Abstract
Studies of subjective and genital sexual arousal in monosexual (i.e. heterosexual and homosexual) men have repeatedly found that erotic stimuli depicting men’s preferred sex produce strong responses, whereas erotic stimuli depicting the other sex produce much weaker responses. Inconsistent results have previously been obtained in bisexual men, who have sometimes demonstrated distinctly bisexual responses, but other times demonstrated patterns more similar to those observed in monosexual men. We used fMRI to investigate neural correlates of responses to erotic pictures and videos in heterosexual, bisexual, and homosexual men, ages 25–50. Sixty participants were included in video analyses, and 62 were included in picture analyses. We focused on the ventral striatum (VS), due to its association with incentive motivation. Patterns were consistent with sexual orientation, with heterosexual and homosexual men showing female-favoring and male-favoring responses, respectively. Bisexual men tended to show less differentiation between male and female stimuli. Consistent patterns were observed in the whole brain, including the VS, and also in additional regions such as occipitotemporal, anterior cingulate, and orbitofrontal cortices. This study extends previous findings of gender-specific neural responses in monosexual men, and provides initial evidence for distinct brain activity patterns in bisexual men.
Sexual Orientation and Sexual Motivation
The term sexual orientation is used to indicate an individual’s sexual arousal and attraction patterns, sexual behavior patterns, or sexual identity1, which often go together but can also differ from each other2,3,4,5,6. While most individuals identify as heterosexual, a significant number of individuals also report identifying as homosexual (1.9–2% of the US population) or bisexual (2–4% of the US population), with even greater proportions reporting some degree of same-sex behavior or attraction7,8.
If the term sexual orientation is used to describe a pattern of arousal and attraction1, then genital assessment has high face validity for studying sexual orientation in men. Neuroimaging, however, may have a variety of methodological advantages, including the potential for greater sensitivity in detecting motivational responses to stimuli that are psychologically significant yet unlikely to result in noticeable physiological changes9 or even subjective responses10. Even with briefly-presented erotic pictures, fMRI has demonstrated a high degree of sensitivity and specificity in measuring sexual orientation11,12,13.
Furthermore, genital arousal is capable only of indicating degree of increase or decrease along a single dimension of tumescence, and thus provides little qualitative information on the mental states underlying sexual arousal and desire. Neuroimaging techniques, in contrast, can localize activity within various brain structures, and therefore suggest and test hypotheses about diverse psychological processes influencing sexuality. The present research focuses on reward-related brain regions.
Neural Correlates of Sexuality and Reward
Numerous studies have examined the neuroimaging correlates of responses to sexual stimuli since the first investigations by Rauch et al.14 and Stoléru et al.15. Specific results vary by experimental paradigm, but findings generally suggest that mechanisms underlying the response to erotic stimuli overlap with those involved in responding to arousing and rewarding stimuli more generally16,17,18. This is unsurprising, since to the extent that individuals are oriented to seek out particular sexual interactions19, they are probably—although not necessarily5—motivated by the anticipation that such interactions will be rewarding20,21,22.
However, since a particular brain area can activate for multiple reasons, caution is needed in making inferences about functional significance from observed activity23,24,25. When sexual stimuli produce significant fMRI responses, it can be difficult to determine the extent to which different brain regions indicate general arousal, sexual arousal, or both. In studies that have attempted to distinguish sexual arousal and general arousal (i.e., non-sexual and potentially non-valenced autonomic, neuroendocrine, and neuromodulatory changes), only activity within the ventral striatum (VS) and hypothalamus have been specifically associated with the experience of stimuli as erotic, and in addition correlating with degree of sexual intensity26,27.
The VS—and in particular the nucleus accumbens subsection—is a neural epicenter for selecting actions on the basis of their relative valuations28,29. As such, it may be the ideal region of interest for investigating sexual preferences. VS activity is the most widely-used measure of preferences for choices and outcomes in the neuroeconomics literature30,31. The VS has been associated with motivational processes in a wide variety of neuroimaging studies, ranging from the desire to breathe when deprived of air32, or drink when thirsty33, to cravings for food34 and drugs35, to feelings of aesthetic appreciation and attraction36, to compulsive videogame playing37, social approval38,39, monetary rewards39,40,41, and more. The underlying factor across all of these paradigms is reward, suggesting that the VS contributes to a “common neural currency” of value42.
Notably, dopaminergic stimulation of the VS does not produce subjective feelings of pleasure43,44,45, but rather seems to be more closely related to desire, craving, or wanting. Indeed, one study found that relative VS responses to food and erotic images predicted relative levels of weight gain and sexual desire in the months following fMRI assessment46.
Thus, while VS activity does not necessarily indicate sexual arousal, it can nonetheless be used to assess relative degrees of motivation while viewing sexual stimuli28,47,48. That is, relatively greater VS activity in response to either male or female erotic images would be consistent with relative androphilic or gynephilic preferences, respectively.
Support for VS as a measure of male sexual orientation is also evidenced by earlier studies of heterosexual and homosexual men’s neural responses to visual erotica13,49,50,51,52. All of these studies found the VS to be included among brain regions with greater activity in response to erotic stimuli featuring participants’ preferred sexes (i.e. women for heterosexual males and men for homosexual males) relative to their non-preferred sexes (i.e. men for heterosexual males and women for homosexual males).
These neuroimaging results are consistent with findings from the genital arousal literature53,54,55,56, wherein heterosexual or homosexual men both tend to exhibit category-specific patterns of subjective and genital arousal. That is, heterosexual and homosexual men exhibit—with a high degree of correspondence between genital and subjective measures—substantial arousal to erotic stimuli depicting their preferred sex, and little arousal to their non-preferred sex. Neuroimaging research suggests that these category-specific arousal patterns are also reflected in category-specific patterns of activation in the brain’s reward system13,49,50,51,52,57,58.
What do these patterns look like for bisexual men? Studies of bisexually-identified men have produced inconsistent findings59,60. Bisexual men consistently exhibit bisexual patterns of subjective arousal, but have sometimes shown category-specific patterns of genital arousal4,61,62. More recently, bisexual genital arousal patterns have been found in a study that used particularly strict inclusion criteria for bisexuality, requiring bisexual participants to have had at least two sexual partners and one romantic partner (of three months or greater duration) of each sex63,64. The present study is the first to study bisexuality using neuroimaging.
The Present Research
First, we examined the category specificity of sexual motivation among monosexual (i.e., homosexual and heterosexual) men by comparing VS activation patterns to erotic pictures and videos depicting males versus those depicting females. These data were also compared with subjective responses to those same stimuli. Then, we investigated the degree to which bisexual men showed different VS responses to erotic pictures and videos compared with monosexual men. Of note, our bisexual male subjects were a subset of those from our previous genital arousal research (finding support for bisexual arousal patterns) (Rosenthal et al.)63,64. Thus, they comprise a promising sample to investigate neurally. Finally, we explored the degree to which heterosexual, homosexual, and bisexual men exhibited category-specific activation patterns across the entire brain, including neural regions for which we did not have a priori hypotheses. If the VS is a particularly valid indicator of positive incentive value, then we would expect the whole brain analyses to show it to be a relatively robust indicator of sexual orientation.
In all analyses, we examined activation patterns in response to both erotic pictures and videos. Although these patterns can be expected to be largely similar65,66, picture and video stimuli have different strengths and weaknesses. For example, picture stimuli may be better for exploring the initial appraisal of stimuli, with less influence from higher-order processing18,67. Video stimuli, in contrast, might be better for exploring deeper emotional states or for assessing the effects of more elaborative processing on sexual responses.
Method
Participants
26 heterosexual men, 28 bisexual men, and 25 homosexual men were recruited using internet advertisements on Craigslist. Bisexual participants were required to have had at least two sexual partners and one romantic partner (of three months or greater duration) of each sex, as described in a previous study of genital arousal in this population63,64. The data described here are from a slightly smaller subset of the men from Rosenthal et al.63,64, which included 28 heterosexual, 30 bisexual, and 27 homosexual men, because two heterosexual, five bisexual, and two homosexual men did not undergo fMRI assessment; three additional bisexual men were identified and participated in the experiment subsequent to our initial publication63,64.
After responding to advertisements, participants were screened for inclusion using online questionnaires. Participants provided information about sexual orientation, sexual interests, and personality, in addition to answering screening questions relevant to medical eligibility for fMRI research. Participants were informed of the risks and nature of the study, and agreed to participate in questionnaire, fMRI, and genital arousal portions of the research. Genital arousal data were previously reported in Rosenthal et al.63,64. All methods were approved by the Institutional Review Board of Northwestern University and carried out in accordance with its guidelines. Informed consent was obtained from each participant for every portion of the study in which they participated.
Participants’ sexual orientation was assessed using a modified Kinsey score68, which asked participants about their sexual fantasies throughout adulthood as well as in the past year53,69. The scale ranged from 0 to 6, with 0 corresponding to an exclusively heterosexual orientation and 6 corresponding to an exclusively homosexual orientation. Responses to the questions about adulthood and about the past year were averaged to create a Kinsey score for each participant. The average Kinsey score was 0.4 for heterosexual men (SD = 0.46, range = 0–1.5), 3.2 for bisexual men (SD = 0.85, range = 2–4.5), and 5.7 for homosexual men (SD = 0.45, range = 5–6). Self-reported sexual identities (i.e., “Homosexual”/“Gay”, “Bisexual”/“Bi”, “Heterosexual”/“Straight”) corresponded with the Kinsey score ranges for all participants.
Participants’ ages ranged from 25 to 50 years old. Mean ages were 32.3 for heterosexual men (SD = 6.75, range = 25–48), 37.5 for bisexual men (SD = 8.32, range = 26–50) and 33.2 for homosexual men (SD = 6.4, range = 26–50). The sample was racially and ethnically diverse, with Caucasian (60.76%), Latino (10.13%), African American (12.66%), and Asian American (6.33%) participants, as well as participants who identified otherwise or who identified as multiracial (10.13%).
Stimuli and Procedure
Stimuli and assessment
Picture runs
The present study employed a subset of the picture stimuli used in Safron et al.13 and Sylva et al.70. Pictures depicted a nude man, a nude woman, or a same-sex couple (i.e, either two men or two women) engaged in explicit sexual contact. Erotic stimuli featuring same-sex pairs engaging in explicit sexual interaction is common in research on sexual arousal and sexual orientation53,61,69. Such stimuli are similar to pictures of nude individuals, in the sense that only men or women, but not both, are depicted in a given picture. Thus, sexual arousal induced by them is relatively unambiguous. However, erotic stimuli featuring explicit sexual activity in couples tends to be substantially more arousing compared with pictures of nudes69.
In each of two 10.5-minute runs (ordering counterbalanced), participants viewed 40 erotic pictures featuring male actors and 40 erotic pictures featuring female actors. Each picture was shown for 3.5 seconds, followed by a variable-duration fixation cross presented for either 1.5, 6.5, or 11.5 seconds. During the presentation of each picture, participants used buttons held in their right hands to rate that image on a scale of −2 to +2 (respectively: “strongly disliked,” “disliked,” “liked,” “strongly liked”), with no option of 0 for neutral ratings.
Video runs
Following picture assessment, participants were shown six video clips depicting individual masturbating men and six video clips depicting individual masturbating women. Depicted individuals appeared sexually aroused but did not reach orgasm. To estimate baseline responses, six natural landscape videos were shown.
In each of two 9.25-minute runs (ordering counterbalanced), videos were presented for 15 seconds each, followed by a 15-second distraction task requiring participants to indicate via button-press when a number in a series decreased by an interval other than seven. This task was intended to facilitate a return to emotional and physiological baseline.
After leaving the scanner, participants viewed the videos once more and provided ratings of each clip. Videos were rated using a 5-point scale for degree of sexual appeal, ranging from “not at all” (0) to “very much” (4), with a midpoint of “somewhat” (2).
fMRI signal extraction methods
Image acquisition
A Siemens Trio 3 T magnet and 12-channel RF head coil were used to collect T2*-weighted gradient-recalled EPI images from the whole brain (32 3-mm slices with a 0.99-mm interslice gap; TR = 2500 ms; TE = 20 ms; flip angle = 80°; FOV = 200 × 220 mm, 120 × 128 matrix). Slices were taken along the plane connecting the anterior and posterior commissures, with a 1.72 mm × 1.72 mm × 3.99 mm resolution, with more refined axial dimensions intended to produce less distortion and signal dropout in sub-cortical areas, although possibly at the expense of signal-to-noise ratio. During each picture run, 250 whole-brain volumes were collected, and during each video run, 220 whole-brain volumes were collected, with the first four volumes discarded to account for initial magnetization effects. For anatomical localization, a structural MRI scan consisting of T1-weighted images was conducted after the testing runs (160 1-mm axial slices; TR = 2.1 ms; TE = 4.38 ms; flip angle = 15°; FOV = 220 mm; 256 × 192 matrix).
Image pre-processing
Image pre-processing and analysis was performed using SPM 12b (Wellcome Trust Centre for Neuroimaging, London, UK)71, and implemented in Matlab v 8.1.604 (The MathWorks Inc., MA, USA).
Functional (EPI) volumes were first corrected for slice timing. Each participant’s volumes were then registered to the mean slice, after which the registered volumes were resliced, used to create a mean resliced image, and then co-registered to the mean structural (T1) image. All EPI images, including the mean resliced image, as well as the structural (T1) scans were then spatially normalized to Montreal Neurological Institute (MNI) space, and re-sampled to 3 × 3 × 3 mm (27 mm3) resolution. Normalized functional images were then smoothed to an 8 mm full-width-at-half-maximum Gaussian kernel.
Signal to noise ratio and head coverage exclusions
To exclude participants with poor signal due to either head motion or scanner conditions, average signal-to-noise ratio (SNR) over time was calculated for each subject (after preprocessing, using a mask that included only voxels with appreciable EPI signal). The SNR ratio for each voxel (mean divided by standard deviation) was averaged across all voxels in the brain72,73. Participants whose picture data SNR was less than one standard deviation below the mean were excluded from picture analyses. Similarly, participants whose video data SNR was less than one standard deviation below the mean were excluded from video analyses.
Based on these criteria, eleven participants (two heterosexual, seven bisexual, and two homosexual) were excluded from fMRI and subjective picture analyses, and ten participants (four heterosexual, five bisexual, and one homosexual) were excluded from fMRI and subjective video analyses. An additional six participants (one heterosexual, four bisexual, and one homosexual) were excluded from subjective picture rating analyses due to insufficient subjective data resulting from a data-recording error. An additional five participants (three heterosexual, four bisexual, and two homosexual) were excluded from subjective video rating analyses for the same reason. Thus, after exclusions were performed for both SNR and insufficient subjective data, we included a total of 23 heterosexual men, 17 bisexual men, and 22 homosexual men in picture analyses, and included 19 heterosexual men, 19 bisexual men, and 22 homosexual men in video analyses.
To check the validity of our SNR-exclusion criterion, head motion plots were visually inspected for all participants72. Excluded participants were confirmed to have highly variable head positions as compared to included participants. An additional validity-check was performed using evoked responses to erotic pictures minus a fixation-cross baseline. Excluded participants had substantially reduced activity in visual cortices as compared to included participants.
For whole-brain analyses, mean functional scans were individually examined to identify participants with substantial cutoffs in head coverage. As a result, one homosexual male who had substantial frontal lobe cutoff was excluded from whole-brain analyses in addition to those participants excluded for SNR.
First-level analyses
For both the video and picture assessments, a standard general linear model (GLM)71 was used to identify hemodynamic changes for each participant, and a high-pass filter (cutoff 128 s) was used to remove low-frequency temporal noise.
For the picture assessment, each participant’s responses to each stimulus contrast of interest were concatenated within stimulus type, using data from both runs. Estimated average activity was calculated for each participant’s separate responses to male pictures, female pictures, male videos, and female videos (contrasted with fixation cross for pictures and nature scenes for videos). These estimates were used for region of interest analyses. For whole-brain analyses, estimated average activity was also calculated for each participant’s response to male compared with female pictures and videos.
Ventral striatum region of interest analyses
An a priori region of interest (ROI) analysis was performed on the VS—centered on the nucleus accumbens—as this was the area most likely to indicate reward. The VS and hypothalamus are the only two areas that have been shown to be specifically associated with sexual (as opposed to general) arousal26,27. We focused on the VS because it likely has higher validity for reflecting reward compared with the hypothalamus, which contains a variety of nuclei with heterogeneous functions (including sexual arousal) that would be difficult to disambiguate with the limited spatial resolution of 3 T fMRI.
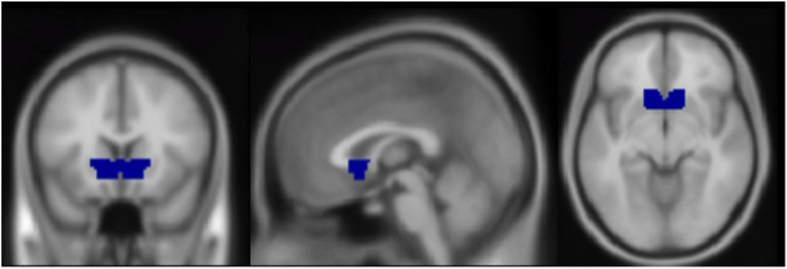
The VS ROI mask used in the present study was drawn on an MNI template brain using the WFU PickAtlas toolbox for SPM 874. It was anatomically defined as a dilated intersection of the ventral anterior caudate and putamen. The resulting VS ROI is shown in Fig. 1.
Figure 1. Mask used as the ventral striatum (VS) ROI, drawn using an average brain in the WFU PickAtlas toolbox for SPM 8.
MNI coordinates displayed: x = 0, y = 17, z = −8.
Estimates of average VS activity for each participant were extracted using the MarsBar toolbox for SPM875. Extracted VS ROI data were analyzed using JMP Pro v11 (SAS Institute, Cary, NC).
Planned contrasts
Category-specificity in heterosexual and homosexual men
First we examined how well VS activity predicts sexual orientation in monosexual men via differential responding to male versus female erotic stimuli within our paradigm. For each participant we separately calculated differential VS activation for erotic pictures and videos by subtracting activation to female erotic stimuli from activation to male erotic stimuli. The straightforward prediction is that on average, these contrasts should yield positive values for homosexual men and negative values for heterosexual men.
Comparing bisexual and monosexual men
What comprises evidence for bisexual neural activation patterns in men? In exploring whether bisexually-identified men have greater propensity to bisexual responding compared with monosexual men, it is inadequate to show that on average, bisexual men tend to exhibit significant VS activity to both sexes. This is because a group average showing a high response to both sexes could be the result of particular members of the group responding strongly to women and others to men, even if no individual in the group is attracted to both. This would happen if a sample of bisexually-identified men comprised a mixture of men who individually have either heterosexual or homosexual response patterns.
An adequate test of the hypothesis that bisexual-men are uniquely bisexual in their response patterns requires a dependent variable that gauges bisexual responses within individuals. Two related dependent variables have been proposed. The more intuitive is that compared with monosexual men, bisexual men should show a smaller difference, on average, between their responses to men and women63,64. The dependent variable in this case is the absolute value of the difference between responses to men and responses to women; henceforth we refer to this variable as |Male–Female|. A slightly different test is motivated by recognizing that regardless of which sex a bisexual man responds less to, he should respond more to that sex compared with a monosexual man’s response to his less activating sex61. This test uses response to the less activating sex as its dependent variable; henceforth we refer to this variable as Minimum(Male, Female). The two tests provide complementary information, and ideally both should be analyzed in studies of bisexually-identified men’s arousal patterns. These analyses were conducted via one-way ANOVA, implemented via a single planned contrast within a multiple regression model (Judd, McClelland, & Ryan, 2011). Each planned contrast compared the mean of the bisexual subsample with the unweighted pooled means of the other two subsamples.
Whole brain analyses
Finally, we examined overall patterns of differential activation in response to male compared with female erotic stimuli across the entire brain. If the VS is a particularly valid indicator of desire, then it is also likely to be a particularly robust discriminator of male and female sexual stimuli in monosexual men in whole brain contrasts. Further, if bisexual men have less specific arousal patterns, then they are likely to exhibit less extensive differential activity between male and female stimuli compared with the activity patterns expected for heterosexual and homosexual men. However, with respect to establishing bisexual patterns of brain activity, it is important to remember methodological limitations discussed in the previous section. Accordingly, instead of being used as strong tests of bisexual arousal, these whole brain tests could provide information on average responses to male or female stimuli, with an additional possibility that bisexual men may recruit a unique set of neural regions in differentiating between male and female stimuli.
Tests of average group responses to stimulus conditions were performed using one-sample contrasts. Each group (heterosexual men, bisexual men, and homosexual men) was tested individually for clusters of greater activity for male stimuli compared with female stimuli, and female stimuli compared with male stimuli for pictures and videos separately, using a corrected statistical threshold (p < 0.05 FWE).
For these analyses, cluster reports were generated in SPM. Peak activations and cluster extents (extent threshold k = 5) were visually examined as overlays on slice and render maps. Neuroanatomical descriptions were determined based on agreement between two trained investigators, and checked against designations from the WFU Atlas74.
Results
Planned Contrasts
Category-specificity of heterosexual and homosexual men
Consistent with our prediction, there were large and statistically significant VS activation differences between homosexual and heterosexual men when male–female stimuli difference scores were compared (Table 1). For both standardized subjective ratings and VS responses, differences between male and female stimuli (both picture and video) were in the expected directions, with negative scores for heterosexual men and positive scores for homosexual men. These results, consistent with our prediction for all stimulus types, support the VS’s ability to signify sexual orientation for the monosexual men within our sample. If these results had not been consistent with our predictions, they may have called to question the appropriateness of either our ROI or our stimuli for our subsequent tests of bisexual men.
Table 1. Male-Female Stimulus Contrasts in Heterosexual and Homosexual Men.
| Heterosexual Men | Homosexual Men | t (df) | Cohen’s d | |
|---|---|---|---|---|
| Subjective Ratings: Pictures | Mean: −1.141 SD: 0.381 | Mean: 1.317 SD: 0.304 | 23.863** (43) | −9.095 |
| Subjective Ratings: Videos | Mean: −1.219 SD: 0.359 | Mean: 1.450 SD: 0.385 | 24.010** (43) | −7.170 |
| VS Activity: Pictures | Mean: −0.715 SD: 0.665 | Mean: 1.100 SD: 0.692 | 9.067** (44) | −2.674 |
| VS Activity: Videos | Mean: −0.341 SD: 1.210 | Mean: 0.767 SD: 0.561 | 4.040** (44) | −1.175 |
Male-female stimulus difference scores by sexual orientation of monosexual participants for both standardized subjective ratings and standardized VS activations. Positive difference scores indicate subjective ratings or VS activation greater toward male stimuli. df = degrees of freedom. SD = Standard deviation. **Significant at p < 0.001.
Comparing bisexual and monosexual men
Consistent with our predictions (Table 2), bisexual men rated their subjectively less preferred sex significantly higher compared with the ratings of monosexual men (measured using the test Minimum(Male, Female)); further, bisexual men rated male and female stimuli significantly more similarly than did monosexual men (measured using the test |Male–Female|). These results were present for both picture and video stimuli.
Table 2. Planned contrasts comparing bisexual and monosexual men’s subjective responses and VS activation toward both pictures and videos.
| Contrast 1: Minimum (Male, Female) |
Contrast 2: |Male – Female| |
|||
|---|---|---|---|---|
| β | t (df) | β | t (df) | |
| Subjective Ratings: Pictures | 0.979 | 5.869** (60) | 1.53 | 7.584** (60) |
| Subjective Ratings: Videos | 1.30 | 8.495** (64) | 1.526 | 9.153** (64) |
| VS Activity: Pictures | 0.029 | 0.149 (64) | 0.463 | 2.064* (64) |
| VS Activity: Videos | 0.365 | 2.108* (66) | 0.361 | 1.566 (66) |
For Minimum(Male,Female) contrasts, positive betas indicate greater response values in bisexual (vs. non bisexual) men. For the |Male – female| test, positive betas indicate greater response values in monosexual (vs. bisexual) men. df = degrees of freedom. *Significant at p < 0.05. **Significant at p < 0.001.
VS activation results were somewhat more complicated. Compared with monosexual men, bisexual men showed significantly higher VS responses to their less activating sex for video stimuli, but not for pictures. Bisexual men also showed significantly more similar VS responses between male and female stimuli for pictures but not for videos.
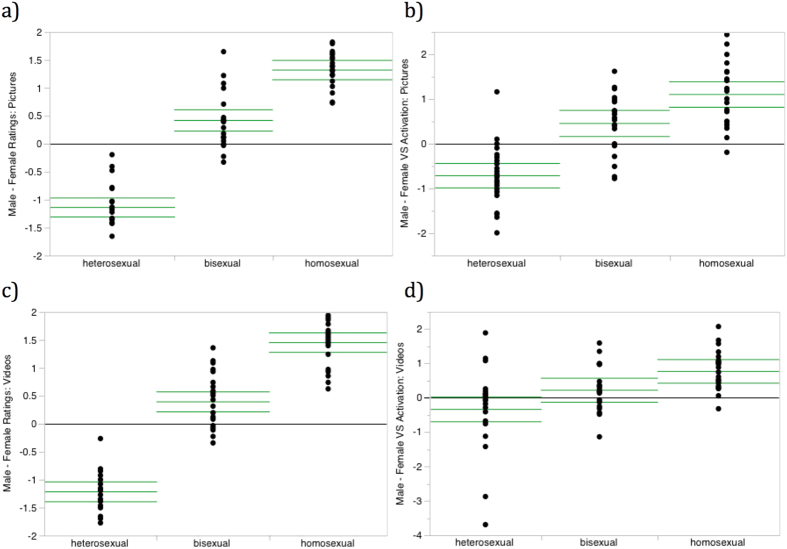
Individual-level male-female stimuli difference scores are shown for all participant groups in Fig. 2. Patterns are consistent with the contrasts described above, with predominantly positive (male favoring) difference scores in homosexual men, and predominantly negative (female favoring) difference scores in heterosexual men. Overall stimulus differentiation was smaller among bisexual men, but with a slightly greater subjective preference and VS response toward male stimuli, consistent with the Kinsey scores reported by our particular bisexual sample (M = 3.2, SD = 0.85, range = 2–4.5).
Figure 2. Male-female stimuli difference scores for subjective ratings and VS responses by sexual orientation.
Difference scores are defined as a participant’s average response to stimuli depicting males minus average response to stimuli depicting females. Points represent individual participants. Horizontal bars indicate group means and 95% confidence interval of the mean. Horizontal lines at 0 indicate no difference between ratings to erotic stimuli depicting each sex. (a) Difference scores for subjective ratings of picture stimuli. (b) Difference scores for VS activation evoked by picture stimuli. (c) Difference scores for subjective ratings of videos stimuli. (d) Difference scores for VS activation evoked by video stimuli.
To test whether indicators of bisexual activation were significant when considered in aggregate, we regressed the dichotomous variable “Bisexual versus Monosexual” on the four indicators: activation to the less activating stimuli (higher in men with a relatively bisexual pattern) and absolute difference of male and female stimuli (lower in men with a relatively bisexual pattern), measured separately for pictures and videos. Together, the four predictors did not reach statistical significance, whether the dependent variable was treated numerically (via least squares regression) or nominally (via logistic regression): respectively, F(4, 59) = 1.82, p = 0.14, R2 = 0.11; χ2(4) = 7.55, p = 0.11.
Whole Brain Analyses
Note: as previously discussed, only the ROI analyses constitute an unambiguous test of bisexual response.
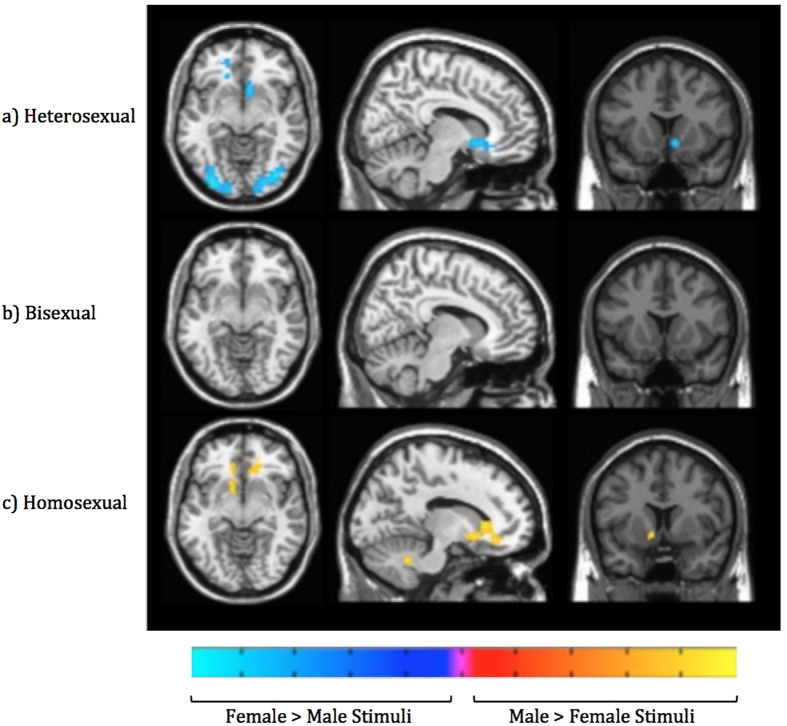
When responses to male and female erotic pictures were compared (Fig. 3 and Table 3), bisexual men did not exhibit significant differences in their responses to male vs. female stimuli. In contrast, heterosexual and homosexual men showed strong (and opposite direction) differential activations between male and female pictures in multiple cortical and subcortical areas, including the VS.
Figure 3. Whole brain activations for the male minus female contrasts when brain activation evoked by viewing neutral stimuli was subtracted from activation toward erotic pictures.
Height threshold is set at p < 0.05 FWE with a cluster threshold of k = 5. Axial slice 32 is shown for heterosexual, bisexual, and homosexual men. Sagittal slice 50 is shown for heterosexual and bisexual men, and sagittal slice 39 is shown for homosexual men to facilitate comparisons with heterosexual men’s patterns and to show ventral striatum activation present on the left rather than right side. Coronal slice 38 is shown for heterosexual and bisexual men, and coronal slice 41 is shown for homosexual men in order to display ventral striatum activity and facilitate comparisons with heterosexual men’s patterns.
Table 3. Differential brain activations between male and female pictures in heterosexual, bisexual and homosexual men.
| R/L | Region | BA | x/y/z | voxels | peak T |
|---|---|---|---|---|---|
| Heterosexual Men | |||||
| Female > Male Pictures | |||||
| R | middle occipital gyrus | 18 | (33, −85, −1) | 313 | 10.07 |
| R | middle occipital gyrus | 18 | (27, −91, 11) | 8.56 | |
| R | primary visual cortex, cuneus, fusiform gyrus, posterior lingual gyrus | 17 | (21, −94, −1) | 8.53 | |
| L | middle occipital gyrus | 18 | (−30, −76, −1) | 323 | 9.5 |
| L | middle occipital gyrus | 18 | (−30, −88, −7) | 9.43 | |
| L | primary visual cortex, cuneus | 17 | (−12, −97, −1) | 7.85 | |
| R | ventral striatum | (9, 17, −7) | 53 | 8.14 | |
| R | subgenual anterior cingulate | 32 | (15, 35, −10) | 7.2 | |
| R | subgenual anterior cingulate | 32, 25 | (9, 26, −10) | 6.53 | |
| L | orbitofrontal cortex | 10 | (−18, 44, −7) | 19 | 7.16 |
| L | precentral gyrus | 4 | (−42, −25, 65) | 24 | 6.94 |
| Male > Female Pictures: no activations | |||||
| Bisexual Men | |||||
| Female > Male Pictures: no activations | |||||
| Male > Female Pictures: no activations | |||||
| Homosexual Men | |||||
| Female > Male Pictures: no activations | |||||
| Male > Female Pictures | |||||
| L | left medial prefrontal cortex | 10 | (−18, 32, 5) | 218 | 9.19 |
| L | ventral striatum, anterior cingulate, medial orbitofrontal cortex | 32, 11 | (−9, 23, 2) | 9.01 | |
| R | anterior cingulate, medial orbitofrontal cortex, ventral striatum | 32, 11 | (21, 32, 8) | 8.08 | |
| R | caudate body | (18, −10, 26) | 47 | 8.79 | |
| L | caudate body | (−21, −10, 26) | 36 | 8.76 | |
| L | dentate nucleus | (−12, −52, −31) | 23 | 8.65 | |
| R | nodule of vermis | (3, −52, −34) | 6.73 | ||
| L | cerebellar tonsil | (−9, −52, −40) | 6.49 | ||
| R/L | culmen, declive | (0, −58, −19) | 44 | 7.96 | |
| R | medial orbitofrontal cortex | 11 | (6, 44, −13) | 15 | 7.94 |
Results are presented separately for each orientation group. L = left, R = right, BA = Brodmann area. Coordinates are in MNI space. All clusters were significant with p < 0.05 FWE corrections.
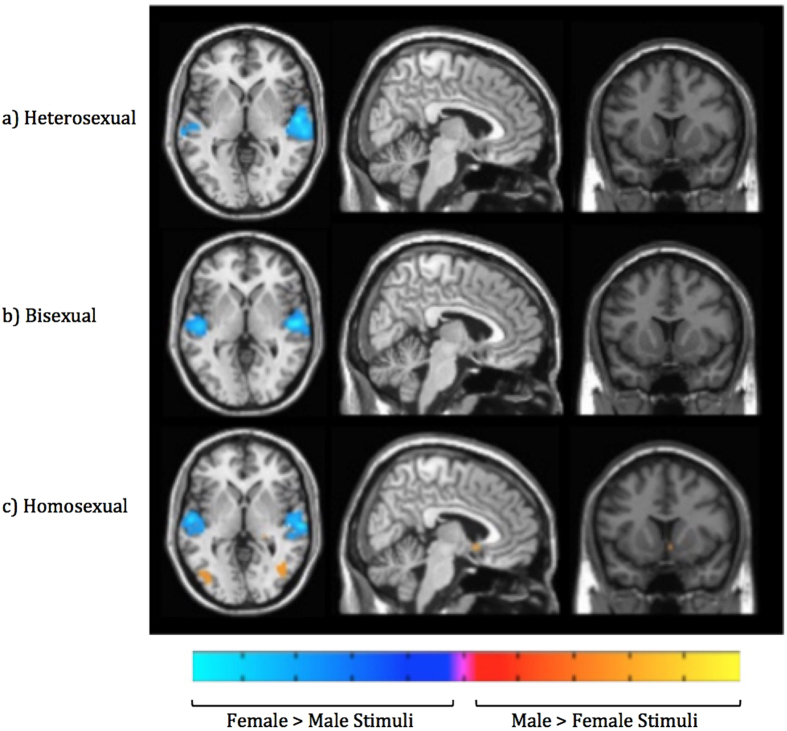
When responses to male and female erotic videos were compared (Fig. 4 and Table 4), all groups showed bilateral superior temporal cortex activations consistent with a stimulus confound in which more extensive and substantial vocalizations were present in female (compared with male) erotic videos76. Complex parietal activations were observed in bisexual and homosexual groups where some areas showed greater activity for male videos, and others showed greater activity for female videos. Only homosexual men exhibited significantly different VS activation in response to their sexually preferred video stimuli.
Figure 4. Whole brain activations for the male minus female contrasts when brain activation evoked by viewing neutral stimuli was subtracted from activation toward erotic videos.
Height threshold is set at p < 0.05 FWE with a cluster threshold of k = 5. Axial slice 36, sagittal slice 48, and coronal slice 39 are shown for all groups.
Table 4. Differential brain activations between male and female videos in heterosexual, bisexual and homosexual men.
| R/L | Region | BA | x/y/z | voxels | peak T |
|---|---|---|---|---|---|
| Heterosexual Men | |||||
| Female > Male Videos | |||||
| R | superior temporal gyrus, secondary auditory cortex, primary auditory cortex | 41, 42, 22 | (60, −10, 2) | 301 | 11.64 |
| R | superior temporal gyrus, secondary auditory cortex | 22 | (63, −22, 2) | 10.63 | |
| R | superior temporal gyrus, primary auditory cortex | 41, 42 | (51, −22, −1) | 8 | |
| L | superior temporal gyrus, primary auditory cortex, secondary auditory cortex | 41, 42 | (−51, −22, 5) | 124 | 11.36 |
| L | superior temporal gyrus, primary auditory cortex, secondary auditory cortex | 41, 42 | (−66, −25, 2) | 8.47 | |
| L | superior temporal gyrus, secondary auditory cortex | 41, 42 | (−63, −31, 11) | 6.4 | |
| Male > Female Videos: no activations | |||||
| Bisexual Men | |||||
| Female > Male Videos | |||||
| R | superior temporal gyrus, primary auditory cortex, secondary auditory cortex | 41, 42, 22 | (57, −13, −1) | 298 | 13.08 |
| R | superior temporal gyrus, primary auditory cortex, secondary auditory cortex | 42 22 | (51, −25, 8) | 10.57 | |
| R | superior temporal gyrus, secondary auditory cortex | 22 | (63, −7, −4) | 10.09 | |
| L | superior temporal gyrus, primary auditory cortex, secondary auditory cortex | 41, 42 | (−45, −22, 8) | 292 | 10.55 |
| L | primrary auditory cortex | 41 | (−48, −16, −1) | 10.5 | |
| L | superior temporal gyrus, primrary auditory cortex, secondary auditory cortex | 41, 42, 22 | (−57, −25, 8) | 9.04 | |
| R | angular gyrus | 39 | (57, −58, 35) | 25 | 8.58 |
| Male > Female Videos | |||||
| R | postcentral sulcus, superior parietal lobule | 2, 5, 7 | (27, −46, 53) | 211 | 8.83 |
| R | postcentral sulcus, superior parietal lobule | 2, 7 | (33, −37, 47) | 8.09 | |
| R | supramarginal gyrus, postcental sulcus | 40, 2 | (42, −31, 41) | 7.83 | |
| R | superior occipital lobe | 19 | (33, −82, 20) | 18 | 7.18 |
| Homosexual Men | |||||
| Female > Male Videos | |||||
| L | superior temporal gyrus, primary auditory cortex, posterior insula | 22, 41, 42 | (−57, −31, 5) | 365 | 12.52 |
| L | superior temporal gyrus, primary auditory cortex | 41 | (−48, −28, 5) | 11.96 | |
| L | superior temporal gyrus | 22 | (−57, −10, −1) | 10.65 | |
| R | superior temporal gyrus, middle temporal gyrus | 22, 42, 41 | (63, −19, −4) | 318 | 11.71 |
| R | superior temporal gyrus | 22 | (63, −25,8) | 11.7 | |
| R | primary auditory cortex, secondary auditory cortex | 42, 41 | (48, −19, 5) | 9.82 | |
| R | supramarginal gyrus | 40 | (63, −43, 38) | 44 | 8.61 |
| R | angular gyrus | 40 | (60, −52, 29) | 6.82 | |
| Male > Female Videos | |||||
| R | postcentral gyrus, postcentral sulcus, supramarginal gyrus | 3, 1, 2, 40 | (30, −34, 47) | 663 | 10.04 |
| R | superior parietal lobule, postcentral gyrus | 7, 5, 3, 1, 2 | (39, −37, 56) | 9.92 | |
| R | superior parietal lobule, precuneus, superior occipital lobe, precuneus, cuneus, middle occipital lobe, primary visual cortex | 7, 19, 18, 17 | (24, −61, 62) | 9.33 | |
| R | middle frontal gyrus | 6 | (27, −4, 56) | 93 | 9.82 |
| R | middle frontal gyrus | 6 | (36, −4, 62) | 9.8 | |
| R | middle frontal gyrus | 6 | (36, −4, 53) | 9.77 | |
| L | primary visual cortex | 17 | (−12, −82, 8) | 30 | 8.59 |
| R | inferior temporal gyrus, occipitotemporal junction | 37 | (45, −70, −4) | 63 | 8.2 |
| R | inferior temporal gyrus, lateral occipital gyrus | 19 | (48, −58, −10) | 6.9 | |
| L | superior parietal lobule | 7 | (−21, −58, 56) | 104 | 8.12 |
| L | superior parietal lobule | 7 | (−30, −49, 56) | 7.77 | |
| L | postcentral gyrus, postcentral sulcus | 3, 1, 2 | (−24, −34, 44) | 7.7 | |
| R | fusiform gyrus | 37 | (33, −52, −16) | 21 | 7.88 |
| L | middle occipital gyrus | 19 | (−24, −97, 14) | 80 | 7.17 |
| L | middle occipital gyrus | 19 | (−39, −79, 11) | 7.16 | |
| L | middle occipital gyrus, extending into occipitotemporal junction | 19, 37 | (−36, −79, −1) | 6.77 | |
| L | superior occipital | 19 | (−24, −82, 32) | 10 | 6.96 |
Results are presented separately for each orientation group. L = left, R = right, BA = Brodmann area. Coordinates are in MNI space. All clusters were significant with p < 0.05 FWE corrections.
Discussion
In the present investigation, patterns of brain activation were generally consistent with those of previous research in showing that heterosexual and homosexual men’s responses to male and female erotic stimuli are quite distinct4,53,54. Furthermore, our results are consistent with recent past studies of male bisexuality (e.g. Rosenthal et al.63,64) in showing that bisexual men’s responses to male and female stimuli tend to be less distinct compared with monosexual men’s responses63,64. Here, however, these response patterns were demonstrated using fMRI activations in the ventral striatum (VS), providing an objective measure of valence.
Category Specificity in Heterosexual and Homosexual Men
Heterosexual men showed greater VS responses to erotic stimuli featuring women, and homosexual men showed greater VS responses to erotic stimuli featuring men. Further, these responses were consistent with subjective assessments of the same stimuli.
Compared with videos, pictures produced somewhat stronger differential VS responses between male and female stimuli, as indicated by larger effect sizes in monosexual region of interest analyses (Table 1) and the robustness of whole-brain findings. One potential explanation for this difference may be that the reliability of the pictures paradigm benefited from the larger number of stimuli used. Alternatively, it may be the case that the VS is more sensitive to briefly presented stimuli, which is consistent with both reward-prediction error77,78,79,80 as well as incentive salience models43 of dopamine and VS functioning. Indeed, while the VS is frequently active during both reward anticipation and consumption, the association is particularly strong when consumed rewards occur unexpectedly or are uncertain30, as might be the case for randomly presented pictures. Additionally, for some participants it could have been the case that wanting or desire responses were strongest at the beginning of a video presentation, but then decreased—or fluctuated—over the subsequent 15 seconds.
Comparing Bisexual and Monosexual Men
Compared with monosexual men, bisexual men reported relatively bisexual subjective response patterns for all stimuli. With respect to VS activity, significantly bisexual patterns were observed for both picture and video stimuli, but in different tests. Compared with monosexual men, bisexual men showed significantly greater VS activity to their less activating sex for video stimuli, but not for pictures. Bisexual men also showed significantly less differential activation between male and female sexual stimuli for picture stimuli, but not for videos. Thus, although not all relevant fMRI test results were statistically significant, overall VS activation patterns support the idea that the bisexual men had a more bisexual reward responses compared with the monosexual men.
Whole brain activation patterns
This study focused on the VS as the primary region of interest, but whole brain activation patterns in each group revealed similar category-specific responses in monosexual men, as well as non-specific responses in bisexual men. These findings are described below along with tentative reverse inferences regarding potential functional significances.
Neural responses to erotic pictures
Heterosexual men had no areas where male pictures produced significantly greater activity than did female pictures. The reverse direction subtraction of female > male pictures, however, revealed extensive activations spanning multiple brain areas. Activity in the right VS likely indicated positive incentive value towards female stimuli (see introduction). This focal VS cluster in the nucleus accumbens exhibited the most robust differential activation compared with all other clusters in the brain, outside of occipital cortex, which likely indicated visual attention81,82. Right fusiform and adjacent extrastriate cortices may have indicated face and body perception83,84,85. Subgenual anterior cingulate and left orbitofrontal cortices may have indicated positive valence27,86,87,88. Additionally, motor strip (i.e. precentral gyrus) may have indicated preparation of motor sequences or perhaps motor imagery89,90,91,92.
Similarly to the heterosexual group, homosexual men had no areas where nonpreferred pictures (i.e., female erotic images) produced significantly greater activity than did male pictures. The reverse direction subtraction of male > female pictures, however revealed extensive activations spanning multiple brain areas. Similarly to the results for heterosexual men, some of the most robust activity was observed in the VS (again, focally in the nucleus accumbens), albeit more bilaterally, and particularly in the left hemisphere. Anterior cingulate and medial orbitofrontal cortices may have indicated positive valence27,86,87,88. Bilateral caudate body and cerebellum may have indicated imagination and preparation of motor sequences90,93,94,95,96.
In contrast to the activation patterns obtained for heterosexual men, the bisexual group had no brain areas with significantly different activation between male and female erotic pictures. While this sort of pattern could result from averaging over a mixture of heterosexual and homosexual response profiles, evidence for distinctly bisexuality responses is suggested by region of interest analyses in the VS.
Neural responses to erotic videos
Heterosexual men had no areas where male videos produced significantly greater activity than did female videos. The reverse direction subtraction of female > male videos, however, revealed activations in bilateral superior temporal cortices, which likely indicated an auditory confound from more extensive and substantial vocalizations being present in female erotic videos76.
Similarly to heterosexual men, homosexual men showed auditory activations in response to female compared with male stimuli. However, they also showed activity in the parietal lobe toward female compared with male stimuli, with clusters observed in both the right angular and right supramarginal gyri. The functional significance of these activations is unclear, but could be tentatively interpreted as indicating either mentalizing or mirroring-related-cognition97,98,99,100,101,102,103.
More extensive activations were observed in homosexual men for areas of the brain responding more to male than female erotic videos. A small focal cluster was observed in the medial orbitofrontal cortex, which may indicate positive valence86. Activity was observed bilaterally (albeit was somewhat right-lateralized) in a larger cluster spanning both early and higher order visual areas including fusiform and extrastriate cortices, and parietal areas indicative of spatial attention, mental imagery, bodily sensations, and possibly mirroring84,85,97,98,99,100,101,102,103. Bilateral (but primarily right-lateralized) pre-motor cortex potentially indicated imagination and preparation of motor sequences90. It is unclear why homosexual men showed more extensive category-specific responses than did heterosexual men in response to erotic videos.
In contrast to the patterns obtained for heterosexual and homosexual men viewing erotic videos, bisexual men showed extensive activation patterns for both the female > male and male > female subtractions. For the female > male subtraction, similar auditory-related activity was observed as in heterosexual and homosexual men. An additional parietal cluster was observed in the right angular gyrus, with possible interpretations including mentalizing97,98,99,100,101,102,103. For the male > female subtraction, activity in the superior occipital cortex may have indicated visual attention81,82. Activity in the postcentral sulcus may have indicated bodily sensations105,106. Additionally, superior parietal lobule and supramarginal gyrus—more anteriodorsomedial than observed in the female > male contrast for homosexual men—may have indicated perception or imagination related to depicted sexual activities107,108. For bisexual men, erotic videos did not produce clear category-specific brain activity in one direction or another. It is further notable that there were significant findings not present for either heterosexual or homosexual participants, suggesting that bisexuals represent a group with distinct response profiles.
Limitations
While our initial sample size was large by the standards of neuroimaging studies of human sexuality, a number of subjects were excluded in order to ensure data quality. Consequently, our study may have been somewhat underpowered for detecting small (but potentially meaningful) effects.
The establishment of an appropriate baseline for activation represents an ongoing challenge for studies of the neural bases of sexual responses. An ideal baseline should produce similar affective (and cognitive) responses between groups. While fixation cross presentations and nature scenes likely represented adequate control conditions for erotic pictures and videos (respectively), some participants could have had significant processing differences during these baseline measurements. For example, since we did not measure genital responses in the scanner, we cannot rule out the possibility that some participants (and perhaps in particular bisexual participants) experienced significant erectile activity that continued after the presentation of arousing stimuli. This could have had the effect of reducing the magnitude of evoked responses for our minimum arousal tests.
Future work may benefit from using other kinds of appetitive stimuli as controls (e.g. appealing food or monetary rewards). This could potentially provide an overall measure of reward sensitivity. Additionally, these appetitive stimuli could also be used to create individually customized functional regions of interest. Theoretically, by using a localizer task to determine the most strongly reward-sensitive voxels in each person’s brain, it may be possible to generate a particularly sensitive measure of erotic preferences.
Future Directions
Consistency among neural measures of incentive value, subjective and genital arousal, and sexual identity supports the idea that male sexual orientation (in heterosexual, bisexual, and homosexual men) is motivated by patterns of sexual arousal and attraction1. One question that remains to be examined is the stability of these neural responses over time, both in terms of early development109, as well as throughout life110. Although it may seem ethically problematic to conduct such research in younger participants, one advantage of fMRI over other measures of sexual interest is the ability to both objectively and non-invasively measure valenced responses to non-explicit stimuli109,111.
It may be the case that attraction patterns (and patterns of VS activation to male versus female stimuli) of bisexual individuals change based on a variety of contextual factors112. Conceivably, for example, a bisexual person may exhibit more gynephilic or androphilic preferences depending on whether they have recently been romantically or sexually involved with women or men. Research measuring individuals over multiple time-points is needed to examine this (admittedly speculative) possibility. Furthermore, it would be especially informative to include fMRI measures alongside measures of genital and subjective responses in a longitudinal study.
In the present study, we focused on the VS as a measure of positive incentive value. Exploring the role of sexual aversion may also be an informative avenue for future investigations113. For example, is bisexuality partially related to lower levels of sexual aversion? A major challenge for conducting these investigations would be finding a brain area in which activity is specific to aversion, rather than general arousal or overall salience. In the past, regions such as the anterior insula activity have been used as a metric of disgust114, when general visceral emotionality would likely be a more valid inference with respect to this region115. Additionally—and not limited in its applications to studying aversion—multi-voxel classifier techniques might be valuable for inferring valence from more sparsely distributed activation patterns116,117,118.
Future investigations should also explore the degree of specificity of reward-related (and possibly aversion-related) brain activity in women of different sexual orientations. Women’s subjective and genital responses to erotic stimuli suggest that relatively bisexual patterns may be observed in women compared to men53,69,119. Bisexual erotic responses in women are further suggested by preliminary fMRI evidence in which monosexual women—contrasted with monosexual men—exhibited greater activity toward non-preferred erotic pictures in posterolateral putamen, dorsal striatum, and thalamus70. Functional neuroimaging focusing on the VS may be a particularly valuable converging line of evidence for studying sexual responses in women, as physiological arousal patterns have been difficult to interpret in light of their low correlation with subjective report120. In addition, neuroimaging may have unique advantages for directly comparing men and women in their sexual response patterns, as the same type of objective measurement can be performed on the same brain structures in both men and women.
Conclusion
Here we replicated previous findings of category-specific neural responses in heterosexual and homosexual men, and further provided evidence that bisexual men exhibit distinctly bisexual neural responses. This sample exhibited bisexual arousal patterns in prior analyses of subjective and genital measures63,64, and has now been shown to exhibit relatively bisexual ventral striatum (VS) activation patterns. Although mixed results have been obtained in studies of genital arousal patterns in bisexual men, this investigation found bisexual patterns of VS activity in a sample in which participants had been specifically screened for bisexual romantic and sexual histories. However, it should be noted that these findings were variable across different tests, with some tests failing to find evidence for distinct patterns in bisexual participants. Nonetheless, convergence between multiple modalities suggests that for the men studied here, bisexual identities are consistent with bisexual patterns of arousal and desire, similarly to monosexual men.
Additional Information
How to cite this article: Safron, A. et al. Neural Correlates of Sexual Orientation in Heterosexual, Bisexual, and Homosexual Men. Sci. Rep. 7, 41314; doi: 10.1038/srep41314 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
The authors would like to thank Dr. Joan Linsenmeier for providing invaluable feedback on previous versions of this manuscript. This work was supported by a National Science Foundation Graduate Fellowship Research Program award to Adam Safron as well as by the American Institute of Bisexuality.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.M.B., A.S., D.S., and A.R. were responsible for study concept and design. D.S. and A.R. were responsible for data collection. A.S. and V.K. analyzed the data and wrote the manuscript. M.L. and M.W. provided neuroimaging analysis support. J.M.B. provided domain expertise and guidance through all stages of research and manuscript preparation. All authors have read and approved the final manuscript version.
References
- Bailey J. M. What is sexual orientation and do women have one? Neb. Symp. Motiv. Neb. Symp. Motiv. 54, 43–63 (2009). [DOI] [PubMed] [Google Scholar]
- Cass V. C. Homosexual identity formation: a theoretical model. J. Homosex. 4, 219–235 (1979). [DOI] [PubMed] [Google Scholar]
- Diamond L. M. Sexual Fluidity: Understanding Women’s Love and Desire (Harvard University Press, 2008). [Google Scholar]
- Freund K. In Understanding Homosexuality: Its Biological and Psychological Bases (ed. Loraine D. J. A.) 25–81 (Springer Netherlands, 1974). [Google Scholar]
- Meston C. M. & Buss D. M. Why Women Have Sex: Understanding Sexual Motivations from Adventure to Revenge (Times Books, 2009). [Google Scholar]
- Troiden R. R. The formation of homosexual identities. J. Homosex. 17, 43–73 (1989). [DOI] [PubMed] [Google Scholar]
- A third of young Americans say they aren’t 100% heterosexual. YouGov U.S. Available at: https://today.yougov.com/news/2015/08/20/third-young-americans-exclusively-heterosexual/. (Accessed: 10th January 2017).
- Copen C. E., Chandra A. & Febo-Vazquez I. Sexual Behavior, Sexual Attraction, and Sexual Orientation Among Adults Aged 18–44 in the United States: Data From the 2011–2013 National Survey of Family Growth. Natl. Health Stat. Rep. 1–14 (2016). [PubMed] [Google Scholar]
- Kuban M., Barbaree H. E. & Blanchard R. A Comparison of Volume and Circumference Phallometry: Response Magnitude and Method Agreement. Arch. Sex. Behav. 28, 345–359 (1999). [DOI] [PubMed] [Google Scholar]
- Gillath O. & Canterberry M. Neural correlates of exposure to subliminal and supraliminal sexual cues. Soc. Cogn. Affect. Neurosci. 7, 924–936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J. et al. Assessment of sexual orientation using the hemodynamic brain response to visual sexual stimuli. J. Sex. Med. 6, 1628–1634 (2009). [DOI] [PubMed] [Google Scholar]
- Ponseti J. et al. Assessment of Pedophilia Using Hemodynamic Brain Response to Sexual Stimuli. Arch. Gen. Psychiatry, doi: 10.1001/archgenpsychiatry.2011.130 (2011) [DOI] [PubMed] [Google Scholar]
- Safron A. et al. Neural correlates of sexual arousal in homosexual and heterosexual men. Behav. Neurosci. 121, 237–248 (2007). [DOI] [PubMed] [Google Scholar]
- Rauch S. L. et al. Neural activation during sexual and competitive arousal in healthy men. Psychiatry Res. 91, 1–10 (1999). [DOI] [PubMed] [Google Scholar]
- Stoléru S. et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch. Sex. Behav. 28, 1–21 (1999). [DOI] [PubMed] [Google Scholar]
- Georgiadis J. & Kringelbach M. L. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Prog. Neurobiol. 98, 49–81 (2012). [DOI] [PubMed] [Google Scholar]
- Pfaus J. G. et al. Who, What, Where, When (and Maybe Even Why)? How the Experience of Sexual Reward Connects Sexual Desire, Preference, and Performance. Arch. Sex. Behav. 41, 31–62 (2012). [DOI] [PubMed] [Google Scholar]
- Stoléru S., Fonteille V., Cornélis C., Joyal C. & Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: A review and meta-analysis. Neurosci. Biobehav. Rev. 36, 1481–1509 (2012). [DOI] [PubMed] [Google Scholar]
- Ikemoto S. & Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 31, 6–41 (1999). [DOI] [PubMed] [Google Scholar]
- Pfaus J. G. Pathways of sexual desire. J. Sex. Med. 6, 1506–1533 (2009). [DOI] [PubMed] [Google Scholar]
- Toates F. An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. J. Sex Res. 46, 168–193 (2009). [DOI] [PubMed] [Google Scholar]
- Toates F. How Sexual Desire Works: The Enigmatic Urge (Cambridge University Press, 2014). [Google Scholar]
- Poldrack R. A. Can cognitive processes be inferred from neuroimaging data? Trends Cogn. Sci. 10, 59–63 (2006). [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. The role of fMRI in cognitive neuroscience: where do we stand? Curr. Opin. Neurobiol. 18, 223–227 (2008). [DOI] [PubMed] [Google Scholar]
- Poldrack R. A. The future of fMRI in cognitive neuroscience. NeuroImage, doi: 10.1016/j.neuroimage.2011.08.007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark R. et al. Erotic and disgust-inducing pictures–differences in the hemodynamic responses of the brain. Biol. Psychol. 70, 19–29 (2005). [DOI] [PubMed] [Google Scholar]
- Walter M. et al. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. NeuroImage 40, 1482–1494 (2008). [DOI] [PubMed] [Google Scholar]
- Knutson B., Wimmer G. E., Kuhnen C. M. & Winkielman P. Nucleus accumbens activation mediates the influence of reward cues on financial risk taking. Neuroreport 19, 509–513 (2008). [DOI] [PubMed] [Google Scholar]
- Mannella F., Gurney K. & Baldassarre G. The nucleus accumbens as a nexus between values and goals in goal-directed behavior: a review and a new hypothesis. Front. Behav. Neurosci. 7, 135 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof E. K., Kaps L., Falkai P. & Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - An activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia 50, 1252–1266 (2012). [DOI] [PubMed] [Google Scholar]
- Haber S. N. & Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology 35, 4–26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans K. C. et al. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J. Neurophysiol. 88, 1500–1511 (2002). [DOI] [PubMed] [Google Scholar]
- de Araujo I. E. T., Kringelbach M. L., Rolls E. T. & McGlone F. Human cortical responses to water in the mouth, and the effects of thirst. J. Neurophysiol. 90, 1865–1876 (2003). [DOI] [PubMed] [Google Scholar]
- Tang D. W., Fellows L. K., Small D. M. & Dagher A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol. Behav. 106, 317–324 (2012). [DOI] [PubMed] [Google Scholar]
- Kühn S. & Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci, doi: 10.1111/j.1460-9568.2010.07590.x (2011). [DOI] [PubMed] [Google Scholar]
- Kühn S. & Gallinat J. The neural correlates of subjective pleasantness. NeuroImage 61, 289–294 (2012). [DOI] [PubMed] [Google Scholar]
- Ko C.-H. et al. Brain activities associated with gaming urge of online gaming addiction. J. Psychiatr. Res. 43, 739–747 (2009). [DOI] [PubMed] [Google Scholar]
- Izuma K., Saito D. N. & Sadato N. Processing of the incentive for social approval in the ventral striatum during charitable donation. J. Cogn. Neurosci. 22, 621–631 (2010). [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer K. N. et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Soc. Cogn. Affect. Neurosci. 4, 158–165 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks Z. A. et al. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci. 1, 506–516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G., Redouté J. & Dreher J.-C. The architecture of reward value coding in the human orbitofrontal cortex. J. Neurosci. Off. J. Soc. Neurosci. 30, 13095–13104 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D. J. & Glimcher P. W. The root of all value: a neural common currency for choice. Curr. Opin. Neurobiol. 22, 1027–1038 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge K. C. Motivation concepts in behavioral neuroscience. Physiol. Behav. 81, 179–209 (2004). [DOI] [PubMed] [Google Scholar]
- Kringelbach M. L. & Berridge K. C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 13, 479–487 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. S., Berridge K. C. & Aldridge J. W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA 108, E255–264 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demos K. E., Heatherton T. F. & Kelley W. M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. Off. J. Soc. Neurosci. 32, 5549–5552 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Jeong B. S., Choi J. & Kim J.-W. Sex differences in interactions between nucleus accumbens and visual cortex by explicit visual erotic stimuli: an fMRI study. Int. J. Impot. Res., doi: 10.1038/ijir.2015.8 (2015). [DOI] [PubMed] [Google Scholar]
- Oei N. Y. L., Both S., van Heemst D. & van der Grond J. Acute stress-induced cortisol elevations mediate reward system activity during subconscious processing of sexual stimuli. Psychoneuroendocrinology 39, 111–120 (2014). [DOI] [PubMed] [Google Scholar]
- Hu S.-H. et al. Patterns of brain activation during visually evoked sexual arousal differ between homosexual and heterosexual men. AJNR Am. J. Neuroradiol. 29, 1890–1896 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagerer S. et al. Neural activation toward erotic stimuli in homosexual and heterosexual males. J. Sex. Med. 8, 3132–3143 (2011). [DOI] [PubMed] [Google Scholar]
- Paul T. et al. Brain response to visual sexual stimuli in heterosexual and homosexual males. Hum. Brain Mapp. 29, 726–735 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponseti J. et al. A functional endophenotype for sexual orientation in humans. NeuroImage 33, 825–833 (2006). [DOI] [PubMed] [Google Scholar]
- Chivers M. L., Rieger G., Latty E. & Bailey J. M. A sex difference in the specificity of sexual arousal. Psychol. Sci. 15, 736–744 (2004). [DOI] [PubMed] [Google Scholar]
- Freund K. A laboratory method for diagnosing predominance of homo- or hetero- erotic interest in the male. Behav. Res. Ther. 1, 85–93 (1963). [DOI] [PubMed] [Google Scholar]
- Janssen E. Sexual arousal in men: a review and conceptual analysis. Horm. Behav. 59, 708–716 (2011). [DOI] [PubMed] [Google Scholar]
- Sakheim D. K., Barlow D. H., Beck J. G. & Abrahamson D. J. A Comparison of Male Heterosexual and Male Homosexual Patterns of Sexual Arousal. J. Sex Res. 21, 183–198 (1985). [Google Scholar]
- Kranz F. & Ishai A. Face perception is modulated by sexual preference. Curr. Biol. CB 16, 63–68 (2006). [DOI] [PubMed] [Google Scholar]
- Savic I., Berglund H. & Lindström P. Brain response to putative pheromones in homosexual men. Proc. Natl. Acad. Sci. USA 102, 7356–7361 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Rieger G. & Rosenthal A. M. Still in Search of Bisexual Sexual Arousal: Comment on Cerny and Janssen (2011). Arch. Sex. Behav. 40, 1293–1295 (2011). [DOI] [PubMed] [Google Scholar]
- Cerny J. A. & Janssen E. Patterns of sexual arousal in homosexual, bisexual, and heterosexual men. Arch. Sex. Behav. 40, 687–697 (2011). [DOI] [PubMed] [Google Scholar]
- Rieger G., Chivers M. L. & Bailey J. M. Sexual arousal patterns of bisexual men. Psychol. Sci. J. Am. Psychol. Soc. APS 16, 579–584 (2005). [DOI] [PubMed] [Google Scholar]
- Tollison C. D., Adams H. E. & Tollison J. W. Cognitive and physiological indices of sexual arousal in homosexual, bisexual, and heterosexual males. J. Behav. Assess. 1, 305–314 (1979). [Google Scholar]
- Rosenthal A. M., Sylva D., Safron A. & Bailey J. M. Sexual arousal patterns of bisexual men revisited. Biol. Psychol. 88, 112–115 (2011). [DOI] [PubMed] [Google Scholar]
- Rosenthal A. M., Sylva D., Safron A. & Bailey J. M. The male bisexuality debate revisited: Some bisexual men have bisexual arousal patterns. Arch. Sex. Behav. 41, 135–147 (2012). [DOI] [PubMed] [Google Scholar]
- Bühler M., Vollstädt-Klein S., Klemen J. & Smolka M. N. Does erotic stimulus presentation design affect brain activation patterns? Event-related vs. blocked fMRI designs. Behav. Brain Funct. BBF 4, 30 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti A. et al. Dynamics of male sexual arousal: distinct components of brain activation revealed by fMRI. NeuroImage 26, 1086–1096 (2005). [DOI] [PubMed] [Google Scholar]
- Rupp H. A. & Wallen K. Sex differences in response to visual sexual stimuli: a review. Arch. Sex. Behav. 37, 206–218 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsey A. C., Pomeroy W. R. & Martin C. E. Sexual Behavior in the Human Male. Am. J. Public Health 93, 894–898 (1948). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers M. L., Seto M. C. & Blanchard R. Gender and sexual orientation differences in sexual response to sexual activities versus gender of actors in sexual films. J. Pers. Soc. Psychol. 93, 1108–1121 (2007). [DOI] [PubMed] [Google Scholar]
- Sylva D. et al. Neural correlates of sexual arousal in heterosexual and homosexual women and men. Horm. Behav. 64, 673–684 (2013). [DOI] [PubMed] [Google Scholar]
- Friston K. J. et al. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 2, 189–210 (1994). [Google Scholar]
- Parrish T. B., Gitelman D. R., LaBar K. S. & Mesulam M. M. Impact of signal-to-noise on functional MRI. Magn. Reson. Med. Off. J. Soc. Magn. Reson. Med. Soc. Magn. Reson. Med. 44, 925–932 (2000). [DOI] [PubMed] [Google Scholar]
- Van Dijk K. R. A., Sabuncu M. R. & Buckner R. L. The Influence of Head Motion on Intrinsic Functional Connectivity MRI. NeuroImage 59, 431–438 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J. A., Laurienti P. J., Kraft R. A. & Burdette J. H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 19, 1233–1239 (2003). [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R. & Poline J.-B. Region of interest analysis using an SPM toolbox. in (2002).
- Samson F., Zeffiro T. A., Toussaint A. & Belin P. Stimulus complexity and categorical effects in human auditory cortex: an activation likelihood estimation meta-analysis. Front. Psychol. 1, 241 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray S. & O’Doherty J. Neural coding of reward-prediction error signals during classical conditioning with attractive faces. J. Neurophysiol. 97, 3036–3045 (2007). [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998). [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav. Brain Funct. BBF 6, 24 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenner M.-P. et al. No unified reward prediction error in local field potentials from the human nucleus accumbens: evidence from epilepsy patients. J. Neurophysiol. jn.00260.2015, doi: 10.1152/jn.00260.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Flaisch T., Bradley M. M., Fitzsimmons J. R. & Lang P. J. Affective picture perception: gender differences in visual cortex? Neuroreport 15, 1109–1112 (2004). [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. & Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 837–855 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo-Merino B., Urgesi C., Orgs G., Aglioti S. M. & Haggard P. Extrastriate body area underlies aesthetic evaluation of body stimuli. Exp. Brain Res. Exp. Hirnforsch. Expérimentation Cérébrale 204, 447–456 (2010). [DOI] [PubMed] [Google Scholar]
- Peelen M. V., Glaser B., Vuilleumier P. & Eliez S. Differential development of selectivity for faces and bodies in the fusiform gyrus. Dev. Sci. 12, F16–25 (2009). [DOI] [PubMed] [Google Scholar]
- Schwarzlose R. F., Baker C. I. & Kanwisher N. Separate face and body selectivity on the fusiform gyrus. J. Neurosci. Off. J. Soc. Neurosci. 25, 11055–11059 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach M. L. The human orbitofrontal cortex: linking reward to hedonic experience. Nat. Rev. Neurosci. 6, 691–702 (2005). [DOI] [PubMed] [Google Scholar]
- O’Doherty J. et al. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41, 147–155 (2003). [DOI] [PubMed] [Google Scholar]
- Rolls E. T. The functions of the orbitofrontal cortex. Brain Cogn. 55, 11–29 (2004). [DOI] [PubMed] [Google Scholar]
- Cross E. S., Hamilton A. F. de C. & Grafton S. T. Building a motor simulation de novo: observation of dance by dancers. NeuroImage 31, 1257–1267 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorey B. et al. Activation of the parieto-premotor network is associated with vivid motor imagery–a parametric FMRI study. PloS One 6, e20368 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oullier O., Jantzen K. J., Steinberg F. L. & Kelso J. A. S. Neural substrates of real and imagined sensorimotor coordination. Cereb. Cortex N. Y. N 1991 15, 975–985 (2005). [DOI] [PubMed] [Google Scholar]
- Tomasino B., Maieron M., Guatto E., Fabbro F. & Rumiati R. I. How are the motor system activity and functional connectivity between the cognitive and sensorimotor systems modulated by athletic expertise? Brain Res. 1540, 21–41 (2013). [DOI] [PubMed] [Google Scholar]
- Hanakawa T. et al. Functional properties of brain areas associated with motor execution and imagery. J. Neurophysiol. 89, 989–1002 (2003). [DOI] [PubMed] [Google Scholar]
- Lacourse M. G., Orr E. L. R., Cramer S. C. & Cohen M. J. Brain activation during execution and motor imagery of novel and skilled sequential hand movements. NeuroImage 27, 505–519 (2005). [DOI] [PubMed] [Google Scholar]
- Stoodley C. J. The Cerebellum and Cognition: Evidence from Functional Imaging Studies. Cerebellum Lond. Engl., doi: 10.1007/s12311-011-0260-7 (2011). [DOI] [PubMed] [Google Scholar]
- Timmann D. et al. The human cerebellum contributes to motor, emotional and cognitive associative learning. A review. Cortex J. Devoted Study Nerv. Syst. Behav. 46, 845–857 (2010). [DOI] [PubMed] [Google Scholar]
- Padmala S., Lim S.-L. & Pessoa L. Pulvinar and Affective Significance: Responses Track Moment-to-Moment Stimulus Visibility. Front. Hum. Neurosci. 4 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann Y. B., Pinsk M. A., Wang L., Li X. & Kastner S. The Pulvinar Regulates Information Transmission Between Cortical Areas Based on Attention Demands. Science 337, 753–756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert U. et al. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus–a DTI tractography study. Hum. Brain Mapp. 33, 2627–2637 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granziera C. et al. In-vivo magnetic resonance imaging of the structural core of the Papez circuit in humans. Neuroreport 22, 227–231 (2011). [DOI] [PubMed] [Google Scholar]
- Metzger C. D. et al. High field FMRI reveals thalamocortical integration of segregated cognitive and emotional processing in mediodorsal and intralaminar thalamic nuclei. Front. Neuroanat. 4, 138 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger C. D., van der Werf Y. D. & Walter M. Functional mapping of thalamic nuclei and their integration into cortico-striatal-thalamo-cortical loops via ultra-high resolution imaging—from animal anatomy to in vivo imaging in humans. Front. Neurosci. 7, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papez J. W. A proposed mechanism of emotion. Arch. Neurol. Psychiatry 38, 725–743 (1937). [Google Scholar]
- Marson L. Lesions of the periaqueductal gray block the medial preoptic area-induced activation of the urethrogenital reflex in male rats. Neurosci. Lett. 367, 278–282 (2004). [DOI] [PubMed] [Google Scholar]
- Kelly Y. T., Webb T. W., Meier J. D., Arcaro M. J. & Graziano M. S. A. Attributing awareness to oneself and to others. Proc. Natl. Acad. Sci. USA 111, 5012–5017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krach S. et al. Are women better mindreaders? Sex differences in neural correlates of mentalizing detected with functional MRI. BMC Neurosci. 10, 9 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J. P. Mentalizing and Marr: an information processing approach to the study of social cognition. Brain Res. 1079, 66–75 (2006). [DOI] [PubMed] [Google Scholar]
- Mouras H. et al. Activation of mirror-neuron system by erotic video clips predicts degree of induced erection: an fMRI study. NeuroImage 42, 1142–1150 (2008). [DOI] [PubMed] [Google Scholar]
- Perry D., Walder K., Hendler T. & Shamay-Tsoory S. G. The gender you are and the gender you like: sexual preference and empathic neural responses. Brain Res. 1534, 66–75 (2013). [DOI] [PubMed] [Google Scholar]
- Saxe R. & Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia 43, 1391–1399 (2005). [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. & Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. NeuroImage 48, 564–584 (2009). [DOI] [PubMed] [Google Scholar]
- Ben-Yakov A., Rubinson M. & Dudai Y. Shifting gears in hippocampus: temporal dissociation between familiarity and novelty signatures in a single event. J. Neurosci. Off. J. Soc. Neurosci. 34, 12973–12981 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Encoding and retrieval along the long axis of the hippocampus and their relationships with dorsal attention and default mode networks: The HERNET model. Hippocampus 25, 500–510 (2015). [DOI] [PubMed] [Google Scholar]
- Ritchey M., Wing E. A., LaBar K. S. & Cabeza R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb. Cortex N. Y. N 1991 23, 2818–2828 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C. M., Oakes T. R. & Davidson R. J. The voice of emotion: an FMRI study of neural responses to angry and happy vocal expressions. Soc. Cogn. Affect. Neurosci. 1, 242–249 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S. & Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. J. Sex. Med. 8, 2269–2275 (2011). [DOI] [PubMed] [Google Scholar]
- Farah M. J. Why does the somatosensory homunculus have hands next to face and feet next to Genitals? A hypothesis. Neural Comput. 10, 1983–1985 (1998). [DOI] [PubMed] [Google Scholar]
- Parpia P. Reappraisal of the Somatosensory Homunculus and Its Discontinuities. Neural Comput., doi: 10.1162/NECO_a_00179 (2011). [DOI] [PubMed] [Google Scholar]
- Bartolo A. et al. Contribution of the motor system to the perception of reachable space: an fMRI study. Eur. J. Neurosci. 40, 3807–3817 (2014). [DOI] [PubMed] [Google Scholar]
- Filimon F., Rieth C. A., Sereno M. I. & Cottrell G. W. Observed, Executed, and Imagined Action Representations can be Decoded From Ventral and Dorsal Areas. Cereb. Cortex N. Y. N 1991, doi: 10.1093/cercor/bhu110 (2014). [DOI] [PubMed] [Google Scholar]
- Burke S. M., Cohen-Kettenis P. T., Veltman D. J., Klink D. T. & Bakker J. Hypothalamic response to the chemo-signal androstadienone in gender dysphoric children and adolescents. Neuroendocr. Sci. 5, 60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. W. et al. Brain activation by visual erotic stimuli in healthy middle aged males. Int. J. Impot. Res. 18, 452–457 (2006). [DOI] [PubMed] [Google Scholar]
- Ishai A. Sex, beauty and the orbitofrontal cortex. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 63, 181–185 (2007). [DOI] [PubMed] [Google Scholar]
- Diamond L. M. The Desire Disorder in Research on Sexual Orientation in Women: Contributions of Dynamical Systems Theory. Arch. Sex. Behav. 41, 73–83 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang M. et al. Neural circuits of disgust induced by sexual stimuli in homosexual and heterosexual men: an fMRI study. Eur. J. Radiol. 80, 418–425 (2011). [DOI] [PubMed] [Google Scholar]
- Calder A. J. et al. Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. Eur. J. Neurosci. 25, 3422–3428 (2007). [DOI] [PubMed] [Google Scholar]
- Craig A. D. B. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 1225, 72–82 (2011). [DOI] [PubMed] [Google Scholar]
- Chikazoe J., Lee D. H., Kriegeskorte N. & Anderson A. K. Population coding of affect across stimuli, modalities and individuals. Nat. Neurosci. 17, 1114–1122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. D., Gottfried J. A., Tobler P. N. & Kahnt T. Identity-specific coding of future rewards in the human orbitofrontal cortex. Proc. Natl. Acad. Sci. 112, 5195–5200 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J., Zelano C., Gottfried J. A. & Mohanty A. Human Amygdala Represents the Complete Spectrum of Subjective Valence. J. Neurosci. 35, 15145–15156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivers M. L. & Bailey J. M. A sex difference in features that elicit genital response. Biol. Psychol. 70, 115–120 (2005). [DOI] [PubMed] [Google Scholar]
- Chivers M. L., Seto M. C., Lalumière M. L., Laan E. & Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Arch. Sex. Behav. 39, 5–56 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]