58 | 7.86 | 540 | 2036.55 | 2241.37 | 2.13 |
| (0.58) | (0.03) | (13.77) | (2.06) | (0.05) | ||
| Sump | 25.58 | 7.86 | 540 | 2036.34 | 2241.15 | 2.11 |
| (0.59) | (0.04) | (12.57) | (8.41) | (0.02) | ||
| Ambient | ||||||
| Tank | 25.58 | 8.16 | 380 | 1862.20 | 2236.27 | 3.74 |
| (0.58) | (0.02) | (12.09) | (3.96) | (0.08) | ||
| Sump | 25.58 | 8.16 | 380 | 1865.6 | 2240.11 | 3.74 |
| (0.59) | (0.03) | (12.45) | (4.65) | (0.02) | ||
PCO2 = partial pressure of CO2; DIC = Total dissolved inorganic carbon; TA = Total alkalinity; ΩArag: Aragonite saturation state. Salinity of 34.46 was used for the calculations using the CO2SYS computer software. Values are means [n = 12 (3 samples collected every 4 hours during a 24 hr period)] ± SE.
Three replicate 20 l tanks were used for each combination of competition, CO2, and macroalgal treatment levels, each tank holding four experimental sub-samples (see Supplementary Fig. S2). Species of macroalgae were not intermixed in each tank but kept separate. Coral control and coral-algal mimic levels were the same for the three algal species and due to logistic constrains, no attempt was made to mimic individual algal morphology. Sub-samples were randomly allocated to replicate tanks and were suspended in the water column on a fishing line. A small power head in each tank assured water circulation, and all tanks were cleaned every 3 days to reduce biofilms. Shading screens were placed over the experimental area to reduce light intensity and heating, approximating field conditions. Underwater light measurements in the tanks averaged 242.11 ± 84.78 (SEM) μM at 4:00 pm (in situ light measurements in the field ranged between 220 and 488 μM at 4 m depth at 4:00 pm; all light measurements were obtained using a LI-1400 data logger, LI-192SA sensor). Seawater temperature averaged 25.87 ± 0.14 (SEM) °C and salinity was 34.46 ppm (measured using a portable refractometer) during the experiment. The experiment was carried out for 25 days during March 2013. The response variable included a measurement of coral tissue loss (an indicator of partial colony mortality), which was recorded daily in the tanks as the amount of sloughed tissue using four categories: 0, 1–33, 34–66 and 67–100% per coral branch. Coral branches exhibiting partial tissue loss were examined under the stereomicroscope confirming the absence of coral tissue in the affected areas. Coral tissue loss generally progressed from the base upwards along the branch.
Effects of elevated CO2 on the potency of macroalgal allelopathy
To test whether elevated CO2 enhanced the allelopathic potency of seaweeds, the macroalgae C. cervicornis, A. glomerata and Ch. fastigiata were exposed to elevated CO2 concentrations, their surface metabolites extracted, these extracts applied to corals in situ, and the damage on the corals compared to control extracts from algae grown at ambient CO2 concentrations. Macroalgal collections and CO2 manipulations were conducted in the same way as described above. Macroalgae were exposed to two levels of CO2, ambient (control, pH: 8.16 ± 0.02 SEM) and high (936 CO2-ppm, pH: 7.69 ± 0.02 SEM) for 14 days (February 25th to March 10th, 2013). Ambient and high CO2 treated seawater from the mixing 200 l sumps continuously fed the experimental tanks at a flow rate of 1.79 l/min (see sumps carbonate chemistry data in Table 3). Each macroalgal species was grown separately in 200-l plastic sumps exposed to natural sunlight. Experimental conditions including light levels, temperature, and water circulation were the same as for the previous experiment. Following the two week CO2 treatment, surface allelochemicals from the experimental macroalgae were extracted following existing protocols70. In short, approximately 20 ml displacement volume of each algal species (which included several individuals) was cleaned of fouling organisms and spun with a salad spinner to remove excess water; algae were then submerged in hexane (98%) and vortexed for 30 seconds. Algal tissue was removed, the solvent removed via rotary evaporation, and the hexane-soluble surface extract retained, and incorporated at natural volumetric concentration into 1 × 1 cm Phytagel pads (following methods of22,38). Previous assays with allelopathic seaweeds showed this method was entirely sufficient to obtain bioactivity from the algal surface that equalled activity of the entire extract22,38,44. Gel pads impregnated with macroalgal extracts from each species and CO2 level were put into contact with the surface of A. intermedia branches (n = 15) in situ at a depth of 6 m on the fore reef of Heron Island (Harrys Bommie). Gel pads were placed approximately 3–5 cm from the tip of the coral branch and gently secured with a plastic cable tie. 15 replicates per CO2 and algal treatment combination were used in addition to 15 control pads (same solvent used but no extracts, i.e. Phytagel controls) to test for possible effects of the gels, solvents, and extraction protocol on the corals. Each coral branch contained only one gel and gel pads from the different algal species were not intermixed in each coral branch. In total, we used 105 gel pads that were placed on coral colonies distributed over an area of approx. 150 m2. Gel pads were removed after 24 hours and the symbiotic dinoflagellate chlorophyll fluorescence (Effective Quantum Yield, EQY) assessed in situ beneath the area that had been covered by the gel pad using a Pulse-Amplitude-Modulation Fluorometer [Walz, Model: Diving-PAM with red measuring light LED, 650 nm; settings: damp = 2 and gain = 1–3; fiberoptics: DIVING-F, active diameter 5.5 mm; all measurements (one per coral branch) were made at a distance of 2 mm from the coral surface and to standardise that distance we used a plastic tube adapter]. EQY has been previously used to estimate photosynthetic efficiency and coral health38,41. The 24 hour exposure period to the gel pads was insufficient to induce coral tissue loss or considerable bleaching (authors’ per. obs.), therefore EQY was chosen for this experiment. All PAM measurements were conducted between 11:00 and 13:00 hrs, with treatment types interspersed through time.
Data analyses
The rate of coral tissue loss was calculated on a daily basis, and values averaged by the number of days until complete loss of coral tissue, or until the end of the experiment at day 25 (if living tissue remained at the end of the experiment) (Fig. 1). This rate of tissue loss was statistically compared among treatments with a two-way nested ANOVA, with CO2 and coral-algal competition as fixed factors, tanks as the nested component, and branches as replicates. There was no significant effect of the nested (i.e. tank) component on the rate of coral tissue loss (p = 0.584). We therefore pooled the data to increase the power of the analyses71 and subsequently conducted a two-way (non-nested) ANOVA with 12 replicate branches per treatment combination, followed by post hoc Tukey tests (Table 1).
EQY values of corals were compared with a two-way ANOVA with CO2 (two levels: extracts of algae grown under ambient CO2, and extracts from algae grown under high CO2) and algal treatments (three algal species) as fixed factors. The analyses included 15 coral branches as replicates. Significant interactions occurred between treatments, therefore we conducted subsequent one-way ANOVAs within treatment combinations followed by post hoc Tukey tests. EQY values for each CO2 and algal treatment combination were also compared against the phytagel control using a 2-sided Dunnett test using SYSTAT software. Data were tested for normality using Shapiro-Wilk test and for homogeneity of variances using Levene-Equality of Several Variance (for fluorescence values) and Cochran tests (for coral tissue loss) and data were not transformed for the analyses.
Additional Information
How to cite this article: Del Monaco, C. et al. Effects of ocean acidification on the potency of macroalgal allelopathy to a common coral. Sci. Rep. 7, 41053; doi: 10.1038/srep41053 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We thank C. Barrón, A. Ordoñez, C. Birrell and B. Lewis for assistance in the lab, S. Byrnes for providing the rotovap, and GBRMPA for providing the permit to conduct this research (G35391.1). This project was funded by the Australian Research Council (DP-120101778) and the Great Barrier Reef Foundation.
Footnotes
The authors declare no competing financial interests.
Author Contributions C.D.M. participated in the design of the study, collected aquaria experimental data, conducted field work and carried out lab and statistical analyses. G.D.-P. conceived, designed and coordinated the study, participated in lab and field work, collected field data and conducted statistical analyses. M.E.H. participated in the design of the study, conducted lab and field work and guided the allelopathy work. P.G. participated in collection of aquaria and field data and lab work. P.J.M. participated in the design of the study. C.D., G.D.-P., M.E.H. drafted initial manuscript and P.J.M., P.G. contributed to the writing of the paper. All authors gave final approval for publication.
References
- Dodge R. E. et al. A call to action for coral reefs. Science 322, 189–190 (2008). [DOI] [PubMed] [Google Scholar]
- De’ath G., Fabricius K. E., Sweatman H. & Puotinen M. The 27–year decline of coral cover on the Great Barrier Reef and its causes. Proc. Natl. Acad. Sci. USA 109, 17995–17999 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B. C., Donovan M. K., Cramer K. L. & Lam V. V. Status and Trends of Caribbean Coral Reefs: 1970–2012 (Global Coral Reef Monitoring Network, IUCN, Gland, Switzerland, 2014). [Google Scholar]
- Bruno J. F., Sweatman H., Precht W. F., Selig E. R. & Schutte V. G. Assessing evidence of phase shifts from coral to macroalgal dominance on coral reefs. Ecology 90, 1478–1484 (2009). [DOI] [PubMed] [Google Scholar]
- Hughes T. P., Graham N. A. J., Jackson J. B. C., Mumby P. J. & Steneck R. S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evol. 25, 633–642 (2010). [DOI] [PubMed] [Google Scholar]
- McCook L. J., Jompa J. & Diaz-Pulido G. Competition between corals and algae on coral reefs: a review of evidence and mechanisms. Coral Reefs 19, 400–417 (2001). [Google Scholar]
- Mumby P. J. & Steneck R. S. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 991, 1–9 (2008). [DOI] [PubMed] [Google Scholar]
- Roff G. & Mumby P. J. Global disparity in the resilience of coral reefs. Trends Ecol. Evol. 27, 404–413 (2012). [DOI] [PubMed] [Google Scholar]
- Jackson J. B. C. et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 293, 629–638 (2001). [DOI] [PubMed] [Google Scholar]
- Gardner T. A., Cote I. M., Gill J. A., Grant A. & Watkinson A. R. Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (2003). [DOI] [PubMed] [Google Scholar]
- Diaz-Pulido G. & McCook L. J. The fate of bleached corals: patterns and dynamics of algal recruitment. Mar. Ecol. Prog. Ser. 232, 115–128 (2002). [Google Scholar]
- Dixson D. L., Abrego D. & Hay M. E. Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345, 892–897 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- IPCC, 2. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, 2014). [Google Scholar]
- Anthony K. R. N. et al. Ocean acidification and warming will lower coral reef resilience. Glob. Change Biol. 17, 1798–1808 (2011). [Google Scholar]
- Diaz-Pulido G., Gouezo M., Tilbrook B., Dove S. & Anthony K. R. N. High CO2 enhances the competitive strength of seaweeds over corals. Ecol. Lett. 14, 156–162 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabricius K. E. et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nature Clim. Change 1, 165–169 (2011). [Google Scholar]
- Hofmann L. C. et al. CO2 and inorganic nutrient enrichment affect the performance of a calcifying green alga and its noncalcifying epiphyte. Oecologia 177, 1157–1169 (2015). [DOI] [PubMed] [Google Scholar]
- Johnson M. D., Price N. N. & Smith J. E. Contrasting effects of ocean acidification on tropical fleshy and calcareous algae. PeerJ 2, e411 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S. & Hoegh-Guldberg O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell S. D., Kroeker K. J., Fabricius K. E., Kline D. I. & Russell B. D. The other ocean acidification problem: CO2 as a resource among competitors for ecosystem dominance. Philos. Trans. R. Soc. Lond. 368, 20120442 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Spencer J. M. et al. Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99 (2008). [DOI] [PubMed] [Google Scholar]
- Rasher D. B. & Hay M. E. Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. USA 107, 9683–9688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery M. & Lesser M. P. Allelopathy in the tropical alga Lobophora variegata (Phaeophyceae): Mechanistic basis for a phase shift on mesophotic coral reefs? J. Phycol. 50, 493–505 (2014). [DOI] [PubMed] [Google Scholar]
- Vieira C. et al. Allelopathic interactions between the brown algal genus Lobophora (Dictyotales, Phaeophyceae) and scleractinian corals. Sci. Rep. 6, 18637 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. E. et al. Indirect effects of algae on coral: algae-mediated, microbe induced coral mortality. Ecol. Lett. 9, 835–845 (2006). [DOI] [PubMed] [Google Scholar]
- Morrow K. M., Ritson-Williams R., Ross C., Liles M. R. & Paul V. J. Macroalgal extracts induce bacterial assemblage shifts and sublethal tissue stress in Caribbean corals. PLoS One 7, e44859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugues M. M., Smith G. W., Hooidonk R. J. v., Seabra M. I. & Bak R. P. M. Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923 (2004). [Google Scholar]
- Vega Thurber R. et al. Macroalgae decrease growth and alter microbial community structure of the reef-building coral, Porites astreoides. Plos One 7, e44246 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidart-Bouzat M. G. & Imeh-Nathaniel A. Global change effects on plant chemical defenses against insect herbivores. J. Integr. Plant Biol. 50, 1339–1354 (2008). [DOI] [PubMed] [Google Scholar]
- Landosky J. M. & Karowe D. N. Will chemical defenses become more effective against specialist herbivores under elevated CO2? Glob. Change Biol. 3159–3176 (2014). [DOI] [PubMed] [Google Scholar]
- Arnold T. et al. Ocean acidification and the loss of phenolic substances in marine plants. PLoS One 7, e35107 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancor S., Tuya F., Gil-Díaz T., Figueroa F. L. & Haroun R. Effects of a submarine eruption on the performance of two brown seaweeds. J. Sea Res. 87, 68–78 (2014). [Google Scholar]
- Campbell J. E., Craft J. D., Muehllehner N., Langdon C. & Paul V. P. Responses of calcifying algae (Halimeda spp.) to ocean acidification: implications for herbivores. Mar. Ecol. Prog. Ser. 514, 43–56 (2014). [Google Scholar]
- Barott K. L. & Rohwer F. L. Unseen players shape benthic competition on coral reefs. Trends Microbiol. 12, 621–628 (2012). [DOI] [PubMed] [Google Scholar]
- Wangpraseurt D. et al. In situ oxygen dynamics in coral-algal interactions. PLoS One 7, e31192 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. L. & Carpenter R. C. Water-flow mediated oxygen dynamics within massive Porites-algal turf interactions. Mar. Ecol. Prog. Ser. 490, 1–10 (2013). [Google Scholar]
- Hay M. E. & Fenical W. Marine plant-herbivore interactions: the ecology of chemical defense. Ann. Rev. Ecol. Syst. 19, 111–145 (1988). [Google Scholar]
- Rasher D. B., Paige Stout E., Engel S., Kubanek J. & Hay M. E. Macroalgal terpenes function as allelopathic agents against reef corals. Proc. Natl. Acad. Sci. USA 108, 17726–17731 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi M. P., Sneed J. M., Sharp K. H. & Paul V. J. Marine chemical ecology in benthic environments. Nat. Prod. Rep. 31, 1510–1553 (2014). [DOI] [PubMed] [Google Scholar]
- Rasher D. B. & Hay M. E. Competition induces allelopathy but suppresses growth and anti-herbivore defence in a chemically rich seaweed. Proc. R. Soc. B 281, 20132615 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. S. & Deheyn D. D. Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Sci. Rep. 3, 1421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes T. P. et al. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 1–6 (2007). [DOI] [PubMed] [Google Scholar]
- Bonaldo R. M. & Hay M. E. Seaweed-coral interactions: variance in seaweed allelopathy, coral susceptibility, and potential effects on coral resilience. PLoS One 9, e85786 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andras T. F. et al. Seaweed allelopathy against coral: Surface distribution of a seaweed secondary metabolite by imaging mass spectrometry. J. Chem. Ecol. 38, 1203–1214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul V. J. et al. Chemically mediated interactions between macroalgae Dictyota spp. and multiple life-history stages of the coral Porites astreoides. Mar. Ecol. Prog. Ser. 426, 161–170 (2011). [Google Scholar]
- Vroom P. S. & Braun C. L. Benthic composition of a healthy subtropical reef: Baseline species-level cover, with an emphasis on algae, in the Northwestern Hawaiian Islands. PLoS One 5, e9733 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaneveld J. R. et al. Overfishing and nutrient pollution interact with temperature to disrupt coral reefs down to microbial scales. Nat. Commun. 7, 11833 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pulido G. & Díaz J. M. Algal assemblages in lagoonal reefs of Caribbean oceanic atolls. Proc. 8th Int. Coral Reef Symp. 1, 827–832 (1997). [Google Scholar]
- Cheal A. J. et al. Coral-macroalgal phase shifts or reef resilience: links with diversity and functional roles of herbivorous fishes on the Great Barrier Reef. Coral Reefs 29, 1005–1015 (2010). [Google Scholar]
- Renken H., Mumby P. J., Matsikis I. & Edwards H. J. Effects of physical environmental conditions on the patch dynamics of Dictyota pulchella and Lobophora variegata on Caribbean coral reefs. Mar. Ecol. Prog. Ser. 403, 63–74 (2010). [Google Scholar]
- Agrell J., McDonald E. P. & Lindroth R. L. Effects of CO2 and light on tree phytochemistry and insect performance. Oikos 88, 259–272 (2000). [Google Scholar]
- Burdett H. L., Donohue P. J. C., Hatton A. D., Alwany M. A. & Kamenos N. A. Spatiotemporal variability of dimethylsulphoniopropionate on a fringing coral reef: the role of reefal carbonate chemistry and environmental variability. PLoS One 8, e64651 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuelas J. & Llusią J. Effects of carbon dioxide, water supply and seasonality on terpene content and emission by Rosmarinus officinalis. J. Chem. Ecol. 23, 979–993 (1997). [Google Scholar]
- Sun Z., Hüve K., Vislap V. & Niinemets U. Elevated [CO2] magnifies isoprene emissions under heat and improves thermal resistance in hybrid aspen. J. Exp. Bot. 64, 5509–5523 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radecker N., Meyer F. W., Bednarz V. N., Cardini U. & Wild C. Ocean acidification rapidly reduces dinitrogen fixation associated with the hermatypic coral Seriatopora hystrix. Mar. Ecol. Prog. Ser. 511, 297–302 (2014). [Google Scholar]
- Morrow K. M. et al. Natural volcanic CO2 seeps reveal future trajectories for host-microbial associations in corals and sponges. ISME J. 9, 894–908 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo K. R. Carbon Cycle - Marine Manipulations. Nature 450, 491–492 (2007). [DOI] [PubMed] [Google Scholar]
- Diaz-Pulido G. et al. In Climate change and the Great Barrier Reef (ed. Johnson J. E. & Marshall P. A.) 153–192 (Great Barrier Reef Marine Park Authority & Australian Greenhouse Office, Townsville, 2007). [Google Scholar]
- Lima-Filho J. V. M., Carvalho A. F. F. U., Freitas S. M. & Melo V. M. M. Antibacterial activity of extracts of six macroalgae from the northeastern Brazilian coast. Braz. J. Microbiol. 33, 311–313 (2002). [Google Scholar]
- Hughes T. P. Catastrophes, phase-shifts and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 (1994). [DOI] [PubMed] [Google Scholar]
- Diaz-Pulido G. et al. Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS One 4, e5239 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson V. R., Russell B. D., Fabricius K. E., Brownlee C. & Hall-Spencer J. M. Temperate and tropical brown macroalgae thrive, despite decalcification, along natural CO2 gradients. Glob. Change Biol. 18, 2792–2803 (2012). [DOI] [PubMed] [Google Scholar]
- Enochs I. C. et al. Shift from coral to macroalgae dominance on a volcanically acidified reef. Nature Clim. Change 1083–1088 (2015). [Google Scholar]
- Hoegh-Guldberg O. et al. Coral reefs under rapid climate change and ocean acidification. Science 318, 1737–1742 (2007). [DOI] [PubMed] [Google Scholar]
- Olsen K., Paul V. J. & Cliff R. Direct effects of elevated temperature, reduced pH, and the presence of macroalgae (Dictyota spp.) on larvae of the Caribbean coral Porites astreoides. Bull. Mar. Sci. 91, 255–270 (2015). [Google Scholar]
- Bender D., Diaz-Pulido G. & Dove S. Effects of macroalgae on corals recovering from disturbance. J. Exp. Mar. Biol. Ecol. 429, 15–19 (2012). [Google Scholar]
- Diaz-Pulido G. et al. Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs. Nat. Commun. 5, 3310 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. & Wallace D. W. R. Program Developed for CO2 System Calculations (ORNL/CDIAC-105, Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US Department of Energy, Oak Ridge, Tennessee, 1998).
- Dickson A. G., Sabine C. L. & Christian J. R. Guide to best practices for ocean CO2 measurements (North Pacific Marine Science Organization (PICES Special Publication, 3), Sidney, British Columbia, 2007).
- Nylund G. M., Gribben P. E., De Nys R., Steinberg P. D. & Pavia H. Surface chemistry versus whole-cell extracts: antifouling tests with seaweed metabolites. Mar. Ecol. Prog. Ser. 329, 73–84 (2007). [Google Scholar]
- Underwood A. J. Experiments in Ecology: Their Logical Design and Interpretation Using Analysis of Variance (Cambridge University Press, Cambridge, UK., 1997). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


