Abstract
Women exhibit more rapid escalation from casual drug taking to addiction, exhibit a greater withdrawal response with abstinence, and tend to exhibit greater vulnerability than men in terms of treatment outcome. In rodents, short-term estradiol intake in female rats enhances acquisition and escalation of drug taking, motivation for drugs of abuse, and relapse-like behaviors. There is also a sex difference in the dopamine response in the nucleus accumbens. Ovariectomized female rats exhibit a smaller initial dopamine increase after cocaine treatment than castrated males. Estradiol treatment of ovariectomized female rats enhances stimulated dopamine release in the dorsolateral striatum, but not in the nucleus accumbens, resulting in a sex difference in the balance between these two dopaminergic projections. In the situation where drug-taking behavior becomes habitual, dopamine release has been reported to be enhanced in the dorsolateral striatum and attenuated in the nucleus accumbens. The sex difference in the balance between these neural systems is proposed to underlie sex differences in addiction.
Keywords: addiction, amphetamine, cocaine, drug abuse, sex difference, substance use
Abstract
Las mujeres presentan una escalada más rápida desde una ingesta casual de una droga hasta la adicción, una mayor respuesta de privación con la abstinencia, y tienden a presentar una mayor vulnerabilidad que los hombres respecto a los resultados del tratamiento. En roedores, el consumo de estradiol a corto plazo en ratas hembra refuerza la adquisición y escalada de la ingesta de drogas, la motivación por las drogas de abuso y las conductas tipo recaída. También hay una diferencia por sexo en la respuesta de dopamina en el núcleo accumbens. Las ratas ovariectomizadas presentan un menor incremento inicial de dopamina después del tratamiento con cocaína respecto a los machos castrados. El tratamiento con estradiol de las ratas ovariectomizadas aumenta la liberación de dopamina por estimulación del estriado dorsolateral, pero no del núcleo accumbens, lo que determina una diferencia por sexo en el balance entre estas dos vías dopaminérgicas. En situaciones en que la conducta de ingestión de la droga se hace habitual, se ha encontrado que la liberación de dopamina está aumentada en el estriado dorsolateral y atenuada en el núcleo accumbens. Se ha propuesto que la diferencia por sexo en el balance entre estos sistemas neurales está a la base de las diferencias por sexo en las adicciones.
Abstract
Les femmes passent plus rapidement de la consommation occasionnelle de drogue à l'addiction, souffrent d'un syndrome de sevrage plus sévère et sont plus vulnérables que les hommes en termes de résultat thérapeutique. Chez les rongeurs, la prise d'estradiol à court terme chez les rates augmente la propension pour la drogue et l'escalade de sa prise, la motivation pour les drogues illicites et les tendances à la rechute. Il y a aussi une différence selon le sexe dans la réponse à la dopamine dans le noyau accumbens. Des rates ovariectomisées présentent une plus faible augmentation de la dopamine initiale après traitement par cocaïne que des mâles castrés. Le traitement par estradiol de rates ovariectomisées favorise la libération de dopamine dans le striatum dorsolatéral, mais pas dans le noyau accumbens, conduisant à une différence selon le sexe dans l'équilibre entre les deux projections dopaminergiques. Dans les situations de prises de drogue habituelles, la libération de dopamine est augmentée dans le striatum dorsolatéral et atténuée dans le noyau accumbens. La différence selon le sexe dans l'équilibre entre ces systèmes neuronaux sous-tendrait les différences selon le sexe dans l'addiction.
Introduction
The chromosomes and hormones in the developing fetus contribute to sex differences in the brain.1,2 The fact that we can find similar sex differences in the brains of humans and rodents suggests that the basic biology is different for females and males, and that not all sex differences in brain and behavior are due to sociocultural experiences that differ for men and women. This is true for sex differences in addiction, even though there are also effects of experience and culture on vulnerability to addiction that can differentially affect males and females.3
Engaging in pleasurable activities or eating sweet or highly palatable foods activate the reward system.4,5 Drugs of abuse all produce their effects by causing changes in neurotransmitter function that increase neural activity in the reward system, and there are sex differences in this regard.6-8 These ideas will be developed further below. Intriguingly, only about 10% to 16% of humans9 and other species10,11 become addicted to drugs. This is in spite of the fact that all individuals will exhibit long-term changes in the brain after being exposed to a drug of abuse.12-14 In the laboratory, all rodents will eventually learn to self-administer cocaine or morphine if they are alone in a self-administration chamber for a few hours a day, but only about 10% to 16% of male rats will develop behaviors that are similar to the characteristics of addiction in humans.10,11 Thus, even though there are changes in the brain in all individuals that receive a drug, the changes in the brains of those who become addicted are different from those who do not. Individual differences in genetics, personality traits, experiences during development, and whether one is male or female are all thought to contribute to how one responds to drugs or food or gambling and whether one develops compulsive behaviors associated with an addiction.
Sex differences in addiction-like behavior
Women who are vulnerable to addiction will tend to escalate use more rapidly to the point of addiction than men will. This is true for alcohol, most illicit drugs, and gambling.6,15,16 In other words, for those women and men who are vulnerable to addiction, women escalate to addiction more rapidly than men. Similarly, in laboratory experiments, female rats acquire drug self-administration in an operant conditioning chamber more rapidly than male rats.17-19 This is primarily due to circulating ovarian hormones in females, where short-term estradiol exposure enhances the rate of acquisition of drug taking.19-24 In these laboratory experiments, subjects are trained to lever press or nose poke to receive an injection of a drug such as cocaine or morphine. Acquisition is measured by the number of days it takes for the animal to reach a stable level of responding for the drug. This animal model is useful for identifying factors that make an individual vulnerable to drug taking. The test for self-administration of drugs has inherent face validity for studying addiction. Using this behavioral test, female rats will also escalate drug taking more rapidly than males, and females will work harder than male rats to get a single injection.25-29
To examine motivation for a drug in the laboratory, animals are put on a reinforcement schedule that increases with each successive reinforcement delivered, a progressive ratio (PR) schedule that continues until the animals fail to complete the response requirement (referred to as the breaking point). Females exhibit a higher breaking point on a PR than males.26,27,30 Estradiol treatment also enhances motivation in ovariectomized (OVX) rats.31 These results suggest that estradiol is involved in the acquisition of and motivation for drug taking.
During attempts to quit (abstinence) use of drugs such as cocaine and amphetamine, women exhibit withdrawal symptoms that are more unpleasant than those of men.32,33 Similarly, female smokers report increased negative affect during withdrawal and experience a greater stress response.34 On the other hand, men exhibit greater withdrawal symptoms when quitting alcohol consumption.35 During abstinence, women report greater craving induced by cues, and this is thought to result in greater vulnerability in women than in men, in terms of treatment outcomes.36
In studies with rodents, after self-administration is established, animals undergo an extinction process to examine the effect of abstinence on responding under conditions when the drug was previously available. During extinction, rats are placed in an operant chamber, but no drug is delivered even though the animal responds for the drug. This continues until the animal stops responding for drug. After that, the investigator examines both whether the animal will reinitiate self-administration and what increases reinitiation behavior. What is found is that there is greater drug-, cue-, and stress-induced reinstatement for cocaine and morphine in females than in males.37-39 Reinstatement is also hormone-dependent in females, where estradiol enhances and progesterone attenuates reinstatement in females.37,40-43
Finally, although drug self-administration in laboratory animals resembles drug-taking behavior in humans, the animals do not show symptoms of addiction. More recent tests have been devised where animals exhibit symptoms more typically associated with addiction, such as responding for drug even when not being rewarded, responding for drug in the presence of adverse consequences, high motivation to take drug on a PR schedule, and loss of motivation for previously desired reinforcers. Under conditions in which responding for drug can be assessed for these attributes, addiction-like behavior can be studied in the rat. Interestingly, only 10% to 20% of male rats exhibit addiction-like behavior, which is similar to the percentage of humans who become addicted.10,44,45
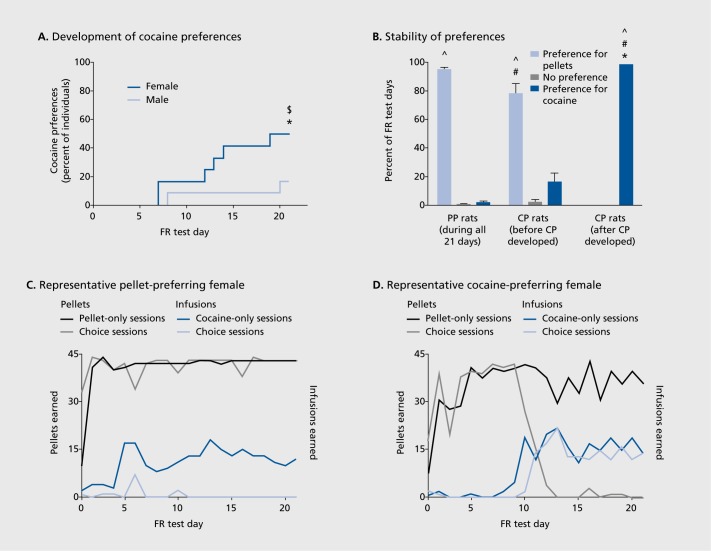
In the Becker laboratory, we use a choice paradigm where male and female rats are given a choice between palatable sugar pellets and intravenous cocaine. Early in the testing, all animals choose pellets, but after 3 to 7 weeks, a certain proportion of rats choose cocaine over pellets, and more females than males choose cocaine (Figure 1).44,46 The rats that choose cocaine do so at the expense of the pellets that were previously found to be rewarding by these rats. Other laboratories have also found that more females than males choose cocaine over food reinforcement, and that this is not due to the dose of cocaine or the amount of food received.47,48
Figure 1. Figure 1. Females are more likely to develop a preference for cocaine. (A) The development of cocaine preferences in male (open circles) and female (filled circles) rats (n =12 per sex). Significant increase in the proportion of cocaine-preferring (CP) females between the second and last fixed-ratio 5 (FR5) tests (*P=0.05). The proportion of CP females was greater than males ($P=0.05). (B) The stability of preferences in pellet-preferring (PP) rats and CP rats (both before and after CP developed). Significant difference between PP and CP rats within same preference category (#P=0.05). Significant difference in preference category before and after CP developed (*P=0.05). Significant difference between the “preference for cocaine” and “preference for pellets” categories within a given group (^P=0.05). PP rats (n = 16) and CP rats (n = 8). (C) Representative self-administration behavior in a PP rat over the 21 FR sessions, displaying the number of infusions (gray) and pellets (black) earned each day during the cocaine-only or pellet-only sessions (closed symbols) and the choice session (open symbols). (D) Representative self-administration behavior in a CP rat (CP criteria met on day 11 in this case). Reproduced from ref 44: Perry AN, Westenbroek C, Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE. 2013;8(11):e79465. Reproduced under the Creative Commons Attribution License.
The reward system
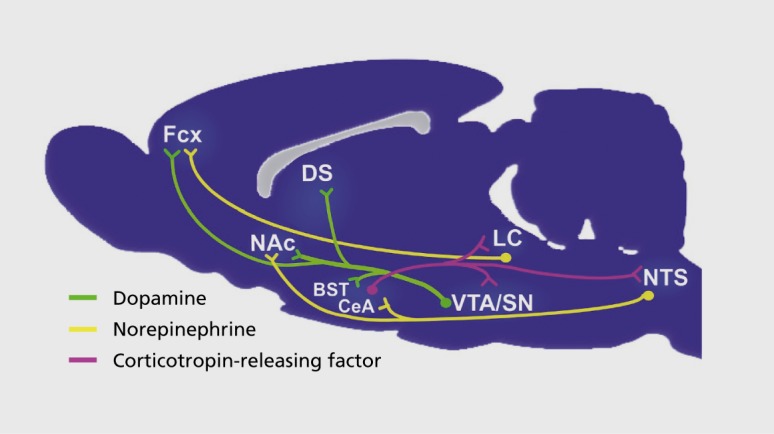
The reward system is necessary for animals and humans to engage in behaviors such as eating, drinking, or mating.7,49 Some of the key neurotransmitter projections of the reward system are illustrated schematically in Figure 2. Evidence for sex differences in the reward system has focused on the forebrain regions that receive input from neurons in the midbrain that use the neurotransmitter dopamine (DA).8 This review will focus on the nucleus accumbens (NAc) and the dorsal striatum (DS). The NAc is important for engaging in behaviors and learning that they are rewarding,50 whereas the striatum is involved in escalated drug taking and automatic or compulsive behaviors.51 DA in the NAc and DS is important for the development of craving or “wanting”; the endogenous opiates in the NAc are important for the pleasure or “liking.”4,5 The feeling of pleasure associated with a drug may be transient, but wanting is not.4,5 When use switches from being a casual pleasure to being avid and compulsive intake of the drug or food, the pattern of neural activation has shifted from the NAc to the DS.51 Thus, addiction is associated with greater DA activation in the DS and reduced DA activity in the NAc.52,53
Figure 2. A sagittal section of the rat brain depicting some of the neural systems involved in the reward system. Not shown are the glutamate projections from frontal cortex and other brain regions, as well as the GABAergic neurons and the enkaphalin and dynorphin neurons in the nucleus accumbens and dorsal striatum. BST, bed nucleus of the stria terminalis; CeA, central nucleus of the amygdala; DS, dorsal striatum; Fcx, frontal cortex; LC, locus coeruleus; NAc, nucleus accumbens; NTS, nucleus tractus solitarus; VTA/SN, ventral tegmental area/substantia nigra. Reproduced from ref 8: Becker JB, Perry AN, Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):14. Reproduced under the Creative Commons Attribution License. Copyright © 2012, Becker et al, licensee BioMed Central Ltd.
In the NAc and dorsolateral striatum (DLS), the activity of γ-aminobutyric acid (GABA)ergic medium spiny projection neurons is modulated by DA receptors B1 and D2 (D1DR and D2DR, respectively).54-56 DA release activates D1DR-containing medium spiny neurons in the direct pathway and inhibits D2DR-containing neurons in the indirect pathway, collectively resulting in appetitive/approach responses.57,58 Reduced DA release results in less inhibition of D2DR and attenuation of the ability to disrupt ongoing behavioral activities.59,60 The direct pathway is important for initiation of appetitive behaviors, and the indirect pathway inhibits competing repetitive behaviors.59,60 Within the striatum, plasticity in the direct and indirect pathways is thought to occur in opposite directions during addiction, with greater activation of the direct pathway driving motivated behaviors and reduced activity in the indirect pathway resulting in greater addiction-like behaviors, especially when the DLS becomes involved. This is referred to as “reciprocal plasticity,” and it has been found to be associated with acquisition of goal-directed behavior in the dorsomedial striatum.61
In the laboratory, self-administration or repeated treatment with drugs such as cocaine, amphetamine, or morphine produce long-term changes in the structure and function of the neurons in the reward system.12-14,62 Brain imaging studies in humans find DA release in the NAc is reduced in cocaine addicts,63 and similar results are found in animal studies using in vivo microdialysis.46,64 Next, the evidence indicating that sex differences in the organization of the reward system mediate important aspects of sex differences in addiction will be discussed.
Sex differences in the reward system
As noted above, there are sex differences in drug-taking behavior, and estradiol treatment is thought to mediate these effects in part. Estradiol has been shown to enhance the behavioral response to amphetamine and cocaine, as indicated by greater stereotypy, locomotion, and rotational behavior after OVX rats are treated with estradiol.65-70 In OVX female rats, estradiol enhances acquisition of, escalation of, motivation for, and reinstatement of drug-taking behavior. These effects are thought to be mediated by the action of estradiol in DLS, where estradiol treatment of OVX female rats enhances the amphetamine- or cocaine-induced increase of DA in females, but not in males.71,72 On the other hand, progesterone can attenuate the effect of estradiol to enhance acquisition of cocaine-taking behavior in female rats20,73 and attenuates cocaine intake in women.74,75
A greater behavioral response to amphetamine and cocaine by females than by males is only part of the sex difference in the DLS. The behaviors exhibited are also different, and the neural response to amphetamine, as indicated by c-fos activation, is sexually dimorphic.71,76 These findings suggest that the difference between males and females is not simply a quantitative difference, where females show a greater response than males. The sex differences in the response to cocaine and amphetamine may also reflect different unerlying neural mechanisms mediating the response to these drugs—a phenomenon referred to as a “divergent” sex difference.3
The increase in stimulated striatal DA release is a direct effect of estradiol on the striatum that is blocked by selected estradiol receptor antagonists.77-79 However, estradiol receptors are not found on the striatal DA terminals, but are found on medium spiny GABA projection neurons that have reciprocal collaterals onto the presynaptic DA terminals.80 These neuroanatomical results are consistent with patch-clamp electrophysiology showing that estradiol blocks L-type calcium channels in medium spiny striatal neurons and with in vivo microdialysis studies showing that estradiol attenuates potassium-stimulated striatal GABA release,81,82 Thus, the rapid effects of estradiol on stimulated striatal DA release are apparently indirectly mediated by an attenuation of GABAergic inhibition.
In the NAc, the cocaine-induced increase in DA in OVX females is lower than the cocaine-induced increase in DA from NAc of male rats, with or without estradiol treatment.71 A similar sex difference in rats has been reported in the NAc core DA response to amphetamine.83,84 In nicotine-addicted humans, the ventral striatal DA response to nicotine is greater in men than women.53 Thus, both the DLS and NAc exhibit different responses in males and females, and there are different processes mediating sex differences in DLS versus NAc regions. Interestingly, changes in glutamate function, associated with an addiction-like phenotype, are the same in males and females.85
Male rats with lower NAc DA are more impulsive and more likely to develop addiction-like behaviors.10,50 We find that, on average, females have lower NAc DA than males.71 If both males and females that are found to have lower NAc DA are at greater risk of developing cocaine self-administration behavior and addiction-like behavior, then the sex difference in NAc DA release induced by cocaine may reflect a population difference. In other words, if more females have this characteristic than males, then it appears that females as a group are more at risk; the overall sex difference reflects the greater number of females than males with lower NAc DA. This still needs to be empirically determined and is currently under investigation in the Becker laboratory.
Expression of striatal D1DR is 10% higher in males than in females, and although there are no sex differences in D2DR densities, there is a sexually dimorphic effect of estradiol on D2DR, where estradiol rapidly downregulates D2 binding in females, but not males.86 In cell culture, pretreatment with estradiol reduces D2DR inhibition of adenylate cyclase activity and enhances D1DR activation of adenylate cyclase,87,88 Additionally, estradiol treatment decreases expression of regulator of G-protein signaling 9-2 (RGS9-2), a GTPase-activating protein associated with D2DR signaling.89
It is proposed that females acquire the cocaine-taking behavior more rapidly than males and that they take more cocaine because there is less of an increase in DA/infusion in NAc. More cocaine is needed to achieve comparable increases in DA. We also suggest that the transition to compulsive drug taking in females is facilitated by enhanced DA transmission in DLS relative to NAc. It seems likely that in females, estradiol is involved in exacerbating the rate of escalation of drug taking by increasing the reinforcing effects of many drugs of abuse during the initial stages of acquisition and transition to addiction.
Implications for addiction treatment and other human conditions
These sex differences in the reward system and effects of estradiol on dopaminergic function in the DLS have implications for development of treatments for addiction, as well other psychiatric conditions. Even though estradiol enhances acquisition of drug taking and the transition to addictive-like behavior in rats, once animals are avidly self-administering cocaine, the estrous cycle does not modulate drug-taking behavior.44,46 This suggests that ovarian hormones may be helpful as adjunctive treatments early in the acquisition of drug-taking behavior, before compulsive behavior develops, when progesterone may attenuate some of the subjective effects of drugs of abuse and thereby decrease drug intake.20,75 Later in the addiction process, based on this model of addiction, drugs that restore the balance between the direct and indirect pathways in DS are most likely to be beneficial.
In terms of other disorders, it has long been noted that there are sex differences in schizophrenia, with women having later onset of symptoms and a slight increase in incidence associated with menopause,90,91 leading to the suggestion that estradiol is protective in schizophrenia. This may seem counterintuitive if estradiol enhances stimulated DA release in the DS, as schizophrenia is thought of as a disorder associated with hyperdopaminergic activity. Most studies of the role of ovarian hormones in schizophrenia, using animal models, have focused on the possible antipsychotic effects of high doses of estradiol.92,93 It should be noted that the effects of estradiol on drug taking and DA release discussed above are mediated by physiological doses of estradiol and that a high dose of estradiol downregulates presynaptic DA release,77 On the other hand, estradiol is not the only hormone secreted during the menstrual cycle, and progesterone may also conribute to sex differences in schizophrenia.90 Finally, we know that schizophrenia is not due solely to disordered DA function, and although the ovarian hormones may contribute to symptomatology in some way, it is yet to be determined whether it is modulation of DA function that mediates this effect.
Conclusions
Drug-taking behavior is thought to be initiated by the activation of the NAc and dorsomedial striatum, where the rewarding properties of the drug and the cues that predict the drug are first learned. It is proposed that the transition to compulsive drug taking is mediated by enhanced activation of DA release in DLS and by attenuation of the DA response in the NAc and dorsomedial striatum. Women exhibit more rapid escalation of drug taking to addiction, exhibit a greater withdrawal response with abstinence, and tend to exhibit relapse due to cue-induced craving more than men. In rodents, acquisition and escalation of drug taking, motivation for drugs of abuse, and relapse-like behaviors are greater in females than in males. Estradiol enhances these sex differences in females. In the drug-naive rat, there is a sex difference in the DA response in the NAc, with OVX females exhibiting a lower initial DA increase after cocaine treatment than castrated males. Estradiol treatment of OVX female rats enhances stimulated DA release in DLS, but not NAc, resulting in a sex difference in the balance between the NAc and DLS. It has been reported that when drug-taking behavior becomes habitual, enhanced DA release in the DLS and attenuated NAc DA release occur. The sex difference in the balance between these neural systems is proposed as a mechanism that mediates sex differences in addiction.
Acknowledgments
This work was supported by a grant from the National Institute on Drug Abuse to JBB (R01-DA-039952). The author declares no conflict of interest.
Selected abbreviations and acronyms
- D1DR
D1 dopamine receptor
- D2DR
D2 dopamine receptor
- DA
dopamine
- DLS
dorsolateral striatum
- DS
dorsal striatum
- NAc
nucleus accumbens
- OVX
ovariectomized
REFERENCES
- 1.McCarthy MM., Arnold AP., Ball GF., Blaustein JD., De Vries GJ. Sex differences in the brain: the not so inconvenient truth. J Neurosci. 2012;32(7):2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCarthy MM., Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker JB., McClellan M., Reed BG. Sociocultural context for sex differences in addiction. Addict Biol. 2016;21(5):1052–1059. doi: 10.1111/adb.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 5.Berridge KC., Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646–664. doi: 10.1016/j.neuron.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobzean SA., DeNobrega AK., Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 7.Becker JB., Taylor JR. Sex differences in motivation. In: Becker JB, Berkley K, Geary N, Hampson E, Herman JP, Young EA, eds. Sex Differences in the Brain: From Genes to Behavior. Oxford, UK: Oxford University Press; 2008:177–199. [Google Scholar]
- 8.Becker JB., Perry AN., Westenbroek C. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3(1):14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady KT., Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22(2):241–252. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 10.Deroche-Gamonet V., Belin D., Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305(5686):1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 11.Belin D., Mar AC., Dalley JW., Robbins TW., Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson TE., Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17(21):8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson TE., Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11(5):1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Robinson TE., Kolb B. Morphine alters the structure of neurons in the nucleus accumbens and neocortex of rats. Synapse. 1999;33(2):160–162. doi: 10.1002/(SICI)1098-2396(199908)33:2<160::AID-SYN6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 15.Tavares H., Martins SS., Lobo DS., Silveira CM., Gentil V., Hodgins DC. Factors at play in faster progression for female pathological gamblers: an exploratory analysis. J Clin Psychiatry. 2003;64(4):433–438. doi: 10.4088/jcp.v64n0413. [DOI] [PubMed] [Google Scholar]
- 16.Moran-Santa Maria MM., Flanagan J., Brady K. Ovarian hormones and drug abuse. Curr Psychiatry Rep. 2014;16(11):511. doi: 10.1007/s11920-014-0511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roth ME., Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology (Berl). 2004;172(4):443–449. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- 18.Lynch WJ. Sex differences in vulnerability to drug self-administration. Exp Clin Psychopharmacol. 2006;14(1):34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 19.Hu M., Crombag HS., Robinson TE., Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 20.Jackson LR., Robinson TE., Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 21.Lynch WJ., Roth ME., Mickelberg JL., Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacol Biochem Behav. 2001;68(4):641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 22.Perry AN., Westenbroek C., Becker JB. Impact of pubertal and adult estradiol treatments on cocaine self-administration. Horm Behav. 2013;64(4):573–578. doi: 10.1016/j.yhbeh.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roth ME., Casimir AG., Carroll ME. Influence of estrogen in the acquisition of intravenously self-administered heroin in female rats. Pharmacol Biochem Behav. 2002;72(1-2):313–318. doi: 10.1016/s0091-3057(01)00777-8. [DOI] [PubMed] [Google Scholar]
- 24.Hu M., Becker JB. Acquisition of cocaine self-administration in ovariectomized female rats: effect of estradiol dose or chronic estradiol administration. Drug Alcohol Depend. 2008;94(1-3):56–62. doi: 10.1016/j.drugalcdep.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reichel CM., Chan CH., Ghee SM., See RE. Sex differences in escalation of methamphetamine self-administration: cognitive and motivational consequences in rats. Psychopharmacology (Berl). 2012;223(4):371–380. doi: 10.1007/s00213-012-2727-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JA., Gowl BA., Westenbroek C., Clinton SM., Akil H., Becker JB. Effects of a selectively bred novelty-seeking phenotype on the motivation to take cocaine in male and female rats. Biol Sex Differ. 2011;2:3. doi: 10.1186/2042-6410-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westenbroek C., Perry AN., Becker JB. Pair housing differentially affects motivation to self-administer cocaine in male and female rats. Behav Brain Res. 2013;252:68–71. doi: 10.1016/j.bbr.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll ME., Batulis DK., Landry KL., Morgan AD. Sex differences in the escalation of oral phencyclidine (PCP) self-administration under FR and PR schedules in rhesus monkeys. Psychopharmacology (Berl). 2005;180(3):414–426. doi: 10.1007/s00213-005-2182-x. [DOI] [PubMed] [Google Scholar]
- 29.Lynch WJ., Taylor JR. Sex differences in the behavioral effects of 24-h/ day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29(5):943–951. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DC., Bennett SA., Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl). 1989;98(3):408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 31.Becker JB., Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29(1):36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosten TA., Gawin FH., Kosten TR., Rounsaville BJ. Gender differences in cocaine use and treatment response. J Subst Abuse Treat. 1993;10(1):63–66. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- 33.Hudson A., Stamp JA. Ovarian hormones and propensity to drug relapse: a review. Neurosci Biobehav Rev. 2011;35(3):427–436. doi: 10.1016/j.neubiorev.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Hogle JM., Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43(4):344–356. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 35.Devaud LL., Alele P., Ritu C. Sex differences in the central nervous system actions of ethanol. Crit Rev Neurobiol. 2003;15(1):41–59. doi: 10.1615/critrevneurobiol.v15.i1.20. [DOI] [PubMed] [Google Scholar]
- 36.Fox HC., Sofuoglu M., Morgan PT., Tuit KL., Sinha R. The effects of exogenous progesterone on drug craving and stress arousal in cocaine dependence: impact of gender and cue type. Psychoneuroendocrinology. 2013;38(9):1532–1544. doi: 10.1016/j.psyneuen.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feltenstein MW., Henderson AR., See RE. Enhancement of cue-induced reinstatement of cocaine-seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl). 2011;216(1):53–62. doi: 10.1007/s00213-011-2187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anker JJ., Carroll ME. Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology (Berl). 2010;208(2):211–222. doi: 10.1007/s00213-009-1721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fuchs RA., Evans KA., Mehta RH., Case JM., See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl). 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- 40.Feltenstein MW., See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89(2-3):183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swalve N., Smethells JR., Zlebnik NE., Carroll ME. Sex differences in reinstatement of cocaine-seeking with combination treatments of progesterone and atomoxetine. Pharmacol Biochem Behav. 2016;145:17–23. doi: 10.1016/j.pbb.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anker JJ., Carroll ME. Sex differences in the effects of allopregnanolone on yohimbine-induced reinstatement of cocaine seeking in rats. Drug Alcohol Depend. 2010;107(2-3):264–267. doi: 10.1016/j.drugalcdep.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larson EB., Carroll ME. Estrogen receptor β, but not, mediates estrogen's effect on cocaine-induced reinstatement of extinguished cocaine-seeking behavior in ovariectomized female rats. Neuropsychopharmacology. 2007;32(6):1334–1345. doi: 10.1038/sj.npp.1301249. [DOI] [PubMed] [Google Scholar]
- 44.Perry AN., Westenbroek C., Becker JB. The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS ONE. 2013;8(11):e79465. doi: 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belin D., Balado E., Piazza PV., Deroche-Gamonet V. Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry. 2009;65(10):863–868. doi: 10.1016/j.biopsych.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Perry AN., Westenbroek C., Jagannathan L., Becker JB. The roles of dopamine and α1-adrenergic receptors in cocaine preferences in female and male rats. Neuropsychopharmacology. 2015;40(12):2696–2704. doi: 10.1038/npp.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerstetter KA., Ballis MA., Duffin-Lutgen S., Carr AE., Behrens AM., Kippin TE. Sex differences in selecting between food and cocaine reinforcement are mediated by estrogen. Neuropsychopharmacology. 2012;37(12):2605–2614. doi: 10.1038/npp.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerstetter KA., Kippin TE. Impact of sex and gonadal hormones on cocaine and food reinforcement paradigms. J Addict Res Ther. 2011;S4(2):2963. doi: 10.4172/2155-6105.s4-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hone-Blanchet A., Fecteau S. Overlap of food addiction and substance use disorders definitions: analysis of animal and human studies. Neuropharmacology. 2014;85:81–90. doi: 10.1016/j.neuropharm.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 50.Belin D., Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57(3):432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 51.Willuhn I., Burgeno LM., Everitt BJ., Phillips PE. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc Natl Acad Sci USA. 2012;109(50):20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volkow ND., Fowler JS., Wang GJ., Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 53.Cosgrove KP., Wang S., Kim SJ., et al Sex differences in the brain's dopamine signature of cigarette smoking. J Neurosci. 2014;34(50):16851–16855. doi: 10.1523/JNEUROSCI.3661-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Owesson-White C., Belle AM., Herr NR., et al Cue-evoked dopamine release rapidly modulates D2 neurons in the nucleus accumbens during motivated behavior. J Neurosci. 2016;36(22):6011–6021. doi: 10.1523/JNEUROSCI.0393-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Centonze D., Picconi B., Baunez C., et al Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology. 2002;26(2):164–175. doi: 10.1016/S0893-133X(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 56.Surmeier DJ., Ding J., Day M., Wang Z., Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30(5):228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson SM., Eskenazi D., Ishikawa M., et al Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14(1):22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soares-Cunha C., Coimbra B., Sousa N., Rodrigues AJ. Reappraising striatal D1- and D2-neurons in reward and aversion. Neurosci Biobehav Rev. 2016;68:370–386. doi: 10.1016/j.neubiorev.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 59.Vicente AM., Galvão-Ferreira P., Tecuapetla F., Costa RM. Direct and indirect dorsolateral striatum pathways reinforce different action strategies. Curr Biol. 2016;26(7):R267–R269. doi: 10.1016/j.cub.2016.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gerfen CR., Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shan Q., Ge M., Christie MJ., Balleine BW. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci. 2014;34(28):9196–9201. doi: 10.1523/JNEUROSCI.0313-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor JR., Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4- methylenedioxymethamphetamine (“ecstasy”). Biol Psychiatry. 2001;50(2):137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 63.Moeller SJ., Tomasi D., Honorio J., Volkow ND., Goldstein RZ. Dopaminergic involvement during mental fatigue in health and cocaine addiction. Transl Psychiatry. 2012;2:e176. doi: 10.1038/tp.2012.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Calipari ES., Ferris MJ., Jones SR. Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem. 2014;128(2):224–232. doi: 10.1111/jnc.12452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peris J., Decambre N., Coleman-Hardee ML., Simpkins JW. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated striatal [3H]dopamine release. Brain Res. 1991;566(1-2):255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 66.Schultz KN., von Esenwein SA., Hu M., et al Viral vector-mediated overexpression of estrogen receptor-α in striatum enhances the estradiol-induced motor activity in female rats and estradiol-modulated GABA release. J Neurosci. 2009;29(6):1897–1903. doi: 10.1523/JNEUROSCI.4647-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker JB., Molenda H., Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Ann N Y Acad Sci. 2001;937:172–187. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 68.Sell SL., Thomas ML., Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67(3):281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 69.Hu M., Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23(2):693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 1990;118(2):169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 71.Cummings JA., Jagannathan L., Jackson LR., Becker JB. Sex differences in the effects of estradiol in the nucleus accumbens and striatum on the response to cocaine: neurochemistry and behavior. Drug Alcohol Depend. 2014;135:22–28. doi: 10.1016/j.drugalcdep.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Castner SA., Xiao L., Becker JB. Sex differences in striatal dopamine: in vivo microdialysis and behavioral studies. Brain Res. 1993;610(1):127–134. doi: 10.1016/0006-8993(93)91225-h. [DOI] [PubMed] [Google Scholar]
- 73.Larson EB., Anker JJ., Gliddon LA., Fons KS., Carroll ME. Effects of estrogen and progesterone on the escalation of cocaine self-administration in female rats during extended access. Exp Clin Psychopharmacol. 2007;15(5):461–471. doi: 10.1037/1064-1297.15.5.461. [DOI] [PubMed] [Google Scholar]
- 74.Evans SM. The role of estradiol and progesterone in modulating the subjective effects of stimulants in humans. Exp Clin Psychopharmacol. 2007;15(5):418–426. doi: 10.1037/1064-1297.15.5.418. [DOI] [PubMed] [Google Scholar]
- 75.Evans SM., Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31(3):659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 76.Castner SA., Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712(2):245–257. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- 77.Becker JB. Direct effect of 17 β-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5(2):157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- 78.Xiao L., Becker JB. Effects of estrogen agonists on amphetamine-stimulated striatal dopamine release. Synapse. 1998;29(4):379–391. doi: 10.1002/(SICI)1098-2396(199808)29:4<379::AID-SYN10>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 79.Xiao L., Jackson LR., Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77(4):239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- 80.Almey A., Milner TA., Brake WG. Estrogen receptor and G-protein coupled estrogen receptor 1 are localized to GABAergic neurons in the dorsal striatum. Neurosci Lett. 2016;622:118–123. doi: 10.1016/j.neulet.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mermelstein PG., Becker JB., Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16(2):595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu M., Watson CJ., Kennedy RT., Becker JB. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59(2):122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- 83.Virdee K., McArthur S., Brischoux F., et al Antenatal glucocorticoid treatment induces adaptations in adult midbrain dopamine neurons, which underpin sexually dimorphic behavioral resilience. Neuropsychopharmacology. 2014;39(2):339–350. doi: 10.1038/npp.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gillies GE., Virdee K., McArthur S., Dalley JW. Sex-dependent diversity in ventral tegmental dopaminergic neurons and developmental programing: a molecular, cellular and behavioral analysis. Neuroscience. 2014;282:69–85. doi: 10.1016/j.neuroscience.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Doyle SE., Ramôa C., Garber G., Newman J., Toor Z., Lynch WJ. A shift in the role of glutamatergic signaling in the nucleus accumbens core with the development of an addicted phenotype. Biol Psychiatry. 2014;76(10):810–815. doi: 10.1016/j.biopsych.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bazzett TJ., Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637(12):163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 87.Maus M., Bertrand P., Drouva S., et al Differential modulation of D1 and D2 dopamine-sensitive adenylate cyclases by 17β-estradiol in cultures striatal neurons and anterior pituitary cells. J Neurochem. 1989;52(2):410–418. doi: 10.1111/j.1471-4159.1989.tb09136.x. [DOI] [PubMed] [Google Scholar]
- 88.Maus M., Cordier J., Glowinski J., Premont J. 17β-Oestradiol pretreatment of mouse striatal neurons in culture enhances the responses to adenylate cyclase sensitive tobiogenic amines. Eur J Neurosci. 1989;1(2):154–161. doi: 10.1111/j.1460-9568.1989.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 89.Silverman JL., Koenig Jl. Evidence for the involvement of ERβ and RGS9-2 in 17-β estradiol enhancement of amphetamine-induced place preference behavior. Horm Behav. 2007;52(2):146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun J., Walker AJ., Dean B., van den Buuse M., Gogos A. Progesterone: the neglected hormone in schizophrenia? A focus on progesterone-dopamine interactions. Psychoneuroendocrinology. 2016;74:126–140. doi: 10.1016/j.psyneuen.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 91.Gur RE., Petty RG., Turetsky BI., Gur RC. Schizophrenia throughout life: sex differences in severity and profile of symptoms. Schizophr Res. 1996;21(1):1–12. doi: 10.1016/0920-9964(96)00023-0. [DOI] [PubMed] [Google Scholar]
- 92.Arad M., Weiner I. Sex-dependent antipsychotic capacity of 17(3-estradiol in the latent inhibition model: a typical antipsychotic drug in both sexes, atypical antipsychotic drug in males. Neuropsychopharmacology. 2010;35(11):2179–2192. doi: 10.1038/npp.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Häfner H., Behrens S., De Vry J., Gattaz WF. An animal model for the effects of estradiol on dopamine-mediated behavior: implications for sex differences in schizophrenia. Psychiatry Res. 1991;38(2):125–134. doi: 10.1016/0165-1781(91)90038-q. [DOI] [PubMed] [Google Scholar]