Abstract
Objective: The use of bioactive extracellular matrix (ECM) grafts such as amniotic membranes is an attractive treatment option for enhancing wound repair. In this study, the concentrations, activity, and distribution of matrix components, growth factors, proteases, and inhibitors were evaluated in PURION® Processed, micronized, dehydrated human amnion/chorion membrane (dHACM; MiMedx Group, Inc.).
Approach: ECM components in dHACM tissue were assessed by using immunohistochemical staining, and growth factors, cytokines, proteases, and inhibitors were quantified by using single and multiplex ELISAs. The activities of proteases that were native to the tissue were determined via gelatin zymography and EnzChek® activity assay.
Results: dHACM tissue contained the ECM components collagens I and IV, hyaluronic acid, heparin sulfate proteoglycans, fibronectin, and laminin. In addition, numerous growth factors, cytokines, chemokines, proteases, and protease inhibitors that are known to play a role in the wound-healing process were quantified in dHACM. Though matrix metalloproteinases (MMPs) were present in dHACM tissues, inhibitors of MMPs overwhelmingly outnumbered the MMP enzymes by an overall molar ratio of 28:1. Protease activity assays revealed that the MMPs in the tissue existed primarily either in their latent form or complexed with inhibitors.
Innovation: This is the first study to characterize components that function in wound healing, including inhibitor and protease content and activity, in micronized dHACM.
Conclusion: A variety of matrix components and growth factors, as well as proteases and their inhibitors, were identified in micronized dHACM, providing a better understanding of how micronized dHACM tissue can be used to effectively promote wound repair.
Keywords: : amniotic membrane, wound healing, MMP, protease inhibitors

Jennifer Lei, PhD
Introduction
The treatment of large, chronic wounds (e.g., foot ulcers, venous leg ulcers) is challenging, particularly in diabetic patient populations. Approximately one-quarter of diabetic patients will develop a chronic wound in his or her lifetime, which, if not managed successfully, may require amputation. In fact, 81% of amputations in diabetic patients have been attributed to inadequate wound healing.1
Physiological wound healing is a well-coordinated sequence of events in which signaling and remodeling of the extracellular matrix (ECM) are integral components. After injury, the wound progresses through hemostasis, inflammation, proliferation, and repair/remodeling, ultimately resulting in the regeneration of functional tissue. In contrast, a chronic wound may result from delayed resolution of the inflammatory phase. Inflammatory cells recruited to the site of injury produce elevated reactive oxygen species and secrete an abundance of proinflammatory cytokines, which, in turn, recruit additional inflammatory cells, resulting in a perpetual cycle of inflammation.2 The combination of excessive proinflammatory cytokines and reactive oxygen species induce upregulation of proteases and matrix metalloproteinases (MMPs) that not only degrade ECM but also alter the activity of growth factors and proteins that are necessary for progression to tissue repair/regeneration.3 A large clinical study involving a variety of complex wounds in a diverse patient population measured elevated MMP levels in 28% of chronic wounds.4 In a prolonged inflammatory state, tissue inhibitors of metalloproteinases (TIMPs) and other inhibitors may not be sufficient to modulate the effects of the disproportionately high concentration of MMPs.
MMPs are a class of proteinases with diverse roles in tissue remodeling. Aside from the well-known role of ECM proteolysis, the downstream functions of MMPs include release, activation, and inactivation of growth factors and cytokines, all of which influence cell–cell and cell–matrix interactions, and ultimately cell proliferation, migration, and differentiation.5 MMP expression is increased during physiological tissue remodeling, including bone turnover and fracture healing, pregnancy, angiogenesis, and wound healing.5,6 However, unregulated MMP expression can result in excessive tissue breakdown and pathological conditions; therefore, inhibitors of MMPs are necessary to modulate MMP activity.7
Successful closure of chronic wounds often requires advanced therapies to resolve the persistent inflammatory state and to repair the lost and/or damaged tissue. However, standard compression bandaging is often inadequate, as noted when progress toward wound closure is not observed after 3–4 weeks of treatment.8 In these patients, treatment with naturally derived, bioactive ECM allografts such as amniotic membrane is an attractive alternative therapy to accelerate repair.
During pregnancy, the amniotic sac forms an adjustable biocapsule that supports and protects the developing fetus.9 The complex role of the amniotic sac is facilitated by the unique structure and functionality of the ECM components that are contained within this specialized tissue. The dynamic and biologically active tissue matrix provides tensile strength and integrity, anchors cells, and influences cell morphology and function via interaction with cell surface receptors, and it is a reservoir for growth factors. The amniotic sac is composed of amnion and chorion layers that are constantly remodeling through synthesis and degradation of the ECM. The amnion layer is a thin (35–60 μm) avascular membrane that is composed of epithelium, basement membrane, compact layer, and fibroblast layer. Each contains many ECM macromolecules (e.g., collagen types I and IV, fibronectin, laminin, heparan sulfate proteoglycans [HSPGs], hyaluronic acid [HA], and glycoproteins) that provide mechanical support for the tissue, provide sites for cell attachment and motility, and sequester growth factors.10 The chorion layer ECM is composed of fibronectin, laminin, and types I and IV collagen that support the cells and sequester growth factors/cytokines of the chorion.11 An intermediate spongy layer separates the amnion and the chorion. Throughout pregnancy, the amniotic membrane ECM undergoes physiological remodeling—a highly coordinated process involving the secretion of growth factors, cytokines, and matrix proteins—to maintain structural integrity while allowing for expansion during growth of the fetus. As in response to injury, the balance of MMPs and their inhibitors is crucial for maintenance of the ECM during pregnancy.
The amniotic membrane has a long history of use in wound-healing applications due to the dynamic nature of the tissue and its growth factor/cytokine-rich ECM. In particular, dehydrated human amnion/chorion membrane (dHACM) has demonstrated clinical efficacy in the enhancement of wound repair compared with standard compression therapy.12 dHACM contains an array of growth factors, cytokines, and chemokines, and it has been shown to promote proliferation and migration of bone marrow-derived mesenchymal stem cells, adipose-derived stem cells (both healthy and diabetic), and hematopoietic stem cells.13–17
The treatment of complex, full-thickness wounds may be augmented by the use of a micronized formulation of dHACM (in addition to membrane dHACM) to fill the wound bed and to provide additional bioactive molecules to the site of injury. Moreover, the larger surface area afforded by the micronized dHACM may facilitate cell–tissue interactions and growth factor presentation/availability, ultimately promoting processes such as angiogenesis, keratinocyte migration, and stem cell recruitment. Micronized dHACM has been applied directly in powder form to wounds, and it is injected after rehydration at wound edges. Micronized dHACM was previously used as an adjuvant to membrane dHACM in three patients with chronic wounds that had not healed after aggressive treatment with other wound care strategies. In these challenging clinical scenarios, this combination therapy resulted in successful closure of the wounds.18
Clinical Problem Addressed
The tissue-healing effects of the amniotic membrane on chronic wounds are believed to be due, at least in part, to the inherent capacity of the membrane to coordinate ECM remodeling, an essential function not only during pregnancy but also for wound healing. The amniotic membrane presents specialized ECM components in their native structure, and key regulatory molecules in the form of growth factors, cytokines, and proteins contained therein. This work evaluated the concentrations and distribution of vital ECM components and growth factors, along with MMPs and their inhibitors, in micronized dehydrated human amnion/chorion membrane (dHACM), and it serves as the first exploration into the structure and composition of the micronized form of the tissue. Ultimately, an understanding of how amniotic membrane therapies such as dHACM are efficacious in the treatment of chronic wounds is crucial to advancing the standards of wound care.
Materials and Methods
dHACM Tissue Processing
Membrane and micronized formulations of dehydrated human amnion/chorion membranes (dHACM, EpiFix®; MiMedx Group, Inc.) processed by a proprietary PURION® Process19 were used in this study. Briefly, amnion and chorion were isolated from human placentas that were donated under informed consent from scheduled Cesarean section births. The tissue layers were gently cleansed, laminated, and dehydrated. For micronized dHACM, the tissue was subsequently cryomilled and sieved by using filters of 180 and 25 μm pore size.
Immunohistochemistry of dHACM
For ease of maintaining tissue orientation during histological sectioning, membrane dHACM tissue samples (n = 3) were used for immunohistochemistry (IHC). Tissues were hydrated in 0.9% NaCl for 10 min, embedded in TissueTek optimum cutting temperature compound (Sakura Finetek), and cryosectioned at a thickness of 5 μm. Sections were fixed by incubation in chilled acetone for 10 min and dried at room temperature for at least 30 min. The sections were washed with phosphate-buffered saline (PBS), blocked, and incubated with primary antibodies (mouse anti-human collagen IV, 1:100; Abcam, mouse anti-human collagen I, 1:1,000; sheep anti-human HA, 1:100; Abcam) overnight at 4°C. After washing with PBS, sections were stained with a secondary antibody (Alexa Fluor® 660 goat anti-mouse; Alexa Fluor 555 donkey anti-mouse; Alexa Fluor 647 donkey anti-sheep, respectively; 1:100; Life Technologies) for 30 min at room temperature and mounted in Fluorogel (Electron Microscopy Sciences).
Micronized dHACM Extraction and Digestion for ECM Quantification
Micronized dHACM (n = 4–7) samples were extracted in 4 M guanidine hydrochloride (Sigma) and 0.1 M sodium acetate (Sigma) containing Protease Inhibitor Cocktail Set III (Calbiochem; EMD Millipore) at 4°C overnight and pH 6.0. Solid material was removed by centrifugation and the supernatant was frozen at −20°C, whereas the pellet was resuspended in a second volume of extraction buffer overnight. Supernatants from both extractions were combined and dialyzed in 50 kDa MWCO dialysis tubing (Spectra/Por® 6 Standard RC, Spectrum) against deionized water for 2 days and, subsequently, frozen and lyophilized. Samples were analyzed for fibronectin, laminin, and HSPGs by using single-factor enzyme-linked immunosorbent assays (ELISAs) (fibronectin, R&D Systems; laminin, Abcam; HSPGs, Cusabio).
Collagen was extracted from the remaining micronized dHACM pellets and membrane dHACM pellets that had been processed in the same manner (n = 3). Pellets were rinsed and acidified before an overnight pepsin (Worthington) digestion. Collagen type I was purified from the pepsin digests through a 0.7 M salt precipitation. The precipitated collagen was frozen, lyophilized, and prepared for separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (ThermoFisher) by addition of a tris-glycine-dithiothreitol sample buffer (ThermoFisher) and heating at 100°C for 5 min. The volume loaded was based on the measured bulk mass after lyophilization. Separated proteins contained within the gel were stained with Coomassie Blue (ThermoFisher).
To determine levels of HA in micronized dHACM, tissues (n = 4) were digested in a 1 mg/mL papain (Worthington) buffer containing 0.2 M sodium acetate, 1.1 mM EDTA, 5.5 mM cysteine hydrochloride, and 0.067 mM β-merceptoethanol overnight at 60°C. Solutions were boiled and cooled before dialysis in 50 kDa MWCO tubing against deionized water overnight. Solutions were collected, frozen, and lyophilized. HA content in dry samples was evaluated by using a single-factor ELISA (R&D Systems).
Analyses of Growth Factors, Cytokines, Proteases, and Inhibitors
Micronized dHACM (n = 4–5) was incubated in lysis buffer (RayBiotech) containing Protease Inhibitor Cocktail Set III at a concentration of 35 mg/mL with gentle agitation overnight at 4°C. Lysates were centrifuged and filtered (0.22 μm pore size; EMD Millipore). Growth factors, cytokines, proteases, and protease inhibitors were evaluated by using multiplex ELISA Quantibody® arrays (Human Cytokine Array Q1000 and Human MMP Array Q1, RayBiotech), and single-factor ELISAs (alpha 2-macroglobulin [α2 M] and alpha-1 antitrypsin [A1AT]; RayBiotech). Amounts of each molecule were reported as average moles/mg of tissue.
Detection of MMPs by Gelatin Zymography
Micronized dHACM (n = 4) was incubated in Tris glycine SDS (ThermoFisher) overnight at 4°C with gentle agitation at a concentration of 40 mg/mL. Samples were centrifuged and filtered before dilution to concentrations of 20, 10, and 1 mg/mL for each donor. Ten nanograms of recombinant MMP-2 (R&D Systems) that had been activated for 1 h at 37°C with 4-aminophenylmercuric acetate (APMA) was used as a control, and a Benchmark Pre-stained protein ladder (Novex) was included with each gel. Each sample was loaded into individual wells of a 10% gelatin zymogram gel (Novex) and run at 125 V for 110 min. Gels were collected and incubated in 1 × Renaturing buffer (Novex) for 30 min, followed by 1 × Developing buffer (Novex) overnight at 37°C. Gels were stained with SimplyBlue™ SafeStain (ThermoFisher) for 30 min and destained in 20% NaCl for 24 h. Images were taken by using an Amersham Imager 600 (GE Healthcare Life Sciences) and analyzed by using ImageQuantTL software. Band densities were quantified and normalized to the recombinant MMP-2 control.
Gelatinase/Collagenase Activity Assay
Micronized dHACM (n = 5) was incubated in the Enzchek® Gelatinase/Collagenase Activity Assay kit reaction buffer (ThermoFisher) at 40 mg/mL overnight at 4°C with gentle agitation. Extract samples were collected, centrifuged, and filtered before dilution to concentrations of 20, 10, and 1 mg/mL. Extracts were assayed alone and in combination with Clostridium collagenase (ThermoFisher) at 0.0625 U/mL, or with 0.1 mM of the inhibitor 1, 10-phenathroline (ThermoFisher). All samples were incubated with 10 μg/mL DQ gelatin substrate for 45 min and read with a plate reader (Synergy M3; Biotek) at an excitation wavelength of 485 nm and an emission wavelength of 528 nm. Readings from samples with added collagenase were normalized to a Clostridium collagenase control at 0.0625 U/mL. Readings from samples only and samples with added inhibitor were normalized to a control that contained both inhibitor (0.1 mM) and collagenase (0.0625 U/mL).
Statistical Analysis
All values were reported as mean ± standard deviation, and statistical analyses were performed in Minitab (v17.1). Zymography data and the comparison of gelatinase/collagenase activity to collagenase controls were evaluated by one-way analysis of variance (ANOVAs). The comparison of gelatinase/collagenase activity to inhibitor + collagenase controls was performed by using a two-way ANOVA with factors of extract concentration and inhibitor condition. For each ANOVA, pairwise comparisons were made by using a Tukey's post hoc test. Significant differences were assigned when p ≤ 0.05.
Results
Composition of ECM Collagen and HA in dHACM
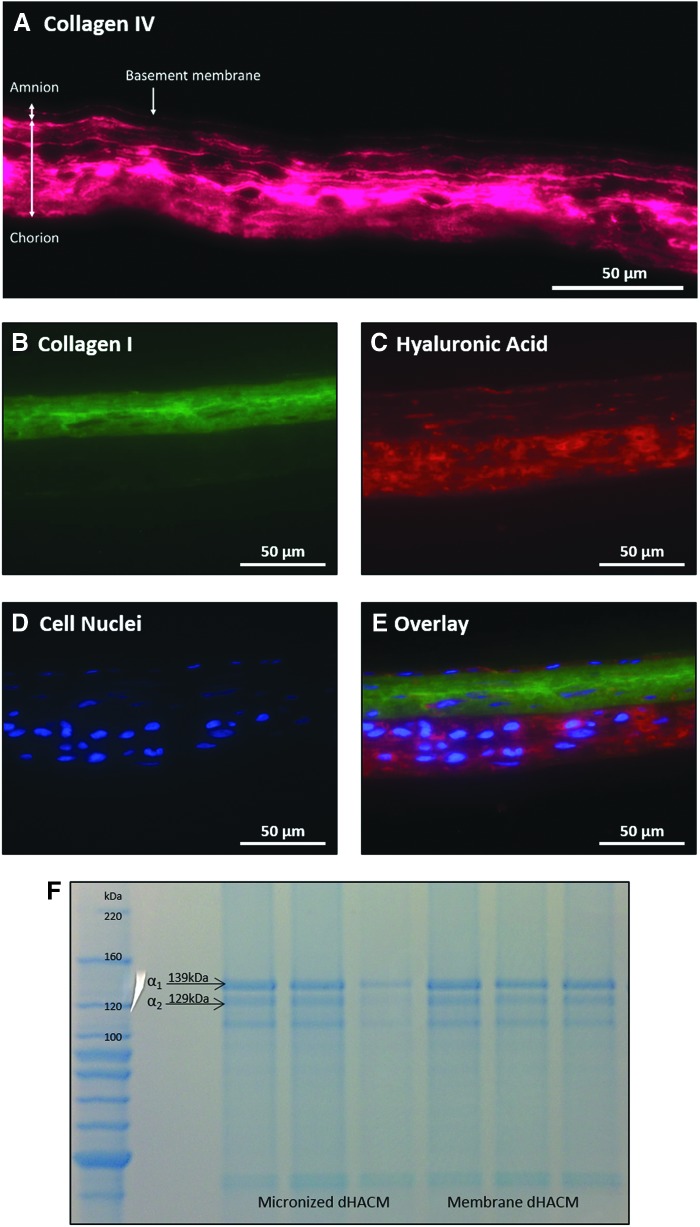
To visualize ECM components found in dHACM tissue, immunofluorescent staining was performed for collagen IV, collagen I, and HA. Collagen IV was observed in the amnion basement membrane and throughout the entire chorion layer of the dHACM membrane tissue (Fig. 1A). Collagen I was localized mainly to the amnion layer and the reticular portion of the chorion layer (Fig. 1B), whereas HA was primarily observed in the chorion trophoblast layer (Fig. 1C).
Figure 1.
Visualization of collagen and ECM components through immunohistochemistry and SDS-PAGE. (A) Immunofluorescent staining of collagen IV observed in pink. (B) Collagen I staining observed in green. (C) HA staining observed in red. (D) DAPI staining of cell nuclei observed in blue. (E) Overlay of collagen I, HA, and DAPI staining. (F) SDS-PAGE of extracted collagen from micronized and membrane dHACM, n = 3, scale bar = 50 μm. dHACM, dehydrated human amnion/chorion membrane; ECM, extracellular matrix.
SDS-PAGE was run on digested micronized and membrane dHACM tissues to confirm the presence of collagen and to further identify collagen subunits. All donors of both dHACM formulations exhibited bands at both 139 and 129 kDa (Fig. 1F), molecular weights that correspond to the α1 and α2 collagen subunits of collagen type I. Although some donors exhibited fainter bands of the two units, overall, collagen I α1 and α2 subunits were observed.
Quantification of Matrix Components
It was determined that 1.84 ± 1.05 × 103 ng/mg of fibronectin, 1.82 ± 1.71 × 103 ng/mg of HA, 4.54 ± 4.14 ng/mg of HSPGs, and 1.46 ± 0.91 ng/mg of laminin were measured in the micronized tissue extract (Table 1).
Table 1.
Quantified amounts of fibronectin, HA, heparin sulfate proteoglycan, and laminin in guanidine-extracted micronized dHACM
| ECM Component | ng/mg Tissue |
|---|---|
| Fibronectin | 1.84 ± 1.05 × 103 |
| Hyaluronic acid | 1.82 ± 1.71 × 103 |
| Heparan sulfate proteoglycan | 4.54 ± 4.14 |
| Laminin | 1.46 ± 0.908 |
Values reported as moles of that molecule/mg of tissue ± standard deviation, n = 4–7.
dHACM, dehydrated human amnion/chorion membrane; ECM, extracellular matrix; HA, hyaluronic acid.
Analysis of Growth Factors, MMPs, and Inhibitors
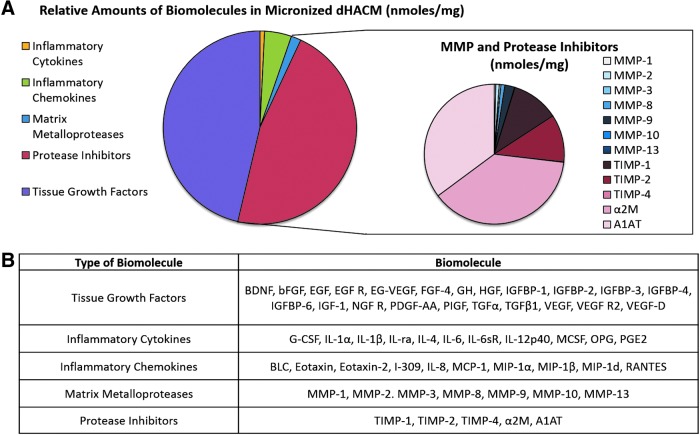
Multiplex and single-factor ELISAs were utilized to identify cytokines and regulatory proteins (nmol/mg of tissue) in micronized dHACM. A table of these growth factors, cytokines, and regulatory proteins is provided in Figure 2B. Of the factors identified, 46.7% are classified as tissue growth factors that can promote wound healing. This includes insulin-like growth factor binding protein-3 (IGFBP-3), placental growth factor (PlGF), platelet-derived growth factor-AA (PDGF-AA), transforming growth factor beta 1 (TGF-β1), and vascular endothelial growth factor (VEGF). Inflammatory cytokines and chemokines were 0.8% and 4.5%, respectively, of the total concentration of factors. Lastly, protease inhibitors such as TIMP-1, TIMP-2, α2M, and A1AT accounted for 46.3% of the identified factors; whereas MMPs such as MMP-2, MMP-8, and MMP-9 comprised 1.7% of the detected molecules (Fig. 2). Further analysis measured plasma protease inhibitors and TIMPs in high quantities: α2M at 92.18 moles/mg, and TIMP-1 and TIMP-2 at ∼13 moles/mg each (Table 2). In contrast, MMP-9, MMP-8, and MMP-2 were the most prevalent proteases, at concentrations of 2.67 ± 1.99, 1.03 ± 0.79, and 0.94 ± 0.97 moles/mg, respectively.
Figure 2.
Growth factors, cytokines, protease, and inhibitors found in micronized dHACM. (A) Relative amounts of different classes of the 55 growth factors found in micronized dHACM and breakdown of MMPs and inhibitors identified. Reported amounts calculated from nmoles of growth factor/mg tissue. (B) Table of biomolecules detected in micronized dHACM, n = 5. MMP, matrix metalloproteinase.
Table 2.
Quantified amounts of MMPs, TIMPs, and protease inhibitors found in micronized dHACM
| Moles/mg Tissue | |
|---|---|
| Proteases | |
| MMP-1 | 0.42 ± 0.18 |
| MMP-2 | 0.94 ± 0.97 |
| MMP-3 | 0.47 ± 0.29 |
| MMP-8 | 1.03 ± 0.79 |
| MMP-9 | 2.67 ± 1.99 |
| MMP-10 | 0.10 ± 0.15 |
| MMP-13 | 0.01 ± 0.0008 |
| Inhibitors | |
| TIMP-1 | 12.91 ± 1.22 |
| TIMP-2 | 12.90 ± 4.22 |
| TIMP-4 | 0.11 ± 0.06 |
| α2M | 92.18 ± 53.33 |
| A1AT | 41.30 ± 23.91 |
Values reported as moles of that molecule/mg of tissue ± standard deviation, n = 4.
MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
MMP Gelatin Zymography
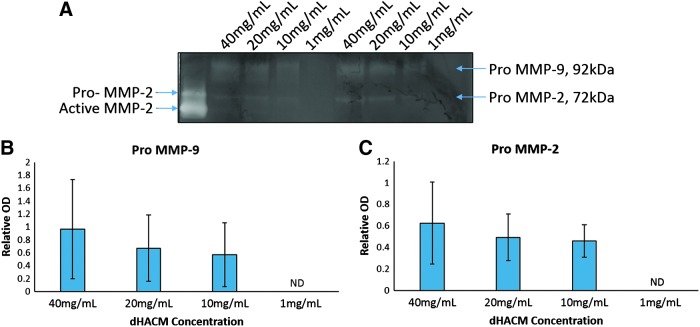
To demonstrate the activity of the MMPs found in micronized dHACM, a gelatin zymogram gel was run on dHACM extracts at concentrations ranging from 40 to 1 mg/mL. This assay denatures and dissociates complexed gelatinases within the sample before renaturing after gel electrophoresis; therefore, this technique is able to detect MMPs in both their latent and inhibitor-bound form. The MMP-2 control revealed one band at approximately 62 kDa and one band at 72 kDa, corresponding to the molecular weights of pro MMP-2 and active MMP-2, respectively. For extract concentrations of 40, 20, and 10 mg/mL, bands were observed at 92 and 72 kDa (Fig. 3A), which correspond to the molecular weights of MMP-9 and MMP-2 in their inactive (pro) form. No bands were observed in 1 mg/mL samples. When the band density was quantified and normalized to the optical density of the control MMP-2 sample (band at 72 kDa), no significant effect of concentration was observed for either MMP (Fig. 3B, C).
Figure 3.
Detection of MMP-2 and MMP-9 by gelatin zymography. (A) Representative image from zymogram containing recombinant MMP-2 control and two donors at different concentrations of micronized dHACM extracts. (B) Quantification of pro-MMP-9 bands observed at 92 kDa. (C) Quantification of pro-MMP-2 bands observed at 72 kDa. Values reported as average OD normalized to control pro-MMP-2 band with error bars representing standard deviation, n = 4. OD, optical density.
MMP Activity
To characterize gelatinase and collagenase activity in micronized dHACM, degradation of a fluorescently tagged gelatin substrate was measured when treated with the following: dHACM extract, extract with added inhibitor (+I), and extract with added collagenase (+C). After normalization to the control inhibitor + collagenase, in which minimal collagenase activity was expected, extract-only groups at multiple concentrations were not significantly different than the inhibitor + collagenase control (Fig. 4A). In addition, activity levels were not significantly different between extract-only groups and their corresponding extract + inhibitor group. When extract groups were spiked with collagenase, higher extract concentrations exhibited lower collagenase activity: 0.72 ± 0.14 U/mL at 1 mg/mL extract concentration, and 0.002 ± 0.1 U/mL at 40 mg/mL extract concentration (Fig. 4B). Normalized activity levels for 40, 20, and 10 mg/mL were significantly lower than the collagenase-only control.
Figure 4.
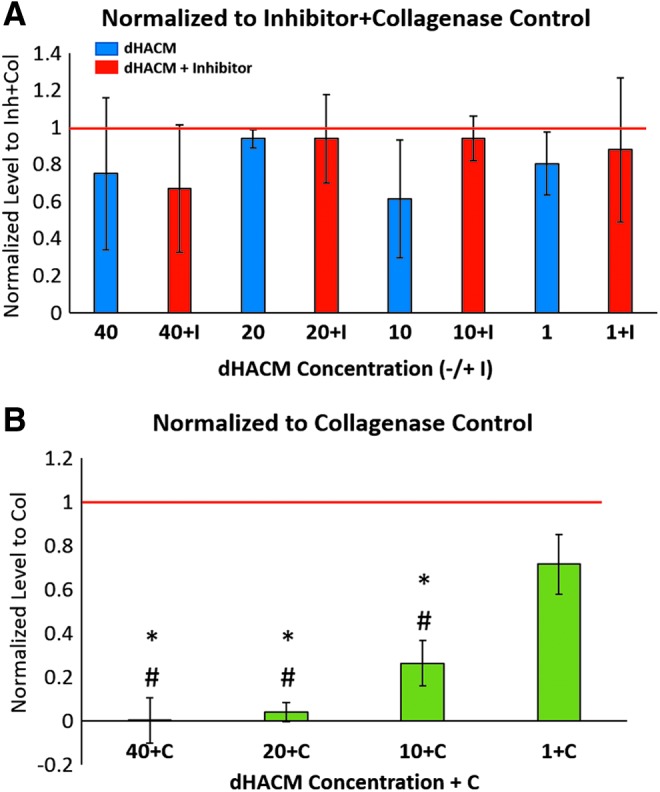
MMP activity in varying doses (40, 20, 10, and 1 mg/mL) of micronized dHACM extracts determined by Encheck® gelatinase/collagenase activity assay. (A) Activity levels of extract only (blue) and extract with added inhibitor (+I, red) normalized to inhibitor + collagenase control (red line). (B) Activity levels of extract with added collagenase (+C, green) normalized to collagenase-only control (red line). Values reported as average normalized activity level with error bars representing standard deviation, *indicates significant difference compared with collagenase-only control, and #indicates significant difference compared with 1 + C group, p < 0.05, n = 5.
Discussion
This study identified ECM components, growth factors, cytokines, and MMPs/inhibitors in micronized dHACM that can play a role in the wound-healing process. In addition, the activity of MMPs and protease inhibitors in the tissue was analyzed. Evaluation of ECM components in dHACM tissue revealed, in particular, the localization of collagens I and IV and HA as well as fibronectin, laminin, HA, and HSPGs in high quantities. A broad array of factors (growth factors, cytokines, chemokines, proteases, and inhibitors) were detected in the tissue. The zymogram assay revealed MMPs in their latent form, and the Enzchek gelatinase/collagenase activity assay suggested an excess of active inhibitors in the tissue. Overall, the results from these studies demonstrate that the tissue is composed of an array of structural materials, signaling molecules, and remodeling enzymes that are usually involved in a metabolically active, growing tissue, and provide a better understanding of potential roles of micronized dHACM in wound-healing applications.
In amniotic membranes, the primary ECM components in the amnion layer include a basement membrane that is composed of collagen types IV and VII, fibronectin, and laminin. The adjacent compact and fibroblast layers also contain collagens and provide further mechanical support to the tissue.20 In addition to their roles in situ, these ECM components may provide critical structural and regulatory cues to support and enhance healing in chronic wounds that are treated with dHACM grafts. Immunostaining allowed for visualization and localization of ECM components in the original amnion/chorion membranes before micronization and confirmed that dHACM tissue contains collagens I and IV, and HA, molecules that are present in the native amniotic membrane. Collagen I is found in the basement membrane, compact, and fibroblast layers of amnion, as well as the reticular layer of the chorion, as observed from localized immunostaining (Fig. 1). In addition, collagen IV was observed in the basement membrane of the amnion and throughout the chorion regions of dHACM, similar to its orientation in the native amniotic membrane.20 Gel separation of collagen extracts revealed comparable banding patterns between micronized and membrane dHACM, indicating that both formulations of dHACM tissue contain similar collagen content.
Other ECM components present in micronized dHACM include fibronectin, laminin, HA, and HSPGs, all of which have been shown to play specific roles in wound healing. Fibronectin is a key component of the ECM and functions as an adhesive molecule that provides binding sites for cells, promoting growth and migration.21 Laminin, found in the basement membrane, mediates binding of bioactive molecules and cells, which, in turn, regulates re-epithelialization, cell shape and movement, and angiogenesis during the wound-healing process.22,23 Immunostaining of HA demonstrated that the majority of the HA in dHACM was localized to the chorion layer, with relatively weak staining observed in the amnion. HA, a nonsulfated glycosaminoglycan, is a primary component of the ECM and is known to activate fibroblast proliferation and angiogenesis during the process of wound closure.24 HSPGs are cell surface proteoglycans that can bind growth factors such as fibroblast growth factor-2 (FGF-2), resulting in protection from degradation and enhancing cell response and activation.25 The roles of these ECM components are pertinent in wound-healing processes, where it has been shown that heparin sulfate-deficient mice displayed impaired angiogenesis in a corneal micropocket assay, as well as decreased granulation tissue and delayed closure of wounds.26
Previous studies characterizing membrane dHACM tissue have identified growth factors and cytokines that were also observed in micronized dHACM tissue in this study (Fig. 2).13,15 The tissue growth factors detected in micronized dHACM are known to regulate cell migration, proliferation, and synthesis of ECM components such as collagen, fibronectin, and HA.27 A variety of these growth factors, including epidermal growth factor, FGF, VEGF, and PDGF, are capable of initiating cell signaling and transcriptional activation of MMPs.28 In addition, many of the cytokines and chemokines that are identified participate in the inflammatory phase to activate monocytes and regulate leukocyte migration and proliferation, all of which are vital processes in wound healing.27
During pregnancy, the amniotic membrane ECM must continue to expand and remodel to maintain structural integrity while accommodating the developing fetus and amniotic fluid through term. Furthermore, tissue remodeling requires protease activity to break down existing ECM components, whereas tissue degradation is offset by a balance of TIMPs and ECM production that supports overall tissue growth. Notably, MMP-9-deficient mice exhibited impaired wound neovascularization and collagen deposition, along with increased inflammatory cell infiltration.29 Due to their important roles naturally, as well as in wound healing, this study evaluated MMPs and TIMPs released from micronized dHACM tissue and quantified overall MMP activity in tissue extracts. Collagenases (MMP-1, MMP-8, MMP-13), gelatinases (MMP-2, MMP-9), and stromelysins (MMP-3, MMP-10) were detected in the tissue via ELISAs, whereas activity assays revealed that MMPs may exist either in their inactive form or complexed with inhibitors contained within the tissue. Typically, MMPs are synthesized in a latent form, at which point they are either secreted or bound to cell surfaces.28 Thus, the zymogram assay was employed to separate latent and active forms of MMPs based on molecular weight, and it revealed the activity of the enzymes after activation in a renaturing buffer.30 This was observed with pro MMP-9 and pro MMP-2 bands at 92 and 72 kDa, respectively, in micronized dHACM extracts (Fig. 3).31 Although significant differences were not observed as a function of dose, the number of samples used may have limited the ability to detect differences.
From the Enzchek activity assay, no differences in MMP activity were observed (at any concentration of tissue extract) between the extract only and extract + inhibitor, or between these groups and the inhibitor + collagenase control, suggesting minimal protease activity in dHACM tissue (Fig. 4A). Active MMPs play critical roles in wound healing, including regulation of epithelial tissue architecture by degradation of the basement membrane and disruption of intercellular junctions,32 along with support of keratinocyte migration33 and wound contraction.34 However, spatiotemporal control of MMP activity is necessary to maintain the balance of degradation and production of ECM, to prevent further breakdown that could contribute to a chronic wound environment.32
TIMPs are known to bind different MMPs; for example, TIMP-2 has been shown to bind MMP-2 and MMP-9,35 resulting in modulation of MMP activity and regulation of cell migration during wound healing.33 With ELISAs, multiple TIMPs and two large plasma protease inhibitors (α2M and A1AT) were detected, and each (except TIMP-4) was present at molar ratios in excess of all MMPs measured (Table 2). α2 M is known to inhibit most proteinases of all classes (metallo-, serine, carboxyl, thiol),36 and A1AT can act on serine proteases, collagenases, and elastase.37 The effect of extract concentration on MMP activity, whereby greater collagenase activity was observed at the lowest concentration of extract (1 mg/mL), further implied the presence of active inhibitors in dHACM tissue extract (Fig. 4B). It has previously been shown that immediate intervention of alkali burns of the cornea with amniotic membrane resulted in lower proteinase activity and subsequent epithelial healing compared with groups without amniotic membrane administration.38 Furthermore, in both normal acute wounds and chronic leg ulcers, MMP activity decreased significantly as wound closure occurred.39 Therefore, the administration of protease inhibitors may promote epidermal healing. With the delivery of micronized dHACM, inactive MMPs in the tissue could be activated by MMPs or growth factors in the wound bed; however, the complexation of excess TIMPs and plasma protease inhibitors with both the endogenous MMPs and the MMPs present in the wound is expected to promote resolution.
Innovation
Due to the dynamic nature of the tissue and its biologic factor-rich ECM, amniotic membranes have demonstrated efficacy in wound-healing applications. These results identified ECM components and characterized a variety of growth factors, cytokines, proteases, and inhibitors present in the dHACM tissue. We believe that at an injury site, the administration of micronized dHACM tissue will deliver matrix molecules, a cocktail of growth factors and cytokines, and protease inhibitors that collectively promote healing. Ultimately, the use of micronized dHACM in the treatment of chronic wounds may advance the current standards of wound care.
Abbreviations and Acronyms
- ANOVA
analysis of variance
- dHACM
dehydrated human amnion/chorion membrane
- ECM
extracellular matrix
- ELISA
enzyme-linked immunosorbent assay
- FGF
fibroblast growth factor
- HA
hyaluronic acid
- HSPGs
heparan sulfate proteoglycans
- MMP
matrix metalloproteinase
- PAGE
polyacrylamide gel electrophoresis
- SDS
sodium dodecyl sulfate
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors would like to acknowledge Dr. Heather Bara for a critical review of this article. This work was funded by MiMedx Group, Inc.
Author Disclosure and Ghostwriting
J.L., L.B.P., J.J.L., M.M., and T.J.K. disclose that they are employees of MiMedx. The content of this article was expressly written by J.L., L.B.P., J.J.L., M.M., and T.J.K. No ghostwriters were used to write this article.
About the Authors
Jennifer Lei, PhD, and Lauren B. Priddy, PhD, are Biomedical Engineers in the R&D department of MiMedx. They received their PhDs in Bioengineering from the Georgia Institute of Technology. Jeremy J. Lim, PhD, is Senior Biomedical Engineer within the R&D department of MiMedx. He received his PhD in Biomedical Engineering from the Georgia Institute of Technology. Michelle Massee is the R&D Department Biomedical Project Manager. She received her bachelor's degree in Biomedical Engineering from the Georgia Institute of Technology. Thomas J. Koob, PhD, is the Chief Scientific Officer of MiMedx. He is the senior executive responsible for the overall research direction and oversight of the company's product and tissue offerings. Dr. Koob received his PhD in Biochemistry from Washington University School of Medicine in St. Louis, Missouri. Dr. Koob has published more than 145 biomedical and biological articles and 12 book chapters, and he is listed as an inventor on more than 80 issued and pending patents.
Key Findings.
• dHACM tissue contained the ECM components collagens I and IV, HA, heparin sulfate proteoglycans, fibronectin, and laminin, as well as numerous growth factors, cytokines, chemokines, proteases, and protease inhibitors that are known to participate in the wound-healing process.
• Though MMPs were present in dHACM tissues, inhibitors of MMPs overwhelmingly outnumbered the MMP enzymes by a ratio of more than 28:1.
• MMPs in the tissue existed in their latent form and may have been complexed with active inhibitors.
• Overall, this work provides a better understanding of how micronized dHACM tissue can be used to effectively promote wound repair.
References
- 1.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–521 [DOI] [PubMed] [Google Scholar]
- 2.Schafer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165–171 [DOI] [PubMed] [Google Scholar]
- 3.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 1: normal and chronic wounds: biology, causes, and approaches to care. Adv Skin Wound Care 2012;25:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serena T, Cullen B, Bayliff S, Gibson M, DeMarco D, Galbraith J, et al. Protease Activity Levels Associated with Healing Status of Chronic Wounds. Vienna, Austria: European Wound Management Association, 2012 [Google Scholar]
- 5.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007;8:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001;17:463–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker AH, Edwards DR, Murphy G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. J Cell Sci 2002;115. [DOI] [PubMed] [Google Scholar]
- 8.Tallman P, Muscare E, Carson P, Eaglstein WH, Falanga V. Initial rate of healing predicts complete healing of venous ulcers. Arch Dermatol 1997;133:1231–1234 [PubMed] [Google Scholar]
- 9.Devlieger R, Millar LK, Bryant-Greenwood G, Lewi L, Deprest JA. Fetal membrane healing after spontaneous and iatrogenic membrane rupture: a review of current evidence. Am J Obstet Gynecol 2006;195:1512–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parry S, Strauss JF., III Premature rupture of the fetal membranes. N Engl J Med 1998;338:663–670 [DOI] [PubMed] [Google Scholar]
- 11.Strauss JF., III Extracellular matrix dynamics and fetal membrane rupture. Reprod Sci 2013;20:140–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT, EpiFix VLUSG. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen 2014;22:688–693 [DOI] [PubMed] [Google Scholar]
- 13.Koob TJ, Lim JJ, Massee M, Zabek N, Denoziere G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater 2014;102:1353–1362 [DOI] [PubMed] [Google Scholar]
- 14.Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, et al. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell 2014;6:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 2015;103:1133–1140 [DOI] [PubMed] [Google Scholar]
- 16.Massee M, Chinn K, Lei J, Lim JJ, Young CS, Koob TJ. Dehydrated human amnion/chorion membrane regulates stem cell activity in vitro. J Biomed Mater Res B Appl Biomater 2015. [Epub ahead of print]; DOI: 10.1002/jbm.b.33478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Massee M, Chinn K, Lim JJ, Godwin L, Young CS, Koob TJ. Type I and II diabetic adipose-derived stem cells respond to dehydrated human amnion/chorion membrane allograft treatment by increasing proliferation, migration, and altering cytokine secretion. Adv Wound Care (New Rochelle) 2016;5:43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjorn S, Graves D, Kim A. Novel approach to expedite chronic wound healing by utilizing injectable dehydrated amniotic membrane. The Desert Foot Conference, Phoenix, AZ, 2012 [Google Scholar]
- 19.Daniel J, Tofe R, Spencer R, Russo J. Placental Tissue Grafts, Assignee: MiMedx Group, Inc., Kennesaw, GA: USA Patent 8,409,626; 2012 [Google Scholar]
- 20.Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian M. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cells Mater 2008;15:88–99 [DOI] [PubMed] [Google Scholar]
- 21.Lenselink EA. Role of fibronectin in normal wound healing. Int Wound J 2015;12:313–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iorio V, Troughton LD, Hamill KJ. Laminins: roles and utility in wound repair. Adv Wound Care (New Rochelle) 2015;4:250–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colognato H, Yurchenco PD. Form and function: the Laminin family of heterotrimers. Dev Dyn 2000;218:213–234 [DOI] [PubMed] [Google Scholar]
- 24.Prosdocimi M, Bevilacqua C. Exogenous hyaluronic acid and wound healing: an updated vision. Panminerva Med 2012;54:129–135 [PubMed] [Google Scholar]
- 25.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009;17:153–162 [DOI] [PubMed] [Google Scholar]
- 26.Zhou S, Wang J, Cao R, Morita J, Soininen F, Chan K, et al. Impared angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparain sulfate-deficient mice. Cancer Res 2004;64:4699–4702 [DOI] [PubMed] [Google Scholar]
- 27.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen 2008;16:585–601 [DOI] [PubMed] [Google Scholar]
- 28.Yan C, Boyd DD. Regulation of matrix metalloproteinase gene expression. J Cell Physiol 2007;211:19–26 [DOI] [PubMed] [Google Scholar]
- 29.Cho H, Balaji S, Hone NL, Moles CM, Sheikh AQ, Crombleholme TM, et al. Diabetic wound healing in a MMP9-/- mouse model. Wound Repair Regen 2016. [Epub ahead of print]; DOI: 10.1111/wrr.12453 [DOI] [PubMed] [Google Scholar]
- 30.Frankowski H, Gu YH, Heo JH, Milner R, Del Zoppo GJ. Use of gel zymography to examine matrix metalloproteinase (gelatinase) expression in brain tissue or in primary glial cultures. Methods Mol Biol 2012;814:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toth M, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9 by gelatin zymography. Methods Mol Med 2001;57:163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol 2008;40:1334–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins VL, Caley M, O'Toole EA. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res 2013;351:255–268 [DOI] [PubMed] [Google Scholar]
- 34.Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, et al. Impaired wound contraction in stromelysin-1-deficient mice. Ann Surg 1999;230:260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandal M, Mandal A, Das S, Chakraborti TSC. Identification, purification and partial characterization of tissue inhibitor of matrix metalloproteinase-2 in bovine pulmonary artery smooth muscle. Mol Cell Biochem 2003;254:275–287 [DOI] [PubMed] [Google Scholar]
- 36.Rehman AA, Ahsan H, Khan FH. alpha-2-Macroglobulin: a physiological guardian. J Cell Physiol 2013;228:1665–1675 [DOI] [PubMed] [Google Scholar]
- 37.Morse JO. Alpha1-antitrypsin deficiency. N Engl J Med 1978;299:1045–1048 [DOI] [PubMed] [Google Scholar]
- 38.Kim JS, Kim JC, Na BK, Jeong JM, Song CY. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp Eye Res 2000;70:329–337 [DOI] [PubMed] [Google Scholar]
- 39.Trengove N, Stacey M, MaCauley S, Bennett N, Gibson J, Burslem F, et al. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen 1999;7:442–452 [DOI] [PubMed] [Google Scholar]