Abstract
The severe muscle weakness and atrophy measured after human spinal cord injury (SCI) may relate to chronic muscle denervation due to motoneuron death and/or altered muscle use. The aim of this study was to estimate motoneuron death after traumatic human SCI. The diameter and number of myelinated axons were measured in ventral roots post-mortem because ventral roots contain large diameter (> 7 μm) myelinated axons that typically arise from motoneurons and innervate skeletal muscle. In four cases (SCI levels C7, C8, T4, and L1) involving contusion (n = 3) or laceration (n = 1), there was a significant reduction in the number of large diameter myelinated axons at the lesion epicenter (mean ± standard error [SE]: 45 ± 11% Uninjured), one level above (51 ± 14%), and one (27 ± 12%), two (45 ± 40%), and three (54 ± 23%) levels below the epicenter. Reductions in motoneuron numbers varied by side and case. These deficits result from motoneuron death because the gray matter was destroyed at and near the lesion epicenter. Muscle denervation must ensue. In seven cases, ventral roots at or below the epicenter had large diameter myelinated axons with unusually thin myelin, a sign of incomplete remyelination. The mean ± SE g ratio (axon diameter/fiber diameter) was 0.60 ± 0.01 for axons of all diameters in five above-lesion ventral roots, but increased significantly for large diameter fibers (≥ 12 μm) in three roots at the lesion epicenter. Motoneuron death after human SCI will coarsen muscle force gradation and control, while extensive muscle denervation will stifle activity-based treatments.
Keywords: : demyelination, motoneuron death, muscle denervation, remyelination
Introduction
Post-mortem examination of injured human spinal cords typically shows contusion or laceration of the cord. Severance of long nerve tracts, demyelination of axons, and maceration of gray matter are common.1–3 These findings show the unequivocal need for axon regeneration, axon sprouting, and remyelination to restore function after injury.4 Although given far less attention, the survival of spinal neurons and their dendritic trees also is crucial to retain spinal circuitry after injury, to provide sites for formation of new synapses, and for activity-based rehabilitation.
Few animal studies have quantified motoneuron (or interneuron) survival after spinal cord injury5–8 despite the fundamental roles that these neurons play in the control of movement. The contributions of chronic muscle denervation (due to motoneuron death and/or ventral root avulsion) and alterations in muscle use to the striking muscle weakness and atrophy seen after human spinal cord injury also remain unclear.9–13 Motoneuron death after human spinal cord injury has largely been inferred from physiological signs of muscle denervation, not from morphological analysis. For example, various muscles innervated from the lesion epicenter have been unresponsive to electrical stimulation of their peripheral nerves, consistent with complete muscle denervation.14–18 Other studies have shown signs of partial denervation (low motor unit counts, positive sharp waves, small amplitude compound muscle action potentials, muscle weakness that exceeds that expected from disuse atrophy, atrophied and angular muscle fibers), including indications of intramuscular motor axon sprouting, an important compensatory mechanism for recovery of muscle innervation after death of some motoneurons in a motor pool (large polyphasic motor unit potentials, stronger than usual motor unit forces, increased motor unit fiber density, increased jitter in re-innervated muscle fibers).12,19–23
In this study, we address an important and often overlooked aspect of SCI—the effect of injury on the lesioned spinal cord segment and denervation of skeletal muscle as a contributor to muscular weakness. Our aim was to estimate the extent of motoneuron death from the number of large diameter myelinated axons in ventral roots taken post-mortem from individuals who had sustained a spinal cord injury at least 6 months earlier. Ventral roots were chosen for analysis because motor axons exit the spinal cord from these roots, all of the axons can be sampled in one section, and root identity can be defined by anatomical landmarks. The skeletal muscles innervated by a given ventral root are therefore relatively predictable. Motoneurons, in contrast, lie in longitudinal columns that may be relocated by compression of the spinal cord, making the original motor pool identity and location less clear. Many serial sections also are needed to adequately sample spinal motoneurons. Greater motoneuron survival after SCI has practical and clinical importance. Muscle innervation, force generating capacity, and function are more likely to be retained. Control of muscle largely determines functional capability after SCI. In addition, only innervated muscles can be used voluntarily, stimulated to restore functional movements, or trained to benefit from activity-based rehabilitation.24
Methods
Collection and processing of ventral root tissue
Ventral root tissue was taken from the human spinal cord injury tissue bank at The Miami Project to Cure Paralysis (University of Miami, FL). Each spinal cord was removed within 24 h of death, preserved in 10% neutral buffered formalin for at least 2 weeks, and then stored in 0.1 M phosphate buffer.1 Ventral roots were taken for the present study provided that: 1) the person was between 18–61 years when they died, which avoided the motoneuron death associated with aging25; 2) the ventral roots were intact for at least 30 mm after exiting the cord, which avoided motoneuron death from root avulsion10 and Wallerian degeneration of motor axons in our root samples26,27; and 3) the root levels could be identified clearly by anatomical landmarks. For each root, 5–10 mm samples were taken, beginning at the point where the rootlets entered the cord caudal to the corresponding spinal segment, since motor axons take a caudal intramedullary course in the spinal cord before exiting into the ventral root.28 In some cases, ventral roots were unavailable, particularly at or near the lesion epicenter. These roots may have been avulsed by the spinal cord injury itself and/or removed during cord extraction. Table 1 shows the history of each case from which tissue was obtained, the location of root tissue examined relative to the lesion epicenter, the injury classification (contusion or laceration) determined anatomically from cord cross-sections stained with solochrome cyanine for myelin and the Sevier-Munger method for axons,1 and information about the spinal cord pathology.
Table. 1.
Case History and Ventral Root Tissue Samples
| Case | Sex | Age at death (year) | SCI level | SCI cause | SCI duration (year) | Ventral root/ motoneuron counts | SCI classification | Cord epicenter | Other hallmarks | Thin ventral root myelin |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 48 | —– | —– | —– | C, T, L Motoneurons |
—– | —– | —– | —– |
| 2 | F | 47 | —– | —– | —– | Motoneurons | —– | —– | —– | —– |
| 3 | M | 56 | —– | —– | —– | Motoneurons | —– | —– | —– | —– |
| 4 | F | 42 | —– | —– | —– | Motoneurons | —– | —– | —– | —– |
| 5 | M | 61 | —– | —– | —– | Motoneurons | —– | —– | —– | —– |
| 6 | M | 38 | Beyond S2 | Gunshot | 20 | A | Laceration | Not viable | FG degeneration: C, T cord | —– |
| 7 | M | 34 | Beyond S2 | Fall | 9 | A | Contusion | —– | FG degeneration: C cord | —– |
| 8 | M | 42 | C7 | Sport | 23 | A,E,B | Contusion | Not viable | FG, posterior and anterior SC, lateral ST tract degeneration; CS tract normal: C cord | B |
| 9 | M | 58 | C8 | MVA | 2 | A,E,B | Contusion | Not viable, Schwannosis | C7, T1: extensive damage, few neurons, glial scar FG degeneration: C cord; lateral CS tract degeneration: T cord | B |
| 10 | M | 24 | T4 | MVA | 3 | A,E,B | Contusion | Not viable, Schwannosis, glial scar | FG, posterior SC, lateral & anterior ST tract degeneration: C cord; FG and cuneatus degeneration: T cord; lateral CS tract degeneration: L cord | E, B Demyelination: T5 |
| 11 | M | 45 | L1 | Gunshot | 15 | A,E,B | Laceration | Not viable | E, B | |
| 12 | M | 41 | T9 | Gunshot | 25 | A,B | Laceration | Compression | B | |
| 13 | M | 24 | C2 | Gunshot | 2 | B | Laceration | Not viable, Schwannosis | Dieback of posterior SC tract: C, T cord | B Demyelination: lateral CS tract |
| 14 | F | 29 | C3 | Gunshot | 0.5 | B | Laceration | Not viable | B |
SCI, spinal cord injury; F, female; C, cervical; T, thoracic; L, lumbar; M, male; S, sacral; Epicenter not viable: no obvious white/gray matter demarcation; FG, fasciculus gracilis; SC, spinocerebellar tract; CS, corticospinal tract; MVA, motor vehicle accident; A, above lesion; E, lesion epicenter; B, below lesion; ST, spinothalamic tract.
Ventral roots were placed in neutralized 10% formalin and 2% osmium tetroxide in 0.1 M phosphate buffer at pH 7.4, dehydrated, and embedded in epoxy resin. Cross-sections (1 μm) were stained with toluidine blue to detect myelinated axons (Fig. 1A).22
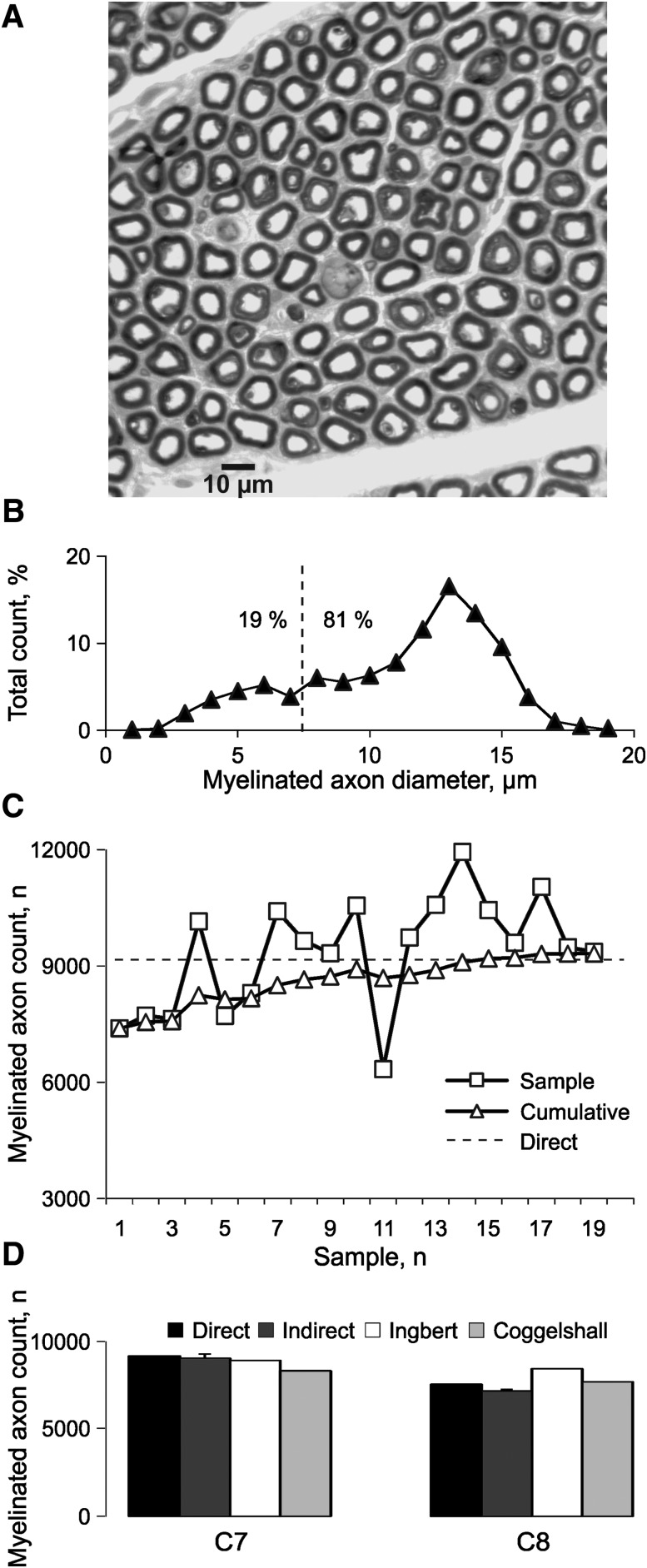
FIG. 1.
Myelinated axon diameters and counts. (A) Toluidine blue–stained myelinated axons in a C7 ventral root. (B) Myelinated axon diameter distribution for the same C7 ventral root to determine the percentage of small (19%) and large diameter fibers (> 7 μm; 81%). (C) Estimated myelinated axon count per sample (squares) and for cumulative samples (triangles), compared with the total direct count (dashed line) for the same C7 ventral root. (D) Total direct and indirect myelinated axon counts for the same C7 ventral root and a C8 ventral root, compared with the values published by Ingbert31 and Coggeshall and colleagues.32
Myelinated axon diameter measurements and counts
Digital images of each root were collected and analyzed using a Zeiss Axiophat II microscope (Carl Zeiss Microscopy, Göttingen, Germany) and a MetaMorph Imaging System (Molecular Devices, Sunnyvale, CA). A grid was laid over each image to sample myelinated axons and to measure their minimum and maximum diameters. Mean myelinated axon diameter was calculated. To estimate the total number of myelinated axons in each root, the number of axons in the sampled areas was scaled according to the total root area. The percentage of large diameter myelinated axons (≥ 7 μm) measured from each root also was scaled to the total root area to give an estimate of the motoneuron count (Fig. 1B), since these large diameter axons typically arise from spinal motoneurons and innervate skeletal muscle.29,30 Since the injury level differed across cases (Table 1), data were compared relative to the lesion epicenter (E). Levels above and below the epicenter were given positive and negative numbers, respectively (e.g. E1, one level above the epicenter; E-1, one level below the epicenter).
The accuracy of the motoneuron estimates was verified in two ways. First, the indirect estimates were compared with direct counts of all of the myelinated axons in one C7 and one C8 ventral root. Initially, the indirect method gave a low estimate of the actual number of myelinated axons in the C7 root (81% of the direct count after one sample; Fig. 1C). However, with additional sampling, the indirect counts approached the direct counts and were within 5% of each other after 9% and 10% of the myelinated axons had been sampled in the respective roots (C7: 9038 ± 247 vs. 9160 and C8: 7154 ± 96 vs. 7537, respectively). Our values also were close to the data reported by Ingbert31 and Coggeshall and colleagues32 (C7: 8913 and 8317 fibers and C8: 8435 and 7687 fibers, respectively; Fig. 1D), and show how many axons had to be measured per root to provide representative counts. Here, 30 ± 2% of the myelinated axons were measured per root, almost three times the number required. Second, motoneurons were counted bilaterally in six spinal cord segments (C7, C8, T1, T4, L2, L3; two to five sections per segment) from five uninjured spinal cords (cases 1–5; Table 1). Motoneuron counts were comparable for each side of the cord (p = 0.46, paired t-test) so data were pooled to calculate a mean count per section. The mean count per segment was calculated then normalized to the data from the segment with the highest mean motoneuron count. The ventral root counts for the corresponding spinal segments were normalized similarly, and the two datasets compared to examine whether both approaches showed high counts for cervical and lumbar spinal cord segments and reduced counts for thoracic segments.
Ventral root sections from seven cases were viewed for myelin abnormalities. To quantify the thin myelin associated with some large diameter axons, the g ratio (axon diameter/fiber diameter, a measure of myelin thickness) was calculated from axon and fiber (axon and myelin) diameter measurements made for all of the axons sampled from eight ventral roots from cases 10 and 11 (three roots at the lesion epicenter, E; one root three levels above the lesion, E3; two roots four levels above the lesion, E4; and two roots five levels above the lesion, E5).
Statistics
Mean (± standard error [SE]) are given unless stated. A two-way repeated measures analysis of variance was used to assess whether the number of motoneurons differed by group (SCI, Uninjured) and spinal level relative to the epicenter, and if the g ratio differed by location relative to the injury (above lesion, epicenter) and axon diameter (1μm bins). Motoneuron and ventral root counts in uninjured cords were compared using a paired t-test. Statistical significance was p < 0.05.
Results
Extensive damage of gray and white matter at the lesion epicenter
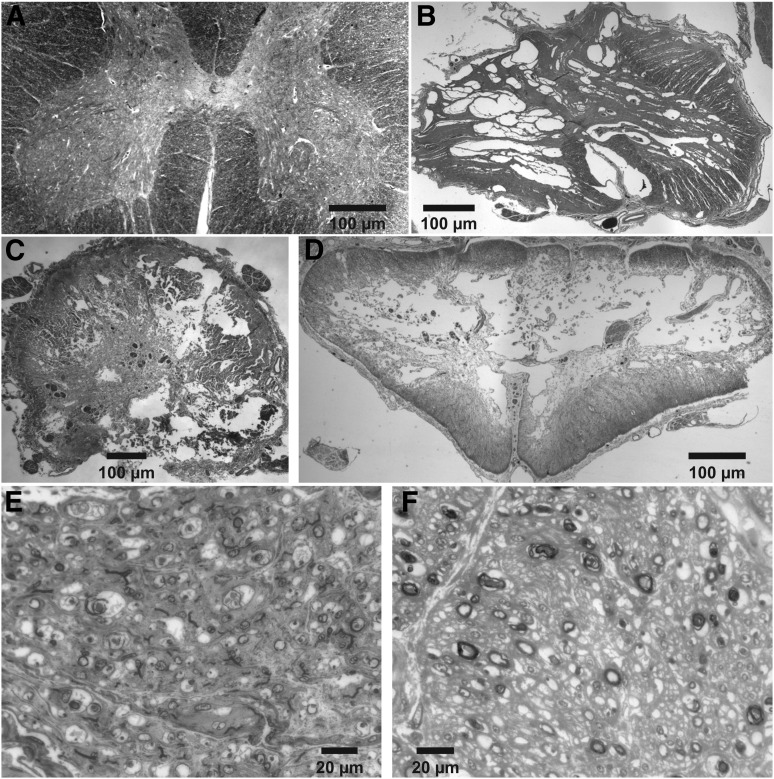
Figure 2 shows four representative human spinal cord sections, one from an uninjured cord at L3 (Fig. 2A), and three from the epicenter of a SCI at L1, T4, and C8 (cases 11, 10, and 9, respectively; Fig. 2B-D). There is no differentiation of the grey matter from the white matter in the three injured sections. Gross tissue sparing occurs primarily around the rim of the cords and there are different degrees of central cavitation. Representative ventral root sections show either an absence of large diameter myelinated axons at the epicenter of the C7 SCI (case 8; Fig. 2E), or few myelinated axons in a L1 ventral root four segments below a T9 SCI, most of which were small in diameter (case 12; Fig. 2F).
FIG. 2.
Spinal cord and ventral roots. Solochrome cyanine–stained spinal cord sections from an uninjured cord at L3 (A), and spinal cords injured at L1 (B; case 11), T4 (C; case 10), or C8 (D; case 9). Toluidine blue–stained sections from a C7 ventral root at the lesion epicenter (E; case 8), and a L1 ventral root four segments below the epicenter at T9 (F; case 12).
High motoneuron numbers in uninjured cervical and lumbar spinal cords
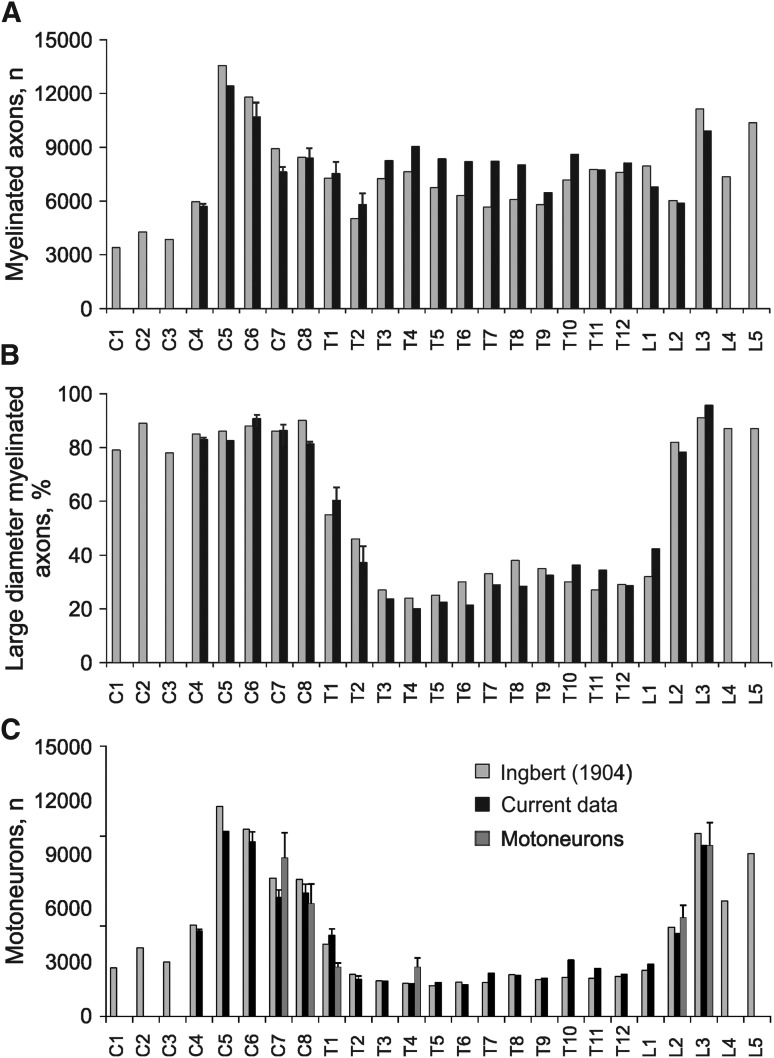
Counts of myelinated axons in ventral roots showed two peaks, one in the cervical cord (∼13,000 and 11,000 fibers at C5 and C6) and the other in the lumbar cord (∼10,500 fibers at L3 and L5), as expected for the cervical and lumbar enlargements. Most other spinal levels had 6000 to 8000 myelinated axons except for C1 to C3, which had 3000–4000 fibers (Fig. 3A). Of these myelinated fibers, ∼80–90 % were large in diameter (> 7 μm) in the cervical ventral roots and from L2 to L5. Between T1 to L2, ∼20–40 % of the myelinated axons were large in diameter (Fig. 3B). Since spinal motoneurons have large diameter myelinated axons, these data suggest that muscles innervated from C5 to C8 and from L2 to L5 were supplied by ∼7000–11,000 motoneurons. Motoneurons numbers were lower at C1 to C4 (∼2,500–5,000 motoneurons) and from T1 to L1 (∼2000–2700 motoneurons; Fig. 3C). These results closely matched the data obtained by Ingbert.31 Comparisons of motoneuron estimates from measuring large diameter myelinated ventral root axons and motoneurons in spinal cord segments also were similar (p = 0.27), suggesting that counts of ventral root axons are a reasonable estimate of motoneuron numbers in uninjured spinal cords.
FIG. 3.
Uninjured myelinated axon and motoneuron estimates by spinal level. (A) Mean (+ standard error) myelinated axon counts (A), percentage of large diameter myelinated axons (> 7 μm; B), and number of motoneurons (C) from C1 to L5 (n = 1 to n = 5 ventral roots per level), compared with motoneuron estimates for two cervical, thoracic and lumbar segments (cases 1–5). Current data from one uninjured spinal cord (case 1), and cervical and thoracic ventral roots taken from two cases of SCI beyond S2 (cases 6 and 7) were similar across cords suggesting that ventral roots remain intact many segments above a SCI. Data from Ingbert31 are plotted for comparison.
Reduced motoneuron numbers above, at, and below cervical, thoracic, or lumbar spinal cord injury
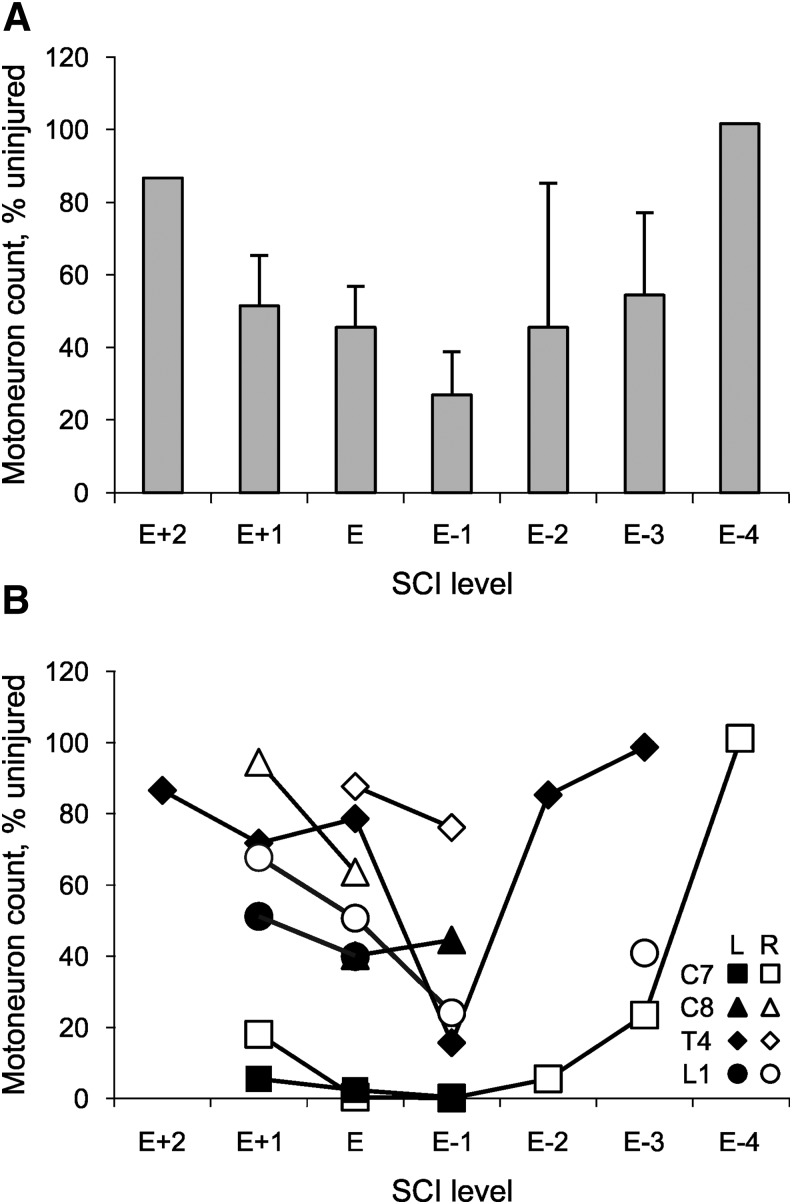
Ventral roots were analyzed above the spinal cord injury, at the injury epicenter, and below the trauma in four cases where the epicenter was either at C7, C8, T4, or L1 (cases 8–11; Fig. 4A). Motoneuron death occurred in all four cases. There were significant differences in the number of motoneurons by group (SCI, Uninjured; p < 0.001), and by level, relative to the epicenter (p = 0.003). There was also an interaction between group and level (p = 0.006). Compared to Uninjured, significantly fewer motoneurons were present in ventral roots arising from one level above the lesion epicenter (E1; mean ± SE, 51 ± 14%; n = 6 roots; p < 0.001), at the lesion epicenter (E; 45 ± 11%; n = 8; p < 0.001), one (E-1; 27 ± 12%; n = 6; p < 0.001), two (E-2; 45 ± 40%; n = 2; p = 0.014) and three levels (E-3; 54 ± 23%; n = 3; p = 0.023) below the lesion epicenter.
FIG. 4.
Motoneuron counts with respect to the lesion epicenter. (A) Mean (+ standard error) motoneuron count from two levels above the epicenter (E + 2) to four levels below the epicenter (E-4) for four SCI cases (epicenter at C7, C8, T4, L1; cases 8–11, respectively), expressed relative to Uninjured data. (B) Motoneuron count for the same four cases by level and side (L, left; R, right).
The reductions in motoneuron numbers were always bilateral but the extent varied by case and side. For example, when the SCI was at C7, < 20% of motoneurons were present from C6 to T1 (range: 0.4–18.2% Uninjured, bilaterally). Recovery only began at T2, three segments below the injury (Fig. 4B). For the C8 SCI, motoneuron numbers were reduced more at the epicenter on the left versus right side of the cord (40% vs. 64% Uninjured; Fig. 4B). When the SCI was at T4, the greatest reductions in motoneuron numbers occurred one level below the lesion, as was typical for 83% of the data (five of six roots). For the SCI at L1, motoneuron numbers were reduced to ∼50% of Uninjured at the epicenter bilaterally and even more below the injury on the right side.
In another case (case 12), where the SCI epicenter was at T9, ventral roots were available above and below the lesion only (Table 1). Motoneuron numbers were lower than usual—two segments above the epicenter on the right and three segments below the SCI at T9 on the left (both 45% Uninjured).
Large diameter axons with thin myelin after spinal cord injury
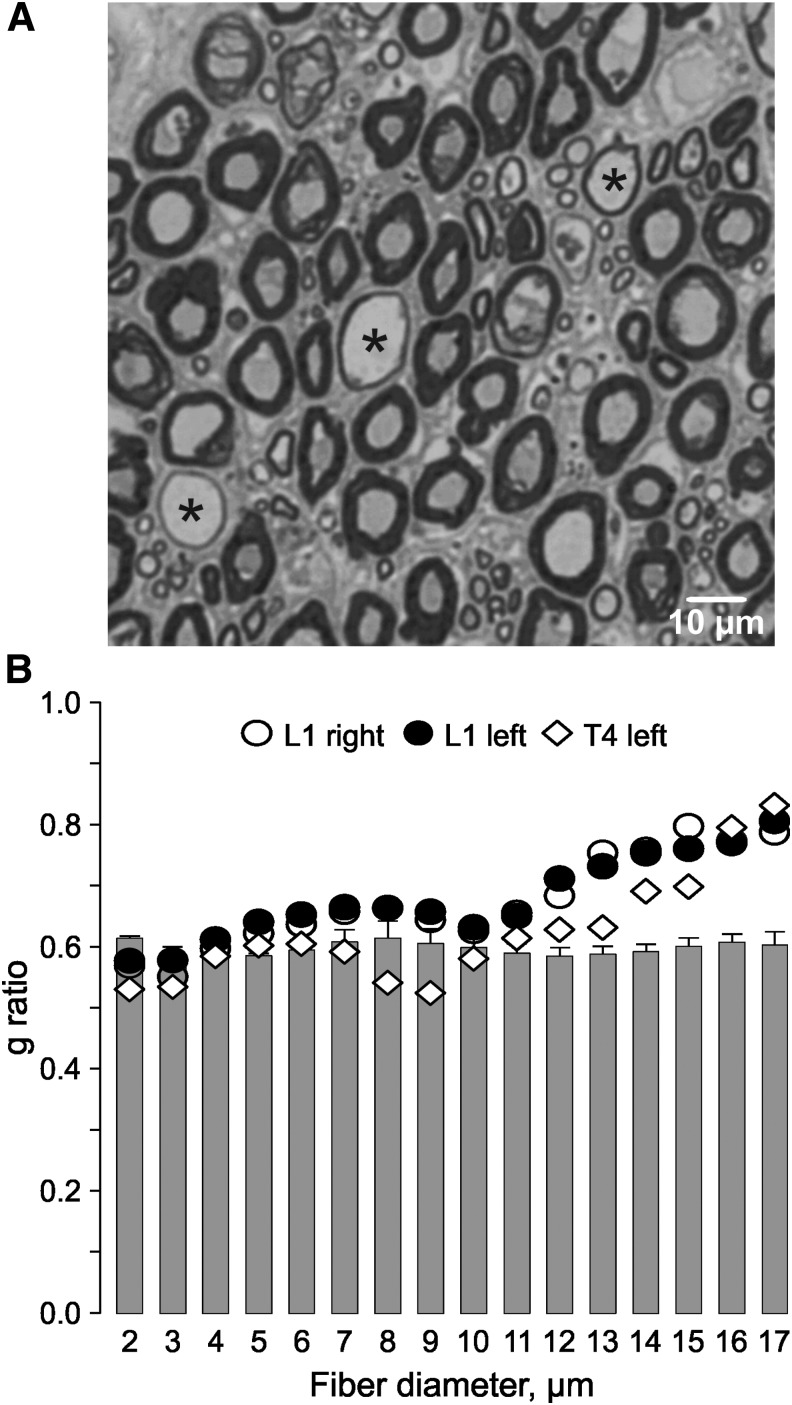
Large diameter axons with thin myelin were evident in ventral roots at and below the lesion epicenter (Fig. 5A; Table 1). The g ratio differed by location (above-level vs. epicenter; p = 0.027) and by fiber diameter (p < 0.001). There was also a significant interaction between diameter and location (p < 0.001). In five above-lesion ventral roots, the mean g ratio was 0.60 ± 0.01 for axons of all diameters. However, the g ratio increased significantly for large diameter fibers (≥ 12 μm) in the roots taken from the lesion epicenter (p ≤ 0.003; Fig. 5B).
FIG. 5.
Remyelination after spinal cord injury. (A) Toluidine blue–stained section from a T1 ventral root (case 13) to show large diameter axons with thin myelin (*). (B) Mean (+ standard error) g ratio by fiber diameter for five above-lesion ventral roots (C7, C8 bilaterally, T1) from case 10 (bars), compared with the mean g ratio for three roots taken from the epicenter of case 10 (left T4 root) and case 11 (L1 root, bilaterally).
Discussion
Our data provide morphological evidence for motoneuron death after injury to human cervical, thoracic, or lumbar spinal cord. Not only did ventral roots at or near the lesion epicenter have reduced numbers of large diameter myelinated axons, there was no viable cord at the epicenter of most injuries. These low axon counts must induce muscle denervation because large diameter myelinated axons typically arise from spinal motoneurons and innervate skeletal muscle. Further, large diameter axons in ventral roots at the lesion epicenter often were myelinated thinly, which is indicative of incomplete remyelination.
Motoneuron death occurs irrespective of the level or cause of human spinal cord injury
The high numbers of motoneuron numbers in both the cervical and lumbar cord (Fig. 3), the high percentage of injuries occurring at these levels (54% and 11%, respectively, in the U.S.; www.nscisc.uab.edu), and the extensive destruction of gray matter (Fig. 2) and motoneurons (Fig. 4) after human SCI, all indicate that denervation of arm and leg muscles must be common after human spinal cord injury. These findings also imply that muscles innervated from the epicenter are likely to be reinnervated, not normally innervated. While inactivity can reduce muscle fiber areas up to 50%,33 motoneuron death and muscle denervation must contribute to the severe atrophy that is measured in muscles after spinal cord injury (e.g. 70% on average in triceps brachii and vastus lateralis).11,12 Survival of thoracic motoneurons is just as important, despite the low proportion of motoneurons at these cord levels because thoracic motoneurons support respiration and trunk control.34,35
Reduced numbers of large diameter motor axons in ventral roots occurred after spinal contusion due to a sports or motor vehicle accident, and after a laceration injury induced by gunshot (Fig. 4; Table 1). The deficits were bilateral but asymmetrical, matching the variance in functional impairment measured across limbs after SCI.36,37 The C7 injury was particularly catastrophic as the number of large diameter motor axons was reduced severely across at least five spinal levels (Fig. 4B). Multi-segmental destruction of motor axons will denervate some muscles completely and irreparably because motor pools typically span 1–3 spinal levels in humans,38 as they do in the macaque.39 Complete, chronic denervation is consistent with the inability to excite various muscles after injury using peripheral nerve stimulation.14–18 Nevertheless, it often is possible to record evoked potentials from the skin in these individuals despite the absence of an evoked muscle compound action potential, suggesting that some large dorsal root ganglion cells remain intact near the lesion epicenter.18
The less extensive reduction in large diameter myelinated axon counts in ventral roots that exited the cord near the epicenter of a C8, T4, or L1 SCI (Fig. 4B) likely induced the physiological signs of partial muscle denervation and reinnervation commonly reported in individuals with SCI (e.g., polyphasic electromyographyic potentials, strong unit forces).22,23 Axons that remain intact can sprout locally to innervate four to six times more muscle fibers,40 which is key to retention of innervation and functional use of these muscles. However, muscles innervated by few motoneurons will likely have coarser force control because each unit will make a relatively greater contribution to whole muscle force. When the sprouting capacity is exceeded (> 75–80% of the motoneurons in a pool die),41 denervation will contribute to whole–muscle weakness. This motoneuron death will delimit the possible benefits that can be achieved with activity-based treatments.
Here, myelinated axon counts were reduced in ventral roots that remained attached to the spinal cord and that were undamaged for at least 30 mm after exiting the cord, therefore, these injuries must have activated neurodegenerative pathways to destroy motoneurons and myelinated axons independently of motoneuron death related to root avulsion.10 Thus, our approach likely underestimates the overall SCI-induced motoneuron death in humans. The direct mechanical impact of the injury may result in primary cell insults that lead to apoptotic, necrotic and/or excitotoxic death of motoneurons.1,42 The low calcium buffering capacity of motoneurons also make it likely that motoneurons cope poorly with injury and die.43
Motoneuron death was greatest one spinal level below the lesion epicenter
In uninjured cases, there was no difference in the estimates for motoneuron numbers when analyzed from large diameter myelinated axons in ventral roots versus counting motoneurons in the ventral horn. This finding supports the idea that assessing ventral root axons provides a reasonable estimate of motoneuron numbers (Fig. 3C). However, no identifiable motoneurons were observed at the epicenter of the T4 SCI (Fig. 2B), but the T4 ventral roots contained near normal numbers of large diameter myelinated axons (79% and 88% Uninjured; Fig. 4B). More striking reductions in large diameter axon counts occurred one level below the epicenter in this case (16% and 76% Uninjured), as well as in the three other cases that also had massive gray matter destruction at the lesion epicenter (Fig. 2). Cavitation, tissue distortion, secondary degradation, and limited availability of tissue to sample can make motoneuron origin, identification, and counting challenging. While neurons may survive, they can be displaced to adjacent segments and/or atrophy, possibly because axonal tears disrupt retrograde signals and neurotrophic support from muscle.44 Thus, we propose that counts of large diameter myelinated axons in ventral roots may provide a more accurate estimate of motoneuron survival after human SCI than motoneuron counts in cord sections. However, even the presence of large diameter axons in ventral roots does not necessarily guarantee that the circuitry and axons remain functional,45 particularly if the injury has displaced neurons and stretched axons. Thus, myelinated axon counts in ventral roots may exceed function, consistent with the low counts of motor units in muscles innervated from near the lesion epicenter.22,23
Clinical assessments show that at-level (one segment) recovery of muscle function occurs in 70% of spinal cord injury cases after 1 year. Recovery of function in two or more spinal segments is less common.46 The more extensive motoneuron death reported here one level caudal to the lesion epicenter may explain why more recovery is not always apparent clinically, even though this is the level where functional return is expected to occur. Further, the extent of muscle denervation will depend on the longitudinal extent of the lesion, which is unexplored clinically. Only the last functional level is evaluated with manual muscle tests.47 Muscles innervated above the lesion also may be impaired given the U-shaped profile of motoneuron survival (Fig. 4A), consistent with muscle function being scored as 5 (normal muscle function) but being associated with weaker than usual maximal voluntary forces.36
Implications for spinal cord repair and recovery of muscle function
These ventral root axon data strongly support the idea that motoneuron death at the lesion epicenter is an important contributor to chronic muscle weakness, atrophy, and dysfunction after human SCI.12 Even though models of spinal cord injury often show only a peripheral rim of spared white matter at the lesion epicenter, the extent varying with lesion severity,48 most repair strategies focus on repair of long fiber pathways or remyelination49,50 without consideration of how the requirements for axon regeneration or sprouting may have to change to accommodate death of spinal neurons. Motoneuron death must induce reorganization of spinal circuitry because motoneurons act as the central hub to coordinate output to muscle. One consequence of fewer motoneurons will be a reduction in the area available for synaptic sites. Axon sprouts or regenerating axons will likely have to grow over longer distances to form new synapses, particularly if all motoneurons die at the lesion epicenter. Synapses on foreign motoneurons are likely to change control of muscle. Further, the functional consequences of a “missing” motor pool is rarely discussed or explored experimentally. It is likely to disrupt limb movement. For example, biceps brachii, an elbow flexor, is paralyzed with a C5 lesion. Bridging or regeneration of spinal axons from C4 to C6 may help wrist extension. However, it will not allow the upper arm to be moved towards or away from the body (elbow flexion and extension primarily arise from C5 and C7, respectively). To move the body towards the hand requires trunk control, which is also absent. Thus, if we are restore limb function, and the balanced amount of excitation and inhibition that intact motoneuron pools typically receive to execute movements,51 there needs to be a focus on studying protection of both motoneurons and interneurons, neuron replacement, axon growth, and remyelination. Current research also shows it is easier to save tissue at the time of SCI than it is to replace or repair it.52
Partial remyelination of peripheral motor axons is common after human spinal cord injury
In seven cases that involved a laceration or a contusion injury, the ventral roots at and below the epicenter contained some large caliber, thinly myelinated axons (Fig. 5A). These changes in myelin were greatest at the lesion epicenter but confined to large diameter axons (Fig. 5B) and therefore did not influence our estimates of motoneuron numbers. The forces associated with contusion and laceration injuries may stretch axons excessively, resulting in both central and peripheral demyelination that is followed by some remyelination.2,53,54 Longitudinal examination of central and peripheral axon conduction latencies revealed improvements over time in hand muscles, which may indicate remyelination of central and/or peripheral axons after human SCI.55 Thinner than usual myelin and shorter internodal distances with remyelination will diminish impulse propagation, which is consistent with the general slowing of single thenar motor axon conduction velocities measured 1.5–19 years after human SCI.56 These changes in axon conduction, as well as chronic motor unit firing at low rates, may induce further adaptations since thenar motor units have low recruitment and maximal firing rates during involuntary and voluntary contractions, and are weak and slow after chronic SCI.18,57–59
Defining the cause of muscle paralysis is important for planning rehabilitation
Muscle denervation is routinely overlooked after human SCI because muscle function is assessed manually in the clinic. Paralyzed muscles are scored as zero without distinguishing between different sources of paralysis: motoneuron death, severance of central nervous system axons, and demyelination of central or peripheral axons.47 While disruption or demyelination of central nervous system axons will leave muscle innervation intact and enable use of patterned electrical stimulation to restore function or to provide activity-based rehabilitation, complete denervation due to motoneuron death will eliminate voluntary control of the affected muscle fibers. It also will exclude use of patterned electrical stimulation to restore function because of the high current needed to excite denervated muscle.60 In these challenging situations, it becomes important to replace motoneurons and re-innervate muscle because innervation will restore muscle excitability and fiber size,61 enable muscle conditioning and all of the benefits of exercise,62 and allow muscles to be stimulated electrically to generate diverse behaviors, such as walking, grasping, respiration, and bladder or bowel control.63
Acknowledgments
This research was funded by National Institutes of Health grant NS-30226 and the Miami Project to Cure Paralysis.
The authors thank Dr. Alex Marcillo for help with tissue retrieval, Dr. Eduardo Sequira for his expert interpretation of the spinal cord pathology, and Mr. Jean-Pierre Brunswhig, Ms. Sharlene Godfrey, and Dr. Michelle Rudinsky for help with tissue processing and analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bunge R. P., Puckett W. R., Becerra J. L., Marcillo A., and Quencer R. M. (1993). Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv. Neurol. 59, 75–89 [PubMed] [Google Scholar]
- 2.Guest J. D., Hiester E. D., and Bunge R. P. (2005). Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp. Neurol. 192, 384–393 [DOI] [PubMed] [Google Scholar]
- 3.Kakulas B. A. and Kaelan C. (2015). The neuropathological foundations for the restorative neurology of spinal cord injury. Clin. Neurol. Neurosurg. 129 Suppl 1, S1–S7 [DOI] [PubMed] [Google Scholar]
- 4.Blesch A. and Tuszynski M. H. (2009). Spinal cord injury: plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 32, 41–47 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez-Argote S., Gransee H. M., Mora J. C., Stowe J. M., Jorgenson A. J., Sieck G. C., and Mantilla C. B. (2016). The impact of mid-cervical contusion injury on diaphragm muscle function. J. Neurotrauma 33, 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collazos-Castro J. E., Soto V. M., Gutierrez-Davila M., and Nieto-Sampedro M. (2005). Motoneuron loss associated with chronic locomotion impairments after spinal cord contusion in the rat. J. Neurotrauma 22, 544–558 [DOI] [PubMed] [Google Scholar]
- 7.Gensel J. C., Tovar C. A., Hamers F. P., Deibert R. J., Beattie M. S., and Bresnahan J. C. (2006). Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma 23, 36–54 [DOI] [PubMed] [Google Scholar]
- 8.Nicaise C., Putatunda R., Hala T. J., Regan K. A., Frank D. M., Brion J. P., Leroy K., Pochet R., Wright M. C., and Lepore A. C. (2012). Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice. J. Neurotrauma 29, 2748–2760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biering-Sorensen B., Kristensen I. B., Kjaer M., and Biering-Sorensen F. (2009). Muscle after spinal cord injury. Muscle Nerve 40, 499–519 [DOI] [PubMed] [Google Scholar]
- 10.Carlstedt T. and Havton L. (2012). The longitudinal spinal cord injury: lessons from intraspinal plexus, cauda equina and medullary conus lesions. Handb. Clin. Neurol. 109, 337–354 [DOI] [PubMed] [Google Scholar]
- 11.Castro M. J., Apple D. F., Jr., Staron R. S., Campos G. E., and Dudley G. A. (1999). Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl. Physiol. 86, 350–358 [DOI] [PubMed] [Google Scholar]
- 12.Thomas C. K., Zaidner E. Y., Calancie B., Broton J. G., and Bigland-Ritchie B. R. (1997). Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Exp. Neurol. 148, 414–423 [DOI] [PubMed] [Google Scholar]
- 13.Thomas C. K. and Zijdewind I. (2006). Fatigue of muscles weakened by death of motoneurons. Muscle Nerve 33, 21–41 [DOI] [PubMed] [Google Scholar]
- 14.Bryden A. M., Kilgore K. L., Lind B. B., and Yu D. T. (2004). Triceps denervation as a predictor of elbow flexion contractures in C5 and C6 tetraplegia. Arch. Phys. Med. Rehabil. 85, 1880–1885 [DOI] [PubMed] [Google Scholar]
- 15.Kern H., Boncompagni S., Rossini K., Mayr W., Fano G., Zanin M. E., Podhorska-Okolow M., Protasi F., and Carraro U. (2004). Long-term denervation in humans causes degeneration of both contractile and excitation-contraction coupling apparatus, which is reversible by functional electrical stimulation (FES): a role for myofiber regeneration? J. Neuropathol. Exp. Neurol. 63, 919–931 [DOI] [PubMed] [Google Scholar]
- 16.Mulcahey M. J., Smith B. T., and Betz R. R. (1999). Evaluation of the lower motor neuron integrity of upper extremity muscles in high level spinal cord injury. Spinal Cord 37, 585–591 [DOI] [PubMed] [Google Scholar]
- 17.Peckham P. H., Mortimer J. T., and Marsolais E. B. (1976). Upper and lower motor neuron lesions in the upper extremity muscles of tetraplegics. Paraplegia 14, 115–121 [DOI] [PubMed] [Google Scholar]
- 18.Thomas C. K. (1997). Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve 20, 788–799 [DOI] [PubMed] [Google Scholar]
- 19.Berman S. A., Young R. R., Sarkarati M., and Shefner J. M. (1996). Injury zone denervation in traumatic quadriplegia in humans. Muscle Nerve 19, 701–706 [DOI] [PubMed] [Google Scholar]
- 20.Marino R. J., Herbison G. J., and Ditunno J. F., Jr. (1994). Peripheral sprouting as a mechanism for recovery in the zone of injury in acute quadriplegia: a single-fiber EMG study. Muscle Nerve 17, 1466–1468 [DOI] [PubMed] [Google Scholar]
- 21.Thomas C. K., Broton J. G., and Calancie B. (1997). Motor unit forces and recruitment patterns after cervical spinal cord injury. Muscle Nerve 20, 212–220 [DOI] [PubMed] [Google Scholar]
- 22.Thomas C. K., Nelson G., Than L., and Zijdewind I. (2002). Motor unit activation order during electrically evoked contractions of paralyzed or partially paralyzed muscles. Muscle Nerve 25, 797–804 [DOI] [PubMed] [Google Scholar]
- 23.Yang J. F., Stein R. B., Jhamandas J., and Gordon T. (1990). Motor unit numbers and contractile properties after spinal cord injury. Ann. Neurol. 28, 496–502 [DOI] [PubMed] [Google Scholar]
- 24.Dietz V. (2011). Neuronal plasticity after a human spinal cord injury: positive and negative effects. Exp. Neurol. 235, 110–115 [DOI] [PubMed] [Google Scholar]
- 25.McComas A. J., Galea V., and de Bruin H. (1993). Motor unit populations in healthy and diseased muscles. Phys. Ther. 73, 868–877 [DOI] [PubMed] [Google Scholar]
- 26.Cavanaugh M. W. (1951) Quantitative effects of the peripheral innervation area on nerves and spinal ganglion cells. J. Comp. Neurol. 94, 181–219 [DOI] [PubMed] [Google Scholar]
- 27.Hoffer J. A., Stein R. B., and Gordon T. (1979) Differential atrophy of sensory and motor fibers following section of cat peripheral nerves. Brain Res. 178, 347–361 [DOI] [PubMed] [Google Scholar]
- 28.Cullheim S. and Kellerth J. O. (1978) A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J. Comp. Neurol. 178, 537–557 [DOI] [PubMed] [Google Scholar]
- 29.Eccles J. C. and Sherrington C. S. (1930). Reflex summation in the ipsilateral spinal flexion reflex. J. Physiol. 69, 1–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feinstein B., Lindegård B., Nyman E., and Wohlfart G. (1955). Morphologic studies of motor units in normal human muscles. Acta Anat. 23, 127–142 [DOI] [PubMed] [Google Scholar]
- 31.Ingbert C. E. (1904). An enumeration of the medullated nerve fibers in the ventral roots of the spinal nerves of man. J. Comp. Neurol. Psychol. 14, 209–270 [Google Scholar]
- 32.Coggeshall R. E., Applebaum M. L., Fazen M., Stubbs T. B., III, and Sykes M. T. (1975). Unmyelinated axons in human ventral roots, a possible explanation for the failure of dorsal rhizotomy to relieve pain. Brain 98, 157–166 [DOI] [PubMed] [Google Scholar]
- 33.Pierotti D. J., Roy R. R., Bodine-Fowler S. C., Hodgson J. A., and Edgerton V. R. (1991). Mechanical and morphological properties of chronically inactive cat tibialis anterior motor units. J. Physiol. 444, 175–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson K. D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 35.Audu M. L., Lombardo L. M., Schnellenberger J. R., Foglyano K. M., Miller M. E., and Triolo R. J. (2015). A neuroprosthesis for control of seated balance after spinal cord injury. J. Neuroeng. Rehabil. 12, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham-Shropshire B. M., Klose K. J., Tucker M. E., and Thomas C. K. (1997). Manual muscle test score and force comparisons after cervical spinal cord injury. J Spinal Cord Med. 20, 324–330 [DOI] [PubMed] [Google Scholar]
- 37.van Hedel H. J. and Curt A. (2006). Fighting for each segment: estimating the clinical value of cervical and thoracic segments in SCI. J. Neurotrauma 23, 1621–1631 [DOI] [PubMed] [Google Scholar]
- 38.Sharrard W. J. (1955). The distribution of the permanent paralysis in the lower limb in poliomyelitis; a clinical and pathological study. J. Bone Joint Surg. Br. 37-B, 540–558 [DOI] [PubMed] [Google Scholar]
- 39.Jenny A. B. and Inukai J. (1983). Principles of motor organization of the monkey cervical spinal cord. J. Neurosci. 3, 567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown M. C., Holland R. L., and Hopkins W. G. (1981). Motor nerve sprouting. Annu. Rev. Neurosci. 4, 17–42 [DOI] [PubMed] [Google Scholar]
- 41.McComas A. J., Sica R. E., Campbell M. J., and Upton A. R. (1971). Functional compensation in partially denervated muscles. J. Neurol. Neurosurg. Psychiatr. 34, 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hagg T. and Oudega M. (2006). Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 23, 264–280 [DOI] [PubMed] [Google Scholar]
- 43.von Lewinski F. and Keller B. U. (2005). Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS. Trends Neurosci. 28, 494–500 [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi N. R., Fan D. P., Giehl K. M., Bedard A. M., Wiegand S. J., and Tetzlaff W. (1997). BDNF and NT-4/5 prevent atrophy of rat rubrospinal neurons after cervical axotomy, stimulate GAP-43 and Talpha1-tubulin mRNA expression, and promote axonal regeneration. J. Neurosci. 17, 9583–9595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin C. S., Macefield V. G., Elam M., Wallin B. G., Engel S., and Kiernan M. C. (2007). Axonal changes in spinal cord injured patients distal to the site of injury. Brain 130, 985–994 [DOI] [PubMed] [Google Scholar]
- 46.Steeves J. D., Kramer J. K., Fawcett J. W., Cragg J., Lammertse D. P., Blight A. R., Marino R. J., Ditunno J. F., Jr., Coleman W. P., Geisler F. H., Guest J., Jones L., Burns S., Schubert M., van Hedel H. J., and Curt A. (2011). Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49, 257–265 [DOI] [PubMed] [Google Scholar]
- 47.Thomas C. K. and Noga B. R. (2003). Physiological methods to measure motor function in humans and animals with spinal cord injury. J. Rehabil. Res. Dev. 40, 25–33 [DOI] [PubMed] [Google Scholar]
- 48.Noble L. J. and Wrathall J. R. (1989). Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp. Neurol. 103, 34–40 [DOI] [PubMed] [Google Scholar]
- 49.Blight A. R. (1983). Cellular morphology of chronic spinal cord injury in the cat: analysis of myelinated axons by line-sampling. Neuroscience 10, 521–543 [DOI] [PubMed] [Google Scholar]
- 50.Crowe M. J., Bresnahan J. C., Shuman S. L., Masters J. N., and Beattie M. S. (1997). Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 3, 73–76 [DOI] [PubMed] [Google Scholar]
- 51.Binder M. D., Heckman C. J., and Powers R. K. (1996). The physiological control of motoneuron activity. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Rowell L.B. and Shepherd J.T. (eds). American Physiological Society: Bethesda, MD, pps. 3–53 [Google Scholar]
- 52.Ramer L. M., Ramer M. S., and Bradbury E. J. (2014). Restoring function after spinal cord injury: towards clinical translation of experimental strategies. Lancet Neurol. 13, 1241–1256 [DOI] [PubMed] [Google Scholar]
- 53.Clark B. D., Barr A. E., Safadi F. F., Beitman L., Al Shatti T., Amin M., Gaughan J. P., and Barbe M. F. (2003). Median nerve trauma in a rat model of work-related musculoskeletal disorder. J. Neurotrauma 20, 681–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James N. D., Bartus K., Grist J., Bennett D. L., McMahon S. B., and Bradbury E. J. (2011). Conduction failure following spinal cord injury: functional and anatomical changes from acute to chronic stages. J. Neurosci. 31, 18543–18555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexeeva N., Broton J. G., Suys S., and Calancie B. (1997). Central cord syndrome of cervical spinal cord injury: widespread changes in muscle recruitment studied by voluntary contractions and transcranial magnetic stimulation. Exp. Neurol. 148, 399–406 [DOI] [PubMed] [Google Scholar]
- 56.Häger-Ross C. K., Klein C. S., and Thomas C. K. (2006). Twitch and tetanic properties of human thenar motor units paralyzed by chronic spinal cord injury. J. Neurophysiol. 96, 165–174 [DOI] [PubMed] [Google Scholar]
- 57.Zijdewind I., and Thomas C. K. (2003). Motor unit firing during and after voluntary contractions of human thenar muscles weakened by spinal cord injury. J. Neurophysiol. 89, 2065–2071 [DOI] [PubMed] [Google Scholar]
- 58.Zijdewind I. and Thomas C. K. (2012). Firing patterns of spontaneously active motor units in spinal cord-injured subjects. J. Physiol. 590, 1683–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zijdewind I., Bakels R., and Thomas C. K. (2014). Motor unit firing rates during spasms in thenar muscles of spinal cord injured subjects. Front. Hum. Neurosci. 8, 922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortimer J. T. (1981). Motor prostheses. In: Handbook of Physiology—A critical, Comprehensive Presentation of Physiological Knowledge and Concepts. Brookhart J.M., Brooks V.B., Geiger S.R., and Mountcastle V.B. (eds). American Physiological Society: Bethesda, MD, pps. 155–187 [Google Scholar]
- 61.Thomas C. K., Erb D. E., Grumbles R. M., and Bunge R. P. (2000). Embryonic cord transplants in peripheral nerve restore skeletal muscle function. J. Neurophysiol. 84, 591–595 [DOI] [PubMed] [Google Scholar]
- 62.Pedersen B. K. (2009). The diseasome of physical inactivity—and the role of myokines in muscle—at cross talk. J. Physiol. 587, 5559–5568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peckham P. H. and Knutson J. S. (2005). Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 7, 327–360 [DOI] [PubMed] [Google Scholar]