Abstract
The recognition that there are fundamental biological sex differences that extend beyond those that define sexual behavior and reproductive function has inspired the drive toward inclusion of both sexes in research design. This is supported by an underlying clinical rationale that studying both sexes is necessary to elucidate pathophysiology and develop treatments for the entire population. However, at a more basic level, sex differences, like genetic differences, can be exploited to better understand biology. Here, we discuss how sex differences at the molecular level of cell signaling and protein trafficking are amplified to create a state of vulnerability that under the right conditions can result in symptoms of neuropsychiatry disease. Although this dialogue focuses on the specific example of corticotropin-releasing factor, the potential for analogous sex differences in signaling and/or trafficking of receptors for other neuromodulators has broad biological and therapeutic implications.
Keywords: amygdala, arousal, β-arrestin, corticotropin-releasing factor, Gs-GTP binding protein, locus coeruleus, norepinephrine, stress
Abstract
El reconocer que hay diferencias biológicas sexuales fundamentales que se extienden más allá de lo que define la conducta sexual y la función reproductiva ha inspirado el camino hacia la inclusión de ambos sexos en los diseños de investigación. Esto se sustenta en una justificación clínica fundamental acerca de la necesidad del estudio de ambos sexos para aclarar la fisiopatología y desarrollar tratamientos para toda la población. Sin embargo, a un nivel más básico, para una mejor comprensión de la biología se puede sacar provecho tanto de las diferencias por sexo como de las diferencias genéticas. En este artículo se discute cómo las diferencias sexuales a nivel molecular de las señales celulares y del tránsito de proteínas son amplificadas para crear un estado de vulnerabilidad que bajo condiciones adecuadas pueden provocar síntomas de una enfermedad neuropsiquiátrica. Aunque este diálogo se concentra en el ejemplo específico del factor liberador de corticotropina, el potencial de las diferencias sexuales análogas en las señales y/o tránsito de los receptores para otros neuromoduladores tiene amplias implicancias biológicas y terapéuticas.
Abstract
L'existence de différences biologiques fondamentales selon le sexe, au-delà de celles qui définissent le comportement sexuel et la fonction reproductrice, est à l'origine de l'inclusion des deux sexes dans les schémas de recherche. Cette conception est soutenue par une justification clinique sous-jacente stipulant que l'étude des deux sexes est nécessaire pour comprendre la physiopathologie et développer des traitements pour toute la population. Toutefois, à un niveau plus basique, les différences selon le sexe, comme les différences génétiques, peuvent être exploitées pour mieux comprendre la biologie. Nous analysons ici comment les différences selon le sexe, au niveau moléculaire de la signalisation cellulaire et de la circulation des protéines, sont amplifiées pour créer un état de vulnérabilité qui peut résulter, dans certaines conditions, en symptômes de maladie neuropsychiatrique. Cette discussion porte sur l'exemple spécifique du facteur de libération de la corticotrophine, mais l'existence de différences analogues selon le sexe dans la signalisation et/ou la circulation des récepteurs d'autres neuromodulateurs, a d'importantes implications biologiques et thérapeutiques.
Introduction
That there are disparities in disease prevalence, severity, and onset determined by sex has long been recognized; however, the underlying mechanisms have not been well delineated. Historically, the conventional thinking has been that circulating sex hormones alter physiological systems in a way that determines these factors. This extended to the concept that the actions of hormones specifically occurring during critical windows of development organize morphology and neural circuitry in a sex-specific manner.1 Sex hormones can regulate gene expression patterns in sexually specific and regionally selective ways that then become expressed as sex-specific behaviors.2 More recent but less wells-studied mechanisms for sex biases involve genetic differences that result from differential encoding of genes on sex chromosomes. Through studies that use the four-core-gene mouse model to distinguish the roles of chromosomal and gonadal sex, evidence has been provided for a genetic basis contributing to sex differences in certain social behaviors, habit formation, nociception, and sensitivity to morphine.3 This model has also suggested that sex differences in the prevalence of certain autoimmune diseases are genetically based.3 A recently identified chromosomal mechanism for conferring sex differences is bias in parent-of-origin selection.4 For example, cortical glutamatergic neurons of female mice preferentially inherit the maternal X chromosome. These examples underscore the diversity and complexity in the mechanisms through which sex differences in disease vulnerability can arise.
Sex disparities in the major psychiatric diseases have been well documented. The disorders that are nearly twice as prevalent in females than in males include posttraumatic stress disorder (PTSD), affective disorders, and anxiety disorders.5-8 Notably, these have all been associated with stress. Stress precipitates or worsens symptoms. Likewise, patients with these diseases often present with end points of hypothalamic-pituitary-adrenal (HPA)-axis dysfunction, such as dysregulated plasma cortisol rhythms and adrenal hypertrophy.9 Substance abuse, and particularly the phenomenon of relapse, has also been linked to stress.10 Although rates of substance abuse disorders are generally higher in males, females may be more vulnerable because they start abusing substances at lower doses, show steeper rates of escalation, and are more prone to relapse.11 Another salient feature of stress-related psychiatric disorders is altered arousal seen as sleep disturbances, inability to concentrate, and inappropriate responses to stimuli. Given that stress and altered arousal are two common threads through the psychiatric disorders that prevail in females, the sex disparity in pathophysiology should lie at the intersection between neural circuits that convey information about stress and those that underlie arousal. A major point of intersection between stress and arousal circuits is at the synapses between axon terminals containing the stress neuropeptide corticotropin-releasing factor (CRF) and dendrites of norepinephrine-containing locus coeruleus (LC) neurons.12 Communication here is the means by which arousal is heightened and attention and cognitive processes are altered to optimally respond to a life-threatening challenge. In this review, we describe how at this node, a convergence of three sex differences in the cellular and molecular substrates of this communication can be amplified and translated to sex differences in behavior and psychopathology.
The corticotropin-releasing factor-locus coeruleus synapse
Although stress is generally considered in terms of the pathophysiology with which it is associated, the stress response is adaptive and critical to survival. CRF orchestrates the stress response through a dual role as a neurohormone within the HPA axis and a neurotransmitter in neural circuits outside of this axis.13 The presentation of an acute life-threatening challenge initiates coordinated CRF release in parallel circuits to integrate endocrine, behavioral, and autonomic responses. A component of the CRF-mediated stress response is its engagement of the forebrain-projecting norepinephrine system through its actions on the pontine nucleus, LC.12 The LC-norepinephrine system is a substrate by which salient stimuli, regardless of valence, initiate arousal and guide attention.14 CRF axon terminals synapse with LC dendrites; through its actions on the CRF subtype 1 receptor (CRF1), CRF shifts the mode of LC discharge from a phasic state, characterized by moderate tonic activity and robust responses to discrete sensory stimuli, to a high tonic state in which cells discharge at a relatively high frequency and are not selectively responsive to discrete stimuli.15-47 Whereas phasic LC discharge is associated with focused attention and maintenance of task performance, the high tonic state is associated with hyperarousal, labile attention, going off-task, and behavioral flexibility.18 In the context of a dynamic environment with life-threatening challenges, these consequences of high tonic LC activity are optimal for survival. However, if they are initiated outside of the context of acute stress or if they are maintained long after stress termination, these same responses would become expressions of the arousal-related pathology that characterizes PTSD, depression, and anxiety. Consistent with this is evidence for excessive CRF and hyperactivity of the brain norepinephrine system in PTSD and depression.19,20
Sex differences in LC dendritic morphology—structural basis for emotional arousal
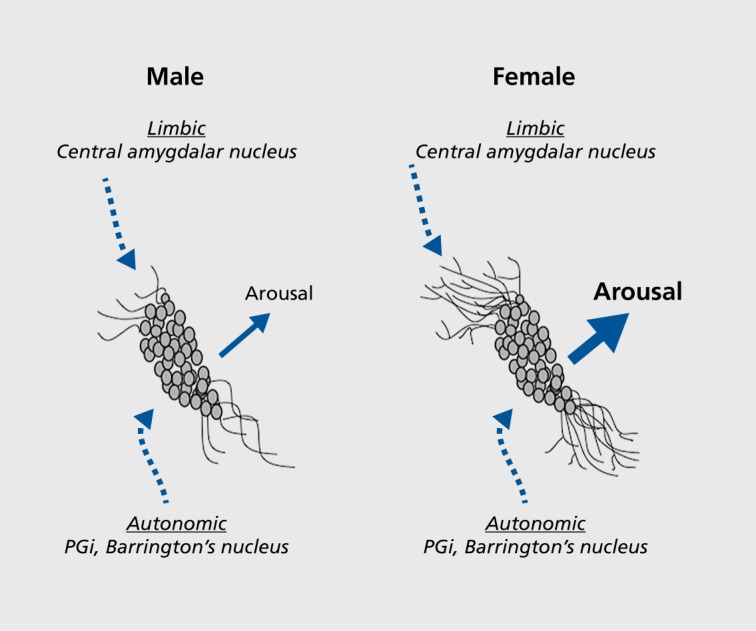
Communication between CRF and LC neurons is topographically organized such that functionally distinct populations of CRF neurons terminate either within the compact nuclear region or in the peri-LC, where LC dendrites extend for hundreds of microns away from the nucleus.21 The LC nuclear core is relatively sparsely innervated by CRF axon terminals, and these arise from autonomic-related CRF neurons. One source of CRF terminals in the nuclear LC is Barrington's nucleus, a nucleus known for its regulation of parasympathetic innervation of the pelvic viscera.22 The other source of CRF that terminates in the LC nucleus is the nucleus paragigantocellularis (PGi) in the ventrolateral medulla.23 The PGi regulates sympathetic preganglionic neurons that control blood pressure. Projections of Barrington's nucleus and PGi CRF neurons to the LC provide a mechanism whereby central noradrenergic activity can be coordinated with autonomic activity in response to an acute stress. CRF neurons in the paraventricular hypothalamic nucleus (PVN) project to the LC and terminate primarily in the medial dendritic zone.24 These neurons are distinct from those that project to the median eminence to initiate adrenocorticotropin release, suggesting that the endocrine and arousal limbs of the stress response can be initiated independently. However, the function of these LC-projecting CRF neurons of the PVN and the stimuli that engage them are currently unknown. In contrast to the sparse CRF innervation of the LC nucleus, a dense CRF terminal field exists in the dorsolateral peri-LC where LC dendrites extend.25 Here, CRF terminals are found in synaptic contact with LC dendrites, indicating proof of direct communication. The CRF terminals that synapse with LC dendrites here derive primarily from the central nucleus of the amygdala (CNA), a nucleus that is central to the generation of emotions.25 As engagement of the LC-norepinephrine system initiates arousal, synapses between CNA axon terminals and LC dendrites can be considered a structural basis for emotional arousal. Notably, unlike the cell-rich compact LC nucleus, the peri-LC is less dense and more heterogeneous, containing neurochemically diverse neurons.26 This provides a potential for complex modulation of this structure through the integration of multiple signals.
LC dendritic processes are more extensive and complex in female than male rats.27 For example, LC dendrites of females have more nodes and ends. Their dendritic trees are longer, have more branches, and have longer branch lengths. A Sholl analysis indicated that the LC dendritic structure is of increased complexity in females versus males.27 Consistent with this, LC dendrites in females make more synaptic contacts than LC dendrites in males, as indicated by an increased density of synaptophysin, a synaptic vesicular protein.27
Because functionally distinct sources of CRF innervate the core and peri-LC dendritic zone, the sexual dimorphism of LC dendrites can bias the type of information that regulates LC activity (Figure 1). By having a dendritic system of higher complexity that extends further into the peri-LC and makes more synaptic contacts, the female LC has a greater probability of communication with CRF terminals from the CNA that are conveying emotion-related information. Thus, as a result of sexual dimorphic dendritic properties, the structure for emotional arousal through which CRF transmits information is potentially greater in females than in males.
Figure 1. Schematic depicting how the topographical arrangement of locus coeruleus (LC) afferent interacts with sex differences in LC dendritic morphology to determine the magnitude of emotional arousal. LC neurons of female rats have longer and more complex dendrites than neurons of males. As a result, the probability that LC dendrites will contact corticotropin-releasing factor (CRF)-containing amgydalar afferents that convey emotion-related information and terminate in the peri-LC rather than the core is greater in females than in males. This would be predicted to result in a greater magnitude of arousal in response to emotion-related stimuli. PGi, paragigantocellularis. Adapted from reference 12: Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583(2-3):194-203. Copyright © Elsevier 2008.
Sex differences in CRF receptor-Gs protein coupling
In the absence of stress, CRF is not tonically released into the LC, and LC neuronal activity is comparable in males and females.28 Spontaneous discharge rates are similar, and they respond to sensory stimuli by a similar magnitude. However, female LC neurons are more sensitive to CRF and are activated by concentrations of CRF that have little effect on male LC neurons.28 As expected, increased sensitivity of female LC neurons to CRF translates to a greater magnitude of activation elicited by stressors that release CRF into the LC.28 Interestingly, this sex difference is unrelated to adult circulating sex hormone levels, suggesting that it has a basis either in an early organizational effect of sex hormones or in sex chromosomes. CRF excites LC neurons by binding to CRF1 on the plasma membrane. The CRF1 is a 7 transmembrane G-protein-coupled receptor that is primarily coupled to the stimulatory G-protein (G1) in brain and signals within the cell through activation of adenylyl cyclase and formation of cyclic adenosine monophosphate (cAMP).29,30 The degree of CRF-Gs coupling determines the magnitude of the neuronal response, and receptor immunoprecipitation studies in which the amount of Gs pulled down with1 was quantified indicated greater CRF1-Gs coupling in females.31 Like the neuronal response to CRF, this sex difference is independent of circulating hormone levels, occurring both in ovariectomized and intact females. This molecular sex difference can account for the functional sex difference expressed as increased neuronal sensitivity to CRF and stressors of female rats. At the time of discovery, this example of a sex difference in coupling of a receptor to its G-protein was unique. However, recent studies using guanosine 5'-O-[-thio]triphosphate (GTPγS) binding as an indicator of receptor G-protein coupling demonstrate similar sex differences with other receptors, including opioidreceptor subtypes.32,33 Given the predominance of G-protein-coupled receptors, their diverse functions, and their ability to serve as therapeutic targets, the potential for sex differences in coupling of some of these receptors to their G-proteins has broad clinical implications.
Sex differences in CRF1-β-arrestin 2 association and CRF1 trafficking
Like other G-protein-coupled receptors, CRF1 becomes internalized into early endosomes after agonist binding.29,34 This process is initiated by phosphorylation of CRF1 at its carboxyl tail and recruitment of β-arrestin 2, which promotes CRF1 internalization into early endosomes. From here, the receptor can either be recycled back to the plasma membrane or recruited to multi-vesicular bodies, where it is degraded with the consequence of downregulation at the plasma membrane. Receptor internalization and subsequent downregulation is an adaptive process that protects cells against overstimulation by agonists. Both agonist- and stress-induced CRF1 internalization have been documented in male LC neurons.35,36 For example, at both 1 hour and 24 hours after swim stress, the percentage of total CRF1labeling that is present in the cytoplasm of LC neurons shifts from 50% in the unstressed state to 80% by 1 hour after swim stress, and this is maintained at 24 hours after stress.36 As time increases after the stress, increasingly more1 is found in multivesicular bodies, consistent with receptor degradation and downregulation. This cellular response has functional consequences that are expressed as a decreased magnitude of LC activation elicited by subsequent CRF exposures (Figure 2) .37,38 In contrast to male LC neurons, female LC neurons do not internalize CRF1 after swim stress.31 Notably, following swim stress, a significantly greater amount of receptor labeling is present on the plasma membrane of female LC neurons, which could reflect either a unique recruitment to the plasma membrane or decreased synthesis. However, the overall CRF1 protein level is not altered. The inability of female LC neurons to internalize CRF1 can account for the observation that unlike male LC neurons, the response of female LC neurons to CRF is not diminished after swim stress.28 Thus, the cellular mechanism for adapting to overstimulation of CRF1 is compromised in female LC neurons. Taken with evidence for a lack of agonist-induced CRF1 internalization in female LC neurons described below, this implies that the molecular mechanisms underlying this important adaptive response to CRF1 stimulation are impaired in females.
Figure 2. Schematic depicting sex differences in corticotropin-releasing factor subtype 1 receptor (CRF1) trafficking. In males, when corticotropin-releasing factor (CRF) released from presynaptic axon terminals (blue) binds to CRF1 on the plasma membrane of locus coeruleus dendrites (yellow), β-arrestin 2 associates with CRF1 and initiates receptor internalization. This results in an attenuated response to subsequent CRF. In females, stress-induced association of β-arrestin 2 with CRF1 is less than in males, perhaps because of the increased CRF1-Gs association. As a result, stress does not induce CRF1 internalization and the response of locus coeruleus neurons to CRF is relatively larger in females. Gs, stimulatory G-protein.
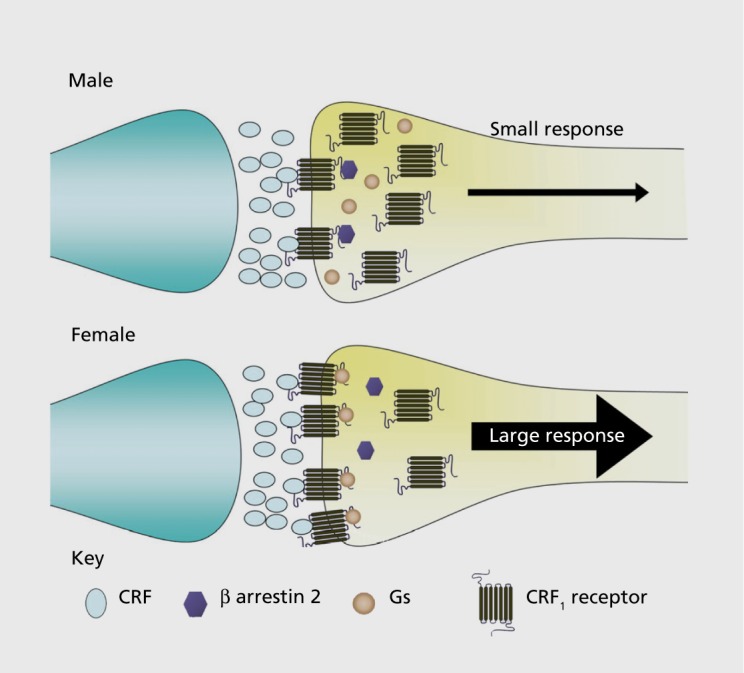
Using the same receptor immunoprecipitation approaches described above that provided evidence for increased CRF1-Gs in females, it was discovered that stress-induced association of CRF1 with β-arrestin 2 was greatly diminished in females compared with males regardless of circulating hormonal status.31 In the absence of stress, CRF1-β-arrestin 2 association is minimal in both males and females. However, shortly after stress, β-arrestin 2 becomes associated with CRF1 selectively in males. This molecular sex difference is not surprising given the spatial competition between Gs and β-arrestin in their association with G -protein-coupled receptors.39,40 Because of this competition, a receptor bias toward Gs, as seen in females, is a bias away from β-arrestin 2. Importantly, the impairment in this molecular step probably contributes to the inability of female LC neurons to internalize CRF1 rendering them more susceptible to hyperstimulation.
Convergence of sex differences at the CRF-LC synapse
The three sex differences discussed above converge on the CRF-LC synapse to make the LC-norepinephrine system of females more sensitive to stress. At the presynaptic level, female LC dendrites are differentially structured so as to receive more CRF-amygdalar input that conveys information about emotion than do male LC dendrites. The neuronal response to this increased CRF influence will then be magnified in females because at the molecular level,CRF1 is designed to be more responsive to agonist stimulation through its enhanced coupling to Gs. Finally, following its activation, CRF1 in female neurons is less able to adapt because of an impairment in β-arrestin 2 association, which compromises internalization. These three sex differences create a stronger structure for emotional arousal in females. However, in spite of these differences, LC neurons of males and females are quite comparable in the unstressed state, underscoring that the expression of sex differences in this system is only apparent under the specific condition of CRF release, Additionally, the differences should be magnified when CRF is present in excess, as has been proposed to occur in stress-related psychiatric disorders such as depression and PTSD. The condition of excessive CRF can be modeled by mice genetically modified to overexpress CRF.
Modeling stress-related psychiatric diseases with CRF-overexpressing mice
That CRF is overexpressed or hypersecreted in PTSD and depression is evidenced by greater levels of CRF in the cerebrospinal fluid of patients, increased numbers of CRF-immunoreactive neurons in the paraventricular hypothalamic nucleus of depressed patients, and increased CRF messenger RNA, as determined by in situ hybridization.41-46 Notably, CRF is also elevated in the LC of depressed patients.47 Taken with evidence for normalization of CRF levels after antidepressant treatment, the findings imply a causal link between elevated CRF and psychopathology.48-51 The presence of estrogen- and androgen-responsive elements in the promoter region of the CRF gene offers a mechanism for sex-specific regulation of CRF levels.52,53 However, there is no evidence for sex differences in CRF levels that can explain female vulnerability to stress-related diseases. CRF levels in patients with depression are comparable between males and females; in healthy human subjects, males actually have more CRF neurons in the paraventricular hypothalamic nucleus than females.42,46,54 These findings implicate postsynaptic mechanisms as a basis for sex differences in stress vulnerability.
The putative link between CRF hypersecretion and psychiatric disorders inspired the development of animal models of CRF hypersecretion to delineate how this condition alters neurons to lead to pathology. The most commonly used models are mice that are genetically altered to overexpress CRF.55-58 These include conditional models in which CRF-overexpression is confined to certain brain regions and/or can temporally be controlled by doxycycline administration. Sex differences have been studied in a transgenic mouse in which CRF expression is under control of the metallothionein (mMTl) promoter.59 In this model, CRF is elevated primarily in neurons that normally express CRF, as well as in certain peripheral tissues, although it is not present in the circulation.57 This may better model the human condition of CRF overexpression than some of the conditional models in which CRF is expressed in neurons and glia and in some regions that do not typically express CRF.
In both male and female CRF-overexpressing mice (CRF-OE), CRF overexpression is apparent in the dense innervation of the LC, compared with wild-type littermates, and there is no sex difference in the density of CRF innervation.59 Although this would be predicted to translate to higher LC firing rates in CRF-OE mice than in wild-type littermates regardless of sex, this is not the case.59 As expected, because wild-type mice should be analogous to the unstressed condition in rats where there is no tonic release of CRF, LC neuronal discharge rates are comparable between males and females. In spite of the massive innervation of LC neurons by CRF in male CRF-OE mice, LC discharge rates are similar to wild-type mice, suggesting that male CRF-OE mice have an adaptation that confers protection from excess CRF. Importantly, this adaptation was not present in female CRF-OE mice, which had LC discharge rates that were nearly three times greater than those of wild-type mice or male CRF-OE mice. This sex difference in CRF-OE mice could be attributed to a different pattern of CRF1 cellular localization.59 Like unstressed male rats, CRF1 is relatively equally distributed between the cytoplasm and plasma membrane in male wild-type mice. Analogous to the stressed male rat, CRF1 is primarily internalized into the cytoplasm in male CRF-OE mice, thereby conferring protection from excess synaptic CRF. Female wild-type and CRFOE mice show an opposite pattern, with greater cytoplasmic localization of CRF1 in wild- type mice and less internalization in CRF-OE mice. The lack of CRF1 internalization in female CRF-OE mice would render their LC neurons vulnerable to the excess CRF in the CRF-LC synapse. Thus, under the specific condition of CRF overexpression, a condition that is thought to be present in stress-related psychiatric disorders, the female LC-norepinephrine system will be tonically hyperactive because of an inability to internalize CRF1. Given that tonic LC hyperactivity is associated with the arousal-related symptoms that characterize anxiety disorders, depression, and PTSD, this could account for the increased prevalence of these disorders in females.
Sex-biased cellular signaling
By regulating CRF1 trafficking and the number of receptors on the plasma membrane, the association of CRF1 with β-arrestin 2 determines the magnitude of the response to CRF released during stress or under pathological conditions. More importantly, it determines the quality of the response by governing, along with Gs, the cellular signaling that transduces the binding of CRF to CRF1. Because β-arrestins serve as a scaffold between receptors and Gs-independent signaling cascades, the degree to which receptors associate with β-arrestins versus Gs defines the cellular signaling that is engaged by ligand-receptor interaction.60 Sex differences in CRF1 coupling to Gs and β-arrestin 2 should translate to sex differences in cellular signaling initiated during stress by the binding of CRF to CRF1. For females, CRF1 signaling will be biased toward Gs-dependent pathways; for males, it will be relatively biased toward β-arrestin 2-related Gs-independent pathways (Figure 3) . In this way, stressors can elicit a series of distinct cellular reactions in males and females that can translate to different physiological and behavioral responses and distinct stress-related pathology. Notably, these sex differences will be magnified when CRF is elevated as in depression and PTSD.
Figure 3. Schematic depicting sex-biased corticotropin-releasing factor subtype 1 receptor (CRF1) signaling. As a result of sex differences in CRF1 coupling to Gs (female bias) and β-arrestin 2 (male bias), corticotropin-releasing factor (CRF) released during stress can engage sexually distinct cellular signaling pathways. These different cellular reactions can translate to sexually distinct stress responses and pathology. β-arr; β-arrestin 2; ERK, extracellular signal-related kinase; Gs, stimulatory G-protein; PKA, protein kinase A; Rho, Rho family of GTPases; Src, proto-oncogene tyrosine protein kinase Src.
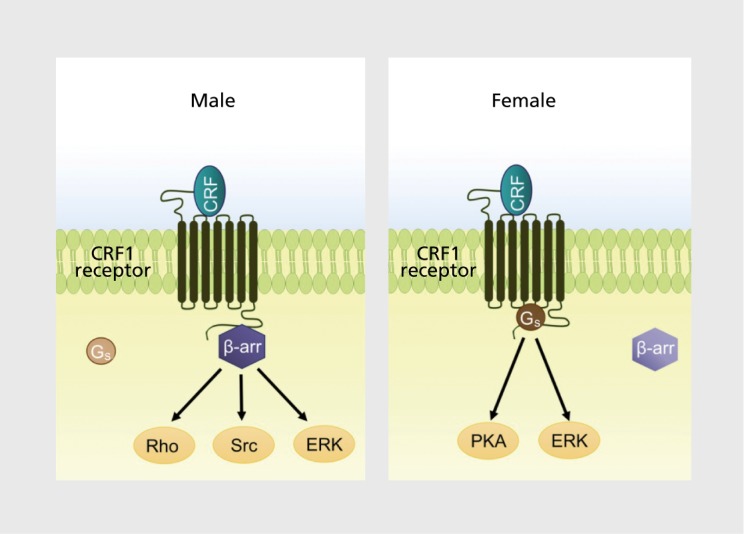
An important function of Gs and β-arrestin-initiated signaling is to regulate the dynamic process of protein phosphorylation that controls the activation and inactivation of cellular proteins. As a result, sex biases in CRF1 signaling should give rise to sexually distinct profiles of phosphorylated proteins when CRF receptors are activated, and the distinctions between male and female phosphoproteomes may reveal the molecular basis for sex differences in stress-related neuropsychiatry disorders. A global phosphoproteomic analysis comparing phosphopeptides in the cortex of male and female CRF-OE mice and their wild-type littermates identified these distinctions in Alzheimer disease-related pathways that may account for an increased vulnerability of females to Alzheimer's disease.61
In addition to psychiatric disorders, Alzheimer disease is an example of a disease that has been associated with stress and that is more prevalent in females.62-64 In animal models, both stress and CRF overexpression accelerate the formation of plaques and cognitive deficits.65-69 This has been attributed in part to activation of protein kinase A (PKA). For example, CRF elicits β-amyloid secretion—a process that contributes to the formation of plaques—in primary cultures of hippocampal neurons of mice that express a human form of amyloid precursor protein, and this effect requires PKA.69 PKA is also involved in tau phosphorylation, a process that has been implicated in the formation of fibrillary tangles.70 Given that PKA activation is a primary component of the Gs signaling cascade, the female bias toward CRF1-Gs signaling would favor Alzheimer disease pathology under conditions of stress or excess CRF. This can explain how stress and sex interact to account for an increased female prevalence of Alzheimer disease. Sex-biased CRF signaling may be a common link underlying the comorbidity between Alzheimer disease and depression.71
Future considerations
Identifying disease processes that arise from the sex bias in signaling is an important translational goal. The comparison of phosphoproteomes under the optimal condition of CRF overexpression described above is one approach toward this goal. Manipulating CRF1-expressing neurons to exhibit a specific signaling bias and determining the consequences of this is another approach that addresses causality. Another important question to address relates to how sex differences in CRF1 coupling to interacting proteins arise. The lack of evidence for a role of circulating hormones implicates an effect that is organized by hormones early in life or a genomic mechanism. Currently, there is no evidence for sex differences in the gene encoding CRF1. Alternatively, the sex difference could be a posttranslational modification of the receptor that affects G, and/or β-arrestin 2 binding. Consistent with this, preliminary findings from the global phosphoproteomic study show sex differences in phosphorylation of the 396S on the carboxyl tail adjacent to a putative binding site for β-arrestin 2.
Although this review has focused on sex-biased CRF1signaling, it would be surprising if this characteristic was unique to CRF1 Evidence is emerging for sex differences in signaling of other receptors, including γ-aminobutyric acid (GABA), dopamine, and opioid receptors.32,33,72-74 Of these, opioid receptors are of interest because stress-induced release of endogenous opioids is thought to mitigate the effects of stress and oppose those of CRF.75 Given the many biological processes mediated by G -protein-coupled receptors and that these are targets of a pharmacopoeia of drugs, identifying sex differences in their signaling has the potential to break new ground in our ability to understand mechanisms underlying disease vulnerability, as well as guide the development of better treatments for psychiatric diseases in men and women.
Acknowledgments
This work was supported by PHS grants MH040008, MH092438, and NSF IOS 1552416. The authors have no competing financial interests to declare.
Selected abbreviations and acronyms
- CRF
corticotropin-releasing factor
- CRF-OE
CRF-overexpressing mice
- CRF1
CRF subtype 1 receptor
- Gs
stimulatory G-protein
- LC
locus coerulcus
Contributor Information
Rita J. Valentino, Department of Anesthesiology and Critical Care Medicine, The Children's Hospital of Philadelphia and University of Pennsylvania, USA.
Debra A. Bangasser, Department of Psychology and Neuroscience Program, Temple University, USA.
REFERENCES
- 1.McCarthy MM., Arnold AP., Ball GF., Blaustein JD., DeVries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang CF., Shah NM. Representing sex in the brain, one module at a time. Neuron. 2014;82(2):261–278. doi: 10.1016/j.neuron.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold AP., Chen X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front Neuroendocrinol. 2009;30(1):1–9. doi: 10.1016/j.yfrne.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg C., Zhang J., Butler JE., Haig D., Dulac C. Sex-specific parent-of-origin allelic expression in the mouse brain. Science. 2010;329(5992):682–685. doi: 10.1126/science.1190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kessler RC., McGonagle KA., Zhao S., et al Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 6.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74(1):5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 7.Kessler RC., McGonagle KA., Nelson CB., Hughes M., Swartz M., Blazer DG. Sex and depression in the National Comorbidity Survey. II: Cohort effects. J Affect Disord. 1994;30(1):15–26. doi: 10.1016/0165-0327(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 8.Breslau N. Gender differences in trauma and posttraumatic stress disorder. J Gend Specif Med. 2002;5(1):34–40. [PubMed] [Google Scholar]
- 9.Gold PW., Goodwin FK., Chrousos GP. Clinical and biochemical manifestations of depression: relationship to the neurobiology of stress (Part 1). N Engl J Med. 1988;316(6):348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- 10.Koob GF., Ahmed SH., Boutrel B., et al Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27(8):739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Becker JB., Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valentino RJ., Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583(23):194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owens MJ., Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43:425–474. [PubMed] [Google Scholar]
- 14.Aston-Jones G., Bloom FE. Norepinephrine-containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non-noxious environmental stimuli. J Neurosci. 1981;1(8):887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Bockstaele EJ., Colago EE., Valentino RJ. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364(3):523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Valentino RJ., Foote SL. Corticotropin-releasing factor disrupts sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987;45:28–36. doi: 10.1159/000124700. [DOI] [PubMed] [Google Scholar]
- 17.Valentino RJ., Foote SL. Corticotropin-releasing factor increases tonic but not sensory-evoked activity of noradrenergic locus coeruleus neurons in unanesthetized rats. J Neurosci. 1988;8(3):1016–1025. doi: 10.1523/JNEUROSCI.08-03-01016.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aston-Jones G., Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- 19.Wong ML., Kling MA., Munson PJ., et al Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97(1):325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Southwick SM., Bremner JD., Rasmusson A., Morgan CA 3rd., Arnsten A., Charney DS. Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46(9):1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 21.Van Bockstaele EJ., Bajic D., Proudfit H., Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73(3):273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- 22.Valentino RJ., Chen S., Zhu Y., Aston-Jones G. Evidence for divergent projections of corticotropin-releasing hormone neurons of Barrington's nucleus to the locus coeruleus and spinal cord. Brain Res. 1996;732(1-2):115. doi: 10.1016/0006-8993(96)00482-9. [DOI] [PubMed] [Google Scholar]
- 23.Valentino RJ., Page M., Van Bockstaele E., Aston-Jones G. Corticotropin-releasing factor innervation of the locus coeruleus region: distribution of fibers and sources of input. Neuroscience. 1992;48(3):689–705. doi: 10.1016/0306-4522(92)90412-u. [DOI] [PubMed] [Google Scholar]
- 24.Reyes BA., Valentino RJ., Xu G., Van Bockstaele EJ. Hypothalamic projections to locus coeruleus neurons in rat brain. Eur J Neurosci. 2005;22(1):93–106. doi: 10.1111/j.1460-9568.2005.04197.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Bockstaele EJ., Colago EE., Valentino RJ. Amygdaloid corticotropin-releasing factor targets locus coeruleus dendrites: substrate for the coordination of emotional and cognitive limbs of the stress response. J Neuroendocrinol. 1998;10(10):743–757. doi: 10.1046/j.1365-2826.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- 26.Sutin EL., Jacobowitz DM. Neurochemicals in the dorsal pontine tegmentum. Prog Brain Res. 1991;88:3–14. doi: 10.1016/s0079-6123(08)63796-6. [DOI] [PubMed] [Google Scholar]
- 27.Bangasser DA., Zhang X., Garachh V., Hanhauser E., Valentino RJ. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav. 2011;103(3-4):342–351. doi: 10.1016/j.physbeh.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtis AL., Bethea T., Valentino RJ. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31(3):544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 29.Hauger RL., Risbrough V., Brauns O., Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5(4):453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalmers DT., Lovenberg TW., Grigoriadis DE., Behan DP., De Souza EB. Corticotropin-releasing factor receptors: from molecular biology to drug design. Trends Pharmacol Sci. 1996;17(4):166–172. doi: 10.1016/0165-6147(96)81594-x. [DOI] [PubMed] [Google Scholar]
- 31.Bangasser DA., Curtis A., Reyes BA., et al Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15(9):877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang YJ., Huang P., Blendy JA., Liu-Chen LY. Brain region - and sex-specific alterations in DAMGO-stimulated [35S]GTPS binding in mice with Oprm1 A112G. Addict Biol. 2014;19(3):354–361. doi: 10.1111/j.1369-1600.2012.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang YJ., Rasakham K., Huang P., Chudnovskaya D., Cowan A., Liu-Chen LY. Sex difference in K-opioid receptor (KOPR)-mediated behaviors, brain region KOPR level and KOPR-mediated guanosine 5'-O-(3-[35S]thiotriphosphate) binding in the guinea pig. J Pharmacol Exp Ther. 2011;339(2):438–450. doi: 10.1124/jpet.111.183905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oakley RH., Olivares-Reyes JA., Hudson CC., Flores-Vega F., Dautzenberg FM., Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and [β-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293(1):R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes BA., Fox K., Valentino RJ., Van Bockstaele EJ. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23(11):2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 36.Reyes BA., Valentino RJ., Van Bockstaele EJ. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149(1):122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conti LH., Foote SL. Effects of pretreatment with corticotropin-releasing factor on the electrophysiological responsivity of the locus coeruleus to subsequent corticotropin-releasing factor challenge. Neuroscience. 1995;69(1):209–219. doi: 10.1016/0306-4522(95)00222-5. [DOI] [PubMed] [Google Scholar]
- 38.Conti LH., Foote SL. Reciprocal cross-desensitization of locus coeruleus electrophysiological responsivity to corticotropin-releasing factor and stress. Brain Res. 1996;722(1-2):19–29. doi: 10.1016/0006-8993(96)00175-8. [DOI] [PubMed] [Google Scholar]
- 39.Ahn S., Shenoy SK., Wei H., Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279(34):35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 40.Lefkowitz RJ., Shenoy SK. Transduction of receptor signals by P-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 41.Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52(3):597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- 42.Nemeroff C., Widerlov E., Bissette GT., et al Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 43.Raadsheer FC., Hoogendijk WJ., Stam FC., Tilders FJ., Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60(4):436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- 44.Chrousos GP., Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244–1252. [PubMed] [Google Scholar]
- 45.Bremner JD., Licinio J., Darnell A., et al Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154(5):624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banki CM., Bissette G., Arato M., O'Connor L., Nemeroff CB. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry. 1987;144(7):873–877. doi: 10.1176/ajp.144.7.873. [DOI] [PubMed] [Google Scholar]
- 47.Bissette G., Klimek V., Pan J., Stockmeier C., Ordway G. Elevated concentrations of CRF in the locus coeruleus of depressed subjects. Neuropsychopharmacology. 2003;28(7):1328–1335. doi: 10.1038/sj.npp.1300191. [DOI] [PubMed] [Google Scholar]
- 48.De Bellis MD., Gold PW., Geracioti TD Jr., Listwak SJ., Kling MA. Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry. 1993;150(4):656–657. doi: 10.1176/ajp.150.4.656. [DOI] [PubMed] [Google Scholar]
- 49.Heuser I., Bissette G., Dettling M., et al Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety. 1998;8(2):71–79. [PubMed] [Google Scholar]
- 50.Kling MA., Geracioti TD., Licinio J., Michelson D., Oldfield EH., Gold PW. Effects of electroconvulsive therapy on the CRH-ACTH-cortisol system in melancholic depression: preliminary findings. Psychopharmacol Bull. 1994;30(3):489–494. [PubMed] [Google Scholar]
- 51.Nemeroff CB., Bissette G., Akil H., Fink M. Neuropeptide concentrations in the cerebrospinal fluid of depressed patients treated with electroconvulsive therapy. Corticotrophin-releasing factor, β-endorphin and somatostatin. Br J Psychiatry. 1991;158:59–63. doi: 10.1192/bjp.158.1.59. [DOI] [PubMed] [Google Scholar]
- 52.Vamvakopoulos NC., Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92(4):1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bao AM., Fischer DF., Wu YH., et al A direct androgenic involvement in the expression of human corticotropin-releasing hormone. Mol Psychiatry. 2006;11(6):567–576. doi: 10.1038/sj.mp.4001800. [DOI] [PubMed] [Google Scholar]
- 54.Bao AM., Swaab DF. Gender difference in age-related number of corticotropin-releasing hormone-expressing neurons in the human hypothalamic paraventricular nucleus and the role of sex hormones. Neuroendocrinology. 2007;85(1):27–36. doi: 10.1159/000099832. [DOI] [PubMed] [Google Scholar]
- 55.Groenink L., Pattij T., De Jongh R., et al 5-HT1A receptor knockout mice and mice overexpressing corticotropin-releasing hormone in models of anxiety. Eur J Pharmacol. 2003;463(1-3):185–197. doi: 10.1016/s0014-2999(03)01281-0. [DOI] [PubMed] [Google Scholar]
- 56.Lu A., Steiner MA., Whittle N., et al Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13(11):1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- 57.Stenzel-Poore MP., Cameron VA., Vaughan J., Sawchenko PE., Vale W. Development of Cushing's syndrome in corticotropin-releasing factor transgenic mice. Endocrinology. 1992;130(6):3378–3386. doi: 10.1210/endo.130.6.1597149. [DOI] [PubMed] [Google Scholar]
- 58.Stenzel-Poore MP., Heinrichs SC., Rivest S., Koob GF., Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14(5 pt 1):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bangasser DA., Reyes BA., Piel D., et al Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2012;18(2):166–173. doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Wire SM., Ahn S., Lefkowitz RJ., Shenoy SK. β-Arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 61.Bangasser DA., Dong H., Carroll J., et al Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer's disease-related signaling. Mol Psychiatry. 2016 Advanced online publication, doi: 10.1038/ mp.2016.185. doi: 10.1038/mp.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson RS., Arnold SE., Schneider JA., Kelly JF., Tang Y., Bennett DA. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27(3):143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- 63.Wilson RS., Evans DA., Bienias JL., Mendes de Leon CF., Schneider JA., Bennett DA. Proneness to psychological distress is associated with risk of Alzheimer's disease. Neurology. 2003;61(11):1479–1485. doi: 10.1212/01.wnl.0000096167.56734.59. [DOI] [PubMed] [Google Scholar]
- 64.Ruitenberg A., Ott A., van Swieten JC., Hofman A., Breteler MM. Incidence of dementia: does gender make a difference? Neurobiol Aging. 2001;22(4):575–580. doi: 10.1016/s0197-4580(01)00231-7. [DOI] [PubMed] [Google Scholar]
- 65.Campbell SN., Zhang C., Roe AD., et al Impact of CRFR1 ablation on amyloid-β production and accumulation in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2015;45(4):1175–1184. doi: 10.3233/JAD-142844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carroll JC., Iba M., Bangasser DA., et al Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J Neurosci. 2011;31(40):14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rissman RA., Staup MA., Lee AR., et al Corticotropin-releasing factor receptor-dependent effects of repeated stress on tau phosphorylation, solubility, and aggregation. Proc Natl Acad Sci U S A. 2012;109(16):6277–6282. doi: 10.1073/pnas.1203140109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong H., Murphy KM., Meng L., et al Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2012;28(3):579–592. doi: 10.3233/JAD-2011-111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dong H., Wang S., Zeng Z., et al Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice. Psychopharmacology (Berl). 2014;231(24):4711–4722. doi: 10.1007/s00213-014-3629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jicha GA., Weaver C., Lane E., et al cAMP-dependent protein kinase phosphorylations on tau in Alzheimer's disease. J Neurosci. 1999;19(17):7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bennett S., Thomas AJ. Depression and dementia: cause, consequence or coincidence? Maturitas. 2014;79(2):184–190. doi: 10.1016/j.maturitas.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Marron Fernandez de Velasco E., Hearing M., Xia Z., Victoria NC., Lujan R., Wickman K. Sex differences in GABABR-GIRK signaling in layer 5/6 pyramidal neurons of the mouse prelimbic cortex. Neuropharmacology. 2015;95:353–360. doi: 10.1016/j.neuropharm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakrabarti S., Liu NJ., Zadina JE., Sharma T., Gintzler AR. Pleiotropic opioid regulation of spinal endomorphin 2 release and its adaptations to opioid withdrawal are sexually dimorphic. J Pharmacol Exp Ther. 2012;340(1):56–63. doi: 10.1124/jpet.111.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bychkov E., Ahmed MR., Gurevich EV. Sex differences in the activity of signalling pathways and expression of G-protein-coupled receptor kinases in the neonatal ventral hippocampal lesion model of schizophrenia. int J Neuropsychopharmacol. 2011;14(1):1–15. doi: 10.1017/S1461145710000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Valentino RJ., Van Bockstaele E. Endogenous opioids: the downside of opposing stress. Neurobiol Stress. 2015;1:23–32. doi: 10.1016/j.ynstr.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


